Abstract
GH plays a major role in the regulation of lipid metabolism and alterations in GH axis elicit major changes in fat distribution and mobilization. For example, in patients with GH deficiency (GHD) or in mice lacking the GH receptor, the percentage of fat is increased. In addition to the direct actions of GH on lipid metabolism, current evidence indicates that ghrelin, a stomach-derived peptide hormone with potent GH secretagogue action, increases lipogenesis in white adipose tissue (WAT) through a hypothalamic-mediated mechanism. Still, the mechanism by which GH tone modulates ghrelin actions on WAT remains unclear. Here we investigated the effect of central ghrelin administration on lipid metabolism in lipogenic tissues (liver and WAT) in the absence of GH, by using a model for the study of GHD, namely the spontaneous dwarf rat, which shows increased body fat. Our data demonstrate that central chronic ghrelin administration regulates adipose lipid metabolism, mainly in a GH-independent fashion, as a result of increased mRNA, protein expression, and activity levels of fatty acid metabolism enzymes. On the contrary, central ghrelin regulates hepatic lipogenesis de novo in a GH-independent fashion but lipid mobilization in a GH-dependent fashion because carnitine palmitoyltransferase 1 was decreased only in wild-type Lewis rats. These findings suggest the existence of a new central nervous system-based neuroendocrine circuit, regulating metabolic homeostasis of adipose tissue. Understanding the molecular mechanism underlying the interplay between GH and ghrelin and their effects on lipid metabolism will provide new strategies for the design and development of suitable drugs for the treatment of GHD, obesity, and its comorbidities.
Central ghrelin favors lipid storage in a GH-independent mode in fat and liver, induces changes in lipid oxidation in a GH-independent fashion in fat, and in a GH-dependent fashion in liver.
The metabolic processes controlled by GH are multiple and complex, and its effects on body composition and intermediary metabolism have been known for many years. GH plays a major role in the regulation of lipid metabolism, and alterations in GH axis elicit major changes in fat distribution and mobilization. This is the reason that patients with GH deficiency (GHD) display increased percentage of fat, which has been recognized as a clinical hallmark that rapidly disappears during the early months of treatment with GH. The adverse lipid profile in subjects with GHD and the mortality associated with this altered lipid profile is the risk factor that has probably attracted most attention in recent years. GHD is associated with conditions related to hyperlipidemia, increased body weight, abnormal body composition, and fat accumulation, and GH replacement in these patients has demonstrated beneficial on cardiovascular risk factors (1,2,3,4,5,6,7).
Ghrelin is a 28-residue peptide hormone from the stomach and acts as the endogenous ligand to GH secretagogue receptor (GHS-R) (8,9,10,11), which is expressed in the brain and peripheral tissues (12). In addition to its role as a stimulator of GH release, ghrelin promotes feeding in humans and rodents, which results in increased body weight and adiposity (11,13,14,15,16). The effects of ghrelin on feeding behavior are believed to be mediated at the level of the hypothalamus by a mechanism involving hypothalamic AMP-activated protein kinase (AMPK), lipid metabolism (Fig. 1A), uncoupling protein-2, and neuropeptide gene expression (17,18,19). In addition, recent evidence has demonstrated that central administration of ghrelin directly increases adiposity by stimulation of the lipogenic program in the white adipose tissue (WAT), via the sympathetic nervous system, in a food intake-independent fashion (16,20). More specifically central ghrelin administration induced the mRNA expression of various fat storage-promoting enzymes in WAT, such as lipoprotein lipase, acetyl-CoA carboxylase (ACC)-α, fatty acid synthase (FAS), and stearoyl-CoA desaturase (SCD)-1, whereas that of the rate-limiting step in fat oxidation, carnitine palmitoyltransferase 1 (CPT1), was decreased (16). This evidence indicates that central ghrelin action is of physiological relevance in the control of adipocyte metabolism and suggests that ghrelin could trigger meal preparation processes in the central nervous system (CNS), preparing metabolic pathways that would lead to a more efficient storage of calories.
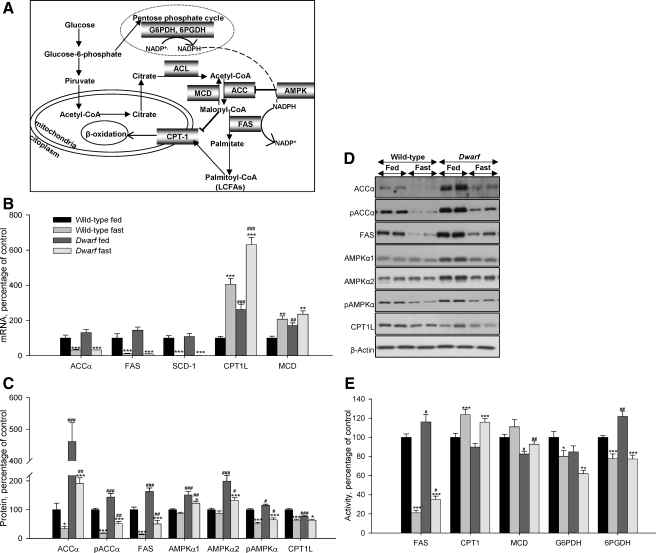
Figure 1.
Squematic representation of the synthesis and oxidation of fatty acids, ACL: ATP-citrate lyase (A), hepatic mRNA (B), protein (C and D), and activity levels (E) of lipid metabolism-related enzymes in fed and fasted rats. Values are means ± sem of eight animals per group. *, **, ***, P < 0.05, 0.01, and 0.001, respectively, vs. fed. #, ##, ###, P < 0.05, 0.01, and 0.001, respectively, vs. wild-type Lewis. ACL, ATP-citrate lyase.
Despite this evidence that links GH axis and ghrelin to lipid metabolism, the relevance of the GH tone on the lipogenic effect of ghrelin remains unclear. Although several studies in GH-deficient rats have demonstrated that weight gain and adiposity caused by ghrelin are independent of its ability to modulate GH secretion (11,13,15,21), GH receptor deficiency blunts the stimulatory effects of ghrelin on feeding in mice (22). The aim of this study was to investigate the effect of central ghrelin administration on lipid metabolism in major lipogenic tissues, such as liver and adipose tissue, in absence of GH. We used the spontaneous dwarf rat, which is a classical model for the study of GHD (23,24). Our data show that central ghrelin regulates adipocyte lipid metabolism in a GH-independent fashion, whereas central ghrelin regulates hepatic lipid mobilization in a GH-dependent fashion. These findings suggest the existence of a new CNS-based neuroendocrine circuit regulating metabolic homeostasis of adipose tissue.
