Abstract
GRP78/BiP is a major endoplasmic reticulum (ER) chaperone protein critical for protein quality control of the ER, as well as controlling the activation of the ER-transmembrane signaling molecules. Through creation of mouse models targeting the Grp78 allele, the function of GRP78 in development and disease has been investigated. These led to the discovery that GRP78 function is obligatory for early embryonic development. However, in adult animals, GRP78 is preferably required for cancer cell survival under pathologic conditions such as tumor progression and drug resistance. The discovery of surface localization of GRP78 in cancer cells reveals potential novel function, interaction with cell-surface receptors, and possible therapeutic implications. Mouse models also reveal that GRP78 controls maturation and secretion of neuronal factors for proper neural migration and offers neuroprotection. Antioxid. Redox Signal. 11, 2307–2316.
Introduction
The endoplasmic reticulum (ER) is a cellular organelle in which secretory and membrane proteins are synthesized and modified. The ER also functions as an intracellular calcium store. The accumulation of unfolded proteins in the lumen of the ER induces a series of adaptive responses, which are collectively termed the unfolded protein response (UPR) (60). A number of pathologic conditions lead to acute ER stress and activate the UPR, such as nutrient deprivation, disruption of calcium homeostasis, viral infection, and secretory protein mutations (60). The UPR is also actively involved in physiologic conditions, such as plasma cell differentiation and pancreatic β-cell function and survival (72). Evidence is accumulating that GRP78 plays important roles in cancer progression, malignancy, drug resistance, and neurologic disorders. This review highlights the recent advances in the study of the role of GRP78 in these emerging areas, with therapeutic implications.
GRP78 as a Master Regulator of the Unfolded Protein Response
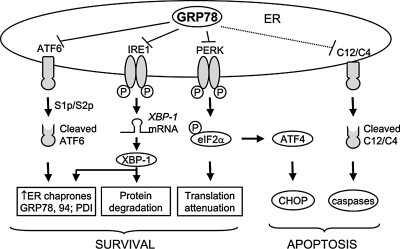
ER chaperones are essential for the normal function of the ER (48). One of the best-characterized ER chaperones is the 78-kDa glucose-regulated protein (GRP78), which is also referred to as BiP or HSPA5. GRP78 is involved in many cellular processes, including translocating the newly synthesized polypeptides across the ER membrane, facilitating the folding and assembly of proteins, targeting misfolded proteins for ER-associated degradation (ERAD), regulating calcium homeostasis, and serving as an ER stress sensor (22, 30, 33). GRP78 is a master regulator for ER stress because of its role as a major ER chaperone with antiapoptotic properties, as well as its ability to control the activation of UPR signaling (30). The interaction of GRP78 with the UPR-induced signaling and apoptotic pathways is summarized in Fig. 1.
FIG. 1.
GRP78 regulates ER stress–signaling pathways leading to UPR survival and apoptosis responses. In nonstressed cells, the ER-transmembrane signaling molecules (ATF6, IRE1, and PERK) and ER-associated caspases (murine caspase-12/human caspase 4) are maintained in an inactive state through binding to GRP78. After ER stress, such as protein misfolding, GRP78 is titrated away, and the survival pathways are activated to block further damage. However, when the stress is too severe, apoptotic responses are triggered, which eventually lead to cell death.
Upon ER stress, GRP78 is released from ER transmembrane signal transducers, including PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6), leading to the activation of these UPR signaling pathways (30). After the disassociation from GRP78, PERK dimerizes to promote its autophosphorylation and activation (60). Activated PERK phosphorylates the eukaryotic translation-initiation factor 2α (eIF2α) to attenuate the rate of general translation initiation and prevent further protein synthesis (20, 64). However, phosphorylated eIF2α can selectively increase the translation of mRNAs containing inhibitory upstream open reading frames (uORFs) in their 5′ untranslated region. The best-studied phosphorylated-eIF2α–dependent translation is activating transcription factor 4 (ATF4) (19, 37). Release from GRP78 allows ATF6 to translocate from ER to Golgi where it is cleaved. The cleaved form of ATF6 migrates into the nucleus and acts as an active transcription factor to upregulate proteins that augment ER folding capacity, like ER chaperones such as GRP78 and GRP94, and folding enzymes, such as PDI (21, 36, 73, 76).
Activated IRE1 has endoribonuclease activity and splices a 26-base intron from the mRNA encoding the X-box binding protein 1 (XBP-1) (75). XBP-1 is a transcriptional factor with target genes including DnaJ, p58, ERdj4, EDEM, and PDI, all involved in protein folding and ERAD (5, 29). Thus, the UPR progresses through transient attenuation of translational and transcriptional induction of ER chaperones, folding enzymes, and proteins involved in ERAD to alleviate protein aggregation in the ER as an adaptive response.
However, under severe and prolonged ER stress, the UPR activates unique pathways that lead to cell death through apoptosis (72). Upon ER stress, the proapoptotic BH3 protein BAX/BAK in the ER membrane undergoes conformational change and permits Ca2+ efflux to the cytosol, which activates m-calpain and subsequently cleaves and activates procaspase 12 and leads to the activation of the caspase cascade (46, 56, 61, 78). CHOP, one of the UPR downstream effectors, inhibits B-cell leukemia/lymphoma 2 (Bcl-2), activates growth arrest and DNA damage–inducible gene 34 (GADD34) and ER oxidoreductin 1 (ERO1), and thus promotes apoptosis (39, 40). However, the role of CHOP in cell death and survival may be context dependent because GADD34 upregulation by CHOP results in feedback inhibition of eIF2α phosphorylation. This could lead to recovery of translation, which may be beneficial; conversely, when translation persists under ER-stress conditions, build up of abnormal proteins may further erode the ER folding capacity, leading to cell death.
