Abstract
A large body of evidence suggests that mitochondrial dysfunction and oxidative damage play a role in the pathogenesis of Parkinson's disease (PD). A number of antioxidants have been effective in animal models of PD. We have developed a family of mitochondria-targeted peptides that can protect against mitochondrial swelling and apoptosis (SS peptides). In this study, we examined the ability of two peptides, SS-31 and SS-20, to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity in mice. SS-31 produced dose-dependent complete protection against loss of dopamine and its metabolites in striatum, as well as loss of tyrosine hydroxylase immunoreactive neurons in substantia nigra pars compacta. SS-20, which does not possess intrinsic ability in scavenging reactive oxygen species, also demonstrated significant neuroprotective effects on dopaminergic neurons of MPTP-treated mice. Both SS-31 and SS-20 were very potent (nM) in preventing MPP+ (1-methyl-4-phenylpyridinium)-induced cell death in cultured dopamine cells (SN4741). Studies with isolated mitochondria showed that both SS-31 and SS-20 prevented MPP+-induced inhibition of oxygen consumption and ATP production, and mitochondrial swelling. These findings provide strong evidence that these neuroprotective peptides, which target both mitochondrial dysfunction and oxidative damage, are a promising approach for the treatment of PD. Antioxid. Redox Signal. 11, 2095–2104.
Introduction
Substantial evidence links mitochondrial dysfunction and oxidative damage to the pathogenesis of Parkinson's disease (PD) (24). There is a decrease in complex I activity in the substantia nigra of PD patients, platelets, and in cybrid cell lines made from platelets (17, 35). Autosomal recessive PD is caused by mutations in parkin, DJ-1, and PINK1, all of which are associated with mitochondria (20), and the first two of which have functions in protecting against oxidative damage (5, 13, 44). Studies with toxin models of PD also strongly implicate mitochondrial dysfunction and oxidative damage in PD. Systemic administration of rotenone to rats, a selective complex 1 inhibitor, produces selective degeneration in the substantia nigra pars compacta as does 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) that produces an irreversible and severe parkinsonian syndrome in humans and nonhuman primates (4, 28). Both toxins have been reported to increase mitochondrial production of reactive oxygen species (ROS) (1, 16, 18, 30). Overproduction of mitochondrial ROS can further impair endogenous antioxidant enzymes such as superoxide dismutase (41). Furthermore, peroxidation of cardiolipin leads to loss of cardiolipin on the inner membrane and dissociation of cytochrome c (27, 31). This will lead to compromised function of cytochrome c oxidase, reduced ATP production, and further increase in ROS generation (9). MPTP is also known to induce mitochondrial permeability transition (MPT) and to result in cytochrome c-mediated, caspase-dependent apoptosis (6, 23).
In view of these observations, there has been great interest in the development of mitoprotective compounds for the treatment of PD. One potential approach is to reduce mitochondrial oxidative stress by administration of antioxidants such as coenzyme Q10 (CoQ10), which protects in the MPTP model of PD, and appears to be promising in initial clinical trials in PD patients (3, 32). A number of other antioxidants, such as azulenyl nitrone spin traps, are also effective in the MPTP model of PD (22, 42). Approaches that block production of ROS such as inhibitors of neuronal nitric oxide synthase and cyclooxygenase 2 also show strong neuroprotective effects against MPTP (21, 29). Conjugating CoQ10 or vitamin E to lipophilic cations, such as triphenylalkylphosphonium ions, can promote their uptake into mitochondria matrix because of the potential gradient across the inner mitochondrial membrane (19). However, it remains to be demonstrated whether this enhanced targeting of antioxidants into the mitochondrial matrix will translate into improved in vivo potency and efficacy in PD.
Another approach is to enhance mitochondrial integrity and prevent apoptotic cell death with MPT inhibitors. Cyclosporin A can attenuate mitochondrial damage, oxidative stress, and apoptosis induced by 1-methyl-4-phenylpyridinium (MPP+), the active metabolite of MPTP (23). However, side effects preclude the use of cyclosporin A for chronic treatment of PD. The neuroprotective effect of minocycline was believed to be, at least in part, due to MPT inhibition (37, 48), but minocycline was unable to prevent MPP+-induced mitochondrial swelling in isolated mitochondria (12) and actually enhanced MPTP toxicity in mice and monkeys (14, 43).
The recent development of a series of mitochondrial targeted peptides that can inhibit MPT provides an opportunity to examine the potential of MPT inhibition as an approach to the treatment of PD (47). The SS (Szeto‱Schiller) peptides are cell-permeable synthetic tetrapeptides that selectively partition to the inner mitochondrial membrane (36, 47). Although these peptides carry 3+ net charge at physiologic pH, their mitochondrial uptake is not dependent on mitochondrial potential, and they do not cause mitochondrial depolarization. These mitochondrial-targeted peptides decrease mitochondrial ROS production, and inhibit mitochondrial swelling and cytochrome c release in isolated mitochondria. The inclusion of a tyrosine or modified tyrosine residue provides additional free radical scavenging properties, and these analogs are very potent in ameliorating ROS-induced cell death (38, 45) and ischemia-reperfusion injury (10, 11, 36). Surprisingly, even the analogs that do not possess intrinsic scavenging ability can significantly reduce oxidative stress and prevent ischemia-reperfusion injury (10, 11, 36). We have reported beneficial effects of one of these mitochondria-targeted peptides, SS-31, in a transgenic mouse model of amyotrophic lateral sclerosis (26). SS-31 treated mice showed significant reduction in loss of motor neurons and oxidative markers in the lumbar spinal cord, and this was accompanied by a significant improvement in motor performance and survival.