Materials and Methods
Animals
We used two male rat models, wild-type (controls) and GH-deficient (spontaneous dwarf rat) Lewis rats (2–3 months old; body weight 365 g ± 4 and 222 g ± 5 g, respectively; Harlan, Bicester, UK). Rats were housed in a temperature-controlled room, with a 12-h light, 12-h dark cycle (lights from 0800 to 2000 h). All experiments and procedures involved in this study were reviewed and approved by the Ethics Committee of the University of Santiago de Compostela, in accordance with European Union Normative for the use of experimental animals.
Fasting experiment
To study hepatic and fat metabolism in a natural situation with high levels of ghrelin and absence/presence of GH, wild-type and GH-deficient rats were fasted during 48 h.
Infusion of ghrelin into lateral ventricle in wild-type and GH-deficient Lewis rats
To assess chronic effects of intracerebroventricular (ICV) ghrelin on epididymal WAT and hepatic metabolism in presence and absence of GH, normal and GH-deficient rats were infused with saline as vehicle (controls) or acyl-ghrelin, 20 μg/d for 8 d.
Implantation of intracerebroventricular (ICV) cannulae
Chronic ICV cannulae were implanted under ketamine/xylazine anesthesia as previously described (17,25). The correct location of the cannulae in the lateral ventricle was confirmed by methylene blue staining. Animals were individually caged and allowed to recover for 1 wk before the experiment. During the postoperative recovery period, the rats were handled regularly under nonstressful conditions.
Chronic ghrelin treatment
Brain infusion cannulae were stereotaxically placed into the lateral ventricle as described above. A catheter tube was connected from the brain infusion cannulae to an osmotic minipump flow moderator (model 2001D or 2ML2; Alzet Corp., Palo Alto, CA). An sc pocket on the dorsal surface of the animal was created using blunt dissection, and the osmotic minipump was inserted. The incision was closed with sutures, and the rats were kept warm until fully recovered. The rats were then infused with either vehicle alone (saline) or vehicle containing acyl-ghrelin (Bachem, Bubendorf, Switzerland; catalog no. H-4864), the pumps released the solutions at a rate of 1 μl/h and 20 μg ghrelin/d. Animals were treated during 8 d.
Acute ghrelin treatment
To study the influence of the acylation state on food intake, wild-type rats were treated with a single ICV injection of 5 μl of either saline or 5 μg acyl-ghrelin and/or 5 μg desacyl-ghrelin (Bachem; catalog no. H-5946). Food intake was measured during 6 h.
Tissue dissection
Rats were killed by cervical dislocation and trunk blood was extracted. The following tissues were dissected and weighed: liver, brown adipose tissue, and visceral, retroperitoneal, omental, and epididymal WAT and somatic index were calculated. Samples were stored at −80 C until further processing and parameters measurement.
Plasma measurements
Plasma total ghrelin and insulin levels were measured by RIA as described previously (17,25) using reagents provided in commercial kits (catalog no. GHRT-89K and RI-13K, respectively; Linco Research Inc., St. Charles, MO). Plasma glucose and triglyceride levels were assessed using a commercial kit based on a colorimetric method (Glucose and Triglyceride Spinreact, Spain).
Real-time quantitative PCR
Expression of mRNA levels of ACCα, CPT1M (muscle type isoform), and CPT1L (liver type isoform), FAS, SCD-1, and malonyl-CoA decarboxylase (MCD) in liver and epididymal WAT were studied by using real-time PCR (TaqMan; Applied Biosystems, Foster City, CA) by using specific primers and probes (supplemental Table S1). All reactions were carried out using the following cycling parameters: 50 C for 2 min, 95 C for 10 min followed by 40 cycles of 95 C for 15 sec, 60 C for 1 min (17,25). For data analysis, the input value of the target gene was standardized to the 18S value for each sample. Data were expressed in comparison with the average value for the vehicle treated rats (control group). We used eight rats per group.
Western blotting
Total protein lysates from liver (20 μg) and epididymal WAT (15 μg) were subjected to SDS-PAGE, electrotransferred on a polyvinylidene difluoride membrane and probed with the indicated antibodies: ACC, phospho-ACC-Ser79 (pACC), AMPKα1 and AMPKα2 (Upstate, Lake Placid, NY); phospho-AMPKα-Thr172 (pAMPKα) (Cell Signaling, Danvers, MA); β-actin (Abcam, Cambridge, UK); CPT1M, FAS, and CPT1L (Santa Cruz Biotechnology, Santa Cruz, CA). For protein detection we used horseradish peroxidase-conjugated secondary antibodies and chemiluminescence (Amersham Biosciences, Little Chalfont, UK). We used eight rats per group and the protein levels were normalized to β-actin for each sample.
Enzyme assays
Tissue samples were homogenized in 10 volumes (liver) or four volumes (adipose tissue) ice-cold buffer: 20 mm Tris-HCl (pH 7.4), 250 mm sucrose, 1 mm EDTA, 1 mm dithiothreitol, 100 mm NaF, and protease inhibitor cocktail (Roche, Stockholm, Sweden). Enzyme activities of FAS, MCD, CPT1, glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH) were determined by spectrophotometry using a microplate reader (Tecan, Sunrise, Switzerland). The reactions were started by the addition of homogenates (30 μl) and substrates (20 μl, omitted in controls) to the reaction mixture (final volume 0.25 ml) and allowing the reactions to proceed at 37 C for preestablished times (5–15 min). FAS (26), G6PDH, 6PGDH (27,28), CPT1 (17,25,29,30), and MCD (31) activities were measured using methods previously described. ACC activity was assayed using an isotopic method (32) by 14CO2 fixation to acid-stable products.
Malonyl-CoA assay
Malonyl-CoA levels were assessed radioenzymatically by a modification of the method of McGarry et al. (33) as described previously (17,34).
Statistical analysis
Date were expresses as percentage of wild-type fed rats or wild type infused with saline (control groups). Data were expressed as mean ± sem. Statistic significance was determined by two-way ANOVA and post hoc Tukey test. P < 0.05 was considered significant.
Results
Effects of fasting on plasma levels
Plasma parameters are shown in supplemental Table S2. In 48-h-fasted wild-type Lewis rats, plasma ghrelin levels increased by 80% when compared with the fed group, whereas in the dwarf group, the levels were increased by just 40%. In the fed normal state, plasma insulin levels in dwarf rats were lower than in normal rats; GH-deficient rats exhibited normoglycemia compared with wild-type Lewis. After 48 h of fasting, plasma insulin, glucose, and triglyceride levels diminished in both animal models.