Activated IRE1 binds to c-Jun–N-terminal inhibitory kinase (JIK) and recruits TRAF2, which leads to the activation of apoptosis signal-regulating kinase 1 and c-Jun amino terminal kinase (ASK1/JNK) and also the release of the procaspase 12 from the ER (49, 74). ER stress also activates the proapoptotic p53-upregulated modulator of apoptosis (PUMA) and NOXA, leading to BAX and BAK activation and apoptosis (34). Furthermore, by monitoring the activation and maintenance of representative UPR pathways in cells treated with low concentrations of chemical ER stress inducers in tissue-culture systems, it was discovered that survival during mild stress is attained through intrinsic instabilities of mRNA and proteins that promote apoptosis, and compared with those that facilitate protein folding and adaptation, such as GRP78 (59). The balance between survival and apoptosis pathways in the UPR is presented and summarized in Fig. 2.
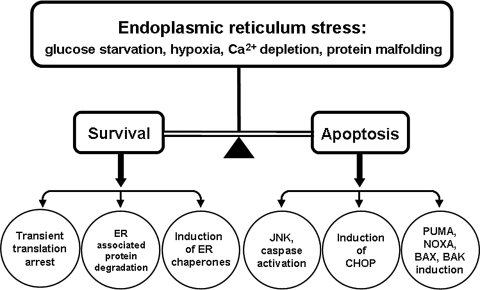
FIG. 2.
Survival and apoptosis responses to ER stress. ER stress can be induced by multiple adverse physiologic conditions, such as those indicated that alter ER homeostasis. When the ER protein load exceeds the ER protein-folding capacity, the UPR signaling pathways will be activated. The stressed cells will try to survive through reducing the nascent proteins influx by transient translational arrest, exporting the malfolded proteins for degradation in a process referred to as ERAD, as well as increasing the folding capacity of the ER by induction of the chaperones and folding enzymes. However, if the stress is too severe to overcome, the ER stress–induced apoptosis pathways will be activated, leading to JNK and caspase activation; induction of CHOP, PUMA, and NOXA; and BAX and BAK activation.
Autophagy is a catabolic process for the degradation and recycling of cytosolic, long-lived, or aggregated proteins and excess or defective organelles. ER stress induces autophagy and promotes cell survival by enabling the use of intracellular resources under starvation conditions (50). GRP78, as a critical component of the ER, is required for ER integrity and ER stress-induced autophagy. In cells in which GRP78 is knocked down by small interfering RNA (siRNA), the ER structure is disrupted, and autophagosome formation under both ER-stress and nutrient-starvation conditions is suppressed (35).
GRP78 is essential for embryonic development
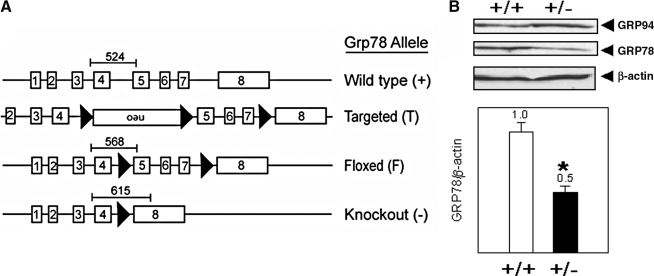
Despite extensive investigation of GRP78 function in regulating the UPR by using tissue-culture systems, important questions pertaining to the physiological function of GRP78 in the context of a whole organism remain to be addressed. To investigate directly the function of GRP78 in vivo, mouse models deficient in GRP78 through gene targeting have been created (38) (Fig. 3A). In the heterozygous Grp78 mice, the level of GRP78 in adult tissues is reduced by about half in adult tissues (Fig. 3B).
FIG. 3.
(A) Schematic drawing of the Grp78 WT and genetically modified alleles. The creation of genetically altered Grp78 mice has been described (38). The loxP sites are indicated by black arrows. Excision of exons 5 through 7, which are flanked by the loxP sites, will disrupt the critical ATPase and peptide-binding domains of GRP78 required for its chaperone function. The location and expected size of PCR products for the WT, floxed, and knockout alleles are indicated. (B) Whole-cell lysate from pooled livers of three WT Grp78 (+/+) and three heterozygous (+/−) littermates were subjected to Western blot with anti-KDEL (that recognized the KDEL C-terminal motif of both GRP78 and GRP94) and anti–β-actin. Quantitation of multiple Western blots with the GRP78 level normalized against β-actin as the loading control is shown below. The 50% reduction of GRP78 level in the Grp78+/− mice is statistically significant (*p value <0.05 by two-sided t test).
Strikingly, homozygous knockout mice of GRP78 demonstrate lethality by embryonic day 3.5, with much-reduced proliferation rate of embryonic cells and massive apoptotic death of the inner cell mass (38). In contrast, the heterozygous Grp78+/− mice are viable and phenotypically normal (38). The Grp78+/− mouse model serves as a valuable resource to examine the effect of partial reduction of GRP78, mimicking potential therapeutic-intervention outcome, on progression of human diseases.
