Abstract
Tumor necrosis factor-α (TNF) is a key cytokine that has been shown to play important physiologic (e.g., inflammation) and pathophysiologic (e.g., various liver pathologies) roles. In liver and other tissues, TNF treatment results in the simultaneous activation of an apoptotic pathway (i.e., TRADD, RIP, JNK) and a survival pathway mediated by NF-κB transcription of survival genes (i.e., GADD45β, Mn-SOD, cFLIP). The cellular response (e.g., proliferation versus apoptosis) to TNF is determined by the balance between the apoptotic signaling pathway and the NF-κB survival pathway stimulated by TNF. Reactive oxygen species (ROS) are important modulators of signaling pathways and can regulate both apoptotic signaling and NF-κB transcription triggered by TNF. ROS are important in mediating the sustained activation of JNK, to help mediate apoptosis after TNF treatment. In some cells, ROS are second messengers that mediate apoptosis after TNF stimulation. Conversely, ROS can cause redox modifications that inhibit NF-κB activation, which can lead to cell death triggered by TNF. Consequently, the redox status of cells can determine the biologic response that TNF will induce in cells. In many liver pathologies, ROS generated extrinsically (e.g., inflammation) or intrinsically (i.e., drugs, toxins) may act in concert with TNF to promote hepatocyte death and liver injury through redox inhibition of NF-κB. Antioxid. Redox Signal. 11, 2245–2263.
Introduction
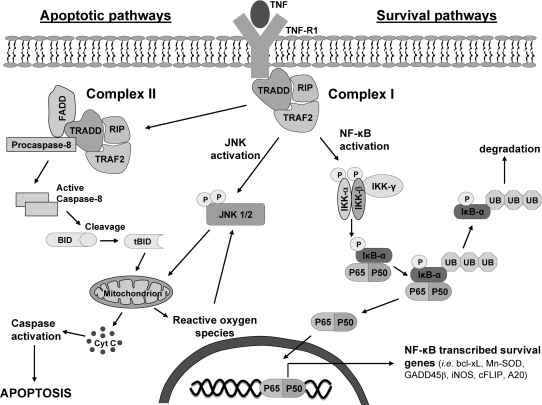
Tumor necrosis factor-α (TNF) is a key cytokine that regulates many biologic responses in cells, including inflammation, proliferation, differentiation, and cell death (14, 151). TNF is an important regulator of inflammation and regulates cytokine production in immune cells. In liver, TNF can trigger both a regenerative and a cell-death response. TNF is essential in liver regeneration after injury, and the inhibition of TNF signaling can prevent liver regeneration (164). Conversely, TNF is also believed to trigger cell death and tissue injury in many liver pathologies, including liver ischemia/reperfusion injury, alcoholic liver disease, viral hepatitis, and liver toxicity caused by toxins such as carbon tetrachloride (118, 136, 166). The pleiotropic biologic effects of TNF can be attributed to its ability to simultaneously activate multiple signaling pathways in cells (Fig. 1). In liver and other tissues, TNF binding to TNF -receptor 1 (TNF-R1) will concurrently activate apoptotic pathways, involving TNF receptor–associated protein with death domain (TRADD), Fas-associated death domain (FADD), caspase-8 and c-Jun N-terminal kinase (JNK), and a survival pathway mediated by the activation of NF-κB, an important transcription factor in the stress response (151, 160). Binding of TNF to TNF-R1 will induce TRADD binding, which in turn promotes association of receptor-interacting kinase (RIP) and FADD to form a complex, which can eventually lead to activation of JNK and caspase-8, important in triggering apoptosis. However, this complex also simultaneously activates NF-κB, which initiates the transcription of survival proteins such as bcl-xl, c-FLIP, X-linked inhibitor of apoptosis (XIAP), and growth arrest DNA damage–inducible gene 45β (GADD45β), which will inhibit key apoptotic proteins activated by TNF. In addition, NF-κB upregulates signaling proteins, such as inducible nitric oxide synthase (iNOS), and antioxidant enzymes such as Mn-superoxide dismutase (SOD) that also modulate cell signaling and survival (62, 157). Consequently, under normal conditions, NF-κB activation will prevent TNF-induced cell death in hepatocytes and other primary cells, and NF-κB–regulated signaling pathways become the predominant biologic response to TNF. The importance of NF-κB in cell survival is underscored by the fact that many cancers have evolved constitutively active NF-κB for survival and growth (132). Conversely, if NF-κB is inhibited, then apoptotic pathways triggered by TNF become dominant, and cell death will ensue (85, 160). Many pathologies associated with TNF-induced injury, such as alcohol-induced liver injury, may be a consequence of inhibition of NF-κB signaling, allowing TNF-stimulated apoptosis to proceed.
FIG. 1.
Activation of apoptotic and survival pathways by TNF. The pleiotropic biologic effects of TNF can be attributed to its ability to activate apoptotic and survival pathways simultaneously. The apoptotic pathway is initialized by the formation of complex I, which is eventually internalized to form complex II. Complex II activates caspase-8, which cleaves Bid to tBid, which translocates to mitochondria and permeabilizes the mitochondrial outer membrane to allow cytochrome c release and possibly to increase mitochondrial ROS generation. The newly released cytochrome c can further activate other caspases, which can target mitochondria, leading to a positive-feedback loop, resulting in extensive caspase activation and ROS generation and ultimately apoptosis. TRAF-2 in complex I also activates the MAP kinase cascade [ASK-1, MKK4/7 (not shown)], leading to the activation of JNK, important in mediating apoptosis in some cells. After activation, JNK translocates to mitochondria and promotes cytochrome c release, mitochondrial permeability transition, and possibly increased ROS generation. Because JNK can be activated by ROS, a positive-feedback loop may ensue in which JNK translocation to mitochondria causes increased ROS generation, which activates more JNK molecules. The survival pathway is mediated by NF-κB transcription of survival genes that block many proteins involved in apoptosis. Complex I will activate IKK, which phosphorylates IκB-α, promoting its ubiquitination and degradation. The degradation of IκB-α releases NF-κB, allowing NF-κB to translocate to the nucleus and promote transcription of survival genes. Consequently, the transcription of NF-κB–regulated proteins, not apoptosis, is the major response to TNF in hepatocytes and other primary cells, under normal conditions.
Key regulators of TNF signaling pathways are reactive oxygen species [ROS; e.g., superoxide (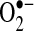 ), hydrogen peroxide (H2O2), and hydroxyl radical (HO•)] (56, 73, 131). First recognized as toxic metabolites of oxygen metabolism, ROS are now recognized as important regulators of many signaling pathways (26, 119). ROS have been suggested to modulate TNF signaling, both the apoptotic signaling pathways and the survival signaling pathways regulated by NF-κB (55, 125). In some cells, ROS have been suggested to act as second messengers that promote sustained activation of JNK and promote apoptotic signaling initiated by TNF (131). ROS also have been shown to inhibit NF-κB activation to prevent transcription of survival genes, particularly when ROS levels are high (30, 55, 131). Many proteins involved in NF-κB activation have redox-sensitive cysteines, which upon being oxidized will inhibit their activity (82, 112). ROS, through promotion of pro-death proteins such as JNK and inhibition of NF-κB, can promote TNF-induced cell death. Consequently, the redox status of cells may determine, to a great extent, the biologic response that TNF will induce in cells. The redox inhibition of NF-κB may be a key component of many pathologies, particularly during inflammation, when both ROS and TNF are simultaneously released by immune cells at high levels (55). Many pathologies associated with TNF, such as alcohol-induced liver injury, may be a consequence of TNF-induced apoptosis caused by a redox disruption in NF-κB signaling by ROS.
), hydrogen peroxide (H2O2), and hydroxyl radical (HO•)] (56, 73, 131). First recognized as toxic metabolites of oxygen metabolism, ROS are now recognized as important regulators of many signaling pathways (26, 119). ROS have been suggested to modulate TNF signaling, both the apoptotic signaling pathways and the survival signaling pathways regulated by NF-κB (55, 125). In some cells, ROS have been suggested to act as second messengers that promote sustained activation of JNK and promote apoptotic signaling initiated by TNF (131). ROS also have been shown to inhibit NF-κB activation to prevent transcription of survival genes, particularly when ROS levels are high (30, 55, 131). Many proteins involved in NF-κB activation have redox-sensitive cysteines, which upon being oxidized will inhibit their activity (82, 112). ROS, through promotion of pro-death proteins such as JNK and inhibition of NF-κB, can promote TNF-induced cell death. Consequently, the redox status of cells may determine, to a great extent, the biologic response that TNF will induce in cells. The redox inhibition of NF-κB may be a key component of many pathologies, particularly during inflammation, when both ROS and TNF are simultaneously released by immune cells at high levels (55). Many pathologies associated with TNF, such as alcohol-induced liver injury, may be a consequence of TNF-induced apoptosis caused by a redox disruption in NF-κB signaling by ROS.
In this review, we examine how ROS and redox changes are important in modulating both apoptotic signaling and NF-κB signaling in response to TNF. Because TNF and NF-κB signaling pathways vary greatly in cells, a great deal of our discussion focuses on TNF signaling in hepatocytes and liver pathologies mediated by TNF.
TNF Signaling Pathways
TNF and TNF receptor
TNF is a 17-kDa cytokine that is released during inflammation by macrophages and other immune cells (14, 151). TNF is the founding member of the TNF superfamily that includes some 19 proteins such as Fas ligand and tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) (87). Two different TNF receptors have been identified in cells: TNF receptor-1 (TNF-R1) and TNF receptor-2 (TNF-R2). A soluble form of TNF receptor (sTNF) also is found in vivo and can reduce free circulating levels of TNF. TNF-R1 is expressed in virtually all cells, whereas TNF-R2 is found primarily in immune cells, where it regulates inflammation (151). TNF-R2 may enhance TNF-R1–induced signaling and apoptotic signaling, but does not directly trigger apoptosis in cells. Consequently, this review focuses on TNF-R1–mediated signaling important in regulating cell differentiation and death in liver and other tissues.
Apoptotic signaling pathway activated by TNF
As previously discussed, the opposing biologic responses (differential and death) that TNF can elicit in tissues, such as liver, are due to TNF activation of both apoptotic and survival signaling pathways in cells (14, 37, 126). The binding of TNF to TNF-R1 will induce an apoptotic response mediated by JNK and caspase-8 and a survival response mediated by NF-κB. However, it must be noted that TNF signaling is very cell specific, and a great degree of variability in signaling pathways activated by TNF can be found in the literature. Consequently, when possible, we focus on TNF signaling in primary hepatocytes or hepatocyte cell lines or both. In most cells, the binding of TNF to TNF-R1 causes a conformation change that recruits TRADD, receptor-interacting kinase (RIP), and TNF-receptor–associated factor 2 (TRAF2) to form a complex, referred to as complex I (Fig. 1) (95). Complex I is believed to be important in triggering both NF-κB activation and JNK activation. Complex I eventually dissociates from TNF-R1 and becomes internalized to the cytoplasm, where it integrates Fas-associated death domain (FADD) and procaspase-8 to form a complex referred to as complex II (95). The formation of complex II is believed to be important in activating apoptotic signaling pathways through activation of caspase-8, which promotes the cleavage of Bid to tBid (truncated Bid) (160). tBid was shown to translocate to mitochondria and permeabilizes the mitochondrial outer membrane to allow cytochrome c release, which can further activate other caspases, leading to a positive-feedback loop resulting in extensive caspase activation and ultimately apoptosis (160, 169). In many cells, ROS have been suggested to increase during or after complex I and II formation (1‱6 h after TNF treatment) to mediate or help potentiate apoptosis after TNF stimulation (24, 36, 121).
TRAF-2 in complex I also is believed to activate the MAP kinase cascade [apoptosis signal-regulating kinase 1 (ASK-1), mitogen-activated protein kinase kinase 4/7 (MKK4/7) that leads to the activation of JNK, a member of the mitogen-activated protein kinase (MAPK) family important in stress response] (104, 160). JNK is important in the stress response and is activated by a wide range of environmental stresses (i.e., ROS, heat) and various cytokines (130). Whereas JNK is important in the stress response, when its activation is prolonged, JNK is believed to mediate both apoptotic and necrotic cell death (51, 85, 130). Sustained JNK activity is a key component of hepatocyte injury during acetaminophen-induced liver injury and ischemia/reperfusion injury in liver (51, 59). JNK activation by complex I after TNF treatment was shown to be transient (30‱60 min), because NF-κB activation is believed to shut down JNK (104, 131). In many cells, including hepatocyte cell lines (e.g., RALA255-10G), prolonged JNK activity (>120 min) is essential for TNF-induced apoptosis to occur (85). In these cells, inhibition of JNK, through chemical or genetic means, prevented TNF-induced apoptosis. However, the requirement of JNK in mediating TNF-induced cell death may be cell specific. JNK 1−/− and JNK 2−/− embryonic fibroblasts were found to have more apoptosis when treated with TNF than did wild-type cells (67). In addition, a recent study showed that concanavalin A (Con A)-induced hepatitis (liver injury mediated through TNF) does not require JNK activation in hepatocytes (29). Thus, JNK activation may be integral for TNF-stimulated apoptosis in some cells, but may not be necessary for apoptosis in all cells.