Effects of fasting on liver lipid metabolism
Gene expression, protein, and activity levels of key enzymes involved in the regulation of lipid metabolism in liver of fed and food-deprived Lewis and dwarf rats are shown in Fig. 1, B–E. As expected, fasting markedly diminished mRNA levels of the fat storage-promoting enzymes, such as ACCα, FAS, and SCD-1 and enhanced mRNA levels of those involved in fatty acid degradation, such as CPT1L and MCD, which were higher in fed dwarf rats compared with fed normal Lewis rats (Fig. 1B). After 48 h of fasting, the protein levels of pAMPKα, pACCα, ACCα, CPT1L, and FAS significantly diminished in normal and GH-deficient rats; however, protein levels of AMPKα1 and AMPKα2 significantly diminished only in the dwarf group. Protein levels of ACCα and FAS were higher in fed dwarf rats, whereas protein levels of CPT1L were lower in this model compared with their controls (Fig. 1, C and D). In normal and GH-deficient rats, FAS, G6PDH, and 6PGDH activities decreased after fasting, whereas the activities of enzymes involved in fatty acid degradation were enhanced. Anew the activity of enzymes related with lipogenesis, such as FAS and 6PGDH, was increased in fed dwarf rats compared with their controls (Fig. 1E).
Effects of fasting on WAT lipid metabolism
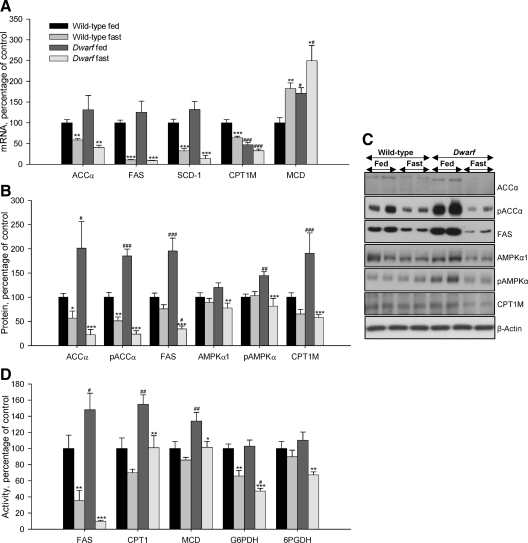
We studied the fasting-induced effect on key enzymes of lipid metabolism in wild-type and GH-deficient Lewis rats. The results are shown in Fig. 2, A–D. As expected, food deprivation clearly diminished mRNA levels of ACCα, FAS, CPT1M, SCD-1, and increased mRNA levels of MCD (Fig. 2A). After fasting, protein levels of pAMPKα and AMPKα1 significantly diminished in GH-deficient rats but not normal rats, whereas protein levels of ACCα, pACCα, FAS, and CPT1M significantly diminished in both models of rats, although the decrease was more striking in dwarf rats. In all cases the protein levels of these enzymes were higher in fed dwarf rats compared with their controls (Fig. 2, B and C). FAS, G6PDH, 6PGDH, CPT1, and MCD activities were lower after 48 h of food deprivation in both animal models, although in normal rats only FAS and G6PDH activities decreased significantly. The activity of FAS, CPT1, and MCD was higher in fed dwarf rats compared with their controls (Fig. 2D).
Figure 2.
Epididymal WAT mRNA (A), protein (B and C), and activity levels (D) of lipid metabolism-related enzymes in fed and fasted Lewis rats. Values are expressed as mean ± sem. *, **, ***, P < 0.05, 0.01, and 0.001, respectively, vs. fed. #, ##, ###, P < 0.05, 0.01, and 0.001, respectively, vs. wild-type Lewis.
Effects of central ghrelin treatment on food intake and body weight gain in wild-type Lewis rats
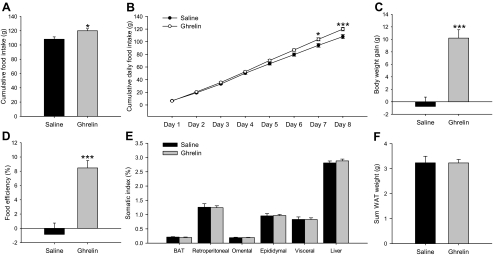
To throw some light on the mechanism involved in fasting-induced changes in lipid metabolism in normal and GH-deficient rats, we assessed the central effects of ghrelin in both experimental models. As expected chronic ICV ghrelin treatment increased food intake (Fig. 3, A and B), body weight gain (Fig. 3C), and food efficiency (Fig. 3D) as well as percent omental and visceral WAT (Fig. 3E) during the 8-d experimental period in wild-type Lewis rats in comparison with their saline-treated controls.
Figure 3.
Effect of an 8-d ICV ghrelin treatment on cumulative food intake (A), cumulative daily food intake (B), body weight gain (C), food efficiency (D), somatic index (E), and sum of retroperitoneal, omental, epididymal, and visceral adipose tissue (F) in wild-type Lewis rats. Values are expressed as mean ± sem. *, **, ***, P < 0.05, 0.01, and 0.001, respectively, vs. saline. Somatic index was calculated as the ratio between tissue weight and body weight and was expressed as a percentage. Food efficiency was calculated as the ratio between body weight gain over the 8-d experimental period and cumulative food intake and was expressed as a percentage.
Effects of central ghrelin treatment on plasma parameters in wild-type Lewis rats
As previously reported (16), ICV ghrelin administration elicited an increase in plasma ghrelin levels in wild-type Lewis rats (supplemental Table S3). On the other hand, plasma insulin, glucose, and triglyceride levels were unchanged in wild-type Lewis rats (supplemental Table S3).