Role of GRP78 in human cancers
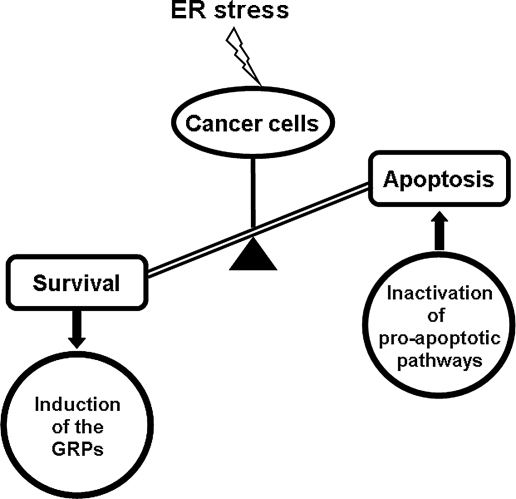
The metabolic environment of tumors is often acidic, hypoxic, and nutrient deprived, having reduced amounts of both amino acids and glucose. This can be due to both poor vascularization and rapid growth of tumor cells, and the intrinsic property of cancer cells with elevated glucose metabolism and higher glycosylation rates. When cells undergo glucose or oxygen deprivation, they activate the UPR. Recent evidence showed that the microenvironment of tumor cells resembles physiologic ER stress, and the UPR is often turned on for cell survival (27). In the case of cancer, mutations resulting from tumorigenesis often inactivate the proapoptotic pathways, thereby suppressing elimination of the cancerous cells. This, coupled with the activation of the prosurvival UPR pathways, offers an advantage for cancer progression (Fig. 4).
FIG. 4.
Inactivation of proapoptotic pathways and activation of prosurvival pathways contribute to cancer cell survival. Cancer cells are subjected to ER stress because of intrinsic factors such as high glucose metabolic rate and extrinsic factors such as nutrient and oxygen deprivation in the tumor microenvironment. This leads to the induction of the GRPs, and, coupled with mutations as a result of tumorigenesis that often inactivate the proapoptotic pathways, shifts the balance to the prosurvival branches of the UPR.
Because of its antiapoptotic property, induction of GRP78 has been reported as a prosurvival factor for cells undergoing ER stress. As discussed in previous reviews (14, 31, 33), it has been well documented that GRP78 is highly elevated in a variety of cancer cell lines, solid tumors, and human cancer biopsies, associating with malignancy and metastasis. The elevation of GRP78 transcription under various stress conditions suggests the involvement of GRP78 in enhanced cell survival. GRP78 has been shown directly to interact with apoptotic pathway intermediates, to block caspase activation, and eventually to lead to apoptosis inhibition and increased cell survival (15, 54, 58). As tumor progression requires proliferation as well as inhibition of tumor cell death, the inherent antiapoptotic properties of GRP78 indicate a potential role in cancer progression. GRP78 also is required for ER integrity and stress-induced autophagy, which may be important for cancer cell survival (35). In support of this, the expression level of GRP78 is markedly higher in primary tumors compared with that in benign tissues. This has been documented in various cancers, including breast cancer (12, 17, 32), hepatocellular carcinoma (66), lung cancer (68), and prostate cancer (7, 53).
Tumor resistance to therapy is a major challenge in cancer treatment. Thus, discovering predictive factors for chemoresistance is crucial for screening and improving adjuvant therapies for cancer patients to avoid unnecessary side effects to treatments, which may turn out to be ineffective or unnecessary. Emerging evidence is suggesting that GRP78 levels may be exploited as a prognostic as well as a diagnostic marker for chemoresponsiveness. The GRP78 expression level was reported to be such a novel predictor of breast cancer patient's responsiveness to doxorubicin (Adriamycin)-based chemotherapy (32). By performing a retrospective cohort study of 127 stage 2 and 3 breast cancer patients treated with doxorubicin-based chemotherapy, tumor specimens of the patients were analyzed for GRP78 expression against the respective drug-resistance status, designated by the time to recurrence (TTR). They found that ∼65% of the subjects expressed high levels of GRP78, which was in concert with previous breast cancer studies (12), and revealed a significant association between GRP78 expression level and resistance to doxorubicin-based chemotherapy.
In prostate cancer, it has been proposed that because of its antiapoptotic and prosurvival properties, GRP78 may be involved in the resistance of prostate cancer cells to chemotherapy or castration resistance (53). In support of this notion, GRP78 expression is upregulated during the transition from localized prostate cancer to metastatic castration resistance based on in vitro evidence and patient cohort studies. In established cell-line models, GRP78 expression is elevated in both castration resistant LNCaP-derived cell line, C42B, and androgen-deprived LNCaP cells compared with LNCaP cells grown in androgen-rich media (53).
Corresponding with the development of castration resistance, a high level of GRP78 expression in prostate tumors is associated with greater relative risk or recurrence and overall survival. It was shown that prostate cancer patients with strong GRP78 immunoreactivity in the primary tumor have a higher risk for clinical recurrence and death compared with those with weak GRP78 expression (7). GRP78 protein expression is significantly higher in malignant prostate cancer tissue than in benign prostatic tissue. The expression intensity is also significantly associated with patient survival and clinical recurrence, demonstrating that GRP78 expression levels may provide prognostic information in tumors (7, 53). This suggests that GRP78 may be a potential target for molecular therapy.
Mechanism for GRP78 in promoting cancer progression
Whereas xenograft studies demonstrated that GRP78 is required for tumor initiation and progression (25), the pathophysiologic role of GRP78 in tumor development is directly elucidated in a recent mouse model (11). As mentioned earlier, homozygous knockout of Grp78 in mouse leads to embryonic lethality by E3.5 (38); nonetheless, the viable and fertile heterozygotes allow direct investigation into the in vivo function of GRP78. Without affecting mouse growth, organ development, and antibody production, Grp78 heterozygosity strikingly prolongs the latency and retards the progression of the oncogene-induced mammary tumors in the well-established MMTVPyVT mouse model (18).