In the present studies, we examined the ability of two SS peptides in the mouse MPTP model. SS-31 contains dimethyltyrosine in its structure and can scavenge ROS, whereas SS-20 cannot (36, 47). Significant neuroprotection was observed with both SS-31 and SS-20 in mice treated with MPTP. To better understand the mechanism of action of these two peptides, we have also compared their ability to prevent MPP+ toxicity in dopamine cells in culture and in isolated mitochondria.
Materials and Methods
Chemicals
SS-31 (D-Arg-(2’6’-dimethyltyrosine)-Lys-Phe-NH2) and SS-20 (Phe-D-Arg-Phe-Lys-NH2) were kindly provided by Dr. Peter W. Schiller (Clinical Research Institute of Montreal, Montreal, Quebec, Canada). The neurotoxic compounds MPTP, MPP+, HPLC standard compounds dopamine, 3, 4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and all other chemicals were bought from Sigma (St. Louis, MO).
MPTP studies in mice
Male C57 black mice (3 months old, 25∼30 grams) were obtained from the Jackson Laboratory (Bar Harbor, ME). MPTP manipulation was carried out in a special animal room restricted for neurotoxin manipulation. All experiments were conducted in accordance with guidelines approved by the Institution for the Care and Use of Animals at Weill Medical College of Cornell University.
MPTP, prepared 2 mg/ml in phosphate buffered saline (PBS), was injected intraperitoneally (i.p.) to C57 black mice with dose of 10 mg/kg for three times in a 2 h interval. SS-31 and SS-20, both dissolved in PBS, were injected i.p. to mice 30 min before each MPTP dose, then 1 and 12 h after the last MPTP dose. Control group of mice were injected with the same volume of PBS vehicle. Mice were sacrificed 7 days after MPTP injection, fresh striata were dissected for HPLC catecholamine analysis, and midbrains were postfixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). A group of mice injected with MPTP 20 mg/kg with or without SS-31 (1 mg/kg) were sacrificed 90 min after the MPTP dose and the striata were dissected for measurement of MPP+ levels (following a protocol as previously reported (40)).
HPLC assay for catecholamines
Dissected striatum was homogenized in chilled 0.1 M perchloric acid (PCA, about 100 μl/mg tissue) by sonication, and an aliquot was taken for protein assay and the rest was centrifuged at 14,000 rpm, 4°C for 15 min. The supernatants were taken for measurements of dopamine and its metabolites DOPAC and HVA by HPLC, as modified from our previously described method (42). Briefly, 15 μl supernatant was isocratically eluted through an 80 × 4.6 mm C18 column (ESA, Inc Chelmsford, MA) with a mobile phase containing 0.1 M LiH2PO4, 0.85 mM 1-octanesulfonic acid, and 10% (vol/vol) methanol, and detected by a 2-channel Coulochem II electrochemical detector (ESA, Inc.). Concentrations of dopamine, DOPAC, and HVA are expressed as nanograms per milligram protein. The protein concentrations of tissue homogenates were measured according to the Bio-Rad protein analyze protocol (Bio-Rad Laboratories, Hercules, CA) and Perkin Elmer Bio Assay Reader (Norwalk, CT).
HPLC assay for MPP+
Striatal tissues were processed in 0.1 M PCA as described above. An aliquot of supernatant was injected onto a Brownlee aquapore × 03-224 cation exchange column (Rainin, Woburn, MA). Samples were eluted isocratically with 20 mM boric acid–sodium borate buffer, pH7.75, containing 3 mM tetrabutylammonium hydrogensulfate, 0.25 mM 1-heptanesulfonic acid and 10% isopropanol. MPP+ was detected with a fluorescence detector set by excitation at 295 nm and emission at 375 nm.
Substantia nigra pars compacta histological analysis
Midbrain tissues fixed in 4% paraformaldehyde for 48 h at 4°C were cryoprotected in 30% sucrose overnight at 4°C. Serial coronal sections (50 μm) were cut through the substantia nigra using a cryostat. Two sets of sections were prepared with each set consisting of seven to eight sections, 100 μm apart. One set of sections was used for Nissl staining (cresyl violet). Another set was processed for tyrosine hydroxylase (TH) immunohistochemistry using avidin–biotin–peroxidase technique. Briefly, free-floating sections were pretreated with 3% H2O2 in PBS for 30 min. The sections were incubated sequentially in (a) 1% bovine serum albumin (BSA)/0.2% Triton X-100 for 30 min, (b) rabbit anti-TH affinity purified antibody (Chemicon, Temecula, CA; 1:4000 in PBS/0.5% BSA) for 18 h, (c) biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA; 1:500 in PBS/0.5%BSA) for 1 h, and (d) avidin–biotin–peroxidase complex (Vector; 1:500 in PBS) for 1 h. The immunoreaction was visualized using 3,3’-diaminobenzidine tetrahydrochloride dihydrate (DAB) with nickel intensification (Vector) as the chromogen. All incubations and rinses were performed with agitation using an orbital shaker at room temperature. The sections were mounted onto gelatin-coated slides, dehydrated, cleared in xylene, and coverslipped. The numbers of Nissl-stained or TH-immunoreactive cells in the substantia nigra pars compacta (SNpc) were counted using the optical fractionator method in the Stereo Investigator (v. 4.35) software program (Microbrightfield, Burlington, VT).