Effects of central ghrelin treatment on liver lipid metabolism in wild-type Lewis rats
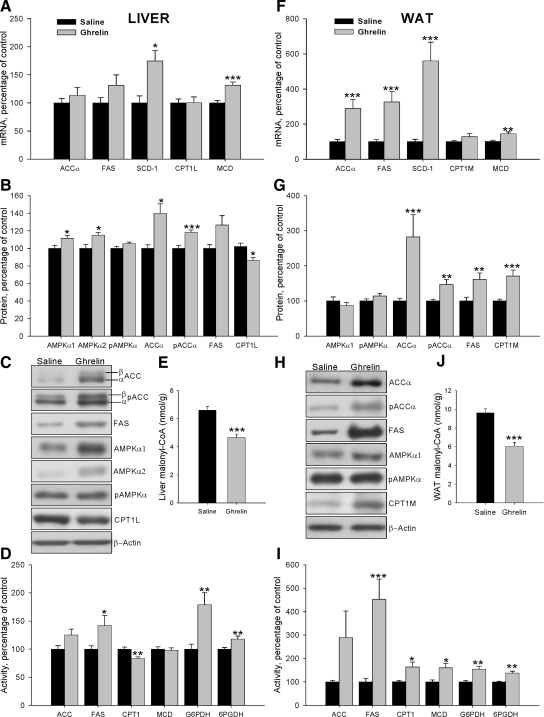
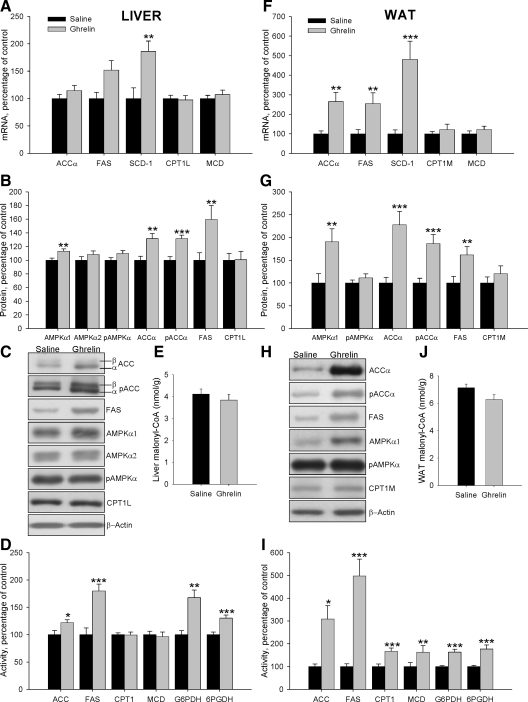
To assess the central effect of ghrelin on hepatic lipogenesis, mRNA, protein, and activity levels of enzymes involved in synthesis and oxidation of lipids were measured in wild-type Lewis rats (Fig. 4, A–D). Chronic ICV ghrelin infusion significantly increased SCD-1 mRNA levels (Fig. 4A). Protein levels of AMPKα1, AMPKα2, pACCα, and ACCα were also significantly increased after the ghrelin treatment (Fig. 4, B and C). Similar results were seen in transcript and protein levels of FAS, although it did not reach statistical significance. However, the treatment induced an increase in FAS, G6PDH, and 6PGDH activities, suggesting an increased lipogenesis de novo (Fig. 4D). On the contrary, CPT1 protein and activity levels were reduced by ghrelin infusion (Fig. 4, B–D). In keeping with, the high levels of FAS activity, malonyl-CoA was decreased in ghrelin-treated rats (Fig. 4E).
Figure 4.
Effect of an 8-d ICV ghrelin treatment on hepatic and WAT mRNA (A and F), protein (B and C and G and H) and activity levels (D and I) of lipid metabolism-related enzymes and malonyl-CoA levels (E and J) in wild-type Lewis rats. Values are expressed as mean ± sem. *, **, ***, P < 0.05, 0.01, and 0.001, respectively, vs. saline.
Effects of central ghrelin treatment on WAT lipid metabolism in wild-type Lewis rats
To study the effects of chronic ICV ghrelin treatment on WAT lipid metabolism, mRNA, protein, and activity levels of the enzymes involved in both synthesis and lipid oxidation were assessed. Ghrelin treatment markedly enhanced mRNA levels of the fat storage-promoting enzymes as ACCα, FAS, and SCD1 as well as MCD expression (Fig. 4F). These results were confirmed by Western blotting, with ICV ghrelin infusion enhancing protein levels of ACCα, pACCα, FAS, and CPT1M (Fig. 4, G and H). The activity of those enzymes, as well as G6PDH and 6PGDH, was significantly increased in ghrelin-treated rats (Fig. 4I), suggesting a higher lipogenesis rate, which is also supported by increased triglyceride content in the WAT of ghrelin-treated animals (vehicle: 110.12 ± 8.52 μmol/g tissue vs. 229.64 ± 15.16 μmol/g tissue: P < 0.001). In line with the increased FAS and MCD activities in ghrelin-treated rats, malonyl-CoA levels were markedly decreased (Fig. 4J), which consequently increased CPT1 activity in ghrelin-treated rats (Fig. 4I).
Effects of central ghrelin treatment on food intake and body weight gain in GH-deficient Lewis rats
As it happened in normal rats, chronic ICV ghrelin treatment increased food intake (Fig. 5, A and B), body weight (Fig. 5C), and food efficiency (Fig. 5D) during the 8-d experimental period in dwarf rats when compared with their saline-treated controls. However, ghrelin did not change the total adipose tissue mass of dwarf rats (Fig. 5, E and F).
Figure 5.
Effect of an 8-d ICV ghrelin treatment on cumulative food intake (A), cumulative daily food intake (B), body weight gain (C), food efficiency (D), somatic index (E), and sum of retroperitoneal, omental, epididymal, and visceral adipose tissue (F) in dwarf rats. Values are expressed as mean ± sem. *, **, ***, P < 0.05, 0.01, and 0.001, respectively, vs. saline.
Effects of central ghrelin treatment on plasma parameters in GH-deficient Lewis rats
ICV ghrelin administration elicited an increase in plasma ghrelin, insulin and glucose levels in GH-deficient Lewis rats. However, triglyceride levels diminished in these animals after ghrelin treatment (supplemental Table S3); these findings agree with other published results (16).
Effects of central ghrelin treatment on liver lipid metabolism in GH-deficient Lewis rats
To assess the central effect of ghrelin on hepatic lipogenesis in absence of GH, mRNA, protein, and activity levels of enzymes involved in synthesis and oxidation of lipids were measured in GH-deficient Lewis rats (Fig. 6, A–D). Chronic ICV ghrelin infusion significantly increased mRNA levels for SCD1 (Fig. 6A). Similarly to normal rats, central ghrelin treatment also induced an increase in the protein levels of AMPKα1, pACCα, ACCα, and FAS (Fig. 6, B and C) as well as an increase in the activity of ACC, FAS, G6PDH, and 6PGDH (Fig. 6D). Overall, these data suggest that central ghrelin action on hepatic lipid metabolism is independent of GH tone. On the other hand, CPT1 protein and activity (Fig. 6, B–D) and malonyl-CoA content (Fig. 6E) did not change in dwarf rats after ghrelin administration.
Figure 6.
Effect of an 8-d ICV ghrelin treatment on hepatic and WAT mRNA (A and F), protein (B and C and G and H), and activity levels (D and I) of lipid metabolism-related and malonyl-CoA levels (E and J) in dwarf rats. Values are expressed as mean ± sem. *, **, ***, P < 0.05, 0.01, and 0.001, respectively, vs. saline.