The underlying mechanisms for dependence on GRP78 on tumor growth were further dissected into three aspects. First, inhibition of tumor cell proliferation by Grp78 heterozygosity supports that GRP78 is critical for cell growth. This could be due to the ER chaperone function of GRP78 in growth-factor secretion or the maturation of growth-factor receptors or both. GRP78 was recently discovered to be expressed on the cell surface of tumor cells, where it acts to enhance AKT signaling, leading to cell survival and proliferation (43). Second, consistent with the observation in the in vitro cell-culture systems (58), GRP78 protects the tumor cells from apoptosis, as tumor cells from the heterozygous mice showed enhanced TUNEL staining and upregulation of CHOP (11). Third, angiogenesis is required for the growth and survival of solid tumors (13). Grp78 heterozygosity remarkably reduces vasculature in tumors, but not in normal organ and tissues, suggesting that tumor angiogenesis preferably depends on GRP78. It would be interesting to determine whether these same mechanisms are used in other cancer models.
Prostate cancer is the most common malignancy in men and the second leading cause of male cancer-related deaths in the Western world. Since its establishment in 2004, the PTEN conditional knockout mouse model that mimics genetic alterations that are found in human prostate cancer has become the gold standard for studying prostate cancer progression (70). GRP78/BiP has recently emerged as a novel biomarker for aggressive prostate cancer (7, 53). Recently, it was reported that homozygous deletion of Grp78 specifically in mouse prostate epithelium suppresses prostate tumorigenesis without affecting postnatal prostate development and growth (16). Mouse prostates with double conditional knockout of GRP78 and PTEN exhibit normal histology and cytology, in contrast to the invasive adenocarcinoma in mouse prostates with Pten inactivation. AKT activation in Pten-null prostate epithelium is inhibited by Grp78 homozygous deletion, corresponding to suppression of AKT phosphorylation by GRP78 knockdown in the prostate cancer cell line. Thus, inactivation of GRP78 may represent a novel approach to stop prostate cancer and potentially other cancers resulting from the loss of PTEN tumor suppression or activation of the oncogenic AKT or both. Nonetheless, how GRP78 downregulates oncogenic AKT signaling remains to be elucidated.
Role of GRP78 in drug resistance
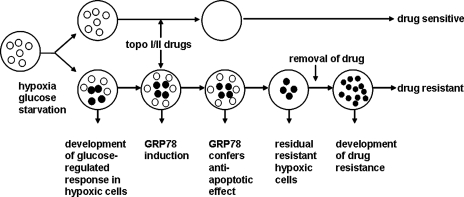
Mounting evidence has shown that the highly elevated expression level of GRP78 is correlated with cancer malignancy, metastasis, and drug resistance in a variety of cancers, including breast cancer, prostate cancer, lung cancer, and glioma (14, 15, 31, 33). Although the development of drug resistance in cancer is likely to be complex and involves multiple mechanisms, the induction of the protective arm of the UPR, such as GRP78 induction in the tumor microenvironment, can be a major contributing factor (Fig. 5).
FIG. 5.
GRP78 induction promotes drug resistance of the tumor. As a consequence of UPR resulting from glucose starvation or hypoxia, induction of GRP78 in cancer cells confers the development of drug resistance during cancer therapy.
This model predicts that when the tumor experiences ER stress, GRP78 will be induced, leading to resistance. Consistent with this, in a human breast cancer model, treatment with the antivascular agent, combretastatin A4P, or the antiangiogenic agent, contortrostatin, leads to high levels of GRP78 protein in viable tumor tissues surviving the drug treatment, particularly in necrotic borders, which are most chemoresistant (10). The same study also revealed that suppression of GRP78 sensitizes human breast cancer cells to etoposide-induced apoptosis, as was observed in bladder cancer cells (58).
Glioma is among the deadliest form of cancer known to be highly chemoresistant. GRP78 is significantly upregulated in malignant glioma specimens and human malignant glioma cell lines compared with a low expression level in normal adult brain (54). Interestingly, among the glioma cell lines, cells with the fastest proliferation rate show the highest levels of GRP78 overexpression and are dependent on GRP78 for proliferation in vitro. Whereas overexpression of GRP78 confers higher resistance to the chemotherapeutic agent temozolomide, knockdown of GRP78 sensitizes glioma cells to temozolomide-, 5-fluorouracil–, and CPT-11–induced apoptosis (54).
One key target for cancer therapy is the tumor vasculature, which plays essential roles in tumor growth and survival. In extending the studies on glioma resistance, it was discovered that GRP78 is highly elevated not only in the glioma cells, but also in the vasculature derived from human glioma specimens, both in situ in tissue and in vitro in primary cell cultures compared with normal brain tissues and blood vessels (69). Primary cultures of human brain endothelial cells derived from blood vessels of malignant glioma tissues (TuBECs) show strong resistance to chemotherapeutic agents such as CPT-11, etoposide, and temozolomide. However, this resistance is blunted when GRP78 expression is suppressed by siRNA or chemical inhibition of its enzyme activity. A previous xenograft study showed that combination therapy with drugs targeting GRP78 with conventional agents enhanced drug efficacy with no deleterious effect on the mice (51). These new studies further revealed that drugs targeting GRP78 will suppress both the tumor and tumor-vasculature development.
