Abstract
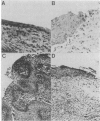
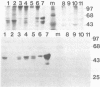
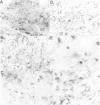
The activated ras oncogene that is present in Harvey sarcoma virus is able to induce malignant transformation of pure cultures of mouse primary keratinocytes. Malignant transformation of these cells is demonstrated by their ability to form carcinomas when grafted back onto syngeneic animals. However, expression of the malignant phenotype by the ras-transformed keratinocytes is drastically inhibited by the presence of normal dermal fibroblasts. This inhibitory effect depends on the ratio of fibroblasts to keratinocytes. It can be observed with mitomycin C-treated growth-arrested dermal fibroblasts and not with other cells, such as normal keratinocytes or established fibroblasts. Thus, a cellular environment approximating normal tissue can suppress tumor formation triggered by a single oncogene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Brown K., Quintanilla M., Ramsden M., Kerr I. B., Young S., Balmain A. v-ras genes from Harvey and BALB murine sarcoma viruses can act as initiators of two-stage mouse skin carcinogenesis. Cell. 1986 Aug 1;46(3):447–456. doi: 10.1016/0092-8674(86)90665-3. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Gilman M. Z., Maruyama M., Weinberg R. A. c-myc and c-fos expression in differentiating mouse primary keratinocytes. EMBO J. 1986 Nov;5(11):2853–2857. doi: 10.1002/j.1460-2075.1986.tb04579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Gerschenson M., Graves K., Carson S. D., Wells R. S., Pierce G. B. Regulation of melanoma by the embryonic skin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7307–7310. doi: 10.1073/pnas.83.19.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins. Cell. 1983 Jul;33(3):915–924. doi: 10.1016/0092-8674(83)90034-x. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Herschman H. R., Brankow D. W. Ultraviolet irradiation transforms C3H10T1/2 cells to a unique, suppressible phenotype. Science. 1986 Dec 12;234(4782):1385–1388. doi: 10.1126/science.3787250. [DOI] [PubMed] [Google Scholar]
- Land H., Chen A. C., Morgenstern J. P., Parada L. F., Weinberg R. A. Behavior of myc and ras oncogenes in transformation of rat embryo fibroblasts. Mol Cell Biol. 1986 Jun;6(6):1917–1925. doi: 10.1128/mcb.6.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I. C., Fusenig N. E. Regeneration of organized epithelial structure. J Invest Dermatol. 1983 Jul;81(1 Suppl):189s–194s. doi: 10.1111/1523-1747.ep12541093. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Bertram J. S., Loewenstein W. R. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. 1986 Jan 17;44(1):187–196. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- Pierce G. B., Lewis S. H., Miller G. J., Moritz E., Miller P. Tumorigenicity of embryonal carcinoma as an assay to study control of malignancy by the murine blastocyst. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6649–6651. doi: 10.1073/pnas.76.12.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop D. R., Lowy D. R., Tambourin P. E., Strickland J., Harper J. R., Balaschak M., Spangler E. F., Yuspa S. H. An activated Harvey ras oncogene produces benign tumours on mouse epidermal tissue. 1986 Oct 30-Nov 5Nature. 323(6091):822–824. doi: 10.1038/323822a0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Saiag P., Coulomb B., Lebreton C., Bell E., Dubertret L. Psoriatic fibroblasts induce hyperproliferation of normal keratinocytes in a skin equivalent model in vitro. Science. 1985 Nov 8;230(4726):669–672. doi: 10.1126/science.2413549. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Kacinski B. M., Kohorn E. I., Merino M. J., Carter D., Blakemore K. J. Demonstration of myc and ras oncogene expression by hybridization in situ in hydatidiform mole and in the BeWo choriocarcinoma cell line. Am J Obstet Gynecol. 1986 Feb;154(2):390–393. doi: 10.1016/0002-9378(86)90677-0. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worst P. K., Mackenzie I. C., Fusenig N. E. Reformation of organized epidermal structure by transplantation of suspensions and cultures of epidermal and dermal cells. Cell Tissue Res. 1982;225(1):65–77. doi: 10.1007/BF00216219. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Vass W., Scolnick E. Altered growth and differentiation of cultured mouse epidermal cells infected with oncogenic retrovirus: contrasting effects of viruses and chemicals. Cancer Res. 1983 Dec;43(12 Pt 1):6021–6030. [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]