Survival pathways activated by TNF–NF-κB activation
NF-κB is an essential transcription factor that regulates inflammation, stress response, and survival. NF-κB is a dimer (homo or hetero) composed of members of the NF-κB subfamily (i.e., p50, p52) and Rel subfamily [i.e., p65 (Rel A), c-Rel, RelB]. In most cells, the most prominent form of NF-κB is the p50/p65 heterodimer, which binds to the κB sites in DNA. Normally NF-κB is found anchored in the cytoplasm with IκB-α. When TNF binds TNF receptor, complex I forms and will activate IκB kinase (IKK) (47, 151). IKK is a complex composed of two catalytic subunits (i.e., IKK-α, IKK-β) and a regulatory subunit, IKK-γ. Once activated, IKK will phosphorylate IκB-α, which triggers ubiquitination and degradation of IκB-α. The degradation of IκB-α releases NF-κB, allowing NF-κB to translocate to the nucleus and to promote transcription of survival genes. NF-κB is essential in survival, and mice lacking the p65 subunit of NF-κB or various IKK subunits will die during embryogenesis because of hepatocyte apoptosis (47). NF-κB upregulates antiapoptotic proteins such as bcl-xL, c-FLIP, and XIAP, which can inhibit proapoptotic bcl-2 family members and caspases (151, 160). For example, c-FLIP will inhibit caspase-8, central in mediating TNF-induced apoptosis (94). XIAP, A20, and GADD45β (105, 160) are NF-κB–transcribed genes that have been suggested to inhibit JNK activated by complex I after TNF stimulation. The importance of GADD45β in regulating JNK (through MKK7, the MAPK kinase upstream of JNK) in liver was recently observed in GADD45β(−/−) mice (105). GADD45β(−/−) mice were found to have increased sustained JNK activity, decreased hepatocyte proliferation, and increased cell death during liver regeneration (a process mediated by TNF) than did wild-type mice (106). In addition to GADD45β, iNOS, A20, and bcl-xl transcribed by NF-κB contribute in protecting liver from cell death induced by TNF (8, 62). NF-κB also upregulates antioxidant enzymes such as Mn-SOD (SOD1‱mitochondria isoform) and ferritin, which are essential in inhibiting TNF-induced apoptosis in some cancer cells (65, 108, 157). NF-κB was suggested to inhibit ROS generated after TNF treatment in many cells (121), although this mechanism remains unclear. TNF can induce both apoptosis and necrosis in cells, with FADD/caspase 8 important in mediating apoptosis and RIP important in mediating necrosis (104). For this review, we focus mainly on TNF-induced apoptosis, the most common consequence after TNF treatment when NF-κB is inhibited. Although NF-κB activation primarily activates prosurvival pathways in cells, in some examples, NF-κB activation induces apoptosis in cells (104).
The activation of NF-κB and transcription of NF-κB genes is a major response to TNF stimulation in cells (151, 160). In immune cells, NF-κB transcribes many cytokines and proteins, such as iNOS, important in inflammation. In liver and other tissue, NF-κB–dependent genes inhibit apoptotic pathways and promote survival and the stress response (73, 126). Liver regeneration requires NF-κB activation to prevent apoptosis and help normal cell progression after injury (16). Because NF-κB is activated, most primary cells are highly resistant to TNF, and very little cell death ensues in culture (36, 41, 55). Consequently, to study apoptotic signaling pathways induced by TNF, NF-κB activation or transcription (or both) of NF-κB–dependent survival genes must be inhibited in most cells (85, 160). NF-κB transcription of survival genes is generally inhibited chemically (i.e., actinomycin, RNA synthesis inhibitor, or cycloheximide, a protein synthesis inhibitor), or through genetic modulation (e.g., knocking down IKK). Co-treatment of hepatocytes with cycloheximide or actinomycin D renders hepatocytes and hepatocyte cell lines susceptible to the cytotoxic effects of TNF by inhibiting expression of NF-κB–transcribed survival genes (36, 85). However, the application of findings from cell-culture studies that use chemical inhibition of NF-κB to situations in vivo is somewhat problematic, because the mechanism of NF-κB inhibition in vivo is likely through very different mechanisms. Thus, some findings from cell-culture studies may not completely reflect the TNF signaling pathways that occur in vivo in TNF-mediated pathologies such as alcohol-induced liver injury.
Another uncertainty in studying TNF signaling in cell culture is the dose of TNF administered to cells. TNF doses used in cell culture can vary greatly, with 5 ng/ml to up to 100 ng/ml of TNF being reported to be used in studies involving cultured primary hepatocytes. Because of NF-κB activation, primary hepatocytes can withstand extremely high doses of TNF (370 ng/ml) without any significant apoptosis occurring (32). However, increasing doses of TNF can cause more apoptosis in hepatocytes sensitized to TNF-induced cell death by treatments such as alcohol, suggesting that varying doses of TNF may modulate the extent of TNF signaling pathways (23). The levels of TNF that cells encounter in vivo during various pathologies are difficult to ascertain. During septic shock episodes in vivo, plasma levels of TNF have been measured to be ∼3 ng/ml (28), suggesting that many doses used in cell culture studies may be on the high side. However, during inflammation, cells such as hepatocytes may encounter highly localized levels of TNF secreted by neighboring macrophages and therefore exposed to higher levels of TNF than seen in plasma. More studies using varying doses of TNF in cell-culture studies must be examined, because in vivo doses of TNF remain uncertain.
Regulation of TNF Signaling by ROS
ROS have been suggested to be important regulators of signaling pathways stimulated by TNF (55, 56, 73, 131). In the past two decades, a great deal of work has shown that both 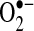 and H2O2 can regulate many signaling pathways.
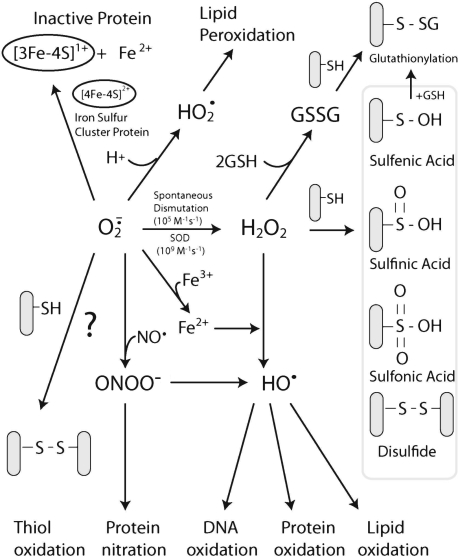
and H2O2 can regulate many signaling pathways. 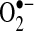 and H2O2 have different chemical properties and thus can regulate different signaling pathways (Fig. 2).
and H2O2 have different chemical properties and thus can regulate different signaling pathways (Fig. 2). 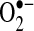 was shown to modulate pathways involving Fas signaling and voltage-dependent anion channels (VDACs) (22, 90), whereas H2O2 was shown to regulate many kinases, including JNK, PKC, Akt, and various tyrosine kinases (119, 127, 170). In some signaling pathways, including apoptotic signaling stimulated by TNF, ROS were suggested as second messengers needed to mediate apoptosis in some cells (104, 131, 160). Because no receptors for ROS have been discovered, it is generally believed that ROS modulate signal pathways through altering redox status of key proteins (56, 139). Thiols in the cysteine residue of proteins or in glutathione (GSH), the major nonprotein thiol in cells, can become oxidized to form a disulfide (reaction 1).
was shown to modulate pathways involving Fas signaling and voltage-dependent anion channels (VDACs) (22, 90), whereas H2O2 was shown to regulate many kinases, including JNK, PKC, Akt, and various tyrosine kinases (119, 127, 170). In some signaling pathways, including apoptotic signaling stimulated by TNF, ROS were suggested as second messengers needed to mediate apoptosis in some cells (104, 131, 160). Because no receptors for ROS have been discovered, it is generally believed that ROS modulate signal pathways through altering redox status of key proteins (56, 139). Thiols in the cysteine residue of proteins or in glutathione (GSH), the major nonprotein thiol in cells, can become oxidized to form a disulfide (reaction 1).
FIG. 2.
Redox changes mediated by 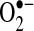 and H2O2 in cells.
and H2O2 in cells. 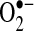 can be generated by many sources in cells, including NADPH oxidase and the mitochondrial respiratory chain.
can be generated by many sources in cells, including NADPH oxidase and the mitochondrial respiratory chain. 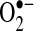 can mediate many reactions that modulate cellular redox status and signaling pathways: (a)
can mediate many reactions that modulate cellular redox status and signaling pathways: (a) 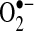 can oxidize proteins with iron-sulfur clusters such as aconitase and other metal groups to modulate activity and function; (b)
can oxidize proteins with iron-sulfur clusters such as aconitase and other metal groups to modulate activity and function; (b) 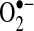 can reduce transition metals (e.g., Fe2+ to Fe3+) important in catalyzing the formation of hydroxyl radical (the most reactive and damaging radical) from H2O2; (c) when nitric oxide is present,
can reduce transition metals (e.g., Fe2+ to Fe3+) important in catalyzing the formation of hydroxyl radical (the most reactive and damaging radical) from H2O2; (c) when nitric oxide is present, 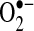 will react with nitric oxide to generate ONOO−, a potent oxidant; (d)
will react with nitric oxide to generate ONOO−, a potent oxidant; (d) 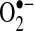 reactivity increases after protonated
reactivity increases after protonated 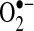 (perhydroxyl radical), which can initiate free radical chain reactions such as lipid peroxidation; and (e)
(perhydroxyl radical), which can initiate free radical chain reactions such as lipid peroxidation; and (e) 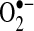 can oxidize thiols to disulfides, but whether the reaction rates are physiologically relevant is debated.
can oxidize thiols to disulfides, but whether the reaction rates are physiologically relevant is debated. 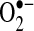 will, spontaneously or through actions of SOD, dismutate into H2O2. H2O2 can readily oxidize cysteines in proteins to disulfides and sulfenic, sulfinic, or sulfonic acids, which can affect protein activity. It is believed that H2O2 modifies signaling pathways through posttranslational modification of cysteine and other amino acids (e.g., methionine) on proteins.
will, spontaneously or through actions of SOD, dismutate into H2O2. H2O2 can readily oxidize cysteines in proteins to disulfides and sulfenic, sulfinic, or sulfonic acids, which can affect protein activity. It is believed that H2O2 modifies signaling pathways through posttranslational modification of cysteine and other amino acids (e.g., methionine) on proteins.
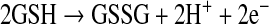 |
In cells, most thiols are in the reduced form with the GSH/GSSG ratio being >100:1 and most proteins being in the reduced thiol form (75, 123). The protein redox status in cells is regulated by GSH along with the proteins, glutaredoxin and thioredoxin (Trx) (56). Glutaredoxin catalyzes the deglutathionylation of proteins by using the reducing power of GSH, whereas Trx (Trx-1 cytoplasmic isoform; Trx-2 mitochondria isoform) contains vicinal thiols that react with and reduce disulfide crosslinks. GSH, along with these redox-regulating proteins, help keep most of the protein in the intracellular environment in the reduced thiol redox state. ROS, conversely, can alter the redox state of cells and oxidize protein thiols. The oxidation of thiols by ROS have been shown to alter the activity of many proteins (56, 123). H2O2 can oxidize cysteines in proteins to disulfides, sulfenic, sulfinic, and sulfonic acids, depending on the concentrations and reactivity of the thiol (Fig. 2). Sulfenic acids (--SOH) can react with GSH and become converted to a glutathionylation bond (--SSG). Glutathionylation of proteins also can occur when GSSG levels increase in response to increased H2O2 levels (due to increased GSH peroxidase activity, which uses the reducing power of GSH to form GSSG during H2O2 detoxification). Not all protein thiols have the same reactivity, and the localized environment (e.g., pKa of neighboring amino acids) will greatly affect thiol reactivity. Thiolates (--S−, deprotonated thiols), being strong nucleophiles, are generally more reactive than thiol groups. It is believed that H2O2 modifies signaling pathways through posttranslational modification of cysteine or other amino acids (i.e., methionine, proline) on proteins (139). 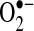 can also oxidize thiols to disulfides, but whether the reaction rates are physiologically relevant is debated (35, 156).
can also oxidize thiols to disulfides, but whether the reaction rates are physiologically relevant is debated (35, 156). 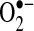 also can oxidize proteins with iron-sulfur clusters such as aconitase and other metal groups to modulate activity and function (46). Many targets of H2O2 and
also can oxidize proteins with iron-sulfur clusters such as aconitase and other metal groups to modulate activity and function (46). Many targets of H2O2 and 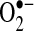 exist inside cells, which could modulate signaling pathways (Fig. 2).
exist inside cells, which could modulate signaling pathways (Fig. 2).
Many pathways activated by TNF, such as JNK and NF-κB, have clearly been shown to be modulated by ROS through redox changes to various regulatory proteins. Proteins, such as Trx-1, involved in JNK activation (through ASK-1 binding), and IKK, involved in NF-κB activation, contain critical cysteines that regulate function (82, 120). Consequently, ROS have been shown to modulate both the apoptotic signaling pathway and NF-κB survival pathways stimulated by TNF, through redox changes to signaling proteins. This has been supported by studies that showed the addition of exogenous ROS or agents that trigger increase in endogenous ROS generation (mitochondria inhibitors that increase ROS) sensitize many cells, including primary hepatocytes, to TNF-induced apoptosis (without actinomycin or cycloheximide treatment) (24, 55, 125, 167). ROS have been shown to sensitize hepatocytes to TNF-induced apoptosis, through inhibition of NF-κB transcription of survival genes (55). The notion that ROS inhibits NF-κB through redox changes to proteins is supported by the observations that redox-modulating agents or GSH-depleting agents [i.e., diamide, diethylmaleate (DEM)] also sensitize hepatocytes to the cytotoxic effects of TNF (55, 88, 92, 99). The sensitization of hepatocytes to TNF by ROS was attributed mainly to redox inhibition of NF-κB, but changes to other redox-sensitive pathways, such as JNK and Akt, also may contribute to increased sensitization to TNF. Redox modulation of TNF signaling may be an important mechanism of sensitizing hepatocytes to the cytotoxic effects of TNF, which may be important in many liver pathologies that are discussed later in the review.
Many works have also suggested that ROS act as second messengers and mediate TNF-induced apoptosis in various cells, when NF-κB transcription of survival genes is inhibited [i.e., actinomyin, cycloheximide, or through genetic means, as reviewed in (104, 131, 160)]. The evidence that ROS are essential in TNF signaling is based on the following observations: (a) an increase in ROS generation [by using fluorescent probes such as dichlorodihydrofluorescein (DCFH)] is detected after TNF treatment to cells, generally before apoptosis (36, 72, 121, 149); (b) antioxidant treatment can prevent TNF-induced cell death in many cells (36, 121); (c) redox changes in proteins (increased disulfide formation) have been observed after TNF treatment to cells (72, 141). Taken together, many researchers have suggested ROS are essential mediators of apoptosis induced by TNF in many cells, including primary hepatocytes. However, the evidence that ROS are second messengers or are necessary during TNF-induced apoptosis has had some methodologic problems. One problem is that the measurement of ROS, because of their reactivity, is extremely difficult (57). Most studies have relied on fluorescence probes such as DCFH to detect ROS, which are nonspecific and prone to artifacts that can generate false-positive signals (153). In addition, studies using antioxidants to demonstrate involvement of ROS are not always conclusive, because antioxidants can have secondary effects, and inhibition of signaling pathways by antioxidants does not necessarily prove ROS involvement (15, 64). Thus, the role of ROS in TNF-stimulated apoptosis may be overstated in some cells, although, in other cells, ROS appear to play an essential role. In the next section, we examine (a) the experimental data implicating ROS as second messengers in TNF signaling, and (b) the proteins involved in TNF signaling that are regulated by redox changes.