Cell culture studies
The substantia nigra-derived dopaminergic cell line (SN4741) was kindly provided by Dr. Jin Son (Burke Medical Research Institute, White Plains, NY) and cultured as described previously (34). Cells were maintained in RF medium that contained DMEM supplemented with 10% fetal calf serum (FCS), 1% glucose, penicillin–streptomycin and l-glutamine. Cells were grown at 37°C and in 5% CO2 to <70% confluence to minimize dedifferentiation. One day before study, SN4741 cells were plated in RF medium containing 0.5% FCS in 96-well plates at a density of 1-2 × 103 cells/well. MPP+ was added at 100 μM alone, or in the presence of SS-31 or SS-20, and incubated for 48 h. Cell viability was evaluated using the Almar blue assay (Biosource, Camarillo, CA). For quantification of apoptosis, SN4741 cells were treated with MPP+ in the absence or presence of SS-31 or SS-20 for 12–24 h. Cells were then stained with 2 mg/ml Hoechst 33342 for 20 min, fixed with 4% paraformaldehyde, and imaged using a Zeiss fluorescent microscope. Nuclear morphology was evaluated using an excitation wavelength of 350 ± 10 nm and a longpass filter of 400 nm for emission. All images were processed and analyzed using the MetaMorph software (Universal Imaging Corp., West Chester, PA). Uniformly stained nuclei were scored as healthy, viable neurons, while condensed or fragmented nuclei were scored as apoptotic.
Mitochondrial respiration studies
Mouse liver mitochondria were isolated from CD-1 mice, as described previously (47). Oxygen consumption was measured with a Clark-type oxygen electrode (Hansatech Instruments, Norfolk, UK). 1.0 mg of mouse liver mitochondria was incubated with 100 μM MPP+ in the absence or presence of SS peptides in 1 ml of respiration buffer (220 mM mannitol, 75 mM sucrose, 2.5 mM K2HPO4, 5 mM HEPES, 2 mM MgCl2, 5 mM glutamate, 2.5 mM malate, and 0.1% BSA, pH 7.4) at RT for 5 min. The oxygen consumption rate was determined at RT and stage 3 respiration was induced by the addition of 800 μM ADP. At 2 min after ADP addition, 5 μl of mitochondrial suspension was collected for ATP determination using a luciferase bioluminescent assay (Sigma-Aldrich, St. Louis, MO). The sample was immediately added to 395 μl of releasing reagent and frozen until analysis.
Mitochondrial swelling assays
Isolated mouse liver mitochondria (0.1 mg) were added to 1.0 ml of buffer (70 mM sucrose, 214 mM mannitol, 5 mM HEPES, 5 mM glutamate, 0.5 mM malate, pH 7.5) containing 50 μM Ca2+ and 0.25 mM Pi, and MPP+ was added alone or together with SS-31 or SS-20. Mitochondrial swelling was measured by decrease in absorbance at 540 nm using a 96-well plate reader.
Statistical analysis
Results were expressed as means ± standard error of means (SEM). Differences were assessed by ANOVA, followed by Tukey–Kramer test for comparison among different means or the Dunnett's test for comparison to a control group. A value of p < 0.05 was taken as being statistically significant.
Results
SS-31 and SS-20 attenuate MPTP-induced depletion of dopamine and its metabolites in mouse striatum
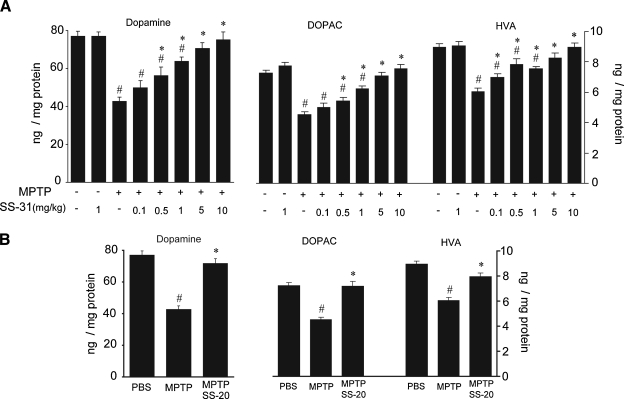
To investigate neuroprotection in MPTP models, it is important to choose proper MPTP dosage to cause dopamine system damage in a moderate range. The C57 black mouse strain is susceptible to MPTP toxin. We injected these mice with three doses of 10 mg/kg of MPTP in a 2-h interval, which caused 44% depletion of dopamine in the striatum. We tested the neuroprotective effects of SS-31 in a range of doses from 0.1 to 10 mg/kg against the moderate MPTP-induced dopamine depletion. SS-31 provided a significant dose-dependent protective effect in rescuing dopamine levels depleted by MPTP toxin. The lowest dose of 0.1 mg/kg SS-31 attenuated MPTP-induced dopamine depletion by 15%, but failed to reach statistical significance. With higher SS-31 doses of 0.5, 1, 5, and 10 mg/kg, statistically significant protective effects were achieved in attenuating MPTP-induced dopamine depletion by 24%, 33%, 39%, and 43%, respectively, and the 5 and 10 mg/kg doses rescued the striatal dopamine from MPTP-induced depletion back to the control levels (Fig. 1A). The dopamine metabolites DOPAC and HVA showed the same dose-dependent protection patterns as dopamine (Fig. 1A).
FIG. 1.
The neuroprotective effect of SS-31 and SS-20 on mice treated with MPTP. (A) In a moderate MPTP modality (10 mg/kg, three doses in 2 h interval), dopamine depletion in striatum by MPTP toxin was 44%, and SS-31 showed a significant dose-respondent protection effect on MPTP-induced dopamine depletion. DOPAC and HVA levels showed dose-respondent protection effects of SS-31. (B) 4 mg/kg SS-20 in a moderate MPTP modality (10 mg/kg, three doses in every 2 h) significantly protected dopamine, DOPAC, and HVA from MPTP-induced depletion. #p < 0.01 compared with normal control. *p < 0.05 compared with MPTP alone, n = 10.