Effects of central ghrelin treatment on WAT lipid metabolism in GH-deficient Lewis rats
To study the effects of central ghrelin treatment on adipose lipid metabolism in absence of GH, mRNA, protein, and activity levels of the enzymes involved in both synthesis and lipid oxidation were assessed in GH-deficient rats (Fig. 6, F–I). Ghrelin treatment enhanced mRNA levels of the fat storage-promoting enzymes as ACCα, FAS, and SCD1 (Fig. 6F). In GH-deficient rats, ICV ghrelin infusion enhanced protein levels of AMPKα1, ACCα, pACCα, and FAS (Fig. 6, G and H). The activity levels of these enzymes, G6PDH and 6PGDH as well as CPT1 and MCD, significantly increased in ghrelin-treated rats (Fig. 6I); in keeping with these observations, triglyceride levels in the adipose tissue of dwarf rats treated with ghrelin have shown a tendency to be up-regulated (vehicle: 300.42 ± 39.71 vs. 401.06 ± 69.71 μmol/g tissue: P = 0.1). Finally, malonyl-CoA levels did not change after central ghrelin treatment in dwarf rats.
Discussion
GH plays a major role in the regulation of lipid metabolism, and impairment in the GH axis elicits major changes in glucose and lipid metabolism. GH-deficient patients (35) and GH receptor knockout mice (GHR-KO) (36,37,38,39,40) display increased insulin sensitivity, insulin secretion, and fasting glucose concentrations and increased fat mass. On the contrary, in conditions of GH excess, such as acromegaly (41) and after GH administration in GH-deficient adults (42,43), insulin antagonistic actions of GH are well described. The present study shows that in GH-deficient rats, lipogenic enzymes are enhanced compared with normal Lewis rats, consistent with the hypothesis that GH decreases adipose tissue accretion (44). Our results provide for first time a clear demonstration that chronic central ghrelin treatment provokes GH-independent up-regulation of fat storage-promoting enzymes in liver and WAT. However, the activity of CPT1, the key enzyme modulating fatty acid oxidation, is enhanced after central ghrelin infusion in a GH-independent fashion in WAT. However, activation of the central ghrelin system specifically decreases hepatic CPT1 activity in wild-type Lewis rats but not the liver of dwarf rats, suggesting GH dependency. Furthermore and contrary to the hypothalamus (17), the present findings indicate that in peripheral tissues the increased ghrelin levels during food deprivation do not mediate the effects of fasting. In these tissues, starvation downregulates the expression of lipogenic enzymes and activates (in liver) or down-regulates (in WAT) CPT1, which are opposite effects to those observed after the ghrelin treatment.
Our results show that after 48 h of fasting, the levels of mRNA, protein, and activity of enzymes related to lipid synthesis were reduced in both liver and WAT. A reduction in the pentose phosphate pathway (based on 6PDGH and G6PDH) was also observed, in accordance with the reduction in the de novo lipogenesis (45,46). To further investigate the role of GH on lipid metabolism, we assayed the levels of AMPK. Our data demonstrate that the hepatic levels of pAMPK are decreased after food deprivation in wild-type Lewis rats, whereas in GH-deficient rats the levels of both active and total protein drop in liver and WAT after 48 h of food deprivation. There are many studies linking food deprivation/restriction and AMPK, and the data are controversial. Several reports have shown that AMPK is increased by fasting and decreased by refeeding (47,48). Contrary, Foretz et al. (49) observed that the overexpression of a constitutively active form of AMPK in liver markedly attenuates increases in the mRNA of lipogenic enzymes, but they did not find a decrease in AMPK activity during refeeding. When mice overexpressing GH and mice lacking GH receptor were subjected to long-term caloric restriction, protein levels of pAMPK were unaffected (40,50). Other studies reported a down-regulation of pAMPK induced by caloric restriction in rat liver, no change in the fed-fasted cycle in normal and transgenic dwarf rats (51) and AMPK activation in rat liver in normal rat fasted by 24 h (52). The exact reasons for these discrepancies between the present study and those previous reports are unclear, although we hypothesize that the phosphorylation of AMPK may be dependent on time, age, species, and duration of the fasting. Further studies are needed to clarify this hypothesis.
Ghrelin is an orexigenic gastrointestinal peptide (19,53,54,55,56) that potently induces GH release (57). Ghrelin binds to the GH secretagogue receptor, which is present in not only the hypothalamus and the pituitary gland but also many other organs and tissues, indicating that ghrelin may also elicit peripheral, GH-independent effects (58). Recent evidence has highlighted that ghrelin acts in the hypothalamus modulating lipid metabolism in peripheral tissues, particularly in the WAT (16). There are several studies highlighting the importance of GH signaling on the effect of ghrelin on metabolism. Although some data from GH-deficient rats have demonstrated that weight gain and adiposity caused by ghrelin are independent of its ability to modulate GH secretion (11,13,15,21), ICV ghrelin treatment did not increase food intake in GH receptor gene-deficient mice (22), and ghrelin failed to increase the expression of GH secretagogue receptor in the hypothalamic arcuate nucleus of dwarf rats (59). Our aim was to determine whether ghrelin’s chronic effects on food intake, body weight, and synthesis and oxidation pathways of lipids are GH independent. Our data show that central ghrelin treatment enhanced body weight, food intake, and food efficiency and increased transcript, protein, and activity levels of enzymes related with lipid synthesis in WAT and to a lesser extent in liver. In previous papers it has been reported that central ghrelin infusion enhances transcript levels of lipogenic enzymes in adipose tissue and liver, but no activity data were shown in those studies (16,60). Here we demonstrated that in addition to mRNA and protein levels, the activity of lipogenic enzymes was increased in both liver and WAT after central ghrelin treatment in a GH-independent fashion. Furthermore, the results obtained for activity G6PDH and 6PGDH support an increased lipogenesis by ghrelin treatment because they produce oxidation of nicotinamide adenine dinucleotide phosphate, which is considered an essential element in de novo lipogenesis by supplying reducing power (46).
Central ghrelin effects are particularly intriguing in the case of AMPK, CPT1, and malonyl-CoA levels. Preceding studies demonstrated that peripheral and central administration of ghrelin to rats affects AMPK activity in a tissue-specific manner. AMPKα is activated in the brain and heart, whereas it is inhibited in liver and adipose tissue, and no effect is detected on skeletal muscle (17,54,60,61,62,63). Our data show for first time that chronic ghrelin treatment enhanced protein levels of AMPKα and pAMPKα. ACC activity was enhanced after central ghrelin infusion in liver and WAT, but the levels of its product, malonyl-CoA, were decreased in both tissues of wild-type Lewis rats. A reasonable explanation for this is that central ghrelin treatment increased the activities of FAS (liver and WAT) and MCD (only in WAT), leading to an increase of malonyl-CoA turnover. Malonyl-CoA acts as negative mediator of fatty acid oxidation by inhibiting CPT-1 and blocking entry of fatty acids into the mitochondria for β-oxidation (64). Interestingly, our results suggest that hepatic CPT1 is regulated in a GH-dependent manner because we observed that chronic infusion of ghrelin directly into the CNS decreased protein and activity levels of CPT1 only in the liver of wild-type Lewis rats and not in dwarf rats. This result suggests that the potential of central ghrelin to promote hepatic lipids storage is higher in a GH-dependent- (favoring lipid deposition and decreasing lipid mobilization) than in a GH-independent manner (favoring only lipid deposition). Contrary to what happens in liver, central ghrelin infusion increased CPT1 protein and activity levels in WAT, independent of GH levels. Nevertheless, activation of the central ghrelin system may increase lipid oxidation in WAT, and our data indicate that fat mass and fat storage enzymes were also stimulated by ghrelin. Thereby, our data suggest that the enhanced β-oxidation in WAT after central ghrelin infusion might be a compensatory mechanism and is a GH-independent effect.