ROS as Second Messengers in TNF-Induced Apoptosis
Methodologic problems
Because of their reactivity and consequent short half-life, ROS are very difficult to detect intracellularly. ROS cannot be directly detected; instead, exogenous probes that react with ROS to form a unique measurable product are commonly used (57, 153). In most, if not all TNF studies, researchers have relied on fluorescent probes that react with ROS and become oxidized to a molecule with a different fluorescent characteristic than the parent compound, which can be readily measured. Two probes, DCFH and hydroethidine (HE), are probably the most widely used probes to measure ROS in cells and have been used in the majority, if not all, of studies involving TNF (24, 36, 72, 121, 149). Although these two probes are convenient and easy to use, they have many inherent problems that make problematic the assessment of ROS in TNF signaling. Both probes can generate many false-positive signals during apoptosis, making results obtained by using these probes very difficult to interrupt (153). In the next section, we review the use of DCFH and HE in measuring ROS in TNF signaling and problems in interpretation that arise from using these probes.
Dichlorodihydrofluorescein (DCFH)
DCFH is probably the most widely used probe to measure H2O2 in cells. Most researchers use DCFH-DA (DCFH diacetate ester form), which is believed to enter cells and become cleaved by esterases to trap DCFH inside cells. DCFH, which is nonfluorescent, is believed to be oxidized by H2O2 to DCF (dichlorofluorescein), which can be measured fluorometrically in cells. DCF fluorescence is usually observed to increase after TNF stimulation before apoptosis (about 3 to 6 h when NF-κB is inhibited), in a wide range of cells, including primary hepatocytes (24, 49, 60, 121, 133). When NF-κB is not inhibited and apoptosis is inhibited, DCF fluorescence is usually not detected or is detected at much lower levels than during TNF-induced apoptosis. Antioxidants such as trolox (water-soluble analogue) and butylated hydroxyanisole (BHA) have been shown to prevent apoptosis and DCF fluorescence, supporting the role of ROS in TNF-induced apoptosis in cells (36, 121). However, many inherent problems exist in using DCFH, and it was widely criticized as a specific measure of H2O2. First, DCFH retention (conversion from DCFH-DA) and diffusion inside cells is dependent on esterase activity and cellular pH. Cellular pH levels frequently change during apoptosis; these are likely to affect DCFH uptake and retention (110, 153). Second, DCFH is not oxidized to DCF by H2O2, but rather by more-reactive oxidants (HO·, ONOO−) and labile iron (117, 142). DCFH is also oxidized by various oxidases and peroxidases through a catalytic process, which does not necessarily depend on changes in H2O2 levels (13, 153). Third, antioxidants, especially exogenously added antioxidants, greatly affect DCF fluorescence independent of H2O2 levels (153). Most problematic in the use of DCFH for TNF studies is that cytochrome c can act as a peroxidase and oxidize DCFH to DCF, independent of changes to H2O2 levels (18, 84). Because cytochrome c is released during TNF-induced apoptosis (when DCF fluorescence is the greatest), DCF oxidation observed after TNF treatment may be a consequence of cytochrome c release that preceded apoptosis rather than changes in H2O2 levels. Free iron and GSH redox, which have been shown to change after TNF stimulation in some cells, have also been shown to affect DCF fluorescence (84, 153). DCFH oxidation may reflect free cytochrome c levels or other redox changes rather than changes in H2O2 or 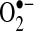 levels in cells (84). Because DCFH was the predominant method used to assess H2O2 in most TNF studies, it is difficult to conclude that TNF treatment increases H2O2 generation in cells. The DCFH data reflect some type of redox change, but whether this is due to H2O2 increase or cytochrome c release or other variables (iron levels, GSH changes) remains to be determined.
levels in cells (84). Because DCFH was the predominant method used to assess H2O2 in most TNF studies, it is difficult to conclude that TNF treatment increases H2O2 generation in cells. The DCFH data reflect some type of redox change, but whether this is due to H2O2 increase or cytochrome c release or other variables (iron levels, GSH changes) remains to be determined.
Hydroethidine
HE (sometimes referred to as dihydroethidine) and its mitochondrial counterpart, MitoSOX (hydroethidine linked to hexy-triphenylphosphonium cation to target mitochondria) are commonly used to assess 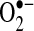 levels inside cells and mitochondria. HE has become a popular probe to measure intracellular
levels inside cells and mitochondria. HE has become a popular probe to measure intracellular 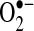 , replacing lucigenin and luminol in popularity, which have been heavily criticized because of their generation of false-positive signals (40, 57). Initially, HE was believed to be specifically oxidized by
, replacing lucigenin and luminol in popularity, which have been heavily criticized because of their generation of false-positive signals (40, 57). Initially, HE was believed to be specifically oxidized by 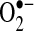 to form ethidine (E+), which has a different fluorescence characteristic than HE that can be monitored (11). Several cell-culture studies, including those involving primary hepatocytes, have shown an increase in E+ formation after TNF treatment, suggesting increases in
to form ethidine (E+), which has a different fluorescence characteristic than HE that can be monitored (11). Several cell-culture studies, including those involving primary hepatocytes, have shown an increase in E+ formation after TNF treatment, suggesting increases in 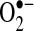 generation in cells (36, 52, 121). However, further analysis of HE revealed that
generation in cells (36, 52, 121). However, further analysis of HE revealed that 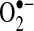 does not oxidize HE to E+; rather, HE is oxidized to E+ by other reactive molecules or through the action of various peroxidases and oxidases (116, 153). Cytochrome c and other mitochondrial heme proteins also have been identified to catalyze HE oxidation to E+ (107).
does not oxidize HE to E+; rather, HE is oxidized to E+ by other reactive molecules or through the action of various peroxidases and oxidases (116, 153). Cytochrome c and other mitochondrial heme proteins also have been identified to catalyze HE oxidation to E+ (107). 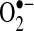 reaction with HE will not generate E+ but rather another product, 2-hydroxyl-ethidine (2-OH-E+). The fluorescence spectrum of 2-OH-E+ overlaps with the fluorescence (excitation, 535 nm; emission, 610 nm) wavelengths used to monitor E+ (172). Consequently, most studies that have used HE have been monitoring both E+ and 2-OH-E+, a
reaction with HE will not generate E+ but rather another product, 2-hydroxyl-ethidine (2-OH-E+). The fluorescence spectrum of 2-OH-E+ overlaps with the fluorescence (excitation, 535 nm; emission, 610 nm) wavelengths used to monitor E+ (172). Consequently, most studies that have used HE have been monitoring both E+ and 2-OH-E+, a 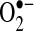 -independent and -dependent product. Taken together, the data suggests that increased E+ fluorescence after TNF stimulation may be a consequence of initial cytochrome c release or other redox changes caused by TNF and not attributable to changes in
-independent and -dependent product. Taken together, the data suggests that increased E+ fluorescence after TNF stimulation may be a consequence of initial cytochrome c release or other redox changes caused by TNF and not attributable to changes in 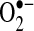 . Measurement of 2-OH-E+ after HE treatment was suggested to be a specific measure of
. Measurement of 2-OH-E+ after HE treatment was suggested to be a specific measure of 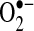 (116, 171). However, because 2-OH-E+ has some fluorescence overlap with E+, additional procedures (i.e., HPLC separation, monitoring dual wavelengths) are required for the specific detection of 2-OH-E+ (115, 171). Further studies using these more-specific methods to measure 2-OH-E+ (a reliable marker of
(116, 171). However, because 2-OH-E+ has some fluorescence overlap with E+, additional procedures (i.e., HPLC separation, monitoring dual wavelengths) are required for the specific detection of 2-OH-E+ (115, 171). Further studies using these more-specific methods to measure 2-OH-E+ (a reliable marker of 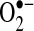 production) are needed to help confirm or negate conclusions from earlier studies that monitored only E+ fluorescence after TNF treatment.
production) are needed to help confirm or negate conclusions from earlier studies that monitored only E+ fluorescence after TNF treatment.
Overall, TNF studies using DCFH and HE do indicate a redox change in cells, but whether the redox change is due to increased 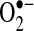 and H2O2 generation or to other changes (i.e., cytochrome c release, free iron, GSH changes) remains to be further investigated. Another problem with the probes is that they remain qualitative, so the extent of the redox changes that occur during these signaling pathways was difficult to assess. Until newer ROS-detection techniques are used in TNF studies, a detailed picture of ROS in TNF signaling will be lacking.
and H2O2 generation or to other changes (i.e., cytochrome c release, free iron, GSH changes) remains to be further investigated. Another problem with the probes is that they remain qualitative, so the extent of the redox changes that occur during these signaling pathways was difficult to assess. Until newer ROS-detection techniques are used in TNF studies, a detailed picture of ROS in TNF signaling will be lacking.
Antioxidants and TNF-Induced Apoptosis
The protective effects of exogenous antioxidants or genetic modulation of antioxidant enzymes against TNF-induced apoptosis have lent support to the notion that ROS are second messengers or mediate apoptosis after TNF. Both the addition of low-molecular-weight antioxidants and the genetic modulation of antioxidant enzymes have been shown to decrease ROS (measured by using DCFH and HE) and in some cases partially prevent TNF-induced apoptosis (15, 37, 38, 121). However, antioxidant treatment, especially at the high doses used in most cell-culture studies, can affect many cellular pathways, independent of antioxidant activity. For example, pyrrolidine dithiocarbamate (PDTC), a metal chelator, was used in many cell-signaling studies to inhibit ROS. However, it was observed that PDTC caused an increase in oxidized glutathione, suggesting that it acts as an oxidizing agent to inhibit NF-κB activation in Jurkat cells (15). Similarly, another commonly used antioxidant, N-acetylcysteine (NAC), was found to inhibit TNF-induced NF-κB activation by reducing the affinity of the TNF receptor (64). Antioxidants also were shown directly to modulate DCFH and HE fluorescence (153). Thus, decreases in the ROS signal observed with antioxidant treatment with DCFH and HE does not necessarily reflect changes in ROS levels in cells. Given that antioxidants work through other mechanisms besides antioxidant action, some caution is required in the interpretation of data that demonstrate the protective effects of antioxidants against TNF-induced apoptosis. These data obtained with antioxidants still have some merit, but additional experiments are needed to confirm that TNF leads to increased ROS generation in cells. In hepatocytes, the antioxidants Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, a water-soluble form of vitamin E) and Mn-TAP (a chemical mimic of superoxide dismutase) prevent apoptosis induced by TNF and cycloheximide (to inhibit synthesis of NF-κB proteins) (37). Because Mn-TAP detoxifies 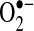 , the data would suggest that
, the data would suggest that 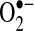 may mediate TNF-induced apoptosis. Trolox, conversely, does not decrease levels of
may mediate TNF-induced apoptosis. Trolox, conversely, does not decrease levels of 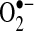 or H2O2, but rather decreases free-radical chain reactions such as lipid peroxidation, suggesting that lipid peroxidation or other free radical chain reactions mediate TNF-induced apoptosis. Based on these antioxidant data, ROS appear to help mediate apoptosis stimulated by TNF in primary hepatocytes, but further validation is necessary.
or H2O2, but rather decreases free-radical chain reactions such as lipid peroxidation, suggesting that lipid peroxidation or other free radical chain reactions mediate TNF-induced apoptosis. Based on these antioxidant data, ROS appear to help mediate apoptosis stimulated by TNF in primary hepatocytes, but further validation is necessary.
In some cells, NF-κB is involved in the transcription of several key antioxidant enzymes, including Mn-SOD, ferritin, and thioredoxin, which play an essential role in preventing apoptosis after TNF treatment. The upregulation of antioxidant enzymes by NF-κB suggests that TNF triggers an increase in ROS generation that is important in mediating apoptosis. Genetic studies modulating antioxidant enzymes, such as Mn-SOD in cells, also support the involvement of ROS in TNF-induced apoptosis in some cells. In several cell lines (i.e., 293 human embryonic cell line, pulmonary adenocarcinoma cells, breast cancer cell lines), Mn-SOD appears to be an essential defense against TNF-induced apoptosis (65, 97, 157). In these cells, NF-κB activation by TNF treatment causes an upregulation of Mn-SOD, which is essential in preventing apoptosis stimulated by TNF. This is supported by the observations that Mn-SOD confers increased resistance to apoptosis induced by TNF-plus-cycloheximide treatment. Conversely, antisense to Mn-SOD made these cells sensitive to TNF, even in the absence of cycloheximide (65, 157).