The mechanisms for the resistance observed in cells with an elevated GRP78 level may be complex; however, some possible explanations exist. GRP78 is the major ER chaperone with multiple cytoprotective functions, including binding of ER-Ca2+, alleviating malfolded protein aggregation in the ER, blocking the activation of ER-associated proapoptotic factors such as BIK and caspase-7, and its requirement for stress-induced autophagy (14, 35, 58). These prosurvival properties may explain the contribution of GRP78 to drug resistance in cancer treatment.
Role of cell-surface GRP78 in cancer therapy
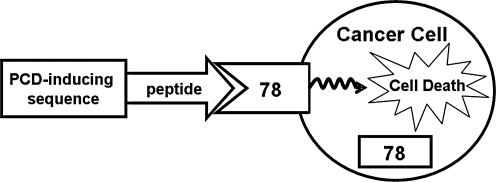
GRP78 is a stress protein that belongs to the HSP70 superfamily. Most of GRP78 has been found in the ER because of the retrieval capacity through the KDEL-retention motif. However, recently it was discovered that a small fraction of the GRP78 cellular pool can escape the ER-retention machinery and localize to the cell surface. Low surface expression of GRP78 on several cell types, including vascular endothelium, has been reported (3, 4, 8, 9). Global profiling of the cell-surface proteome of tumor cells has disclosed a relative abundance of heat-shock chaperones and glucose-regulated proteins, including GRP78 (65). Thus, as proof-of-principle that cell-surface GRP78 in tumors can mediate cancer-specific therapy (Fig. 6), surface GRP78 expression in prostate and breast cancer cells and xenograft models enables tumor targeting by circulating ligands (2).
FIG. 6.
Cell-surface GRP78 is a potential mediator for cancer cell–specific therapy. Preferential expression of GRP78 on the surface of cancer cells provides a mechanism for cancer-specific targeting, with peptides directly and specifically binding to cell-surface GRP78 and transducing proapoptotic agents.
In another study, a taxol-conjugated cell-penetrating peptidic GRP78 ligand was demonstrated to be able to target and kill specifically cancer cells through recognizing surface GRP78 in those cells (26).
In another example, surface GRP78 mediated the antiangiogenic and proapoptotic activity of rK5 through high-affinity binding interaction of rK5 with GRP78 exposed on the surface of stimulated endothelial cells and on hypoxic and cytotoxic stressed tumor cells (8).
GRP78 can form a complex with other proteins on the cell surface and play an important role in signaling transduction. Several GRP78-binding partners have been identified recently. Cell-surface GRP78 was shown to be essential for α2-macroglobulin–induced signal transduction through binding low-density lipoprotein receptor–related protein (LRP) (44). In addition, silencing of GRP78 gene expression attenuated α2-macroglobulin–induced signal transduction, indicating a novel receptor function for cell-surface GRP78 (45). Cell-surface GRP78 may be involved in the promotion of survival and metastasis of prostate cancer by activation of α2-macroglobulin as a receptor. Other cell-surface proteins, like Cripto, a multifunctional cell-surface protein that is a key to vertebrate embryogenesis and human tumor progression, was bound to cell-surface GRP78 (63). The complex of Cripto and GRP78 can enhance tumor growth via inhibition of TGF-β signaling. Therefore, cell-surface GRP78 provides a target with significant therapeutic potential by affecting primarily cancer cells but not normal tissue counterparts. Most recently, it was discovered that GRP78 associates with GPI-anchored T-cadherin on the surface of vascular endothelial cells, providing a novel mechanism by which GRP78 can influence endothelial cell survival as a cell-surface signaling receptor rather that an intracellular chaperone (52). Furthermore, serum from a cancer patient led to the discovery of a new 82-kDa tumor-specific variant of GRP78 that can be a target for antibody-based therapy (57). The epitope is an O-linked carbohydrate moiety and is specific for malignant cells, which may account for shielding this form of GRP78 from immune surveillance and immune response. This interesting finding provides a new approach to targeting tumor cells specifically through the cell-surface GRP78 variant.
Role of GRP78 in neurologic disorders
A common feature of the neurodegenerative diseases is the accumulation and aggregation of misfolded proteins, which can elicit ER stress (55). This leads to the notion that ER stress and the UPR may be involved in neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease (23, 47). However, direct proof of this principle is still emerging. A new spontaneous recessive mutant mouse model, woozy mouse, provided such a link between ER dysfunction and neurodegeneration (77). In the homozygous woozy mutant mouse, adult-onset ataxia and progressive Purkinje cell degeneration develop(77). Genetic mapping identified that the woozy mutant is caused by disruption of the gene encoding SIL1/BAP (BiP-associated protein), a nucleotide exchange factor for GRP78 (6, 77). Both GRP78 and SIL1 are expressed ubiquitously in adult tissues. In the woozy mutant mice, Purkinje cells located in the anterior lobules of the cerebellum (lobule I to VIII) undergo degeneration by age 4 months. However, in caudal lobule IX and lobule X, Purkinje cells and the other types of neuronal cells remain alive after 1 year old (77). Two ER-stress markers, GRP78 and CHOP, are upregulated in the Purkinje cells of lobule I to VIII (77), suggesting that the UPR was activated in the absence of SIL1. Abnormal protein accumulations were detected by both electron and confocal microscopy. Although these observations indicate that protein aggregates could lead to neuronal cell death, it remains to be resolved whether the absence of SIL1 affects other targets in addition to GRP78, and could other nucleotide-exchange factors for GRP78 compensate for the absence of SIL, such that GRP78 function may only be partially impaired in the SIL1-deficient mice? The nonlethal phenotype of the SIL1-deficient mouse strongly suggests that other cofactors exist for GRP78, at least during early embryogenesis, because complete loss of GRP78 function in the GRP78-deficient mice results in embryonic lethality by day 3.5 (38). Furthermore, it was recently reported that GRP170 also can function as the nucleotide-exchange factor for GRP78 (71). Creation of GRP78-specific knockout in Purkinje cells will address these issues.