Another important observation in the present study is that continuous ICV ghrelin infusion resulted in hyperinsulinemia and hyperglycemia only in dwarf rats, although in wild-type Lewis rats, a trend to increase was observed for both parameters. The effects of ghrelin on insulin secretion in experimental animals are inconsistent. It has been shown to either inhibit or stimulate insulin secretion, depending on dose and experimental conditions (65,66,67,68). However, systemic action of exogenous ghrelin to elevate blood glucose levels has been well documented in humans and rodents (69,70,71). Several studies have demonstrated that the ghrelin system is actively involved in the control of insulin sensitivity and glucose metabolism in situations of high-fat diet, GH, and leptin deficiency (72,73,74). In addition, the ghrelin knockout (Ghrl−/−) mice on a high-fat diet showed improved levels of insulin, glucose, and lipids compared with wild-type mice on this diet and exhibited greater glucose tolerance (75). Our results suggest that ghrelin is more important in the control of insulin sensitivity in situations that produce metabolic stress, such as the GH deficiency exhibited by spontaneous dwarf rats. Finally, we observed that plasma total ghrelin levels were increased in ICV ghrelin-treated rats independently of presence/absence of GH.
This increase could be due to an altered ghrelin clearance and/or gastric ghrelin synthesis, a phenomenon reported previously by others (16,73). However, in our opinion, the possibility that increase peripheral ghrelin levels contributed to the observed effects was excluded for several reasons. In a recent work describing the effects of central ghrelin on adipose lipid metabolism, a pair-fed (animals given the same amount of food as consumed by vehicle-treated rats) ghrelin-treated group was included to differentiate between ghrelin effects per se from those related to increased food intake. The results showed that plasma levels of acyl-ghrelin were increased in the ICV ghrelin-ad libitum group, whereas such an increase was absent in the ICV ghrelin pair-fed group. However, despite those differences in plasma values, the ghrelin effects on lipid (and glucose) metabolism occurred independently from ghrelin-induced hyperphagia, discarding a possible interference of peripheral ghrelin action (16). Moreover, in the same study, rats were treated peripherally with the same amount of ghrelin that was given ICV to exclude any potential effect of leaking from the cephalospinal fluid (CFS) after central ghrelin treatment. Under these conditions, no effects on feeding, body weight, adiposity, or lipid and glucose metabolism were detected. Overall, these data exclude and make improbable the existence of leaking from the CFS to the blood and instead suggest the existence of a central pathway modulating ghrelin action on peripheral lipid metabolism. Moreover, in the plasma it is known that acylated ghrelin is quickly deacylated (76). In the CFS this fact is not demonstrated, but to avoid a possible degradation to deacylated ghrelin, we infused acyl-ghrelin directly into the brain continuously. Still, the possibility that the effects here reported are exerted by des-acyl ghrelin, generated after ghrelin infusion, remains open. Further studies assessing the role of nonacylated ghrelin, as well as other peptides generated from the ghrelin, are needed.
In summary, our study indicates that: 1) the effects on lipid metabolism in liver and WAT caused by starvation are independent of ghrelin, 2) central ghrelin treatment favors lipid storage in a GH-independent mode in WAT and liver, and 3) ghrelin induces changes in lipid oxidation in a GH-independent fashion in WAT and in a GH- dependent fashion in liver (decreasing only in normal rats). We propose that ghrelin favors energy stores to minimize negative effects in periods of food scarcity. During fasting, increased ghrelin levels stimulate appetite and favor the recuperation when the food is again available by triggering biological responses that modulate the efficiency of energy storage. However, in situations as GH deficiency or diets rich in fat, which contribute a further increase in positive energy balance and fat mass (77,78), the ghrelin’s actions may constitute a harmful mechanism because it enhances adipose tissue accretion and/or insulin resistance. Although the role of circulating ghrelin levels are not clearly established, (79,80,81), it has been found that ghrelin levels are decreased in GH-deficient patients treated with GH (82). The decreased ghrelin levels are correlated with changes in fat mass and fat-free mass. The present study suggests that central ghrelin effects can mediate such changes in fat metabolism.
Whether a suppression of ghrelin could be useful in controlling adiposity in human obesity associated with GH deficiency remains to be established. In any event, understanding the molecular mechanism underlying the interplay between GH and ghrelin on lipid metabolism will show new strategies for the design and development of suitable drugs for the treatment of GH-deficiency, obesity, and its comorbidities.
Supplementary Material
Footnotes
This work was supported by Grants PGIDIT06PXIB208063PR (to C.D.) and GRC2006/66 (to M.L.) from Xunta de Galicia, Grants PI061700 (to M.L.) and PI051024 and PI070413 (to F.C.) from the Fondo Investigationes Sanitarias, Grants BFU2008 (to C.D.) and RyC-2007-00211 (to M.L.) from the Ministerio de Educacion y Ciencia; European Union Grant Health-F2-2008-223713 (to C.D.), and Grants DK-19514 and DK-67509 (to A.K.S.) from the U.S. Public Health Service. Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición is an initiative of Instituto de Salud Carlos III (ISCIII).
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 16, 2009
Abbreviations: ACC, Acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; CFS, cephalospinal fluid; CNS, central nervous system; CPT1, carnitine palmitoyltransferase 1; CPT1L, CPT1 liver type isoform; CPT1M, CPT1 muscle type isoform; FAS, fatty acid synthase; GHD, GH deficiency; G6PDH, glucose-6-phosphate dehydrogenase; ICV, intracerebroventricular; MCD, malonyl-CoA decarboxylase; pACC, phospho-ACC-Ser79; pAMPKα, phospho-AMPKα-Thr172; 6PGDH,6-phosphogluconate dehydrogenase; SCD, stearoyl-CoA desaturase; WAT, white adipose tissue.