Mn-SOD resides in the mitochondrial matrix, where it catalyzes the dismutation of 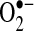 , generated by the respiratory chain, to H2O2 (19). This suggests that in these cell lines, increased
, generated by the respiratory chain, to H2O2 (19). This suggests that in these cell lines, increased 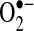 in the mitochondrial matrix, not H2O2, is the key factor in triggering apoptosis stimulated by TNF. In fact, increased expression of Mn-SOD does not decrease H2O2 levels, and, in some cases, may increase H2O2 generation, if the
in the mitochondrial matrix, not H2O2, is the key factor in triggering apoptosis stimulated by TNF. In fact, increased expression of Mn-SOD does not decrease H2O2 levels, and, in some cases, may increase H2O2 generation, if the 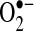 -generating reactions in mitochondria can be pulled forward by Mn-SOD. Thus, it is even possible that increased Mn-SOD expression induced by NF-κB could result in increased H2O2 that may activate or inhibit signaling pathways important in regulating apoptosis, including NF-κB signaling. Conversely, the importance of Mn-SOD in TNF-induced apoptosis more likely suggests that
-generating reactions in mitochondria can be pulled forward by Mn-SOD. Thus, it is even possible that increased Mn-SOD expression induced by NF-κB could result in increased H2O2 that may activate or inhibit signaling pathways important in regulating apoptosis, including NF-κB signaling. Conversely, the importance of Mn-SOD in TNF-induced apoptosis more likely suggests that 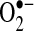 in the mitochondrial matrix is somehow involved in mediating apoptosis stimulated by TNF. This leads to an interesting issue of how
in the mitochondrial matrix is somehow involved in mediating apoptosis stimulated by TNF. This leads to an interesting issue of how 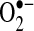 in the mitochondrial matrix is causing apoptosis triggered by TNF in some cells. A number of potential pathways can be affected by
in the mitochondrial matrix is causing apoptosis triggered by TNF in some cells. A number of potential pathways can be affected by 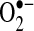 in the matrix, including (a) reduction of transition metals that could generate hydroxyl radical (the most reactive and damaging radical) through Fenton chemistry (19) (although because the mitochondrial matrix is very reduced, it is possible that most free iron may already be reduced, regardless of the presence of
in the matrix, including (a) reduction of transition metals that could generate hydroxyl radical (the most reactive and damaging radical) through Fenton chemistry (19) (although because the mitochondrial matrix is very reduced, it is possible that most free iron may already be reduced, regardless of the presence of 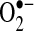 ); (b) inactivation of iron-sulfur cluster proteins such as aconitase, which can also release labile iron that can undergo Fenton chemistry (46); (c) formation of the strong oxidant ONOO−, when nitric oxide is present; and (d) when
); (b) inactivation of iron-sulfur cluster proteins such as aconitase, which can also release labile iron that can undergo Fenton chemistry (46); (c) formation of the strong oxidant ONOO−, when nitric oxide is present; and (d) when 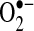 is protonated (HO2·, perhydroxyl radical), it can initiate free-radical chain reactions such as lipid peroxidation (5) (Fig. 2). At present, little experimental evidence supports any particular pathway. However, in rat mesangial cells,
is protonated (HO2·, perhydroxyl radical), it can initiate free-radical chain reactions such as lipid peroxidation (5) (Fig. 2). At present, little experimental evidence supports any particular pathway. However, in rat mesangial cells, 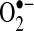 , not ONOO−, was demonstrated to be the important mediator of TNF-induced apoptosis, suggesting that nitric oxide may not play an important role in TNF signaling in these cells (97). Some evidence indicates that
, not ONOO−, was demonstrated to be the important mediator of TNF-induced apoptosis, suggesting that nitric oxide may not play an important role in TNF signaling in these cells (97). Some evidence indicates that 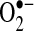 can oxidize thiols to disulfides, similar to H2O2; however, whether the reaction rates are physiologically significant remains controversial (35, 156). The protective effects of Mn-SOD against TNF do not appear universal. In other cell lines, Mn-SOD is not regulated by NF-κB, and Mn-SOD overexpression does not protect against TNF in other cells (104, 108). Thus,
can oxidize thiols to disulfides, similar to H2O2; however, whether the reaction rates are physiologically significant remains controversial (35, 156). The protective effects of Mn-SOD against TNF do not appear universal. In other cell lines, Mn-SOD is not regulated by NF-κB, and Mn-SOD overexpression does not protect against TNF in other cells (104, 108). Thus, 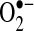 does not appear to be a universal mediator of TNF-induced apoptosis, and a great deal of cell specificity exists regarding the role of
does not appear to be a universal mediator of TNF-induced apoptosis, and a great deal of cell specificity exists regarding the role of 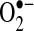 . In hepatocytes, Mn-SOD mRNA appears to be upregulated by TNF (3), but upregulation of Mn-SOD mRNA does not always result in increased translation of Mn-SOD in liver (27). Overexpression of Mn-SOD by using recombinant adenovirus reduced alcohol-induced liver injury in rats (155), a pathology mediated by TNF, suggesting that Mn-SOD could potentially protect against TNF in liver. However, the treatment of mice with hepatotoxin d-galactosamine (GalN) and lipopolysaccharide (LPS), which led to increased TNF expression in liver, did not affect Mn-SOD mRNA levels or expression, suggesting that MnSOD upregulation is not involved in protecting the liver from TNF (27). The importance of Mn-SOD in the liver and TNF-induced liver pathologies remains to be further investigated.
. In hepatocytes, Mn-SOD mRNA appears to be upregulated by TNF (3), but upregulation of Mn-SOD mRNA does not always result in increased translation of Mn-SOD in liver (27). Overexpression of Mn-SOD by using recombinant adenovirus reduced alcohol-induced liver injury in rats (155), a pathology mediated by TNF, suggesting that Mn-SOD could potentially protect against TNF in liver. However, the treatment of mice with hepatotoxin d-galactosamine (GalN) and lipopolysaccharide (LPS), which led to increased TNF expression in liver, did not affect Mn-SOD mRNA levels or expression, suggesting that MnSOD upregulation is not involved in protecting the liver from TNF (27). The importance of Mn-SOD in the liver and TNF-induced liver pathologies remains to be further investigated.
In other cells, ferritin, a protein important in binding and neutralizing labile iron, appears to be important in preventing cell death induced by TNF. In 3DO cells, NF-κB was found to upregulate the ferritin heavy chain (FHC), but not Mn-SOD or thioredoxin (108). Upregulation of FHC by NF-κB was found to be important in preventing ROS generation (measured by using DCFH), JNK activation, and apoptosis caused by TNF (108). In another cell line (human prostate cancer cells, DU145), TNF treatment caused a decrease in levels of the ferritin light chain and increased the levels of labile iron pool (LIP), through a process mediated by JNK (2). Similarly, in L929 cells, an increase in LIP was observed after TNF treatment (161). Ferritin is not normally induced by NF-κB in L929 cells, but the introduction of TNF-inducible FHC prevented TNF-induced ROS generation (measured by using DCFH and HE) and apoptosis (161). In mice, the iron chelator, deferoxamine, increased survival after various TNF-mediated stresses (i.e., LPS, TNF plus galactosamine), suggesting a possible role of iron in mediating TNF-induced liver injury (150).
Taken together, these studies suggest that TNF may increase the LIP to mediate ROS and apoptosis in certain cell lines. LIP can induce the Fenton reaction and cause the formation of the hydroxyl radical, the most reactive and damaging ROS (Fig. 2). 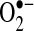 may cause cell damage by reducing ferrous iron (Fe3+) to ferric iron (Fe2+), which catalyzes the breakdown of H2O2 to the hydroxyl radical (Fenton reaction). Thus, a complex network of free-radical reactions involving
may cause cell damage by reducing ferrous iron (Fe3+) to ferric iron (Fe2+), which catalyzes the breakdown of H2O2 to the hydroxyl radical (Fenton reaction). Thus, a complex network of free-radical reactions involving 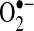 , H2O2, iron, and hydroxyl radical (generated through the Fenton reaction) may be involved in TNF-induced apoptosis in some cells. The hydroxyl radical and LIP can cause the oxidization of DCFH to DCF; thus, changes in DCF fluorescence may be due to increased hydroxyl radicals or iron levels rather than to changes in H2O2 levels (84, 153). Overall, the protective effects of antioxidant enzymes against TNF appear to be cell specific.
, H2O2, iron, and hydroxyl radical (generated through the Fenton reaction) may be involved in TNF-induced apoptosis in some cells. The hydroxyl radical and LIP can cause the oxidization of DCFH to DCF; thus, changes in DCF fluorescence may be due to increased hydroxyl radicals or iron levels rather than to changes in H2O2 levels (84, 153). Overall, the protective effects of antioxidant enzymes against TNF appear to be cell specific.
Protein Redox Changes After TNF Treatment
Additional evidence of increased ROS production during apoptosis comes from studies that observed cellular redox changes and protein redox changes before apoptosis induced by TNF. Several studies reported that a decrease in GSH precedes apoptosis after TNF stimulation (109, 121). As previously mentioned, H2O2 and possibly 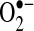 can oxidize protein thiols into disulfides, which can potentially alter protein activity. Several studies have shown that during TNF-induced apoptosis (when NF-κB was inhibited) disulfide bond formation in proteins increases. With a biotinylated GSH probe, glutathionylation of proteins was observed after TNF plus cycloheximide treatment in HeLa cells (141). Proteins identified to be glutathionylated include thioredoxin peroxidase II (a peroxiredoxin) and annexin II. Similarly, thiols in protein phosphatase have been observed to become oxidized (i.e., sulfenic acids and disulfides) after TNF treatment in IKKβ-deficient cells (preventing NF-κB activation) (72). The oxidation of thiols in protein phosphatase leads to decreased dephosphorylation activity and increased duration of JNK phosphorylation, and activity. The thiols in Trx2, Trx reductase, and peroxiredoxin 3 (mitochondrial form) have also been observed to become oxidized after TNF treatment (25, 60, 79). Taken together, these studies suggest an increase in ROS that leads to increased protein thiol oxidation. However, it must be noted that changes in NADPH or various reductases that use NADPH to keep thiols reduced may also increase disulfide formation independent of changes in ROS levels. Whether TNF causes redox changes in primary hepatocytes and other primary cells has not been extensively demonstrated. Protein redox changes are extremely difficult to detect, particularly in vivo, and these protein redox measurements have only recently been achieved. The oxidation of thiols in important signaling proteins such as NF-κB may be the mechanism by which ROS mediates TNF signaling, which is discussed later in the review.
can oxidize protein thiols into disulfides, which can potentially alter protein activity. Several studies have shown that during TNF-induced apoptosis (when NF-κB was inhibited) disulfide bond formation in proteins increases. With a biotinylated GSH probe, glutathionylation of proteins was observed after TNF plus cycloheximide treatment in HeLa cells (141). Proteins identified to be glutathionylated include thioredoxin peroxidase II (a peroxiredoxin) and annexin II. Similarly, thiols in protein phosphatase have been observed to become oxidized (i.e., sulfenic acids and disulfides) after TNF treatment in IKKβ-deficient cells (preventing NF-κB activation) (72). The oxidation of thiols in protein phosphatase leads to decreased dephosphorylation activity and increased duration of JNK phosphorylation, and activity. The thiols in Trx2, Trx reductase, and peroxiredoxin 3 (mitochondrial form) have also been observed to become oxidized after TNF treatment (25, 60, 79). Taken together, these studies suggest an increase in ROS that leads to increased protein thiol oxidation. However, it must be noted that changes in NADPH or various reductases that use NADPH to keep thiols reduced may also increase disulfide formation independent of changes in ROS levels. Whether TNF causes redox changes in primary hepatocytes and other primary cells has not been extensively demonstrated. Protein redox changes are extremely difficult to detect, particularly in vivo, and these protein redox measurements have only recently been achieved. The oxidation of thiols in important signaling proteins such as NF-κB may be the mechanism by which ROS mediates TNF signaling, which is discussed later in the review.
Sources of ROS During TNF-Induced Apoptosis
It is clear that ROS, particularly 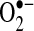 , are important mediators of TNF-induced apoptosis in some cells. Experimental evidence supports the role of ROS in TNF-induced apoptosis in hepatocytes, but further experiments with more specific methods are necessary to validate these findings. The source of ROS that was observed to increase during apoptosis induced by TNF may also be cell specific. Because Mn-SOD is important in preventing apoptosis in some cells, this suggest that mitochondria are the major source of
, are important mediators of TNF-induced apoptosis in some cells. Experimental evidence supports the role of ROS in TNF-induced apoptosis in hepatocytes, but further experiments with more specific methods are necessary to validate these findings. The source of ROS that was observed to increase during apoptosis induced by TNF may also be cell specific. Because Mn-SOD is important in preventing apoptosis in some cells, this suggest that mitochondria are the major source of 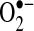 after TNF treatment in these cells. Most studies have suggested that mitochondria are the major sources of ROS during TNF-induced apoptosis, including primary hepatocytes and hepatic cell lines (24, 37, 125, 133). However, several other reports suggest NADPH oxidase as the source of ROS after TNF treatment in fibroblasts and in endothelial and CMVEC cells (10, 80).
after TNF treatment in these cells. Most studies have suggested that mitochondria are the major sources of ROS during TNF-induced apoptosis, including primary hepatocytes and hepatic cell lines (24, 37, 125, 133). However, several other reports suggest NADPH oxidase as the source of ROS after TNF treatment in fibroblasts and in endothelial and CMVEC cells (10, 80).
Mitochondria are major sources of ROS in cells, and it stands to reason that mitochondria are the major source of ROS (both 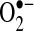 and H2O2) during TNF-induced apoptosis in most cells. Mitochondrial complex I (NADH dehydrogenase) and complex III (ubiquinone oxoreductase) in the respiratory chain have been shown to generate
and H2O2) during TNF-induced apoptosis in most cells. Mitochondrial complex I (NADH dehydrogenase) and complex III (ubiquinone oxoreductase) in the respiratory chain have been shown to generate 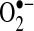 into the mitochondria matrix, where Mn-SOD, residing in the matrix, converts
into the mitochondria matrix, where Mn-SOD, residing in the matrix, converts 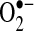 into H2O2. Because H2O2 is freely diffusible (unlike
into H2O2. Because H2O2 is freely diffusible (unlike 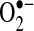 ), a portion of H2O2 diffuses from the mitochondrial matrix, even though GSH peroxidase (important in H2O2 detoxification) also resides in the matrix. Mitochondrial complex III has also been shown to generate
), a portion of H2O2 diffuses from the mitochondrial matrix, even though GSH peroxidase (important in H2O2 detoxification) also resides in the matrix. Mitochondrial complex III has also been shown to generate 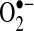 toward the intermembrane space (54, 58, 98), where it can diffuse from mitochondria through VDAC on the outer mitochondrial membrane (53). The basal generation of
toward the intermembrane space (54, 58, 98), where it can diffuse from mitochondria through VDAC on the outer mitochondrial membrane (53). The basal generation of 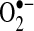 and H2O2 by mitochondria is low, unless the respiratory chain is inhibited by inhibitors such as rotenone (mitochondrial complex I) or antimycin (III) (54). These mitochondria inhibitors greatly enhance ROS generation from the respiratory chain, because electron flow is inhibited, increasing the likelihood of transfer of electrons from respiratory complexes to oxygen to generate
and H2O2 by mitochondria is low, unless the respiratory chain is inhibited by inhibitors such as rotenone (mitochondrial complex I) or antimycin (III) (54). These mitochondria inhibitors greatly enhance ROS generation from the respiratory chain, because electron flow is inhibited, increasing the likelihood of transfer of electrons from respiratory complexes to oxygen to generate 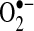 .
.
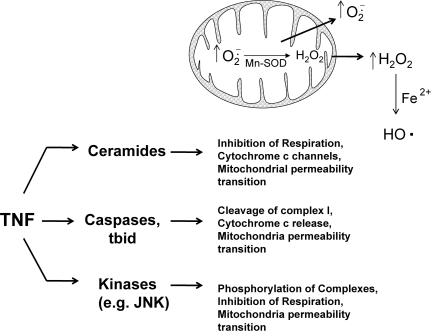
Mitochondria have been reported to swell, aggregate, and undergo other morphologic changes that precede cytochrome c release and loss of membrane potential during TNF-induced apoptosis in cells (31, 125, 145). This suggests that mitochondria are an early target of signaling pathways during TNF-induced apoptosis. Many potential signaling pathways are activated during TNF signaling that can potentially affect mitochondrial respiration and consequently mitochondrial ROS generation. Three potential pathways activated by TNF that could affect mitochondrial bioenergetics and ROS generation are (a) ceramides, (b) kinases such JNK, and (c) caspases and proapoptotic bcl-2 family members (Fig. 3).