In humans, mutations in SIL1 also are nonlethal but cause Marinesco–Sjögren syndrome, an autosomal recessive multisystem disorder characterized mainly by cerebellar ataxia with cataract, myopathy, and mental retardation (1, 62). In contrast with the woozy mouse model, in which only Purkinje cells located in lobules I through VIII are affected, myocytes and lens cells also degenerate in patients with Marinesco–Sjögren syndrome (1, 62). Explanations for this discrepancy include that human cells lack other compensatory mechanisms, are more sensitive to the disruption of ER homeostasis (1), or that other cofactors such as GRP170 can also function as the nucleotide exchange factor for GRP78 (71).
GRP78 has been intensively studied as the master regulator of ER stress in vitro; however, the physiologic and pathologic role of GRP78 in neuron development and neurologic disorders has not been established, partially because of the embryonic lethality of the GRP78 homozygous knockout mice (38). This was solved recently by the creation of a knockin mouse model expressing a mutant form of GRP78 with deletion of the ER-retrieval sequence KDEL by homologous recombination. With this mouse model, in which a low residual level of GRP78 is found in the ER, it is possible to examine the effects of defective ER protein quality control without completely eliminating the function of GRP78. The homozygous GRP78 mutant neonates die soon after birth because of respiratory failure caused by impaired secretion of pulmonary surfactant by alveolar type II epithelial cells (41). The mutant mice display disordered layer formation in the cerebral cortex and cerebellum, a neurologic phenotype of reeler mutant–like malformation (42). Besides that, the whole-brain size and the protein level of reelin, which is secreted by Cajal-Retzius (CR) cells, are remarkably reduced. The maturation and secretion of reelin in CR cells and other factors related to neural migration may be enhanced by GRP78 (42). Collectively these results imply that the mutant form of GRP78 and aberrant ER protein quality control may cause various neurologic disorders. Furthermore, reelin and its mRNA are significantly reduced in patients with schizophrenia (24), and epigenetic aberration of the human reelin gene is associated with psychiatric disorders (67). This indicates that GRP78 and ER protein quality control also may be involved in schizophrenia and psychiatric disorders. Considering the potential important roles of GRP78 in neuronal development and disorders, a deeper understanding of the pathologic role of GRP78 in neurologic disorders and neurodegenerative diseases warrants further investigation through transgenic or tissue- or cell-type–specific knockout mouse models. Most recently, it was reported that a small molecule, BIX, that preferentially induces GRP78 and slight induction of GRP94 and calreticulin can protect neurons from ER stress (28). Thus, a translational potential exists for GRP78 induction as therapy against neurodegenerative diseases and other neurologic disorders.
Abbreviations Used
- ATF6
activating transcription factor 6
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- GADD34
growth arrest and DNA damage-inducible gene 34
- GRP78
78-kDa glucose-regulated protein
- IRE1
inositol-requiring enzyme 1
- PERK
PKR-like ER kinase
- UPR
unfolded protein response
- XBP-1
X-box binding protein 1
Acknowledgments
This work is supported by NCI grants CA027607 and CA111700 to A.S.L.
References
- 1.Anttonen AK. Mahjneh I. Hamalainen RH. Lagier-Tourenne C. Kopra O. Waris L. Anttonen M. Joensuu T. Kalimo H. Paetau A. Tranebjaerg L. Chaigne D. Koenig M. Eeg-Olofsson O. Udd B. Somer M. Somer H. Lehesjoki AE. The gene disrupted in Marinesco-Sjögren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet. 2005;37:1309–1311. doi: 10.1038/ng1677. [DOI] [PubMed] [Google Scholar]
- 2.Arap MA. Lahdenranta J. Mintz PJ. Hajitou A. Sarkis AS. Arap W. Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Berger CL. Dong Z. Hanlon D. Bisaccia E. Edelson RL. A ymphocyte cell surface heat shock protein homologous to the endoplasmic reticulum chaperone, immunoglobulin heavy chain binding protein BIP. Int J Cancer. 1997;71:1077–1085. doi: 10.1002/(sici)1097-0215(19970611)71:6<1077::aid-ijc26>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee G. Ahamed J. Pedersen B. El-Sheikh A. Mackman N. Ruf W. Liu C. Edgington TS. Regulation of tissue factor–mediated initiation of the coagulation cascade by cell surface grp78. Arterioscler Thromb Vasc Biol. 2005;25:1737–1743. doi: 10.1161/01.ATV.0000173419.31242.56. [DOI] [PubMed] [Google Scholar]
- 5.Calfon M. Zeng H. Urano F. Till JH. Hubbard SR. Harding HP. Clark SG. Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 6.Chung KT. Shen Y. Hendershot LM. BAP, a mammalian BiP-associated protein, is a nucleotide exchange factor that regulates the ATPase activity of BiP. J Biol Chem. 2002;277:47557–47563. doi: 10.1074/jbc.M208377200. [DOI] [PubMed] [Google Scholar]
- 7.Daneshmand S. Quek ML. Lin E. Lee C. Cote RJ. Hawes D. Cai J. Groshen S. Lieskovsky G. Skinner DG. Lee AS. Pinski J. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Davidson DJ. Haskell C. Majest S. Kherzai A. Egan DA. Walter KA. Schneider A. Gubbins EF. Solomon L. Chen Z. Lesniewski R. Henkin J. Kringle 5 of human plasminogen induces apoptosis of endothelial and tumor cells through surface-expressed glucose-regulated protein 78. Cancer Res. 2005;65:4663–4672. doi: 10.1158/0008-5472.CAN-04-3426. [DOI] [PubMed] [Google Scholar]