References
- van der Lely AJ 2004 Justified and unjustified use of growth hormone. Postgrad Med J 80:577–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lely AJ 2004 Growth hormone and glucose metabolism: the model of the GH-receptor antagonists. Ann Endocrinol (Paris) 65:81–83 [DOI] [PubMed] [Google Scholar]
- Ghigo E, Arvat E, Giordano R, Broglio F, Gianotti L, Maccario M, Bisi G, Graziani A, Papotti M, Muccioli G, Deghenghi R, Camanni F 2001 Biologic activities of growth hormone secretagogues in humans. Endocrine 14:87–93 [DOI] [PubMed] [Google Scholar]
- Jørgensen JO, Vestergaard E, Gormsen L, Jessen N, Nørrelund H, Christiansen JS, Møller N 2005 Metabolic consequences of GH deficiency. J Endocrinol Invest 28:47–51 [PubMed] [Google Scholar]
- Christiansen JS, Vahl N, Norrelund H, Jørgensen JO 2002 Effects of GH replacement in young patients with childhood onset GH deficiency. Int J Clin Pract Suppl 32–36 [PubMed] [Google Scholar]
- Christiansen JS, Jørgensen JO 1991 Beneficial effects of GH replacement therapy in adults. Acta Endocrinol (Copenh) 125:7–13 [DOI] [PubMed] [Google Scholar]
- Ghigo E, Aimaretti G, Corneli G 2008 Diagnosis of adult GH deficiency. Growth Horm IGF Res 18:1–16 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Kangawa K 2001 Purification and distribution of ghrelin: the natural endogenous ligand for the growth hormone secretagogue receptor. Horm Res 56(Suppl 1):93–97 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Matsuo H, Kangawa K 2001 Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab 12:118–122 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK 2006 Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494:528–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR 2001 Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992–5995 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR 2001 Ghrelin causes hyperphagia and obesity in rats. Diabetes 50:2540–2547 [DOI] [PubMed] [Google Scholar]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schürmann A, Szanto I, Tschöp MH, Rohner-Jeanrenaud F 2006 Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116:1983–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodriguez-Cuenca S, Deoliveira RM, Castañeda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschöp MH, Diéguez C, Vidal-Puig A 2008 Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 7:389–399 [DOI] [PubMed] [Google Scholar]
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR 2008 Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab 7:377–388 [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschöp MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S 2008 UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren AM SC, Thomas EL, Abbott CR, Ghatei MA, Bell JD, Bloom SR, Continuous subcutaneous administration of ghrelin results in accumulation of adipose tissue, independent of hyperphagia or body weight gain. Proc 23rd Joint Meeting of the British Endocrine Societies, Brighton, UK, 2004, (Abstract OC35) [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR 2000 The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325–4328 [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Bjursell M, Ljungberg A, Dickson SL, Kopchick JJ, Bergström G, Svensson L, Oscarsson J, Törnell J, Bohlooly-Y M 2006 Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab 290:E317–E325 [DOI] [PubMed] [Google Scholar]
- López M, Seoane LM, Tovar S, Nogueiras R, Diéguez C, Señarís R 2004 Orexin-A regulates growth hormone-releasing hormone mRNA content in a nucleus-specific manner and somatostatin mRNA content in a growth hormone-dependent fashion in the rat hypothalamus. Eur J Neurosci 19:2080–2088 [DOI] [PubMed] [Google Scholar]
- Charlton HM, Clark RG, Robinson IC, Goff AE, Cox BS, Bugnon C, Bloch BA 1988 Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol 119:51–58 [DOI] [PubMed] [Google Scholar]
- Vázquez MJ, González CR, Varela L, Lage R, Tovar S, Sangiao-Alvarellos S, Williams LM, Vidal-Puig A, Nogueiras R, López M, Diéguez C 2008 Central resistin regulates hypothalamic and peripheral lipid metabolism in a nutritional-dependent fashion. Endocrinology 149:4534–4543 [DOI] [PubMed] [Google Scholar]
- Saggerson ED, Greenbaum AL 1970 The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Effects of altered dietary and hormonal conditions. Biochem J 119:221–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian WN, Braunstein LD, Apse K, Pang J, Rose M, Tian X, Stanton RC 1999 Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol 276:C1121–C1131 [DOI] [PubMed] [Google Scholar]
- Tian WN, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC 1998 Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem 273:10609–10617 [DOI] [PubMed] [Google Scholar]
- Karlic H, Lohninger S, Koeck T, Lohninger A 2002 Dietary l-carnitine stimulates carnitine acyltransferases in the liver of aged rats. J Histochem Cytochem 50:205–212 [DOI] [PubMed] [Google Scholar]
- Shin ES, Cho SY, Lee EH, Lee SJ, Chang IS, Lee TR 2006 Positive regulation of hepatic carnitine palmitoyl transferase 1A (CPT1A) activities by soy isoflavones and l-carnitine. Eur J Nutr 45:159–164 [DOI] [PubMed] [Google Scholar]
- Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB 1998 Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem 273:16146–16154 [DOI] [PubMed] [Google Scholar]
- Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro KL, Ladenheim EE, Ronnett GV, Tu Y, Birnbaum MJ, Lopaschuk GD, Moran TH 2007 Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci USA 104:17358–17363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Stark MJ, Foster DW 1978 Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem 253:8291–8293 [PubMed] [Google Scholar]
- López M, Lelliott CJ, Tovar S, Kimber W, Gallego R, Virtue S, Blount M, Vázquez MJ, Finer N, Powles TJ, O'Rahilly S, Saha AK, Diéguez C, Vidal-Puig AJ 2006 Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes 55:1327–1336 [DOI] [PubMed] [Google Scholar]
- Bougneres PF, Artavia-Loria E, Ferre P, Chaussain JL, Job JC 1985 Effects of hypopituitarism and growth hormone replacement therapy on the production and utilization of glucose in childhood. J Clin Endocrinol Metab 61:1152–1157 [DOI] [PubMed] [Google Scholar]
- Zhou Y, He L, Baumann G, Kopchick JJ 1997 Deletion of the mouse GH-binding protein (mGHBP) mRNA polyadenylation and splicing sites does not abolish production of mGHBP. J Mol Endocrinol 19:1–13 [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ 2003 Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144:3799–3810 [DOI] [PubMed] [Google Scholar]
- Hauck SJ, Bartke A 2001 Free radical defenses in the liver and kidney of human growth hormone transgenic mice: possible mechanisms of early mortality. J Gerontol A Biol Sci Med Sci 56:B153–B162 [DOI] [PubMed] [Google Scholar]
- Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A 2001 Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood) 226:552–558 [DOI] [PubMed] [Google Scholar]
- Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A 2005 Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology 146:851–860 [DOI] [PubMed] [Google Scholar]
- Hansen I, Tsalikian E, Beaufrere B, Gerich J, Haymond M, Rizza R 1986 Insulin resistance in acromegaly: defects in both hepatic and extrahepatic insulin action. Am J Physiol 250:E269–E273 [DOI] [PubMed] [Google Scholar]