Surprisingly, SS-20 also provided significant protection of dopamine neurons at the moderate MPTP exposure (Fig. 1B). 4 mg/kg of SS-20 attenuated 40% of the MPTP-induced dopamine depletion (p < 0.01) to close to the dopamine level of controls (p > 0.05) (Fig. 1B). The dopamine metabolites DOPAC and HVA showed the same protection patterns as dopamine (Fig. 1B).
Neither SS-31 nor SS-20 (data not shown) altered dopamine and its metabolites in mouse striatum when given alone.
SS-31 and SS-20 protect dopaminergic neurons in substantia nigra against MPTP damage
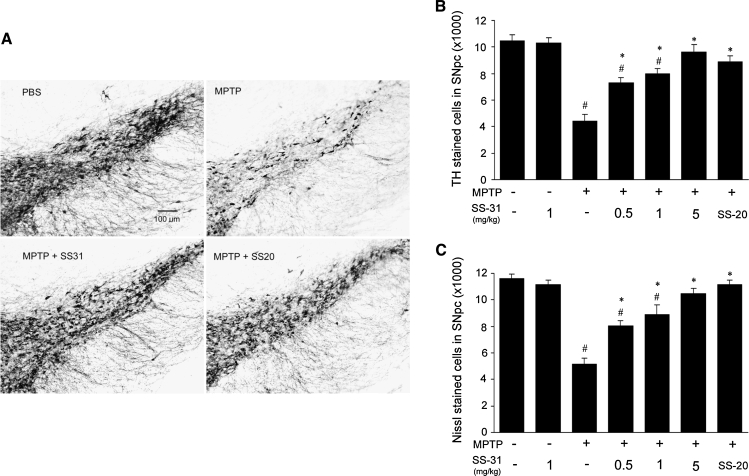
Losses of dopaminergic TH-positive neurons in the SNpc induced by MPTP (10 mg/kg, three doses) were significantly prevented by both SS-31 and SS-20 (Fig. 2A). Dose-dependent protective effects were seen with SS-31 doses of 0.5, 1, and 5 mg/kg. SS-20 also showed significant protection at a dose of 4 mg/kg (Fig. 2B). Nissl stained neuron counts verified the TH-positive neuronal loss (Fig. 2C).
FIG. 2.
SS-31 and SS-20 protect dopaminergic neurons in substantia nigra against MPTP damage. (A) Representative slides showed TH-positive neuronal loss in SNpc caused by MPTP toxicity and protected by either SS-31 or SS-20. (B) TH-positive neuronal counts in SNpc showed SS-31 (0.5, 1, and 5 mg/kg) dose-dependently protected neurons from MPTP (10 mg/kg, three doses in 2 h interval) caused cell loss and SS-20 (4 mg/kg) also attained significant protection. (C) Nissl stained neuron counts in SNpc verified the enzyme stained TH-positive neuronal loss. #p < 0.05 compared with normal control. *p < 0.05 compared with MPTP alone, n = 10.
SS-31 does not interact with MPTP metabolism in the brain
Ninety minutes after intraperitoneal injection of MPTP 20 mg/kg, MPP+ levels in the mouse striatum were not different between the MPTP alone group (4.56 ± 0.6 ng/mg wet tissue) and the group injected with MPTP and 1 mg/kg SS-31 (4.72 ± 0.3 ng/mg wet tissue), indicating that neuroprotective effects of SS-31 are not due to an effect on MPTP uptake or conversion of MPTP to MPP+.
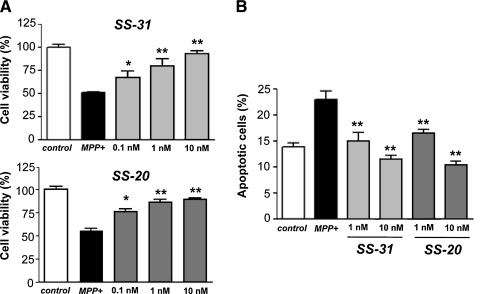
SS-31 and SS-20 prevent cell death elicited by MPP+ in SN4741 dopamine cells
To show that SS peptides are effective when applied in vitro to cells, SN4741 dopamine cells were treated with MPP+ (100 μM) for 48 h in the absence or presence of SS peptides. Incubation with MPP+ alone resulted in a significant reduction in cell viability, while concurrent incubation of these cells with either SS-31 or SS-20 dose-dependently increased cell viability (p < 0.001) (Fig. 3A). The morphologic appearance of cells treated with MPP+ was consistent with apoptosis. Cells treated with 100 μM MPP+ became rounded and shrunken. Staining with Hoechst 33324 showed an increased number of cells with nuclear fragmentation and condensation. Concurrent treatment with either SS-31 or SS-20 dose-dependently reduced the percentage of apoptotic cells (Fig. 3B).
FIG. 3.
SS-31 and SS-20 reduced MPP+-induced cell death in SN4741 dopamine cells. SN4741 cells were treated with MPP+ (100 μM) for 48 h in the absence or presence of SS peptides. Cell viability was quantified by the Almar Blue assay. (A) Concurrent treatment with SS-31 or SS-20 dose-dependently attenuated the reduction in cell viability induced by MPP+ *p < 0.05; **p < 0.01 compared to treatment with MPP+ alone. (B) SS-31 and S-S20 reduced apoptotic cell death induced by 100 μM MPP+. Apoptotic cells were evaluated by microscopy using the Hoechst 33324 dye and cells with fragmented or condensed nuclei were scored as apoptotic. **p < 0.01 compared to treatment with MPP+ alone.
