Abstract
Objective:
To evaluate the safety of Systane® Ultra Lubricant Eye Drops (test solution) in contact lens wearers. A currently marketed contact lens rewetting drop was the control solution.
Participants:
This investigator- and patient-masked, single-site, randomized, and prospective study involved 45 successful contact lens wearers.
Methods:
Eligible subjects’ baseline biomicroscopy findings, visual acuity, and corneal staining score were recorded. Subjects received either the test or control solution with masked labeling. Subjects were instructed to instill their assigned solution in both eyes: 15 minutes prior to lens insertion, at least one drop during lens wear and another drop immediately following lens removal. After 14 days, biomicroscopy results, visual acuity, and corneal staining score were recorded.
Results:
No adverse events were documented for either the test or the control solution. For subjects using Systane® Ultra, no statistically significant change was detected in visual acuity (= 0.7667) or corneal staining score (P = 1.000). For subjects using the control solution, the change in visual acuity (P = 0.0011, mean difference = 1.70 ± 3.22 standard deviation) was not clinically relevant and there was no significant change in corneal staining score (P = 0.5413).
Conclusions:
This clinical study provided evidence of safety and compatibility of Systane Ultra Lubricant Eye Drops in contact lens wearers.
Keywords: lubricant eye drop, contact lens, safety, dry eye
Contact lens wear continues to be a popular method of vision correction, with an estimated 35 million contact lens wearers in North America.1 New lens polymers that promote ocular health, longer-lasting comfort, and flexible wearing modalities attract new wearers and contribute to the success of this form of vision correction.
Despite the fact that the contact lens market is growing, a concerning number of contact lens wearers drop out of contact lens wear every year. While inconvenience, ocular hyperemia, blurry or inconsistent vision as well as cost are all factors in an individual’s decision to discontinue lens wear, contact-lens induced dryness and discomfort are consistently reported as the most common reasons for lens abandonment.2,3 Pritchard and colleagues found 12% of contact lens patients discontinued lens wear within five years of the initial fitting due to dryness and discomfort symptoms.4
For patients, ‘discomfort’ and ‘dryness’ are often synonymous, with a high correlation between comfort and dryness ratings for both symptomatic and asymptomatic patients.1 Among current contact lens wearers, approximately 50% experience dry eye symptoms to varying degrees. And while, in most cases, the dryness is not frequent or noticeable enough for wearers to remove their lenses, as many as 20% have symptoms severe enough for them to reduce their wearing time.5,6 In a study of 1,054 patients surveyed on dry eye symptoms (including 367 contact lens wearers), the authors report an even higher incidence of ocular dryness. Of the contact lens wearers surveyed, 77% reported some degree of dryness symptoms (compared to nearly 44% of nonlens wearers). In addition, 70% to 80% of the contact lens wearers reported frequent eye discomfort and visual changes.7,8
The reported effects of contact lenses on the tear film may precipitate dry eye symptoms. A reduction in the pre-lens tear film lipid layer and an increase in tear film evaporation are associated with contact lens wear.9–13 These changes result in increased tear film osmolality14,15 affecting tear film stability and tear break up time. This disruption of the tear film in contact lens wearers is associated with reduced functional visual acuity,16,17 decreased wear time4 and an increased risk of ocular surface desiccation.18,19
Artificial tear supplements are widely used to relieve dry eye symptoms and sustain ocular comfort during lens wear.20 Begley and colleagues report that 47% of contact lens wearers reported the use of rewetting drops but indicated that these provided only moderate relief.7 Despite their viscosity-enhancing ingredients, instilled drops tend to have a short residency time and require frequent re-instillation throughout the day.