FIG. 3.
Possible pathways activated by TNF that mediate ROS generation from mitochondria. During apoptosis stimulated by TNF, mitochondrial respiration is inhibited, and ROS generation increases in most cells. Several potential signaling pathways are activated during TNF signaling that can potentially affect mitochondrial respiration and ROS generation, including (a) ceramides, signaling lipids that regulate mitochondria function; (b) kinases such JNK, which translocate to mitochondria and phosphorylate mitochondrial proteins; and (c) caspases and proapoptotic bcl-2 family members, which can cleave respiratory complexes and inhibit mitochondrial function. All these pathways can inhibit respiration, potentially to cause increased ROS generation from mitochondria, a major source of both 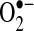 and H2O2 in cells.
and H2O2 in cells.
Ceramides
Ceramides are signaling lipids that have been shown to modulate many signaling pathways, including apoptosis in cells (7, 124). The binding of TNF to TNF receptor was shown to activate sphingomyelinase (SMase), which liberates ceramides from sphingomyelin in the plasma membrane (42). A neutral SMase (NSMase, optimum pH of 7.5) and acidic SMase (ASMase, optimum pH of 4.8) have been identified in cells. The activation of SMase and generation of ceramide have been suggested to mediate, at least in part, ROS generation and apoptosis stimulated by TNF (50). Ceramides have been shown to target mitochondria and form channels in mitochondrial membranes that allow passage of cytochrome c. Ceramides cannot form channels in the plasma membranes (137). The addition of ceramides to isolated heart mitochondria was shown to inhibit respiration and increased ROS generation (measuring DCFH plus horseradish peroxidase, which adds specificity for H2O2) (33). In HUVECs (human umbilical vein endothelial cells), TNF was shown to increase ROS generation from mitochondria (measured by using DCFH) that was inhibited by despiramine (SMase inhibitor), suggesting that ceramides mediate increased ROS generation from mitochondria after TNF treatment (24). In primary hepatocytes, ASMase was suggested to mediate TNF-induced cell death (45). Hepatocytes from ASMase(−/−) mice were resistant to apoptosis and increased ROS generation (measured by using DCFH) caused by TNF and mitochondrial GSH depletion (a redox change that sensitizes hepatocytes to TNF-induced apoptosis).
Phosphorylation by kinases such as JNK
The phosphorylation or glutathionylation of mitochondrial complex I in the respiratory chain was shown to regulate ROS generation from that complex (111, 144). In addition, phosphorylation was shown to regulate mitochondrial complexes such as cytochrome c oxidase (COX), which could theoretically modulate ROS production. TNF treatment of bovine and murine liver homogenates results in the phosphorylation (tyrosine 304, subunit I) and inhibition of COX (60%) (122). Whether this phosphorylation of COX results in an increased generation of ROS from the respiratory chain remains to be determined, but based on other works, inhibition of respiratory complexes often leads to increased ROS generation. The kinase(s) responsible for COX phosphorylation after TNF treatment has not been identified; however, many cytoplasm kinases such JNK have been shown to modulate mitochondria bioenergetics. Recent studies suggested that many kinases (i.e., JNK, PKC) found in cytoplasm translocate to mitochondria after activation to phosphorylate mitochondrial protein and regulate mitochondria bioenergetics (6, 21, 59). For example, after activation, JNK translocates to mitochondria and initiates a signal cascade that leads to pyruvate dehydrogenase phosphorylation and inactivation, loss of membrane potential, and cytochrome c release (6, 59, 170). JNK activated by TRAF-2 after TNF treatment may therefore be important in mediating mitochondrial dysfunction and ROS generation during TNF-induced apoptosis. However, whether JNK is directly responsible for increased mitochondrial ROS production remains to be confirmed experimentally. Initial studies using DCFH observed JNK(−/−) knockout mice have lower ROS generation than do wild-type mice after TNF treatment (149).
Caspases and proapoptotic bcl2 family members
Caspases have been suggested to modulate mitochondria bioenergetics and ROS generation. Caspases have been shown to cleave the NDUFS1 subunit in mitochondrial complex I of the respiratory chain and to inhibit respiration (114). This inhibition of mitochondrial complex I may potentially increase 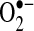 generation, because it is a major site of
generation, because it is a major site of 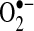 production toward the matrix. In HeLa cells, cytochrome c released from the mitochondria was shown to activate caspase that translocated to mitochondria to inhibit mitochondrial complex I and II respiration and increase ROS (by using HE) (113). Similarly, some proapoptotic members of the bcl-2 family (i.e., bim, tbid, bax, bad) have been suggested to modulate mitochondrial ROS generation. tBid translocation to mitochondria was suggested to cause increased ROS generation (measured by using HE and DCF) in primary hepatocytes treated with TNF and actinomycin D (36). Primary hepatocytes taken from bid(−/−) mice were found to have little ROS generation from mitochondria and apoptosis after TNF and actinomycin D treatment. The fact that tbid and some proapoptotic bcl-2 family members may increase ROS from mitochondria is countered by the fact that antiapoptotic bcl-2 family members (i.e., bcl-2, bcl-xL, mcl-1, bcl-b) may act as antioxidants (68). Bcl-2 was shown to decrease ROS production (measured with DCFH) in cells induced by various treatments. Although most studies regarding bcl-2 have relied on DCFH, one study showed that bcl-2 overexpression decreases lipid peroxidation (measured by using a specific cis-parinaric acid and HPLC method), suggesting that bcl-2 has strong antioxidant properties (147).
production toward the matrix. In HeLa cells, cytochrome c released from the mitochondria was shown to activate caspase that translocated to mitochondria to inhibit mitochondrial complex I and II respiration and increase ROS (by using HE) (113). Similarly, some proapoptotic members of the bcl-2 family (i.e., bim, tbid, bax, bad) have been suggested to modulate mitochondrial ROS generation. tBid translocation to mitochondria was suggested to cause increased ROS generation (measured by using HE and DCF) in primary hepatocytes treated with TNF and actinomycin D (36). Primary hepatocytes taken from bid(−/−) mice were found to have little ROS generation from mitochondria and apoptosis after TNF and actinomycin D treatment. The fact that tbid and some proapoptotic bcl-2 family members may increase ROS from mitochondria is countered by the fact that antiapoptotic bcl-2 family members (i.e., bcl-2, bcl-xL, mcl-1, bcl-b) may act as antioxidants (68). Bcl-2 was shown to decrease ROS production (measured with DCFH) in cells induced by various treatments. Although most studies regarding bcl-2 have relied on DCFH, one study showed that bcl-2 overexpression decreases lipid peroxidation (measured by using a specific cis-parinaric acid and HPLC method), suggesting that bcl-2 has strong antioxidant properties (147).
Modulation of TNF Signaling by ROS: Redox Targets in TNF Signaling
ROS are important second-messenger molecules in some cells, but whether this is a universal mechanism remains to be determined. However, it is clear that many proteins involved in TNF signaling are redox regulated. This has been demonstrated by many studies demonstrating that addition of sublethal levels of exogenous ROS or causing increased endogenous ROS generation (e.g., mitochondria inhibitors) sensitizes cells to TNF-induced cell death (no actinomycin or other NF-κB inhibition treatment is necessary) (55, 125, 159, 167). In primary hepatocytes, we similarly observed that sublethal doses of H2O2 and mitochondrial inhibitors (e.g., antimycin) caused apoptosis when simultaneously treated with TNF (55). The notion that ROS sensitize hepatocytes to TNF-induced apoptosis through redox changes is supported by the fact that redox-modulating agents also sensitized hepatocytes to apoptosis stimulated by TNF. Agents that deplete GSH (i.e., DEM, phorone, or acetaminophen) or oxidizing agents such as diamide at sublethal doses also sensitized hepatocytes to TNF-induced apoptosis (55, 88, 92, 99). In addition, GSH depletion in the liver of mice increased liver injury and mortality caused by galactosamine and lipopolysaccharide (LPS) treatment (a liver-injury model mediated by TNF) (163). These observations do not necessarily support the notion that ROS are second messengers in TNF signaling; rather, these findings demonstrate that many proteins involved in TNF signaling can be redox regulated to promote apoptosis, suggesting that redox changes potentiate proapoptotic signaling pathways or inhibit NF-κB expression of survival genes or both. These redox modulations that activate apoptotic signaling and inhibit NF-κB may be important mechanisms by which TNF induces liver injury in vivo.
Studies from our and other laboratories suggested that redox alteration affects many signaling pathways to modulate survival after TNF treatment. Although many pathways that are redox regulated may be regulated by TNF, we focus on redox regulation of JNK signaling and NF-κB transcription. The redox regulation of JNK and NF-κB may underlie the sensitization of hepatocytes to TNF-induced apoptosis caused by ROS and redox-modulating agents.
JNK
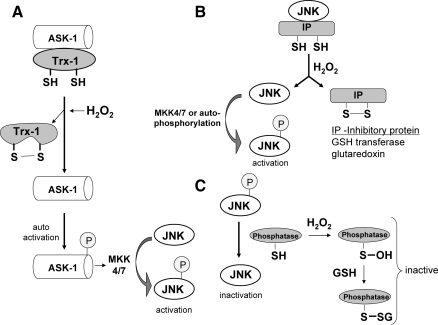
As previously stated, JNK was shown to be activated by various stresses including oxidative stress. Exogenous H2O2 was shown to activate JNK in primary neurons, primary hepatocytes, and various cells lines (59, 130, 170). Similarly, high doses of acetaminophen, which depletes GSH, causes sustained JNK activation, important in mediating hepatocyte death and liver injury (51, 59). Several mechanisms underlie the activation of JNK by ROS (Fig. 4). JNK is phosphorylated primarily by MKK (a MAPK kinase), which in turn is phosphorylated by ASK-1 (a MAPK kinase kinase) in liver. ASK-1 was shown to be inhibited in cytoplasm by an association with thioredoxin, which contains critical redox-sensitive thiols (cysteine 32 or 35) (86, 120, 168). The oxidation of these critical thiols on thioredoxin by H2O2 or other oxidants will cause thioredoxin to disassociate from ASK-1, which subsequently self-activates. Once activated, ASK-1 will phosphorylate MKK, which then activates JNK. Trx-1 association with ASK-1 has also been shown to be important in ubiquitination and degradation of ASK-1 (86). However, other studies showed that JNK is inhibited through association with redox-regulated proteins such as glutaredoxin in human cancer cells (MCF-7/ADR and DU-145) (138), and glutathione S-transferase in the NIH3T3 cell line and mouse embryo fibroblasts (1, 152). ROS was shown to cause the detachment of JNK from these redox-inhibitor proteins, which subsequently become activated by MKK or through autophosphorylation. In addition, sustained JNK activity may be due to redox inhibition of MAP kinase phosphatase, important in dephosphorylating and turning off JNK (72). Whether these redox mechanisms of JNK activation are cell specific and which pathway(s) work in hepatocytes remain to be elucidated. Overall, ROS can alter the redox status of many proteins involved in JNK regulation. Consequently, JNK activation is a common response to ROS in most cells.
FIG. 4.
Redox regulation of JNK. JNK is activated by ROS in most cells through redox regulation of proteins involved in its regulation. The sustained JNK activity by ROS may be due to (A) Trx1 oxidation causing the disassociation from ASK-1. JNK is primarily phosphorylated by MKK (a MAPK kinase), which in turn is phosphorylated by ASK-1 (a MAPK kinase kinase) in liver. ASK-1 is inhibited in cytoplasm by an association with Trx-1, which contains critical redox-sensitive thiols. The oxidation of these critical thiols on Trx-1 by H2O2 or other oxidants will cause Trx-1 to disassociate from ASK-1, which subsequently self-activates. Once activated, ASK-1 will phosphorylate MKK, which then activates JNK. (B) Oxidation of JNK inhibitory proteins. JNK is inhibited through association with redox-regulated proteins such as glutaredoxin and glutathione S-transferase, which contain critical cysteines. ROS can oxidize these inhibitory proteins, liberating JNK, which subsequently becomes activated by MKK or through autophosphorylation. (C) Redox inhibition of MAP kinase phosphatase. MAP kinase phosphatase, important in dephosphorylating and inactivating JNK, contains critical thiols that regulate its activity. Sustained JNK activity by ROS can be a result of redox inhibition of phosphatase through oxidation of its key thiols (i.e., sulfenic acid and disulfide formation, such as glutathionylation).
In most cells after TNF treatment, JNK is transiently activated by TRAF2, probably through ASK-1 (104, 160). It was shown that ASK-1(−/−) embryonic fibroblasts have a severely inhibited (but not complete) JNK activity in response to TNF or H2O2 (146). As previously mentioned, several NF-κB–transcribed proteins (i.e., GAPDD45β, XIAP, A20) may be responsible for turning off JNK activity (105, 143, 160). However, during TNF apoptosis [when NF-κB is inhibited, chemically (e.g., actinomycin D) or genetically (e.g., IKK knockout)], ROS are believed to be important in sustaining JNK activation (121), probably through increased ASK-1 activity (146). A feed-forward loop may exist in which activated JNK translocates to mitochondria and promotes increased ROS generation from the respiratory chain, which in turn will trigger more JNK activation (131, 149). Thus, although TRAF-2 in complex I (TNF-R1, RIP, TRAF2 complex) may be responsible for initial activation of JNK, ROS are believed to be responsible for mediating sustained JNK activity through redox alterations. The increased sensitization of primary hepatocytes to TNF-induced apoptosis by H2O2 and other oxidants may be due in part to the increased sustained JNK activity these agents trigger. Once activated, JNK is believed to target mitochondria and promote cytochrome c release, the mitochondrial permeability transition, and to phosphorylate and inactivate the antiapoptotic bcl-xl protein (6, 39, 59). JNK also was shown to accelerate the degradation of the NF-κB–regulated c-FLIP, an inhibitor of caspase 8, to promote apoptosis in the liver (20). The JNK promotion of c-FLIP degradation shows that prolonged JNK activity may counteract some protective effects of NF-κB, to promote apoptosis stimulated by TNF. In mouse lung epithelial cells, H2O2 treatment inhibited TNF-induced NF-κB activation, but apoptosis still proceeded because of sustained JNK activation (103). Whether an increase in JNK activity caused by ROS alone is enough to sensitize hepatocytes to TNF-stimulated apoptosis, or whether some concurrent redox inhibition of NF-κB also is necessary remains to be determined.