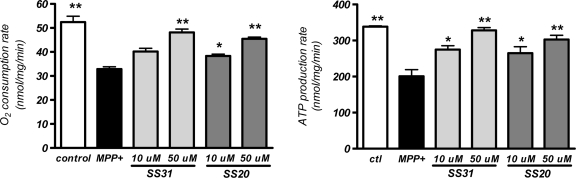
SS-31 and SS-20 prevent MPP+-induced inhibition of mitochondrial O2 consumption and ATP production
MPP+ is known to inhibit mitochondrial complex I activity, and previous studies showed that MPP+ reduces mitochondrial O2 consumption and ATP production (7, 15). Isolated liver mitochondria were therefore used to better understand the mechanisms of cytoprotection provided by SS-31 and SS-20. Treatment of liver mitochondria with 100 μM MPP+ significantly decreased state 3 oxygen consumption, and this reduction was dose-dependently attenuated by the addition of either SS-31 or SS-20 (p < 0.001) (Fig. 4). Oxygen consumption in mitochondria treated with MPP+ in the presence of 50 μM of either SS-31 or SS-20 was not different as compared to untreated mitochondria. There was no difference between the protection provided by SS-31 or SS-20. Neither SS-31 nor SS-20 alone altered oxygen consumption in isolated liver mitochondria (data not shown). The inhibition of mitochondrial oxygen consumption by MPP+ was associated with a significant decrease in ATP production (p < 0.001) (Fig. 4). Treatment with either SS-31 or SS-20 also dose-dependently prevented the reduction in ATP production (Fig. 4). The inhibition of ATP production was almost completely prevented by 50 μM of either SS-31 or SS-20.
FIG. 4.
SS-31 and SS-20 reduced MPP+-induced inhibition of mitochondrial oxygen consumption and ATP production. Isolated mouse liver mitochondria were incubated in respiratory buffer and oxygen consumption was measured by a Clark-type oxygen electrode. State 3 respiration was initiated by the addition of ADP. Co-incubation with 50 μM SS-31 or 10 μM and 50 μM SS-20 significantly attenuated the inhibition of oxygen consumption induced by 100 μM MPP+. ATP production was determined by using luciferase bioluminescent assay. Co-incubation with both doses of 10 μM and 50 μM of either SS-31 or SS-20 significantly attenuated the inhibition of ATP production induced by 100 μM MPP+. *p < 0.05; **p < 0.01 compared to treatment with MPP+ alone. Incubation with SS-31 or SS-20 alone did not alter oxygen consumption and ATP production (data not shown).
SS-31 and SS-20 prevent MPP+-induced mitochondrial swelling
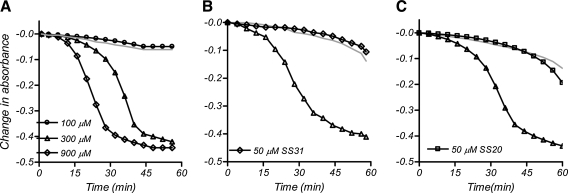
In addition to impairment of ATP production, MPP+ has been shown to cause mitochondrial swelling which can lead to cytochrome c release (6). We, therefore, examined whether the SS peptides can prevent MPP+-induced mitochondrial swelling. The addition of MPP+ induced swelling in liver mitochondria in a dose-dependent manner (Fig. 5A). The swelling caused by 300 μM MPP+ was completely inhibited by the addition of 50 μM SS-31 (Fig. 5B) or SS-20 (Fig. 5C).
FIG. 5.
SS-31 and SS-20 reduced mitochondrial swelling induced by 300 μM MPP+ in the presence of 50 μM Ca2+ and Pi. Mitochondrial swelling was measured by decrease in absorbance at 540 nm. Addition of MPP+ alone dose-dependently increased mitochondrial swelling (A). The light gray line indicates no MPP+ added. The swelling induced by 300 μM MPP+ was prevented by addition of 50 μM SS-31 (B) or 50 μM SS-20 (C).
Discussion
Both mitochondrial dysfunction and oxidative damage are strongly implicated in the pathogenesis of PD (24). The development of agents that can target mitochondria, protect mitochondrial function, and that also exert antioxidant properties is of great potential interest for the treatment of PD. In the present study, we examined the effects of SS-31 and SS-20, two mitochondria-targeted peptides that have been shown to reduce mitochondrial H2O2 production, inhibit mitochondrial swelling, and prevent ischemia-reperfusion injury (36). The major difference between the two peptides is that SS-31 can scavenge ROS because of the phenoxyl group on dimethyltyrosine (36, 47). As a result, SS-31, but not SS-20, has been shown to be very potent in preventing cell death caused by t-butylhydroperoxide (45) or hypochlorous acid (38).
Therapeutics development for neurodegenerative diseases has been hampered by difficulty in delivering compounds across the blood-brain barrier. Brain uptake of these aromatic-cationic tetrapeptides has been shown to be rapid and helps to account for their efficacy in the MPTP model in our present study. The distribution of SS-20 to the brain was determined at various times after subcutaneous injection of 10 mg/kg to rats and brain concentration (determined by HPLC/MS) was found to be 15.1, 7.7, 3.97, and 1.26 ng/g at 0.5, 2, 6, and 16 h after administration (Dajun Yang, personal communication, Stealth Peptides Inc).