Systane® Ultra Lubricant Eye Drops (Alcon Laboratories, Inc., Fort Worth, TX, USA) is a new-generation ocular lubricant for the treatment of dry eye. Clinical results show that Systane® Ultra provides long lasting relief from dry eye.21,22
The loosely cross-linked, droppable gel, after instillation in the eye, reduces in viscosity with the first blink allowing even distribution over the ocular surface. Subsequent interaction with the divalent ions and mucin in the tear film facilitates the development of a viscoelastic, gel-matrix with shear thinning and bioadhesive properties. The gel-matrix promotes retention of the active demulcents for tear film stability, lubrication, and protection of the ocular surface.23,24
The clinical benefits of Systane® Ultra in treating dry eye together with its viscoelastic properties may offer advantages for contact lens wearers in the prevention of contact lens-related dry eye.
The purpose of this clinical study is to evaluate the safety of Systane® Ultra in a group of successful contact lens wearers compared with that of a currently available contact lens rewetting drop: Sensitive Eyes® Rewetting Drops (Bausch and Lomb, Rochester, NY, USA).
Methods
Materials
The test solution was Systane® Ultra Lubricant Eye Drops containing polyethylene glycol 400 (PEG) and propylene glycol (PPG) as demulcents. Bausch and Lomb Sensitive Eyes® Rewetting Drops, an isotonic aqueous solution with low viscosity, currently marketed as a drop to reduce contact lens-related dryness symptoms, was used as the control solution.
Study population
This prospective, randomized, double-masked, single site clinical study of two weeks duration enrolled 45 successful contact lens wearers aged between 18 and 65 years wearing either once or twice per month planned-replacement soft contact lenses (SCL) or gas permeable (GP) lenses. As a prerequisite for enrollment, subjects were required to have contact lens acuity of 20/30 or better in each eye, be in good health and to continue any pre-enrollment systemic medication regimens during the study. Attendance at all clinical study visits and completion of study questionnaires was also required. There were no restrictions to the currently employed contact lens care regimen, rewetting drop usage or lens type (planned replacement soft or GP).
Exclusion criteria included a history of allergy to any study product ingredient and current use of any topical eye medications (with the exception of rewetting drops or artificial tear products). Subjects who had modified their systemic medications within 30 days prior to enrollment were excluded as were subjects with significant active corneal, eyelid or anterior segment infection or inflammation.
The primary objective was to evaluate safety with the use of Systane® Ultra in contact lens wearers. A comparison to a currently available contact lens rewetting drop was included as a control. Safety was established by assessment of visual acuity, corneal staining, biomicroscopy examination and adverse events. The clinical study was performed in compliance with the ethical principles of the Declaration of Helsinki and written informed consent was obtained from all patients enrolled.
Procedures
At the first screening visit (Visit 1 – screening), after a subject’s preliminary eligibility was established, demographic information, medical history and current usage of systemic medication and any topical ocular drops (including rewetting drops, artificial tears and Restasis® [Allergan, Inc., Irvine CA, USA]) was recorded. Baseline measurements of safety parameters, including distance visual acuity with contact lenses and corneal staining score were recorded. Biomicroscopy findings (lids/eyelashes, cornea, and conjunctiva) were recorded as either normal or abnormal.
Subsequently, patients were randomly dispensed either Systane® Ultra or Sensitive Eyes. Both the test and the control solutions were dispensed in 10 and 15 mL bottles with sterile closures and masked labeling supplied in prenumbered kits. Patients were educated in the correct administration of the drops and instructed to instill 1–2 drops of the assigned lubricating eye drop in both eyes on a daily basis, approximately 15 minutes prior to contact lens insertion, at least one drop during the lens wear period and immediately following lens removal. Written instructions were provided.
Follow up visits (Visit 2 – follow up) were conducted two weeks after the first screening visit. Results for distance visual acuity with contact lenses and corneal staining score were recorded. Any changes in biomicroscopy assessment of the lids, cornea, and conjunctiva were recorded. Biomicroscopy findings (lids/eyelashes, cornea, and conjunctiva) were also recorded.
