Abstract
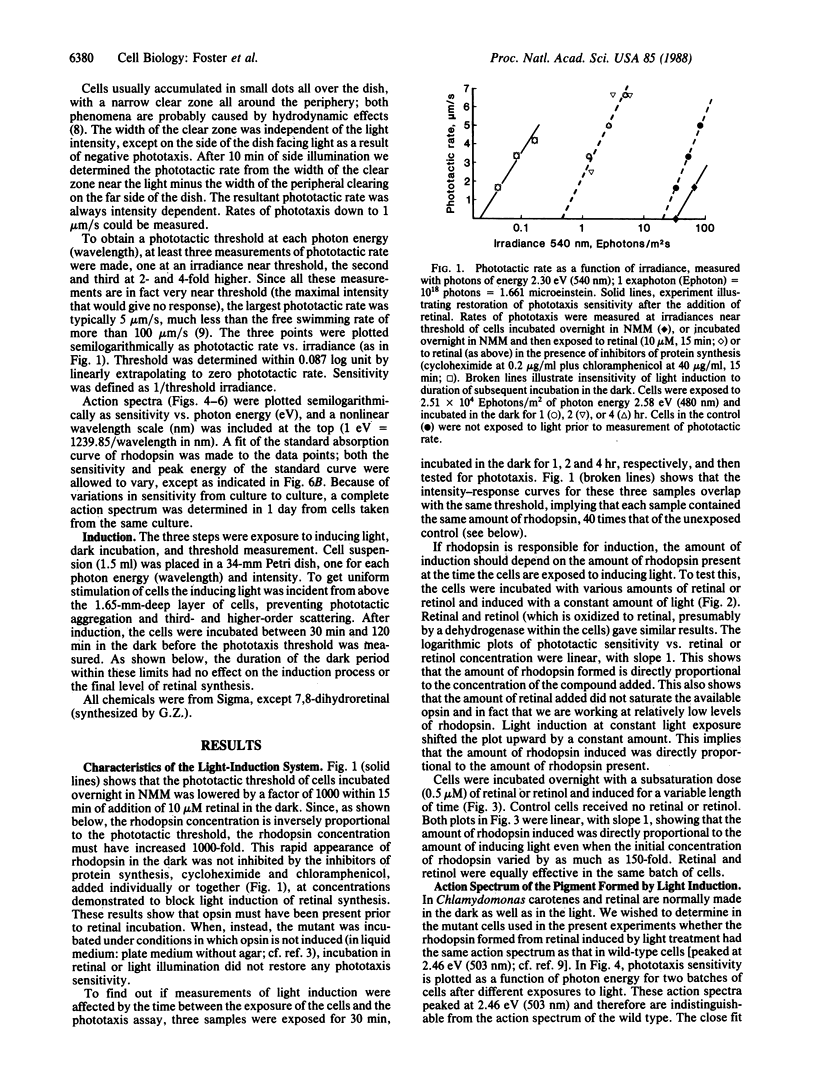
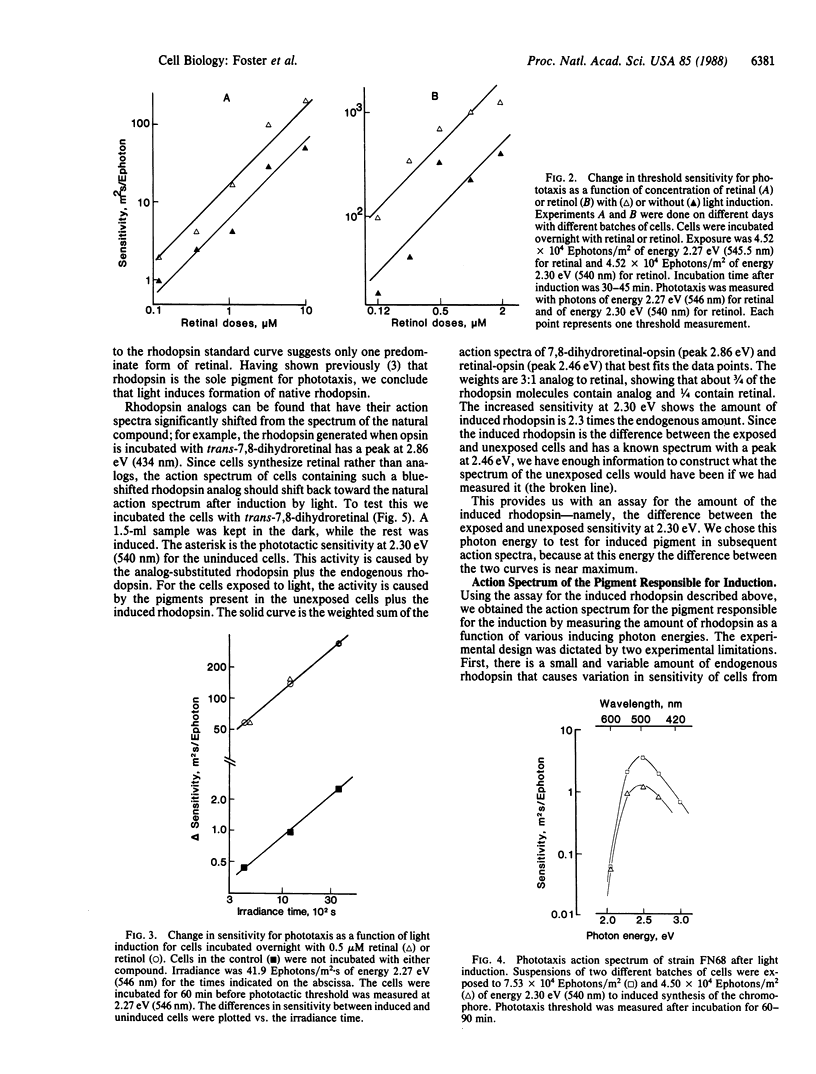
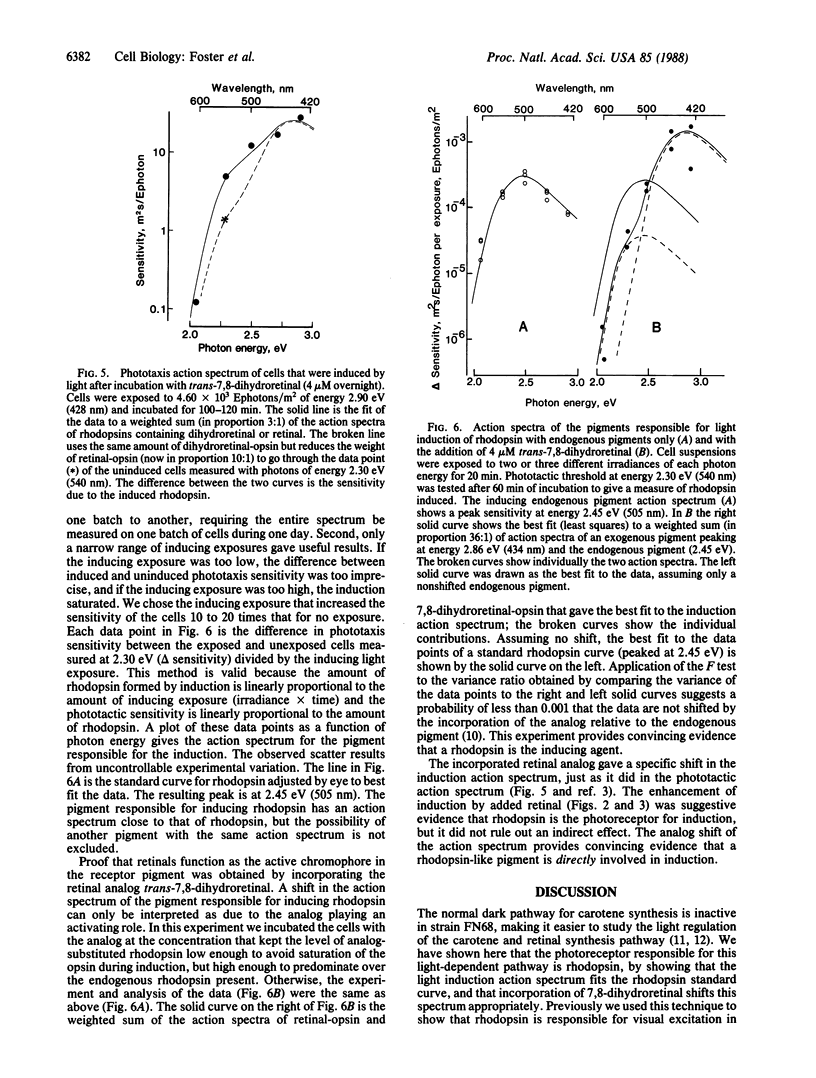
A sensitive assay for the induction of carotenoid and rhodopsin synthesis, based on the phototactic response, has been developed in a mutant of the unicellular alga Chlamydomonas reinhardtii. In the dark, the mutant fails to synthesize carotene and retinal, but it contains the apoprotein opsin. When retinal synthesis is induced by light treatment, the retinal combines with opsin to form rhodopsin, and the cells swim away from a source of light. Since the amount of light required to trigger a phototactic response is inversely proportional to the concentration of rhodopsin, the decrease in amount of light necessary to generate that response can serve as a measure of the amount of retinal synthesized in cells after induction. Using this assay, we found that (i) light induction of retinal depends linearly on light exposure and rhodopsin concentration during the exposure; (ii) the action spectrum of light induction is identical with that for phototaxis for which the receptor pigment is rhodopsin; and (iii) incubation with all-trans-7,8-dihydroretinal before light