The results of the present study show that both SS-31 and SS-20 were equally effective in preventing MPTP neurotoxicity. To investigate neuroprotection in MPTP models, it is important to choose proper MPTP dosage to cause dopamine system damage in a moderate range. We, therefore, chose MPTP at a dose of 10 mg/kg administered three times. This produced approximately a 50% reduction in dopamine. Under these circumstances, SS-31 at 0.5, 1, 5, and 10 mg/kg produced statistically significant dose-dependent neuroprotection, and even the lowest dose of 0.1 mg/kg showed a trend in attenuating MPTP-induced dopamine depletion by 15%. At 5 and 10 mg/kg of SS-31, there was complete protection against loss of dopamine and its metabolites. SS-20 was equally effective in preventing the loss of dopamine, DOPAC, and HVA in the moderate MPTP treatment paradigm, and restored the levels to those seen in controls.
We also examined the ability of SS-31 and SS-20 to protect dopaminergic neurons in the SNpc against MPTP induced damage. We found that SS-31 produced dose-dependent neuroprotective effects, with complete protection at 5 mg/kg. SS-20 at a dose of 4 mg/kg also produced complete neuroprotection against loss of dopaminergic neurons in the SNpc.
The results of this study show that the mitochondria-targeted peptides, SS-31 and SS-20, are highly effective as neuroprotective agents in vivo. It should be noted that the peptides were only administered 30 min before the injection of MPTP. This is consistent with the ability of these small aromatic-cationic peptides to rapidly penetrate cell membranes and diffuse across cellular monolayers (46). Furthermore, our results show that the two peptides do not affect MPTP uptake into the brain, nor do they alter the metabolism of MPTP to MPP+.
The efficacy of SS-20 was unexpected since it is generally thought that ROS play a major role in MPTP neurotoxicity and SS-20 cannot scavenge ROS (36, 47). The ability of SS-20 to protect against MPTP neurotoxicity in the whole animal and prevent MPP+-induced cytotoxicity suggests that the cytoprotective effects of these compounds may occur by mechanisms other than scavenging ROS.
MPP+ inhibits complex I (NADH dehydrogenase) of the electron transport chain (25), and this has been associated with increased superoxide formation by mitochondrial enzymes (1, 18). Later studies, however, found no increase in ROS induced by MPP+ in isolated brain mitochondria when concentrations were kept below 1 mM (2, 15). We also found no increase in ROS production when isolated mitochondria were incubated with up to 300 μM of MPP+ (data not shown). This concentration, however, significantly reduced mitochondrial oxygen consumption and ATP production. A rapid loss of intracellular ATP has also been reported by other investigators (2, 7, 15) and may be the major cause of cell death. Glucose supplementation to enhance anaerobic glycolysis was able to protect against MPP+ toxicity in C6 glioma cells (39), and infusion of the ketone body D-β-hydroxybutyrate also produced protection against MPTP neurotoxicity in mice (33). Interestingly, both SS-31 and SS-20 were able to significantly prevent the inhibition of mitochondrial oxygen consumption and ATP production caused by MPP+, and prevent cell death. These results suggest that the primary cause of cell death elicited by MPP+ may be inhibition of mitochondrial ATP synthesis rather than free radical formation, and that the SS peptides can reduce cell death by preserving mitochondrial ATP synthesis.
In addition to impairment of ATP production, MPP+ may also contribute to neurotoxicity by causing mitochondrial swelling and cytochrome c release (6). In the presence of Ca2+ and Pi, MPP+ induces mitochondrial swelling in both liver and brain mitochondria (6). The effect of MPP+ is inhibited by cyclosporin A, suggesting that MPP+ induces the MPT. Attenuation of MPP+-induced mitochondrial swelling by superoxide dismutase and catalase led to the suggestion that MPP+ caused MPT via oxidative mechanisms. In our study, MPP+ induced mitochondrial swelling was completely inhibited by 50 μM SS-31 or SS-20, concentrations shown to prevent MPP+-induced ATP depletion. ATP depletion is known to potentiate MPT in cultured hepatocytes (8). Upon depletion of ATP, calcium homeostasis cannot be maintained and MPT is induced (33). Thus we believe that the SS peptides prevent MPTP toxicity by two mechanisms—preserving mitochondrial ATP synthesis and inhibiting MPT.
The remarkable potency of the SS peptides in preventing MPP+-induced cell death (nM) may appear to be at odds with the μM concentration required for protecting mitochondrial ATP synthesis and MPT. This may be explained by the selective partitioning of these peptides in the mitochondrial inner membrane. Peptide uptake studies in isolated mitochondria have shown that the concentrations of SS-31 and its analogs in mitochondria are 1,000–5,000 times higher than extramitochondrial concentrations (45, 47). Thus, extracellular concentrations in the nM range could result in >1 μM concentration in the mitochondrial inner membrane. Similar potency has been observed for SS-31 in preventing mitochondrial depolarization and apoptosis caused by t-butylhydroperoxide (45).
We have previously examined the effectiveness of SS-31 for the treatment of amyotrophic lateral sclerosis (ALS) (26). Mouse neuroblastoma cells (N2A) stably transfected with G93A mutant SOD1 show increased sensitivity to oxidative stress induced by H2O2. SS-31 dose-dependently reduced H2O2-induced cell death in G93A cells. We also examined SS-31 in the G93A transgenic mouse model of familial ALS. These mice develop progressive weight loss, impaired motor function eventually paralysis leading to premature death. This is accompanied by a marked loss of motor neurons within the spinal cord. We found that administration of SS-31 led to a significant improvement in survival and motor performance, as well as decreased cell loss and decreased oxidative stress in the lumbar spinal cord.