Distance visual acuity
Contact lens visual acuity was measured for distance. Acuities were measured using a Snellen letter chart. Testing was conducted monocularly under photopic lighting conditions (85 cd/m2).
Biomicroscopy
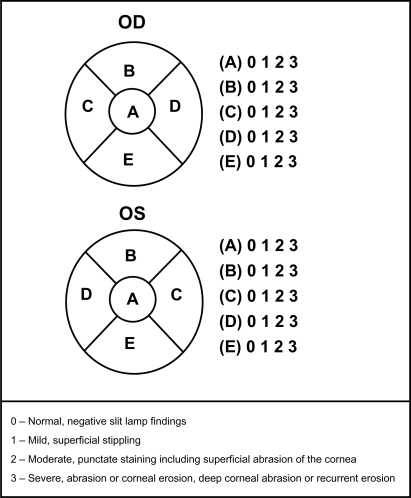
Subjects were assessed for corneal staining and any abnormal findings associated with the lids, cornea, and conjunctiva. Corneal staining was graded using the NEI staining grid in which a score of 0–3 (0 = normal and 3 = severe) was assigned to each of five corneal regions (nasal, central, temporal, inferior, and superior) with a maximum total score of 15 (Figure 1).10
Figure 1. Corneal staining was graded in each of five corneal zones using the NEI grid (superior, nasal, central, inferior, temporal).
Adverse events
Adverse events were considered to be any unfavorable or unexpected medical occurrence in a subject using the test or control solutions. For enrolled subjects, any change from baseline in the clinical findings deemed unfavorable, was considered an adverse event and recorded. Serious adverse events, whether related to use of the respective solution or not, required discontinuation of that solution and appropriate medical treatment.
Statistical methods
Within-subject before and after treatment comparison was conducted using paired t-test. Student’s t-test was used for between-group comparisons in numeric. All tests were twosided with the confidence level set to 95%.
Results
Forty-five subjects were enrolled and successfully completed the study. There were 14 (31%) males and 31 (69%) females ranging in age from 21 to 53 years (mean, 31.2 years; standard deviation [SD], 8.1). Subject ethnicity was White (n = 40. 89%), Asian (n = 2), Hispanic (n = 1), and two were unspecified. Twenty-three subjects were randomized into the Systane® Ultra group, and 22 into the Sensitive Eyes® group. The two treatment groups were comparable in demographic background.
Within-subject changes in visual acuity and the corneal staining score are presented in Table 1. No significant changes in visual acuity were observed in the Systane® Ultra group (P = 0.7667). In the Sensitive Eyes® group (P = 0.0011), an improvement in visual acuity from 22.27 (SD, 3.32) to 20.57 (SD, 1.61) (P = 0011) was observed, although this is not considered clinically relevant.
Table 1.
Within-subject changes in visual acuity (Snellen best-corrected visual acuity) and the sum staining score
| Time |
Systane® Ultra (n = 46 eyes) |
Sensitive Eyes® (n = 44 eyes) |
P-value1 | ||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | ||
| Baseline | |||||
| Visual acuity | 21.20 (2.40) | 20–30 | 22.27 (3.32) | 20–30 | 0.0826 |
| Sum staining score | 0.33 (0.63) | 0–2 | 0.43 (0.76) | 0–2 | 0.4746 |
| Follow-up | |||||
| Visual acuity | 21.30 (2.46) | 20–30 | 20.57 (1.61) | 20–25 | 0.0951 |
| Sum staining score | 0.33 (0.67) | 0–3 | 0.55 (1.02) | 0–5 | 0.2342 |
Notes: P-values of Student’s t-test for between-group comparison. Per paired t-test, no statistically significant within-subject changes were detected for the Systane® Ultra group for visual acuity (P = 0.7667) or sum staining scores (P = 1.0000). For the Sensitive Eyes group, the changes in visual acuity were statistically significant (P = 0.0011, mean difference = 1.70 ± 3.22 SD), but not the sum staining score (P = 0.5413). Clinically, a change of 1.70 in visual acuity is not considered clinically relevant.