- 9.Delpino A. Piselli P. Vismara D. Vendetti S. Colizzi V. Cell surface localization of the 78 kD glucose regulated protein (GRP 78) induced by thapsigargin. Mol Membr Biol. 1998;15:21–26. doi: 10.3109/09687689809027514. [DOI] [PubMed] [Google Scholar]
- 10.Dong D. Ko B. Baumeister P. Swenson S. Costa F. Markland F. Stiles C. Patterson JB. Bates SE. Lee AS. Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res. 2005;65:5785–5791. doi: 10.1158/0008-5472.CAN-05-0754. [DOI] [PubMed] [Google Scholar]
- 11.Dong D. Ni M. Li J. Xiong S. Ye W. Virrey JJ. Mao C. Ye R. Wang M. Pen L. Dubeau L. Groshen S. Hofman FM. Lee AS. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez PM. Tabbara SO. Jacobs LK. Manning FC. Tsangaris TN. Schwartz AM. Kennedy KA. Patierno SR. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992;3:89–96. [PubMed] [Google Scholar]
- 14.Fu Y. Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5:741–744. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y. Li J. Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen-starvation induced apoptosis. Cancer Res. 2007;67:3734–3740. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y. Wey S. Wang M. Ye R. Liao CP. Roy-Burman P. Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of the stress response chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci U S A. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazit G. Lu J. Lee AS. De-regulation of GRP stress protein expression in human breast cancer cell lines. Breast Cancer Res Treat. 1999;54:135–146. doi: 10.1023/a:1006102411439. [DOI] [PubMed] [Google Scholar]
- 18.Guy CT. Cardiff RD. Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding HP. Novoa I. Zhang Y. Zeng H. Wek R. Schapira M. Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 20.Harding HP. Zhang Y. Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 21.Haze K. Yoshida H. Yanagi H. Yura T. Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–297. [PubMed] [Google Scholar]
- 23.Imai Y. Soda M. Inoue H. Hattori N. Mizuno Y. Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 24.Impagnatiello F. Guidotti AR. Pesold C. Dwivedi Y. Caruncho H. Pisu MG. Uzunov DP. Smalheiser NR. Davis JM. Pandey GN. Pappas GD. Tueting P. Sharma RP. Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamora C. Dennert G. Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y. Lillo AM. Steiniger SC. Liu Y. Ballatore C. Anichini A. Mortarini R. Kaufmann GF. Zhou B. Felding-Habermann B. Janda KD. Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry. 2006;45:9434–9444. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 27.Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6:55–69. doi: 10.2174/156652406775574604. [DOI] [PubMed] [Google Scholar]
- 28.Kudo T. Kanemoto S. Hara H. Morimoto N. Morihara T. Kimura R. Tabira T. Imaizumi K. Takeda M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- 29.Lee AH. Iwakoshi NN. Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 32.Lee E. Nichols P. Spicer D. Groshen S. Yu MC. Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 33.Li J. Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 34.Li J. Lee B. Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–7270. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- 35.Li J. Ni M. Lee B. Barron E. Hinton DR. Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M. Baumeister P. Roy B. Phan T. Foti D. Luo S. Lee AS. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol Cell Biol. 2000;20:5096–5106. doi: 10.1128/mcb.20.14.5096-5106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu PD. Harding HP. Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo S. Mao C. Lee B. Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marciniak SJ. Yun CY. Oyadomari S. Novoa I. Zhang Y. Jungreis R. Nagata K. Harding HP. Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullough KD. Martindale JL. Klotz LO. Aw TY. Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mimura N. Hamada H. Kashio M. Jin H. Toyama Y. Kimura K. Iida M. Goto S. Saisho H. Toshimori K. Koseki H. Aoe T. Aberrant quality control in the endoplasmic reticulum impairs the biosynthesis of pulmonary surfactant in mice expressing mutant BiP. Cell Death Differ. 2007;14:1475–1485. doi: 10.1038/sj.cdd.4402151. [DOI] [PubMed] [Google Scholar]
- 42.Mimura N. Yuasa S. Soma M. Jin H. Kimura K. Goto S. Koseki H. Aoe T. Altered quality control in the endoplasmic reticulum causes cortical dysplasia in knock-in mice expressing a mutant BiP. Mol Cell Biol. 2008;28:293–301. doi: 10.1128/MCB.00473-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra UK. Deedwania R. Pizzo SV. Activation and cross-talk between Akt, NF-{kappa}B, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 44.Misra UK. Gonzalez-Gronow M. Gawdi G. Hart JP. Johnson CE. Pizzo SV. The role of Grp 78 in alpha 2-macroglobulin-induced signal transduction: evidence from RNA interference that the low density lipoprotein receptor-related protein is associated with, but not necessary for, GRP 78-mediated signal transduction. J Biol Chem. 2002;277:42082–42087. doi: 10.1074/jbc.M206174200. [DOI] [PubMed] [Google Scholar]