Our present results provide evidence that these mitochondria-targeted peptides, which act at the inner mitochondrial membrane and protect against mitochondrial damage, represent a novel approach to treating neurodegenerative diseases (23, 45). Due to the strong implication of both mitochondrial dysfunction and oxidative damage in PD pathogenesis, this approach may be promising for the treatment of PD.
Abbreviations Used
- CoQ10
coenzyme Q10
- DOPAC
3,4 dihydroxybenzoic acid
- HVA
homovanillic acid
- MPT
mitochondrial permeability transition
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson's disease
- ROS
reactive oxygen species
- SS-20
Phe-D-Arg-Phe-Lys-NH2
- SS-31
D-Arg-(2′6′-dimethyltyrosine)-Lys-Phe-NH2
Acknowledgments
This work was supported by the Parkinson's Disease Foundation, the Michael J. Fox Foundation, the Department of Defense, and the National Institute of Health (PO1 DA08924 (HHS) and R21 NS048295 (HHS)). The editorial assistance of Greta Strong is gratefully acknowledged.
Author Disclosure Statement
Patent applications have been filed by Cornell Research Foundation Inc (CRF) for the technology (SS peptides) described in this article. Hazel H. Szeto is the inventor. CRF, on behalf of Cornell University, has licensed the technology for further research and development to a commercial enterprise in which CRF and Dr. Szeto have financial interests.
References
- 1.Adams JD., Jr. Klaidman LK. Leung AC. MPP+ and MPDP+ induced oxygen radical formation with mitochondrial enzymes. Free Radic Biol Med. 1993;15:181–186. doi: 10.1016/0891-5849(93)90057-2. [DOI] [PubMed] [Google Scholar]
- 2.Bates TE. Heales SJ. Davies SE. Boakye P. Clark JB. Effects of 1-methyl-4-phenylpyridinium on isolated rat brain mitochondria: Evidence for a primary involvement of energy depletion. J Neurochem. 1994;63:640–648. doi: 10.1046/j.1471-4159.1994.63020640.x. [DOI] [PubMed] [Google Scholar]
- 3.Beal MF. Matthews RT. Tieleman A. Shults CW. Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1,2,3,tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 1998;783:109–114. doi: 10.1016/s0006-8993(97)01192-x. [DOI] [PubMed] [Google Scholar]
- 4.Betarbet R. Sherer TB. MacKenzie G. Garcia–Osuna M. Panov AV. Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 5.Canet–Aviles RM. Wilson MA. Miller DW. Ahmad R. McLendon C. Bandyopadhyay S. Baptista MJ. Ringe D. Petsko GA. Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassarino DS. Parks JK. Parker WD., Jr. Bennett JP., Jr. The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 7.Chan P. DeLanney LE. Irwin I. Langston JW. Di Monte D. Rapid ATP loss caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse brain. J Neurochem. 1991;57:348–351. doi: 10.1111/j.1471-4159.1991.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen LJ. Gao YQ. Li XJ. Shen DH. Sun FY. Melatonin protects against MPTP/MPP+ -induced mitochondrial DNA oxidative damage in vivo and in vitro. J Pineal Res. 2005;39:34–42. doi: 10.1111/j.1600-079X.2005.00209.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q. Lesnefsky EJ. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic Biol Med. 2006;40:976–982. doi: 10.1016/j.freeradbiomed.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 10.Cho J. Won K. Wu D. Soong Y. Liu S. Szeto HH. Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis. 2007;18:215–220. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- 11.Cho S. Szeto HH. Kim E. Kim H. Tolhurst AT. Pinto JT. A novel cell-permeable antioxidant peptide, SS-31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- 12.Cornet S. Spinnewyn B. Delaflotte S. Charnet C. Roubert V. Favre C. Hider H. Chabrier PE. Auguet M. Lack of evidence of direct mitochondrial involvement in the neuroprotective effect of minocycline. Eur J Pharmacol. 2004;505:111–119. doi: 10.1016/j.ejphar.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Darios F. Corti O. Lucking CB. Hampe C. Muriel MP. Abbas N. Gu WJ. Hirsch EC. Rooney T. Ruberg M. Brice A. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- 14.Diguet E. Fernagut PO. Wei X. Du Y. Rouland R. Gross C. Bezard E. Tison F. Deleterious effects of minocycline in animal models of Parkinson's disease and Huntington's disease. Eur J Neurosci. 2004;19:3266–3276. doi: 10.1111/j.0953-816X.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- 15.Fonck C. Baudry M. Rapid reduction of ATP synthesis and lack of free radical formation by MPP+ in rat brain synaptosomes and mitochondria. Brain Res. 2003;975:214–221. doi: 10.1016/s0006-8993(03)02675-1. [DOI] [PubMed] [Google Scholar]
- 16.Greenamyre JT. Betarbet R. Sherer TB. The rotenone model of Parkinson's disease: Genes, environment and mitochondria. Parkinsonism Relat Disord. 2003;9(Suppl 2):S59–64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 17.Haas RH. Nasirian F. Nakano K. Ward D. Pay M. Hill R. Shults CW. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Ann Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa E. Takeshige K. Oishi T. Murai Y. Minakami S. 1-Methyl-4-phenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem Biophys Res Commun. 1990;170:1049–1055. doi: 10.1016/0006-291x(90)90498-c. [DOI] [PubMed] [Google Scholar]