Adverse events
There were no adverse events reported from this clinical study. No clinically significant abnormal biomicroscopy findings were reported during follow-up in either group.
Discussion
The safety of Systane® Ultra Lubricant Eye Drops in contact lens wearers was evaluated in this clinical study involving 45 successful lens wearers. Systane® Ultra was well tolerated and demonstrated a favorable safety profile when administered topically at least three times a day in conjunction with contact lens wear. Ocular assessments including visual acuity and corneal staining score showed no significant treatment-related changes and there were no serious ocular or systemic adverse events reported. The safety profile of Systane® Ultra was comparable to that of a currently marketed contact lens rewetting drop. These clinical findings add support for the use of Systane® Ultra by contact lens wearers.
These results provide contact lens wearers with increased confidence in the use of Systane® Ultra during lens wear for the treatment of contact lens-related dry eye. Given that dryness is the most frequent symptom in approximately 75% of contact lens wearers25,26 and contact lens wear can increase the frequency and severity of pre-existing dry eye,25 there is considerable opportunity for contact lens wearers to experience the relief of an effective dry eye therapy. Additional clinical benefits of Systane® Ultra in dry eye patients, namely, immediate patient comfort, enhanced lubrication and minimal blur on instillation,27 are likely to also apply to contact lens wearers. Conclusions drawn by Christensen and colleagues regarding the overall safety profile of an hydroxypropyl guar (HP-Guar)-containing lubricant eye drop in prior research28 add support to the findings of the current study.
Systane® Ultra Lubricant Eye Drops is a new product in the Systane® family of dry eye therapies. It is an aqueous solution comprising PEG (0.4%) and PPG (0.3%) as demulcents and incorporating the benefits of HP-Guar as a gelling agent. The above-mentioned ingredients have a history of use in ophthalmic preparations as well as contact lens care products and have demonstrated compatibility with the ocular surface.28 In addition, Systane® Ultra includes borate and sorbitol as key ingredients. Sorbitol, a water soluble, nonionic compound, interacts with borate29 to control viscosity in the bottle for optimal delivery in the eye. The presence of sorbitol facilitates the even distribution of the eye drop over the ocular surface upon instillation.
Ketelson and colleagues27 demonstrated that this increase in viscoelasticity on-eye and between blinks correlates with a significant reduction in friction. This finding has implications for contact lens wear whereby the reduction in friction between the lens and the lids has the potential to improve lens-wearing comfort. Similar reductions in friction have been reported by Meyer and colleagues in connection with a similar HP-Guar containing ocular lubricant from within the Systane® family.30 In addition, a study involving contact lens wearers showed that the use of an HP-Guar-containing ocular lubricant was associated with improved comfortable lens-wearing times and an improved overall lens-wearing experience.31
The success of contact lens wear depends on the integrity and stability of the tear film. While Systane® Ultra is not considered to be used a re-wetting solution for contact lens wearers, Systane® Ultra is designed to rebuild the tear film for long-term lubrication and extended protection of the ocular surface.22 A good tear film covering a contact lens is thought to provide comfort, vision, lubrication, prevent surface drying, remove debris, and counter infection.6 Chalmers and Begley8 claim that tear film instability is present in almost all contact lens wearers with or without symptoms indicating that the benefits of Systane® Ultra can apply to asymptomatic as well as symptomatic lens wearers.
Moreover, an HP-Guar-containing solution reduced aqueous tear evaporation 30 and 60 minutes after application in dry eye patients. This antievaporative effect may also be achieved in normal eyes when in low relative humidity environments32 and may therefore offer further advantages to contact lens wearers.