- 45.Misra UK. Gonzalez-Gronow M. Gawdi G. Wang F. Pizzo SV. A novel receptor function for the heat shock protein Grp78: silencing of Grp78 gene expression attenuates alpha2M*-induced signalling. Cell Signal. 2004;16:929–938. doi: 10.1016/j.cellsig.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa T. Yuan J. Cross-talk between two cysteine protease families: activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa T. Zhu H. Morishima N. Li E. Xu J. Yankner BA. Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 48.Ni M. Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishitoh H. Matsuzawa A. Tobiume K. Saegusa K. Takeda K. Inoue K. Hori S. Kakizuka A. Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogata M. Hino S. Saito A. Morikawa K. Kondo S. Kanemoto S. Murakami T. Taniguchi M. Tanii I. Yoshinaga K. Shiosaka S. Hammarback JA. Urano F. Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park HR. Tomida A. Sato S. Tsukumo Y. Yun J. Yamori T. Hayakawa Y. Tsuruo T. Shin-ya K. Effect on tumor cells of blocking survival response to glucose deprivation. J Natl Cancer Inst. 2004;96:1300–1310. doi: 10.1093/jnci/djh243. [DOI] [PubMed] [Google Scholar]
- 52.Philippova M. Ivanov D. Joshi MB. Kyriakakis E. Rupp K. Afonyushkin T. Bochkov V. Erne P. Resink TJ. Identification of proteins associating with glycosylphosphatidylinositol-anchored T-cadherin on the surface of vascular endothelial cells: role for Grp78/BiP in T-cadherin-dependent cell survival. Mol Cell Biol. 2008;28:4004–4017. doi: 10.1128/MCB.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pootrakul L. Datar RH. Shi SR. Cai J. Hawes D. Groshen SG. Lee AS. Cote RJ. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–5993. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 54.Pyrko P. Schonthal AH. Hofman FM. Chen TC. Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 55.Rao RV. Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16:653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao RV. Castro-Obregon S. Frankowski H. Schuler M. Stoka V. del Rio G. Bredesen DE. Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program: an Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 57.Rauschert N. Brandlein S. Holzinger E. Hensel F. Muller-Hermelink HK. Vollmers HP. A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab Invest. 2008;88:375–386. doi: 10.1038/labinvest.2008.2. [DOI] [PubMed] [Google Scholar]
- 58.Reddy RK. Mao C. Baumeister P. Austin RC. Kaufman RJ. Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 59.Rutkowski DT. Arnold SM. Miller CN. Wu J. Li J. Gunnison KM. Mori K. Sadighi Akha AA. Raden D. Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rutkowski DT. Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Scorrano L. Oakes SA. Opferman JT. Cheng EH. Sorcinelli MD. Pozzan T. Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 62.Senderek J. Krieger M. Stendel C. Bergmann C. Moser M. Breitbach-Faller N. Rudnik-Schoneborn S. Blaschek A. Wolf NI. Harting I. North K. Smith J. Muntoni F. Brockington M. Quijano-Roy S. Renault F. Herrmann R. Hendershot LM. Schroder JM. Lochmuller H. Topaloglu H. Voit T. Weis J. Ebinger F. Zerres K. Mutations in SIL1 cause Marinesco-Sjögren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet. 2005;37:1312–1314. doi: 10.1038/ng1678. [DOI] [PubMed] [Google Scholar]
- 63.Shani G. Fischer WH. Justice NJ. Kelber JA. Vale W. Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol. 2008;28:666–677. doi: 10.1128/MCB.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi Y. Vattem KM. Sood R. An J. Liang J. Stramm L. Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shin BK. Wang H. Yim AM. Le Naour F. Brichory F. Jang JH. Zhao R. Puravs E. Tra J. Michael CW. Misek DE. Hanash SM. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–7616. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 66.Shuda M. Kondoh N. Imazeki N. Tanaka K. Okada T. Mori K. Hada A. Arai M. Wakatsuki T. Matsubara O. Yamamoto N. Yamamoto M. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–614. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 67.Tamura Y. Kunugi H. Ohashi J. Hohjoh H. Epigenetic aberration of the human REELIN gene in psychiatric disorders. Mol Psychiatry. 2007;12:519, 593–600. doi: 10.1038/sj.mp.4002014. [DOI] [PubMed] [Google Scholar]
- 68.Uramoto H. Sugio K. Oyama T. Nakata S. Ono K. Yoshimastu T. Morita M. Yasumoto K. Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55–62. doi: 10.1016/j.lungcan.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Virrey JJ. Dong D. Stiles C. Patterson JB. Pen L. Ni M. Schönthal AH. Chen TC. Hofman FM. Lee AS. Stress chaperone GRP78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res. 2008;6:1268–1275. doi: 10.1158/1541-7786.MCR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S. Gao J. Lei Q. Rozengurt N. Pritchard C. Jiao J. Thomas GV. Li G. Roy-Burman P. Nelson PS. Liu X. Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 71.Weitzmann A. Volkmer J. Zimmermann R. The nucleotide exchange factor activity of Grp170 may explain the non-lethal phenotype of loss of Sil1 function in man and mouse. FEBS Lett. 2006;580:5237–5240. doi: 10.1016/j.febslet.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 72.Wu J. Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 73.Ye J. Rawson RB. Komuro R. Chen X. Dave UP. Prywes R. Brown MS. Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 74.Yoneda T. Imaizumi K. Oono K. Yui D. Gomi F. Katayama T. Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 75.Yoshida H. Matsui T. Yamamoto A. Okada T. Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida H. Okada T. Haze K. Yanagi H. Yura T. Negishi M. Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao L. Longo-Guess C. Harris BS. Lee JW. Ackerman SL. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet. 2005;37:974–979. doi: 10.1038/ng1620. [DOI] [PubMed] [Google Scholar]
- 78.Zong WX. Li C. Hatzivassiliou G. Lindsten T. Yu QC. Yuan J. Thompson CB. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]