- 19.Kelso GF. Porteous CM. Hughes G. Ledgerwood EC. Gane AM. Smith RA. Murphy MP. Prevention of mitochondrial oxidative damage using targeted antioxidants. Ann NY Acad Sci. 2002;959:263–274. doi: 10.1111/j.1749-6632.2002.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 20.Klein C. Schlossmacher MG. The genetics of Parkinson disease: Implications for neurological care. Nat Clin Pract Neurol. 2006;2:136–146. doi: 10.1038/ncpneuro0126. [DOI] [PubMed] [Google Scholar]
- 21.Klivenyi P. Gardian G. Calingasan NY. Yang L. Beal MF. Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J Mol Neurosci. 2003;21:191–198. doi: 10.1385/jmn:21:3:191. [DOI] [PubMed] [Google Scholar]
- 22.Klivenyi P. Matthews RT. Wermer M. Yang L. MacGarvey U. Becker DA. Natero R. Beal MF. Azulenyl nitrone spin traps protect against MPTP neurotoxicity. Exp Neurol. 1998;152:163–166. doi: 10.1006/exnr.1998.6824. [DOI] [PubMed] [Google Scholar]
- 23.Lee CS. Park WJ. Ko HH. Han ES. Differential involvement of mitochondrial permeability transition in cytotoxicity of 1-methyl-4-phenylpyridinium and 6-hydroxydopamine. Mol Cell Biochem. 2006;289:193–200. doi: 10.1007/s11010-006-9164-0. [DOI] [PubMed] [Google Scholar]
- 24.Lin MT. Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 25.Nicklas WJ. Vyas I. Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 26.Petri S. Kiaei M. Damiano M. Hiller A. Wille E. Manfredi G. Calingasan NY. Szeto HH. Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;98:1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- 27.Petrosillo G. Ruggiero FM. Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 28.Przedborski S. Tieu K. Perier C. Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J Bioenerg Biomembr. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- 29.Schulz JB. Matthews RT. Muqit MM. Browne SE. Beal MF. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- 30.Sherer TB. Betarbet R. Stout AK. Lund S. Baptista M. Panov AV. Cookson MR. Greenamyre JT. An in vitro model of Parkinson's disease: Linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shidoji Y. Hayashi K. Komura S. Ohishi N. Yagi K. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem Biophys Res Commun. 1999;264:343–347. doi: 10.1006/bbrc.1999.1410. [DOI] [PubMed] [Google Scholar]
- 32.Shults CW. Coenzyme Q10 in neurodegenerative diseases. Curr Med Chem. 2003;10:1917–1921. doi: 10.2174/0929867033456882. [DOI] [PubMed] [Google Scholar]
- 33.Simbula G. Glascott PA., Jr. Akita S. Hoek JB. Farber JL. Two mechanisms by which ATP depletion potentiates induction of the mitochondrial permeability transition. Am J Physiol. 1997;273:C479–488. doi: 10.1152/ajpcell.1997.273.2.C479. [DOI] [PubMed] [Google Scholar]
- 34.Son JH. Chun HS. Joh TH. Cho S. Conti B. Lee JW. Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J Neurosci. 1999;19:10–20. doi: 10.1523/JNEUROSCI.19-01-00010.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swerdlow RH. Parks JK. Miller SW. Tuttle JB. Trimmer PA. Sheehan JP. Bennett JP., Jr. Davis RE. Parker WD., Jr. Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 36.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2007;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 37.Wang X. Zhu S. Drozda M. Zhang W. Stavrovskaya IG. Cattaneo E. Ferrante RJ. Kristal BS. Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whiteman M. Spencer JP. Szeto HH. Armstrong JS. Do mitochondriotropic antioxidants prevent chlorinative stress-induced mitochondrial and cellular injury? Antioxid Redox Signal. 2007;10:641–650. doi: 10.1089/ars.2007.1879. [DOI] [PubMed] [Google Scholar]
- 39.Williams ZR. Goodman CB. Soliman KF. Anaerobic glycolysis protection against 1-methy-4-phenylpyridinium (MPP+) toxicity in C6 glioma cells. Neurochem Res. 2007;32:1071–1080. doi: 10.1007/s11064-006-9276-7. [DOI] [PubMed] [Google Scholar]
- 40.Wu DC. Jackson–Lewis V. Vila M. Tieu K. Teismann P. Vadseth C. Choi DK. Ischiropoulos H. Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamakura F. Taka H. Fujimura T. Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- 42.Yang L. Calingasan NY. Chen J. Ley JJ. Becker DA. Beal MF. A novel azulenyl nitrone antioxidant protects against MPTP and 3-nitropropionic acid neurotoxicities. Exp Neurol. 2005;191:86–93. doi: 10.1016/j.expneurol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Yang L. Sugama S. Chirichigno JW. Gregorio J. Lorenzl S. Shin DH. Browne SE. Shimizu Y. Joh TH. Beal MF. Albers DS. Minocycline enhances MPTP toxicity to dopaminergic neurons. J Neurosci Res. 2003;74:278–285. doi: 10.1002/jnr.10709. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L. Shimoji M. Thomas B. Moore DJ. Yu SW. Marupudi NI. Torp R. Torgner IA. Ottersen OP. Dawson TM. Dawson VL. Mitochondrial localization of the Parkinson's disease related protein DJ-1: Implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 45.Zhao K. Luo G. Giannelli S. Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 46.Zhao K. Luo G. Zhao GM. Schiller PW. Szeto HH. Transcellular transport of a highly polar 3+ net charge opioid tetrapeptide. J Pharmacol Exp Ther. 2003;304:425–432. doi: 10.1124/jpet.102.040147. [DOI] [PubMed] [Google Scholar]
- 47.Zhao K. Zhao GM. Wu D. Soong Y. Birk AV. Schiller PW. Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 48.Zhu S. Stavrovskaya IG. Drozda M. Kim BY. Ona V. Li M. Sarang S. Liu AS. Hartley DM. Wu DC. Gullans S. Ferrante RJ. Przedborski S. Kristal BS. Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]