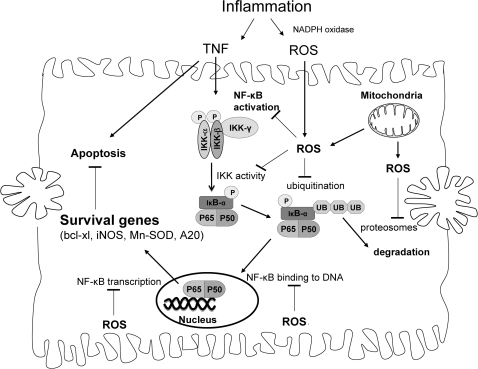
NF-κB signaling
As described by many reviews in this forum, a great deal of cell specificity exists regarding the redox modulation of NF-κB. H2O2 was suggested to activate NF-κB in some cells and to inhibit NF-κB in others cells (48). The dose of H2O2 and delivery method may a play a role in the activation or inhibition of NF-κB in cells (30). Similarly, TNF induction of NF-κB activation was shown to require ROS in some cells (60, 134), whereas ROS were shown to inhibit TNF-stimulated NF-κB activity in other cells (159, 167). In most cells, high doses of ROS are likely to inhibit NF-κB, because of redox inhibition of many proteins involved in NF-κB activation, such as IKK. In hepatocytes, ROS or redox-modulating agents primarily inhibit NF-κB activation after TNF stimulation, leading to a sensitization to TNF-induced apoptosis (55, 88, 92, 99). However, redox changes caused by mitochondrial GSH depletion, by using 3-hydroxy-4-pentenoate (HP), was shown to sensitized hepatocytes to TNF-induced apoptosis, even though NF-κB activation still occurred [high doses of TNF (100 ng/ml) were used in the study] (91). In general, redox changes inhibit NF-κB activity in hepatocytes, and many proteins involved in NF-κB signaling were shown to be regulated by redox changes. Because little evidence exists of activation of NF-κB by ROS after TNF stimulation in hepatocytes and other primary cells, we focus on NF-κB inhibition by ROS after TNF treatment, which may play a pathophysiologic role in many liver diseases.
Many potential sites involved in NF-κB transcription of survival genes that are regulated by redox changes are shown in Fig. 5. NF-κB, like many transcription factors, contains critical cysteine residues that are needed for binding to DNA. The modification of thiols of cysteine residues can potentially affect binding to DNA and thus transcriptional activity. NF-κB was shown to be S-glutathionylated and S-nitrosylated, both modifications causing a decrease in transcriptional activity because of decreased binding to DNA (81). The NF-κB p50 subunit has a critical cysteine-62 thiol that is surrounded by a cationic environment, making the thiol ionized, and highly reactive to thiol-disulfide exchange (93, 100). As previously mentioned, protein redox status is regulated by GSH along with the proteins, glutaredoxin and Trx. In the nucleus, Trx1 serves as a positive regulator of NF-κB and AP-1 transcription, either directly reducing critical cysteines of these proteins, or acting via reduction of GRX and Ref-1 (66). In HeLa cells, TNF was shown to induce a nuclear translocation of Trx1, which directly associates with p50 as well as Ref-1 (61).
FIG. 5.
Potential sites in NF-κB signaling that are redox regulated. Many sites involved in NF-κB signaling are redox regulated: (a) IKK, important in phosphorylation of IκB-α (necessary for its degradation) can be inhibited by redox changes (cysteine-179); (b) proteins involved in ubiquitination of IκB-α and proteasome activity, necessary for its degradation, have been shown to be inhibited by ROS; (c) NF-κB has critical thiols (cysteine-62 on p50) important in binding to DNA; and (d) NF-κB transcription of survival genes also was shown to be inhibited by ROS and GSH depletion. Inflammation, which produces both TNF and ROS, may trigger apoptosis in hepatocytes and other primary cells, through redox inhibition of NF-κB signaling.
Other potential sites of redox regulation of NF-κB activity after TNF stimulation include IKK activity, NF-κB transcriptional activity, ubiquitination of IκB-α, and proteasome activity necessary for IκB-α degradation. The proteins involved in NF-κB signaling that are modulated by redox changes may depend on the redox potential of cells. Work with DEM showed that at different doses, which cause different degrees of redox changes in cells, different sites involved in NF-κB activation were inhibited after TNF simulation. A high dose of DEM (0.5 mM), which depletes cytoplasm and mitochondrial GSH (the latter could potentially increase H2O2 release from mitochondria), inhibited TNF-stimulated NF-κB activation through an IKK-dependent pathway involving decreased IKK activity, IκB-α phosphorylation, and ubiquitination in primary hepatocytes (88). IKK appears to be redox regulated, and H2O2 was shown to inhibit IKK activity through oxidation of cysteine-179 on the IKK-α or IKK-β subunit. (82, 102, 112). Inhibition of IKK by redox changes caused inhibition of IκB-α phosphorylation and degradation, leading to an inhibition of NF-κB translocation to the nucleus (82, 112). RIP in complex I, important in activating IKK after TNF stimulation, also was shown to be downregulated by H2O2 (103). Ubiquitination of IκB-α and proteasomes, necessary for IκB-α degradation after ubiquitination, also appear to be regulated by redox changes (70, 101, 159, 173). IκB-α also was shown to be glutathionylated, which reduces phosphorylation and ubiquitination of the protein (78). Conversely, lower doses of DEM (0.10 mM), which depletes cytoplasmic but not mitochondrial GSH and causes a less-significant redox change in cells, inhibited NF-κB transcriptional activity without inhibiting IKK activity or NF-κB translocation to the nucleus in primary hepatocytes (88). NF-κB was observed bound to DNA, as assessed by the Chip (chromatin immunoprecipitation) assay, suggesting that NF-κB binding to DNA was not inhibited by low-DEM treatment, but rather the NF-κB transcriptional machinery was being affected by redox changes to prevent NF-κB transcription of survival genes (88). The mechanism of how low doses of DEM inhibit the NF-κB transcriptional machinery to prevent transcription of survival genes is being investigated. Buthionine sulfoximine (BSO; a GSH-synthesis inhibitor), which slowly depletes cytoplasmic GSH, inhibited NF-κB transcription triggered by TNF in primary hepatocytes, without affecting NF-κB translocation or binding to DNA, similar to low-DEM treatment (88). In liver, NF-κB also is regulated by Akt (protein kinase B), which is required for maximal NF-κB activation in mouse hepatocytes (63), and Akt signaling was suggested to be redox regulated (83). Taken together, the data suggest that many potential sites exist where redox changes by ROS could inhibit NF-κB transcription of survival genes to promote apoptosis after TNF stimulation. The extent of redox change may be a key determinant of which sites in NF-κB signaling may be redox regulated.
Is redox inhibition of NF-κB a key factor in sensitizing cells to TNF in many diseases?
TNF was shown to play a pathophysiologic role in many diseases, including rheumatoid arthritis, psoriasis, myocardial infarction, and atherosclerosis. In liver, TNF was suggested to mediate various liver pathologies including liver ischemia reperfusion injury, alcoholic liver disease, viral hepatitis, and liver toxicity caused by toxins such as carbon tetrachloride (118, 136, 166). TNF, released during inflammation, is believed to be important in mediating hepatocyte death and tissue injury in these liver pathologies. Because the activation of NF-κB normally confers resistance in hepatocytes and other primary cells to TNF, the mechanism by which TNF is mediating liver injury in these pathologies is not completely known. Based on cell-culture studies, the presumption is that signaling changes occur in hepatocytes that result in sensitization to apoptosis stimulated by TNF. Because NF-κB is the essential pathway preventing TNF-induced apoptosis, inhibition of NF-κB may be an important factor in sensitizing hepatocytes to apoptosis stimulated by TNF. Most of these liver pathologies involving TNF are associated with increased ROS generation from external sources during inflammation (i.e., macrophages and immune cells) or internally (e.g., increased mitochondrial ROS by the herpes virus), that likely causes redox changes in cells. Consequently, redox changes that occur in these pathologies may be an important mechanism of inhibition of NF-κB signaling, leading to sensitization of hepatocytes to TNF-induced cell death.
We examine the possibility that redox inhibition of NF-κB is important in alcoholic liver disease and drug-induced liver injury. Our focus is on NF-κB; however, many important signaling pathways that modulate TNF signaling are also regulated by redox changes (i.e., Akt, PTEN, JNK) and therefore may contribute to sensitization of hepatocytes to apoptosis stimulated by TNF.
Alcoholic liver disease
TNF was shown to play an essential role in the pathogenesis of alcohol-induced liver injury. Alcoholic liver disease also involves extensive redox changes in hepatocytes and consequently may be a disease in which redox changes may lead to inhibition of NF-κB and sensitization to TNF-induced apoptosis. Alcoholic liver disease is associated with hepatocyte apoptosis, believed to be mediated by TNF. The role of TNF in alcoholic liver disease is supported by the observation that patients with alcoholic hepatitis have high serum plasma levels of TNF (12). In addition, mice lacking the TNF-R1 or injected with antibodies to neutralize TNF are more resistant to alcohol-induced liver damage, suggesting the importance of TNF in mediating liver injury (69, 71, 166). Consequently, antisera against TNF are used in the treatment of patients with severe alcoholic liver disease. Because hepatocytes are normally resistant to TNF, this suggests that prolonged alcohol intake causes TNF to have a direct lethal affect on hepatocytes. Long-term alcohol intake appears to cause biochemical changes in hepatocytes, leading to a sensitization to TNF-induced apoptosis and necrosis (14, 34). This is supported by the observation that hepatocytes isolated from rats treated with long-term alcohol are more sensitized to TNF-induced cell death (23, 43).
How does alcohol sensitize hepatocytes to the cytotoxic action of TNF? Alcohol intake is associated with increased ROS generation and redox changes that may lead to redox changes that inhibit NF-κB transcriptional activity and sensitize hepatocytes to TNF. Fernandez-Checa (44) first demonstrated that prolonged alcohol consumption causes a modest loss of cytoplasmic GSH (∼25%) while causing significant mitochondrial GSH depletion [up to 65% (44)], likely to decrease the thiol redox status of both cells and mitochondria. Mitochondrial GSH is important in detoxifying H2O2 formed in the matrix (4), and loss of mitochondrial GSH can cause increased H2O2 release from mitochondria (54). Mitochondria isolated from alcohol-treated mice generated more ROS [measured by using DCFH (9)]. Other sources of ROS during alcohol intake besides mitochondria include Kupffer cells and neutrophils, which generate ROS during inflammation in the liver (154). In addition, alcohol intake causes induction of the cytochrome p450 2E1 (CYP2E1), which is associated with ROS production and generation of reactive products from ethanol oxidation (32, 77, 158). Recent proteomic studies verified that increased ROS generation caused by alcohol intake leads to posttranslational redox changes to proteins (increased proteins disulfides and nitrosylation due to increased iNOS during inflammation) of the liver (96, 140, 148). These proteomic studies identified that many thiols in mitochondrial proteins, such as ATP synthase and 3-ketoacyl-CoA thiolase, become oxidized. Many of these posttranslational redox modifications were associated with a decrease in activity. Whether signaling proteins such as NF-κB undergo redox changes has not yet been determined, but remains a distinct possibility. Some evidence suggests that prolonged alcohol treatment causes the inhibition of NF-κB activation in liver. In alcohol-treated rats, NF-κB activation and translocation to the nucleus was found to be inhibited after partial hepatectomy (165). This supports the idea that alcohol promotes sensitization to TNF, possibly through redox inhibition of NF-κB signaling. Overall, because alcohol is associated with protein redox changes and because many proteins involved in NF-κB signaling are redox regulated, the redox inhibition of NF-κB may be an important mechanism of sensitizing hepatocytes to TNF during alcoholic liver injury, but further studies are needed to confirm this idea. Of course, in addition to redox changes, alcohol treatment affects many metabolic and signaling pathways (i.e., PTEN, Akt, ER stress pathways) that may contribute significantly to sensitization of hepatocytes to TNF-induced apoptosis (76, 135).
Drug-induced liver injury
In addition to alcohol, many other drugs that cause liver injury are believed to cause redox changes in liver. The redox changes caused by drugs may be important in causing hepatocyte apoptosis and liver injury during inflammation when TNF is secreted. Acetaminophen (Tylenol), a widely used analgesic, will cause redox changes in hepatocytes (GSH depletion, increased ROS generation, protein oxidation), even at sublethal doses. The redox changes by acetaminophen and can sensitize hepatocytes to TNF-induced apoptosis, probably through NF-κB inhibition (99). Whether acetaminophen can sensitize hepatocytes to TNF in vivo remains to be determined. Work from Roth's laboratory also shows the possibility of drugs sensitizing hepatocytes to TNF-induced apoptosis in vivo. Most drugs that cause idiosyncratic drug liver injury (rare drug injury that occurs at therapeutic doses) in humans do not cause liver injury in animals. However, work by Roth's group (17, 89) revealed that chlorpromazine (an antipsychotic drug), trovafloxacin (a fluoroquinolone antibiotic), and ranitidine (a histamine 2‱receptor antagonist), which do not normally cause liver injury in mice, cause liver injury when simultaneously injected with a sublethal dose of lipopolysaccharide (LPS; bacteria endotoxin). Sublethal levels of LPS will trigger inflammation and TNF secretion in the liver. Blocking TNF production or activity by using various TNF inhibitors prevented liver injury caused by co-treatment with LPS and trovafloxacin, suggesting an essential role of TNF in hepatocyte injury (128). The liver injury caused by LPS and drugs depended on TNF, suggesting that hepatocytes become sensitized to the cytotoxic effects of TNF. Many drugs have been shown to cause increased ROS generation and redox changes in liver (73, 74); consequently, drug-induced redox changes may inhibit NF-κB signaling to sensitize hepatocytes to the cytotoxic effects of TNF (whose secretion increases in response to LPS). Further studies are needed to investigate the synergistic actions of TNF, secreted during inflammation, and drugs, which can cause redox changes.