- Bramnert M, Segerlantz M, Laurila E, Daugaard JR, Manhem P, Groop L 2003 Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab 88:1455–1463 [DOI] [PubMed] [Google Scholar]
- Rosenfalck AM, Maghsoudi S, Fisker S, Jørgensen JO, Christiansen JS, Hilsted J, Vølund AA, Madsbad S 2000 The effect of 30 months of low-dose replacement therapy with recombinant human growth hormone (rhGH) on insulin and C-peptide kinetics, insulin secretion, insulin sensitivity, glucose effectiveness, and body composition in GH-deficient adults. J Clin Endocrinol Metab 85:4173–4181 [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR 2001 Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806 [DOI] [PubMed] [Google Scholar]
- Kersten S 2001 Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep 2:282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salati LM, Amir-Ahmady B 2001 Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu Rev Nutr 21:121–140 [DOI] [PubMed] [Google Scholar]
- Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB 2005 AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab 289:E794–E800 [DOI] [PubMed] [Google Scholar]
- Hardie DG 2008 AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes 32(Suppl 4):S7–S12 [DOI] [PubMed] [Google Scholar]
- Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B 2005 Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 54:1331–1339 [DOI] [PubMed] [Google Scholar]
- Wang Z, Masternak MM, Al-Regaiey KA, Bartke A 2007 Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology 148:2845–2853 [DOI] [PubMed] [Google Scholar]
- To K, Yamaza H, Komatsu T, Hayashida T, Hayashi H, Toyama H, Chiba T, Higami Y, Shimokawa I 2007 Down-regulation of AMP-activated protein kinase by calorie restriction in rat liver. Exp Gerontol 42:1063–1071 [DOI] [PubMed] [Google Scholar]
- Munday MR, Milic MR, Takhar S, Holness MJ, Sugden MC 1991 The short-term regulation of hepatic acetyl-CoA carboxylase during starvation and re-feeding in the rat. Biochem J 280(Pt 3):733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Horvath TL 2007 Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 30:367–398 [DOI] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M 2005 Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 280:25196–25201 [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S 2004 Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 145:2607–2612 [DOI] [PubMed] [Google Scholar]
- Goto M, Arima H, Watanabe M, Hayashi M, Banno R, Sato I, Nagasaki H, Oiso Y 2006 Ghrelin increases neuropeptide Y and agouti-related peptide gene expression in the arcuate nucleus in rat hypothalamic organotypic cultures. Endocrinology 147:5102–5109 [DOI] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K 2000 Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911 [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Tschöp M, Heiman ML, Ghigo E 2004 Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 25:426–457 [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Tovar S, Mitchell SE, Rayner DV, Archer ZA, Dieguez C, Williams LM 2004 Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes 53:2552–2558 [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G 2005 Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288:E228–E235 [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ 2004 AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279:12005–12008 [DOI] [PubMed] [Google Scholar]
- Kohno D, Sone H, Minokoshi Y, Yada T 2008 Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun 366:388–392 [DOI] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M 2008 The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE 3:e1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Brown NF 1997 The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 244:1–14 [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H, Yada T, Matsukura S 2002 Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes 51:124–129 [DOI] [PubMed] [Google Scholar]
- Adeghate E, Ponery AS 2002 Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. J Neuroendocrinol 14:555–560 [DOI] [PubMed] [Google Scholar]
- Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J 2002 Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 146:241–244 [DOI] [PubMed] [Google Scholar]
- Lee HM, Wang G, Englander EW, Kojima M, Greeley Jr GH 2002 Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 143:185–190 [DOI] [PubMed] [Google Scholar]
- Dezaki K, Yada T 2004 [Ghrelin in the regulation of glucose and lipid metabolism]. Nippon Rinsho 62(Suppl 9):388–391 [PubMed] [Google Scholar]
- Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E 2001 Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86:5083–5086 [DOI] [PubMed] [Google Scholar]
- Broglio F, Benso A, Gottero C, Prodam F, Grottoli S, Tassone F, Maccario M, Casanueva FF, Dieguez C, Deghenghi R, Ghigo E, Arvat E 2002 Effects of glucose, free fatty acids or arginine load on the GH-releasing activity of ghrelin in humans. Clin Endocrinol (Oxf) 57:265–271 [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M 2003 Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 52:947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauna C, Meyler FM, Janssen JA, Delhanty PJ, Abribat T, van Koetsveld P, Hofland LJ, Broglio F, Ghigo E, van der Lely AJ 2004 Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab 89:5035–5042 [DOI] [PubMed] [Google Scholar]
- Sun Y, Asnicar M, Saha PK, Chan L, Smith RG 2006 Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 3:379–386 [DOI] [PubMed] [Google Scholar]
- Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW 2005 Absence of ghrelin protects against early-onset obesity. J Clin Invest 115:3573–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JB, Leite-Moreira AF 2008 Ghrelin, des-acyl ghrelin and obestatin: three pieces of the same puzzle. Peptides 29:1255–1270 [DOI] [PubMed] [Google Scholar]
- Gao J, Ghibaudi L, van Heek M, Hwa JJ 2002 Characterization of diet-induced obese rats that develop persistent obesity after 6 months of high-fat followed by 1 month of low-fat diet. Brain Res 936:87–90 [DOI] [PubMed] [Google Scholar]
- Salomon F, Cuneo RC, Umpleby AM, Sonksen PH 1994 Glucose and fat metabolism in adults with growth hormone deficiency. Clin Endocrinol (Oxf) 41:315–322 [DOI] [PubMed] [Google Scholar]
- Freda PU, Reyes CM, Conwell IM, Sundeen RE, Wardlaw SL 2003 Serum ghrelin levels in acromegaly: effects of surgical and long-acting octreotide therapy. J Clin Endocrinol Metab 88:2037–2044 [DOI] [PubMed] [Google Scholar]
- Malik IA, English PJ, Ghatei MA, Bloom SR, MacFarlane IA, Wilding JP 2004 The relationship of ghrelin to biochemical and anthropometric markers of adult growth hormone deficiency. Clin Endocrinol (Oxf) 60:137–141 [DOI] [PubMed] [Google Scholar]
- Isidro ML, Nemina R, Garcia-Buela J, Sangiao-Alvarellos S, Cordido F 2007 Effect of oral glucose on acylated and total ghrelin secretion in acromegalic patients. Neuro Endocrinol Lett 28:596–603 [PubMed] [Google Scholar]
- Edén Engström B, Burman P, Holdstock C, Karlsson FA 2003 Effects of growth hormone (GH) on ghrelin, leptin, and adiponectin in GH-deficient patients. J Clin Endocrinol Metab 88:5193–5198 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.