exposure shifts the action-spectrum peak for light induction 0.41 eV (-71 nm). We conclude that the photopigment for induction of retinal synthesis is a rhodopsin. The time lag required for induction of retinal synthesis and preliminary experiments with transcription or translation inhibitors suggest that alterations in gene expression could be involved in the induction process. Its control could be similar to other processes in which membrane receptors for hormones, neurotransmitters, or growth factors regulate gene expression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Foster K. W., Saranak J., Patel N., Zarilli G., Okabe M., Kline T., Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature. 1984 Oct 25;311(5988):756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Smyth R. D. Light Antennas in phototactic algae. Microbiol Rev. 1980 Dec;44(4):572–630. doi: 10.1128/mr.44.4.572-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hori H., Lim B. L., Osawa S. Evolution of green plants as deduced from 5S rRNA sequences. Proc Natl Acad Sci U S A. 1985 Feb;82(3):820–823. doi: 10.1073/pnas.82.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M. M., Kirk D. L. Translational regulation of protein synthesis, in response to light, at a critical stage of Volvox development. Cell. 1985 Jun;41(2):419–428. doi: 10.1016/s0092-8674(85)80015-5. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I., Levine R. P. Carotenoids of Wild Type and Mutant Strains of the Green Aiga, Chlamydomonas reinhardi. Plant Physiol. 1964 Jul;39(4):680–687. doi: 10.1104/pp.39.4.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading H. W. Altered biosynthesis in retinal degeneration. Methods Enzymol. 1982;81:794–799. doi: 10.1016/s0076-6879(82)81106-3. [DOI] [PubMed] [Google Scholar]
- SAGER R., ZALOKAR M. Pigments and photosynthesis in a carotenoid-deficient mutant of Chlamydomonas. Nature. 1958 Jul 12;182(4628):98–100. doi: 10.1038/182098a0. [DOI] [PubMed] [Google Scholar]
- Schmidt S. Y., Blanks J. C., Sandberg M. A. Enhancement of (polyA+)RNA synthesis in light in isolated intact photoreceptor cells of the rat. Exp Eye Res. 1985 Aug;41(2):159–170. doi: 10.1016/0014-4835(85)90020-x. [DOI] [PubMed] [Google Scholar]
- Wiechmann A. F. Melatonin: parallels in pineal gland and retina. Exp Eye Res. 1986 Jun;42(6):507–527. doi: 10.1016/0014-4835(86)90042-4. [DOI] [PubMed] [Google Scholar]