Redox regulation of NF-κB in Kupffer cells
Although our focus has been on NF-κB signaling in hepatocytes, NF-κB also is very important in other cells in the liver, including Kupffer cells (resident macrophages in liver). In Kupffer cells, NF-κB regulates TNF production and other inflammatory responses. Interestingly, the activation of NF-κB in Kupffer cells may be redox regulated; iron was shown to prime the activation of NF-κB in Kupffer cells (129, 162). Iron chelators were shown to decrease NF-κB activation caused by LPS in Kupffer cells, whereas addition of Fe2+, but not Fe3+, to media increased NF-κB translocation to the nucleus after LPS treatment (129). Thus, although redox changes alone do not activate NF-κB in Kupffer cells, they modulate the extent of NF-κB activation after LPS stimulation. This suggests that in liver, redox changes in Kupffer cells may help potentiate NF-κB activation that results in increased TNF generation, whereas in hepatocytes, redox changes may inhibit NF-κB activation, leading to increased sensitivity to TNF-induced cell death. Why redox changes potentiate NF-κB activation in Kupffer cells, but inhibit NF-κB activation in hepatocytes, remains uncertain. It would be of great interest to determine the underlying differences in hepatocytes and Kupffer cells responsible for the different NF-κB responses after oxidative stress. Overall, redox changes may have a dual affect in liver, potentiation of NF-κB activation in Kupffer cells and inhibition of NF-κB activation in hepatocytes, to help mediate the pathogenesis of alcohol- and drug-induced liver injury.
Perspective
TNF-induced cell death may be an important component of inflammatory injury in liver and other tissues, particularly when inflammation is prolonged. Because TNF and ROS are secreted simultaneous during inflammation, they may work synergistically to mediate liver injury by promoting hepatocyte apoptosis (Fig. 5). Hepatocytes may become sensitized to the lethal actions of TNF by redox changes induced by ROS that modulate NF-κB and other important signaling pathways. The sensitization of hepatocytes to TNF may be heightened by drugs, viruses, and other foreign agents that induce increased ROS generation and cause redox changes in hepatocytes (73). Overall, much circumstantial data suggest that redox changes in hepatocytes leads to their sensitization to the cytotoxic effects of TNF in many liver pathologies. However, because ROS and redox changes are very difficult to measure in vivo, direct proof has not been established, and further studies are needed.
Abbreviations Used
- ASK-1
apoptosis signal-regulating kinase 1
- BHA
butylated hydroxyanisole
- COX
cytochrome c oxidase
- DCFH
dichlorodihydrofluorescein
- DEM
diethylmaleate
- E+
ethidine
- FADD
Fas-associated death domain
- FHC
ferritin heavy chain
- GADD45β
growth-arrest DNA damage–inducible gene 45β
- GSH
glutathione
- GSSG
oxidized glutathione
- HE
hydroethidine
- IKK
IκB kinase
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LIP
labile iron pool
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MKK
mitogen-activated protein kinase kinase
- RIP
receptor-interacting kinase
- ROS
reactive oxygen species
- SMase
sphinogomyelinases
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-α
- TNF-R1
TNF receptor 1
- TNF-R2
TNF receptor 2
- TRAF-2
TNF receptor–associated factor 2
- TRADD
TNF receptor–associated protein with death domain
- Trx
thioredoxin
- Trx-2
thioredoxin mitochondria isoform
- VDAC
voltage-dependent anion channel
- XIAP
X-linked inhibitor of apoptosis
Acknowledgments
This work is supported by the National Institutes of Health Grant DK067215.
References
- 1.Adler V. Yin Z. Fuchs SY. Benezra M. Rosario L. Tew KD. Pincus MR. Sardana M. Henderson CJ. Wolf CR. Davis RJ. Ronai Z. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antosiewicz J. Ziolkowski W. Kaczor JJ. Herman-Antosiewicz A. Tumor necrosis factor-alpha-induced reactive oxygen species formation is mediated by JNK1-dependent ferritin degradation and elevation of labile iron pool. Free Radic Biol Med. 2007;43:265–270. doi: 10.1016/j.freeradbiomed.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Antras-Ferry J. Maheo K. Morel F. Guillouzo A. Cillard P. Cillard J. Dexamethasone differently modulates TNF-alpha- and IL-1beta-induced transcription of the hepatic Mn-superoxide dismutase gene. FEBS Lett. 1997;403:100–104. doi: 10.1016/s0014-5793(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 4.Antunes F. Han D. Cadenas E. Relative contributions of heart mitochondria glutathione peroxidase and catalase to H(2)O(2) detoxification in in vivo conditions. Free Radic Biol Med. 2002;33:1260–1267. doi: 10.1016/s0891-5849(02)01016-x. [DOI] [PubMed] [Google Scholar]
- 5.Antunes F. Salvador A. Marinho HS. Alves R. Pinto RE. Lipid peroxidation in mitochondrial inner membranes, I: an integrative kinetic model. Free Radic Biol Med. 1996;21:917–943. doi: 10.1016/s0891-5849(96)00185-2. [DOI] [PubMed] [Google Scholar]
- 6.Aoki H. Kang PM. Hampe J. Yoshimura K. Noma T. Matsuzaki M. Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- 7.Arora AS. Jones BJ. Patel TC. Bronk SF. Gores GJ. Ceramide induces hepatocyte cell death through disruption of mitochondrial function in the rat. Hepatology. 1997;25:958–963. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- 8.Arvelo MB. Cooper JT. Longo C. Daniel S. Grey ST. Mahiou J. Czismadia E. Abu-Jawdeh G. Ferran C. A20 protects mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology. 2002;35:535–543. doi: 10.1053/jhep.2002.31309. [DOI] [PubMed] [Google Scholar]
- 9.Bailey SM. Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11–16. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- 10.Basuroy S. Bhattacharya S. Leffler CW. Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF{alpha} in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2009;296:C422–C432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bindokas VP. Jordan J. Lee CC. Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bird GL. Sheron N. Goka AK. Alexander GJ. Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917–920. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- 13.Bonini MG. Rota C. Tomasi A. Mason RP. The oxidation of 2′,7′-dichlorofluorescein to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med. 2006;40:968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Bradham CA. Plumpe J. Manns MP. Brenner DA. Trautwein C. Mechanisms of hepatic toxicity, I. TNF-induced liver injury. Am J Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- 15.Brennan P. O'Neill LA. 2-Mercaptoethanol restores the ability of nuclear factor kappa B (NF kappa B) to bind DNA in nuclear extracts from interleukin 1-treated cells incubated with pyrollidine dithiocarbamate (PDTC): evidence for oxidation of glutathione in the mechanism of inhibition of NF kappa B by PDTC. Biochem J. 1996;320:975–981. doi: 10.1042/bj3200975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner DA. Signal transduction during liver regeneration. J Gastroenterol Hepatol. 1998;13(suppl):S93–S95. doi: 10.1111/jgh.1998.13.s1.93. [DOI] [PubMed] [Google Scholar]
- 17.Buchweitz JP. Ganey PE. Bursian SJ. Roth RA. Underlying endotoxemia augments toxic responses to chlorpromazine: is there a relationship to drug idiosyncrasy? J Pharmacol Exp Ther. 2002;300:460–467. doi: 10.1124/jpet.300.2.460. [DOI] [PubMed] [Google Scholar]
- 18.Burkitt MJ. Wardman P. Cytochrome c is a potent catalyst of dichlorofluorescin oxidation: implications for the role of reactive oxygen species in apoptosis. Biochem Biophys Res Commun. 2001;282:329–333. doi: 10.1006/bbrc.2001.4578. [DOI] [PubMed] [Google Scholar]
- 19.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 20.Chang L. Kamata H. Solinas G. Luo JL. Maeda S. Venuprasad K. Liu YC. Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Churchill EN. Szweda LI. Translocation of deltaPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch Biochem Biophys. 2005;439:194–199. doi: 10.1016/j.abb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Clement MV. Stamenkovic I. Superoxide anion is a natural inhibitor of FAS-mediated cell death. EMBO J. 1996;15:216–225. [PMC free article] [PubMed] [Google Scholar]
- 23.Colell A. Garcia-Ruiz C. Miranda M. Ardite E. Mari M. Morales A. Corrales F. Kaplowitz N. Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 24.Corda S. Laplace C. Vicaut E. Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 25.Cox AG. Pullar JM. Hughes G. Ledgerwood EC. Hampton MB. Oxidation of mitochondrial peroxiredoxin 3 during the initiation of receptor-mediated apoptosis. Free Radic Biol Med. 2008;44:1001–1009. doi: 10.1016/j.freeradbiomed.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis. 2007;27:378–389. doi: 10.1055/s-2007-991514. [DOI] [PubMed] [Google Scholar]
- 27.Czaja MJ. Schilsky ML. Xu Y. Schmiedeberg P. Compton A. Ridnour L. Oberley LW. Induction of MnSOD gene expression in a hepatic model of TNF-alpha toxicity does not result in increased protein. Am J Physiol. 1994;266:G737–G744. doi: 10.1152/ajpgi.1994.266.4.G737. [DOI] [PubMed] [Google Scholar]
- 28.Damas P. Ledoux D. Nys M. Vrindts Y. De Groote D. Franchimont P. Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das M. Sabio G. Jiang F. Rincon M. Flavell RA. Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Oliveira-Marques V. Cyrne L. Marinho HS. Antunes F. A quantitative study of NF-kappaB activation by H2O2: relevance in inflammation and synergy with TNF-alpha. J Immunol. 2007;178:3893–3902. doi: 10.4049/jimmunol.178.6.3893. [DOI] [PubMed] [Google Scholar]
- 31.De Vos K. Goossens V. Boone E. Vercammen D. Vancompernolle K. Vandenabeele P. Haegeman G. Fiers W. Grooten J. The 55-kDa tumor necrosis factor receptor induces clustering of mitochondria through its membrane-proximal region. J Biol Chem. 1998;273:9673–9680. doi: 10.1074/jbc.273.16.9673. [DOI] [PubMed] [Google Scholar]
- 32.Dey A. Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 33.Di Paola M. Cocco T. Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 34.Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160–171. doi: 10.1034/j.1600-0528.2002.017411.x. [DOI] [PubMed] [Google Scholar]
- 35.Dikalov S. Khramtsov V. Zimmer G. Determination of rate constants of the reactions of thiols with superoxide radical by electron paramagnetic resonance: critical remarks on spectrophotometric approaches. Arch Biochem Biophys. 1996;326:207–218. doi: 10.1006/abbi.1996.0067. [DOI] [PubMed] [Google Scholar]
- 36.Ding WX. Ni HM. DiFrancesca D. Stolz DB. Yin XM. Bid-dependent generation of oxygen radicals promotes death receptor activation-induced apoptosis in murine hepatocytes. Hepatology. 2004;40:403–413. doi: 10.1002/hep.20310. [DOI] [PubMed] [Google Scholar]
- 37.Ding WX. Yin XM. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J Cell Mol Med. 2004;8:445–454. doi: 10.1111/j.1582-4934.2004.tb00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djavaheri-Mergny M. Javelaud D. Wietzerbin J. Besancon F. NF-kappaB activation prevents apoptotic oxidative stress via an increase of both thioredoxin and MnSOD levels in TNFalpha-treated Ewing sarcoma cells. FEBS Lett. 2004;578:111–115. doi: 10.1016/j.febslet.2004.10.082. [DOI] [PubMed] [Google Scholar]
- 39.Fan M. Goodwin M. Vu T. Brantley-Finley C. Gaarde WA. Chambers TC. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–29985. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- 40.Faulkner K. Fridovich I. Luminol and lucigenin as detectors for O2. Free Radic Biol Med. 1993;15:447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 41.Feingold KR. Soued M. Grunfeld C. Tumor necrosis factor stimulates DNA synthesis in the liver of intact rats. Biochem Biophys Res Commun. 1988;153:576–582. doi: 10.1016/s0006-291x(88)81134-3. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Checa JC. Colell A. Mari M. Garcia-Ruiz C. Ceramide, tumor necrosis factor and alcohol-induced liver disease. Alcohol Clin Exp Res. 2005;29:151S–157S. [PubMed] [Google Scholar]
- 43.Fernandez-Checa JC. Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Checa JC. Ookhtens M. Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione: compartmentation, efflux, and response to incubation with ethanol. J Clin Invest. 1987;80:57–62. doi: 10.1172/JCI113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Ruiz C. Colell A. Mari M. Morales A. Calvo M. Enrich C. Fernandez-Checa JC. Defective TNF-alpha-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardner PR. Raineri I. Epstein LB. White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S. Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 48.Gloire G. Legrand-Poels S. Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Goossens V. Grooten J. De Vos K. Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci U S A. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guidarelli A. Clementi E. De Nadai C. Bersacchi R. Cantoni O. TNFalpha enhances the DNA single-strand breakage induced by the short-chain lipid hydroperoxide analogue tert-butylhydroperoxide via ceramide-dependent inhibition of complex III followed by enforced superoxide and hydrogen peroxide formation. Exp Cell Res. 2001;270:56–65. doi: 10.1006/excr.2001.5323. [DOI] [PubMed] [Google Scholar]
- 51.Gunawan BK. Liu ZX. Han D. Hanawa N. Gaarde WA. Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 52.Hakoda S. Ishikura H. Takeyama N. Tanaka T. Tumor necrosis factor-alpha plus actinomycin D-induced apoptosis of L929 cells is prevented by nitric oxide. Surg Today. 1999;29:1059–1067. doi: 10.1007/s005950050645. [DOI] [PubMed] [Google Scholar]
- 53.Han D. Antunes F. Canali R. Rettori D. Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 54.Han D. Canali R. Rettori D. Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol. 2003;64:1136–1144. doi: 10.1124/mol.64.5.1136. [DOI] [PubMed] [Google Scholar]
- 55.Han D. Hanawa N. Saberi B. Kaplowitz N. Hydrogen peroxide and redox modulation sensitize primary mouse hepatocytes to TNF-induced apoptosis. Free Radic Biol Med. 2006;41:627–639. doi: 10.1016/j.freeradbiomed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Han D. Hanawa N. Saberi B. Kaplowitz N. Mechanisms of liver injury, III: role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1–G7. doi: 10.1152/ajpgi.00001.2006. [DOI] [PubMed] [Google Scholar]
- 57.Han D. Loukianoff S. McLaughlin L. Oxidative stress indices: analytical aspects and significance. In: Sen CK, editor; Packer L, editor; Hanninen O, editor. Handbook of oxidants and antioxidants in exercise. New York: Elsevier; 2000. pp. 433–484. [Google Scholar]