Conclusion
Systane® Ultra is recommended for the temporary relief of burning and irritation due to dryness of the eye. Use of Systane® Ultra Lubricating Eye Drops in successful contact lens wearers was not associated with any significant change in corneal staining or distance visual acuity and was not associated with any adverse events. While additional clinical evaluation should be conducted to further substantiate the compatibility of Systane Ultra® in this subject group, this clinical study provided evidence of safety and compatibility of Systane® Ultra Lubricant Eye Drops in contact lens wearers.
Acknowledgments
This clinical study was funded by a grant from Alcon Laboratories, Inc., USA. The author thanks Lauren Richard, B. Optom, for her medical writing contributions.
Footnotes
Disclosure
This paper was presented in part at the AOA meeting, June 2009; Washington DC, USA. This clinical study was a sponsored study with requested funding provided by Alcon Laboratories, Inc., Fort Worth, TX, USA. The author has no proprietary interest in any of the devices used in the study.
References
- 1.Fonn D. Targeting contact lens induced dryness and discomfort: what properties will make lenses more comfortable. Optom Vis Sci. 2007;84:279–285. doi: 10.1097/OPX.0b013e31804636af. [DOI] [PubMed] [Google Scholar]
- 2.Schlanger JL. A study of contact lens failures. J Am Optom Assoc. 1993;64:220–224. [PubMed] [Google Scholar]
- 3.Pritchard N, Fonn D. Dehydration, lens movement and dryness ratings of hydrogel contact lenses. Ophthalmic Physiol Opt. 1995;15:281–286. [PubMed] [Google Scholar]
- 4.Pritchard N, Fonn D, Brazeau D. Discontinuation of contact lens wear: a survey. Int Contact Lens Clin. 1999;26:157–162. doi: 10.1016/s0892-8967(01)00040-2. [DOI] [PubMed] [Google Scholar]
- 5.Nichols JJ, Ziegler C, Mitchell GL, Nichols KK. Self-reported dry eye disease across refractive modalities. Invest Ophthalmol Vis Sci. 2005;46:1911–1914. doi: 10.1167/iovs.04-1294. [DOI] [PubMed] [Google Scholar]
- 6.Nichols JJ, Sinnott LT. Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci. 2006;47:1319–1328. doi: 10.1167/iovs.05-1392. [DOI] [PubMed] [Google Scholar]
- 7.Begley C, Chalmers R, Mitchell G, et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea. 2001;20:610–618. doi: 10.1097/00003226-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers R, Begley C. Dryness symptoms among an unselected clinical population with and without contact lens wear. Cont Lens Anterior Eye. 2006;29:25–30. doi: 10.1016/j.clae.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Farris RL. The dry eye: Its mechanisms and therapy, with evidence that contact lens is a cause. CLAO J. 1986;12:234–246. [PubMed] [Google Scholar]
- 10.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 11.Hamano H, Hori M, Mitsunaga S. Measurement of evaporation rate of water from the precorneal tear film and contact lenses. Contacto. 1981;25:7–14. [Google Scholar]
- 12.Cedarstaff TH, Tomlinson A. A comparative study of tear evaporation rates and water content of soft contact lenses. Am J Optom Physiol Opt. 1983;60:167–174. doi: 10.1097/00006324-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Mathers W. Evaporation from the ocular surface. Exp Eye Res. 2004;78:389–394. doi: 10.1016/s0014-4835(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 14.Mackie IA. Contact lenses in dry eyes. Trans Ophthalmol Soc UK. 1985;104:477–483. [PubMed] [Google Scholar]
- 15.Gilbard JP, Gray KL, Rossi SR. A proposed mechanism for increased tear-film osmolarity in contact lens wearers. Am J Ophthalmol. 1986;102:505–507. doi: 10.1016/0002-9394(86)90081-4. [DOI] [PubMed] [Google Scholar]
- 16.Timberlake GT, Doane MG, Bertera JH. Short-term, low-contrast visual acuity reduction associated with in vivo contact lens drying. Optom Vis Sci. 1992;69:755–760. doi: 10.1097/00006324-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gellatly KW, Brennan NA, Efron N. Visual decrement with deposit accumulation of HEMA contact lenses. Am J Optom Physiol Opt. 1988;65:937–941. doi: 10.1097/00006324-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Bruinsma GM, van der Mei HC, Busscher HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials. 2001;22:3217–3224. doi: 10.1016/s0142-9612(01)00159-4. [DOI] [PubMed] [Google Scholar]
- 19.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108:1279–1288. doi: 10.1016/s0161-6420(01)00639-x. [DOI] [PubMed] [Google Scholar]
- 20.Ridder W, Tomlinson A, Paugh J. Effect of artificial tears on visual performance in subjects with dry eye. Optom Vis Sci. 2005 Sep;82(9):835–842. doi: 10.1097/01.opx.0000177803.74120.6f. [DOI] [PubMed] [Google Scholar]
- 21.Lane S, Paugh J, Webb J, Christensen M. An evaluation of the in vivo retention time of a novel artificial tear as compared to a placebo control. Clinical Trials identifier number: NCT 00804791. Poster presented at: ARVO; May 3–7; Fort Lauderdale, FL, USA. 2009. [Google Scholar]
- 22.Torkildsen G, Martin A, Tudor M, Griffin J, et al. Evaluation of functional visual performance using the IVAD method with currently marketed artificial tear products. Poster presented at: ARVO; May 3–7; Fort Lauderdale, FL, USA. 2009. [Google Scholar]
- 23.Hartstein I, Khwarg S, Przydryga J. An open-label evaluation of HP-Guar gellable lubricant eye drops for the improvement of dry eye signs and symptoms in a moderate dry eye adult population. Curr Med Res Opin. 2005;21(2):255–260. doi: 10.1185/030079905x26252. [DOI] [PubMed] [Google Scholar]
- 24.Gifford P, Evans BJW, Morris J. Evaluation of Systane. Cont Lens Anterior Eye. 2006;29:31–40. doi: 10.1016/j.clae.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Begley C, Caffery B, Nichols K, et al. Responses of contact lens wearers to a dry eye survey. Optom Vis Sci. 2000;77:40–46. doi: 10.1097/00006324-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Brennan NA, Efron N. Symptomatology of HEMA contact lens wear. Optom Vis Sci. 1989;66:834–838. doi: 10.1097/00006324-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ketelson HA, Davis J, Meadows DL. Characterization of a novel polymeric artificial tear delivery system. Poster A139 presented at: ARVO; April 27–May 1; Fort Lauderdale, FL, USA. 2008. [Google Scholar]
- 28.Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP-Guar gellable lubricant eye drop for the relief of dryness of the eye. Curr Eye Res. 2004;28(1):55–62. doi: 10.1076/ceyr.28.1.55.23495. [DOI] [PubMed] [Google Scholar]
- 29.Pezron E, Leibler L, Ricard A, Audebert R. Reversible gel formation induced by ion complexation. 2. Phase diagrams. Macromolecules. 1988;21:1126–1131. [Google Scholar]
- 30.Meyer A, Baier R, Chen H, Chowham M. Differential tissue-on-tissue lubrication by ophthalmic formulations. J Biomed Mater Res B Appl Biomater. 2007;82:74–88. doi: 10.1002/jbm.b.30707. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S, Potter W, Christensen MT. Use of Systane to help reduce symptoms of dry eye associated with contact lens wear. Presented at: the 107th Annual American Optometric Association Congress; June 23–27; Orlando, FL, USA. 2004. [Google Scholar]
- 32.Uchiyama E, Di Pascuale M, Butovich I, McCulley J. Impact on ocular surface evaporation of an artificial tear solution containing hydroxypropyl guar. Eye Contact Lens. 2008;34(6):331–334. doi: 10.1097/ICL.0b013e31818c66b5. [DOI] [PMC free article] [PubMed] [Google Scholar]