- 58.Han D. Williams E. Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanawa N. Shinohara M. Saberi B. Gaarde WA. Han D. Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen JM. Zhang H. Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 61.Harper R. Wu K. Chang MM. Yoneda K. Pan R. Reddy SP. Wu R. Activation of nuclear factor-kappa b transcriptional activity in airway epithelial cells by thioredoxin but not by N-acetyl-cysteine and glutathione. Am J Respir Cell Mol Biol. 2001;25:178–185. doi: 10.1165/ajrcmb.25.2.4471. [DOI] [PubMed] [Google Scholar]
- 62.Hatano E. Bennett BL. Manning AM. Qian T. Lemasters JJ. Brenner DA. NF-kappaB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis. Gastroenterology. 2001;120:1251–1262. doi: 10.1053/gast.2001.23239. [DOI] [PubMed] [Google Scholar]
- 63.Hatano E. Brenner DA. Akt protects mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis through NK-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1357–G1368. doi: 10.1152/ajpgi.2001.281.6.G1357. [DOI] [PubMed] [Google Scholar]
- 64.Hayakawa M. Miyashita H. Sakamoto I. Kitagawa M. Tanaka H. Yasuda H. Karin M. Kikugawa K. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirose K. Longo DL. Oppenheim JJ. Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J. 1993;7:361–368. doi: 10.1096/fasebj.7.2.8440412. [DOI] [PubMed] [Google Scholar]
- 66.Hirota K. Murata M. Sachi Y. Nakamura H. Takeuchi J. Mori K. Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus: a two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 67.Hochedlinger K. Wagner EF. Sabapathy K. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 2002;21:2441–2445. doi: 10.1038/sj.onc.1205348. [DOI] [PubMed] [Google Scholar]
- 68.Hockenbery DM. Oltvai ZN. Yin XM. Milliman CL. Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 69.Iimuro Y. Gallucci RM. Luster MI. Kono H. Thurman RG. Antibodies to tumor necrosis factor alpha attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 70.Jaspers I. Zhang W. Fraser A. Samet JM. Reed W. Hydrogen peroxide has opposing effects on IKK activity and IkappaBalpha breakdown in airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:769–777. doi: 10.1165/ajrcmb.24.6.4344. [DOI] [PubMed] [Google Scholar]
- 71.Ji C. Deng Q. Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology. 2004;40:442–451. doi: 10.1002/hep.20309. [DOI] [PubMed] [Google Scholar]
- 72.Kamata H. Honda S. Maeda S. Chang L. Hirata H. Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 73.Kaplowitz N. Biochemical and cellular mechanisms of toxic liver injury. Semin Liver Dis. 2002;22:137–144. doi: 10.1055/s-2002-30100. [DOI] [PubMed] [Google Scholar]
- 74.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 75.Kaplowitz N. Aw TY. Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- 76.Kaplowitz N. Than TA. Shinohara M. Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 77.Kessova I. Cederbaum AI. CYP2E1: biochemistry, toxicology, regulation and function in ethanol-induced liver injury. Curr Mol Med. 2003;3:509–518. doi: 10.2174/1566524033479609. [DOI] [PubMed] [Google Scholar]
- 78.Kil IS. Kim SY. Park JW. Glutathionylation regulates IkappaB. Biochem Biophys Res Commun. 2008;373:169–173. doi: 10.1016/j.bbrc.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 79.Kim JR. Lee SM. Cho SH. Kim JH. Kim BH. Kwon J. Choi CY. Kim YD. Lee SR. Oxidation of thioredoxin reductase in HeLa cells stimulated with tumor necrosis factor-alpha. FEBS Lett. 2004;567:189–196. doi: 10.1016/j.febslet.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 80.Kim YS. Morgan MJ. Choksi S. Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 81.Klatt P. Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 82.Korn SH. Wouters EF. Vos N. Janssen-Heininger YM. Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J Biol Chem. 2001;276:35693–35700. doi: 10.1074/jbc.M104321200. [DOI] [PubMed] [Google Scholar]
- 83.Lahair MM. Howe CJ. Rodriguez-Mora O. McCubrey JA. Franklin RA. Molecular pathways leading to oxidative stress-induced phosphorylation of akt. Antioxid Redox Signal. 2006;8:1749–1756. doi: 10.1089/ars.2006.8.1749. [DOI] [PubMed] [Google Scholar]
- 84.Lawrence A. Jones CM. Wardman P. Burkitt MJ. Evidence for the role of a peroxidase compound I-type intermediate in the oxidation of glutathione, NADH, ascorbate, and dichlorofluorescin by cytochrome c/H2O2: implications for oxidative stress during apoptosis. J Biol Chem. 2003;278:29410–29419. doi: 10.1074/jbc.M300054200. [DOI] [PubMed] [Google Scholar]
- 85.Liu H. Lo CR. Czaja MJ. NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y. Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 87.Locksley RM. Killeen N. Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 88.Lou H. Kaplowitz N. Glutathione depletion down-regulates tumor necrosis factor alpha-induced NF-kappaB activity via IkappaB kinase-dependent and -independent mechanisms. J Biol Chem. 2007;282:29470–29481. doi: 10.1074/jbc.M706145200. [DOI] [PubMed] [Google Scholar]
- 89.Luyendyk JP. Maddox JF. Cosma GN. Ganey PE. Cockerell GL. Roth RA. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther. 2003;307:9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- 90.Madesh M. Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mari M. Colell A. Morales A. Caballero F. Moles A. Fernandez A. Terrones O. Basanez G. Antonsson B. Garcia-Ruiz C. Fernandez-Checa JC. Mechanism of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-kappaB activation. Gastroenterology. 2008;134:1507–1520. doi: 10.1053/j.gastro.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 92.Matsumaru K. Ji C. Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425–1434. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- 93.Matthews JR. Wakasugi N. Virelizier JL. Yodoi J. Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Micheau O. Lens S. Gaide O. Alevizopoulos K. Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Micheau O. Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 96.Moon KH. Hood BL. Kim BJ. Hardwick JP. Conrads TP. Veenstra TD. Song BJ. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 97.Moreno-Manzano V. Ishikawa Y. Lucio-Cazana J. Kitamura M. Selective involvement of superoxide anion, but not downstream compounds hydrogen peroxide and peroxynitrite, in tumor necrosis factor-alpha-induced apoptosis of rat mesangial cells. J Biol Chem. 2000;275:12684–12691. doi: 10.1074/jbc.275.17.12684. [DOI] [PubMed] [Google Scholar]
- 98.Muller FL. Liu Y. Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 99.Nagai H. Matsumaru K. Feng G. Kaplowitz N. Reduced glutathione depletion causes necrosis and sensitization to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology. 2002;36:55–64. doi: 10.1053/jhep.2002.33995. [DOI] [PubMed] [Google Scholar]
- 100.Nishi T. Shimizu N. Hiramoto M. Sato I. Yamaguchi Y. Hasegawa M. Aizawa S. Tanaka H. Kataoka K. Watanabe H. Handa H. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. J Biol Chem. 2002;277:44548–44556. doi: 10.1074/jbc.M202970200. [DOI] [PubMed] [Google Scholar]
- 101.Obin M. Shang F. Gong X. Handelman G. Blumberg J. Taylor A. Redox regulation of ubiquitin-conjugating enzymes: mechanistic insights using the thiol-specific oxidant diamide. FASEB J. 1998;12:561–569. doi: 10.1096/fasebj.12.7.561. [DOI] [PubMed] [Google Scholar]
- 102.Panopoulos A. Harraz M. Engelhardt JF. Zandi E. Iron-mediated H2O2 production as a mechanism for cell type-specific inhibition of tumor necrosis factor alpha-induced but not interleukin-1beta-induced IkappaB kinase complex/nuclear factor-kappaB activation. J Biol Chem. 2005;280:2912–2923. doi: 10.1074/jbc.M409524200. [DOI] [PubMed] [Google Scholar]
- 103.Pantano C. Shrivastava P. McElhinney B. Janssen-Heininger Y. Hydrogen peroxide signaling through tumor necrosis factor receptor 1 leads to selective activation of c-Jun N-terminal kinase. J Biol Chem. 2003;278:44091–44096. doi: 10.1074/jbc.M308487200. [DOI] [PubMed] [Google Scholar]
- 104.Papa S. Bubici C. Zazzeroni F. Pham CG. Kuntzen C. Knabb JR. Dean K. Franzoso G. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–729. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- 105.Papa S. Monti SM. Vitale RM. Bubici C. Jayawardena S. Alvarez K. De Smaele E. Dathan N. Pedone C. Ruvo M. Franzoso G. Insights into the structural basis of the GADD45beta-mediated inactivation of the JNK kinase, MKK7/JNKK2. J Biol Chem. 2007;282:19029–19041. doi: 10.1074/jbc.M703112200. [DOI] [PubMed] [Google Scholar]
- 106.Papa S. Zazzeroni F. Fu YX. Bubici C. Alvarez K. Dean K. Christiansen PA. Anders RA. Franzoso G. Gadd45beta promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J Clin Invest. 2008;118:1911–1923. doi: 10.1172/JCI33913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Papapostolou I. Patsoukis N. Georgiou CD. The fluorescence detection of superoxide radical using hydroethidine could be complicated by the presence of heme proteins. Anal Biochem. 2004;332:290–298. doi: 10.1016/j.ab.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 108.Pham CG. Bubici C. Zazzeroni F. Papa S. Jones J. Alvarez K. Jayawardena S. De Smaele E. Cong R. Beaumont C. Torti FM. Torti SV. Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 109.Pierce RH. Campbell JS. Stephenson AB. Franklin CC. Chaisson M. Poot M. Kavanagh TJ. Rabinovitch PS. Fausto N. Disruption of redox homeostasis in tumor necrosis factor-induced apoptosis in a murine hepatocyte cell line. Am J Pathol. 2000;157:221–236. doi: 10.1016/S0002-9440(10)64533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prosperi E. Intracellular turnover of fluorescein diacetate: influence of membrane ionic gradients on fluorescein efflux. Histochem J. 1990;22:227–233. doi: 10.1007/BF02386009. [DOI] [PubMed] [Google Scholar]
- 111.Raha S. Myint AT. Johnstone L. Robinson BH. Control of oxygen free radical formation from mitochondrial complex I: roles for protein kinase A and pyruvate dehydrogenase kinase. Free Radic Biol Med. 2002;32:421–430. doi: 10.1016/s0891-5849(01)00816-4. [DOI] [PubMed] [Google Scholar]
- 112.Reynaert NL. van der Vliet A. Guala AS. McGovern T. Hristova M. Pantano C. Heintz NH. Heim J. Ho YS. Matthews DE. Wouters EF. Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ricci JE. Gottlieb RA. Green DR. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol. 2003;160:65–75. doi: 10.1083/jcb.200208089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ricci JE. Munoz-Pinedo C. Fitzgerald P. Bailly-Maitre B. Perkins GA. Yadava N. Scheffler IE. Ellisman MH. Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 115.Robinson KM. Janes MS. Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protocol. 2008;3:941–947. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- 116.Robinson KM. Janes MS. Pehar M. Monette JS. Ross MF. Hagen TM. Murphy MP. Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rota C. Chignell CF. Mason RP. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic Biol Med. 1999;27:873–881. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 118.Rudiger HA. Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 119.Saberi B. Shinohara M. Ybanez MD. Hanawa N. Gaarde WA. Kaplowitz N. Han D. Regulation of H(2)O(2)-induced necrosis by PKC and AMP-activated kinase signaling in primary cultured hepatocytes. Am J Physiol Cell Physiol. 2008;295:C50–C63. doi: 10.1152/ajpcell.90654.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saitoh M. Nishitoh H. Fujii M. Takeda K. Tobiume K. Sawada Y. Kawabata M. Miyazono K. Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sakon S. Xue X. Takekawa M. Sasazuki T. Okazaki T. Kojima Y. Piao JH. Yagita H. Okumura K. Doi T. Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Samavati L. Lee I. Mathes I. Lottspeich F. Huttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schafer FQ. Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 124.Schenck M. Carpinteiro A. Grassme H. Lang F. Gulbins E. Ceramide: physiological and pathophysiological aspects. Arch Biochem Biophys. 2007;462:171–175. doi: 10.1016/j.abb.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 125.Schulze-Osthoff K. Bakker AC. Vanhaesebroeck B. Beyaert R. Jacob WA. Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions: evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 126.Schwabe RF. Brenner DA. Mechanisms of liver injury, I: TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 127.Shaw M. Cohen P. Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336:241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaw PJ. Hopfensperger MJ. Ganey PE. Roth RA. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci. 2007;100:259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- 129.She H. Xiong S. Lin M. Zandi E. Giulivi C. Tsukamoto H. Iron activates NF-kappaB in Kupffer cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G719–G726. doi: 10.1152/ajpgi.00108.2002. [DOI] [PubMed] [Google Scholar]
- 130.Shen HM. Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 131.Shen HM. Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 132.Shishodia S. Aggarwal BB. Nuclear factor-kappaB: a friend or a foe in cancer? Biochem Pharmacol. 2004;68:1071–1080. doi: 10.1016/j.bcp.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 133.Shoji Y. Uedono Y. Ishikura H. Takeyama N. Tanaka T. DNA damage induced by tumour necrosis factor-alpha in L929 cells is mediated by mitochondrial oxygen radical formation. Immunology. 1995;84:543–548. [PMC free article] [PubMed] [Google Scholar]
- 134.Shrivastava A. Aggarwal BB. Antioxidants differentially regulate activation of nuclear factor-kappa B, activator protein-1, c-jun amino-terminal kinases, and apoptosis induced by tumor necrosis factor: evidence that JNK and NF-kappa B activation are not linked to apoptosis. Antioxid Redox Signal. 1999;1:181–191. doi: 10.1089/ars.1999.1.2-181. [DOI] [PubMed] [Google Scholar]
- 135.Shulga N. Hoek JB. Pastorino JG. Elevated PTEN levels account for the increased sensitivity of ethanol-exposed cells to tumor necrosis factor-induced cytotoxicity. J Biol Chem. 2005;280:9416–9424. doi: 10.1074/jbc.M409505200. [DOI] [PubMed] [Google Scholar]
- 136.Simeonova PP. Gallucci RM. Hulderman T. Wilson R. Kommineni C. Rao M. Luster MI. The role of tumor necrosis factor-alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol. 2001;177:112–120. doi: 10.1006/taap.2001.9304. [DOI] [PubMed] [Google Scholar]
- 137.Siskind LJ. Kolesnick RN. Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song JJ. Rhee JG. Suntharalingam M. Walsh SA. Spitz DR. Lee YJ. Role of glutaredoxin in metabolic oxidative stress: glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–46575. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- 139.Stadtman ER. Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 140.Suh SK. Hood BL. Kim BJ. Conrads TP. Veenstra TD. Song BJ. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4:3401–3412. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- 141.Sullivan DM. Wehr NB. Fergusson MM. Levine RL. Finkel T. Identification of oxidant-sensitive proteins: TNF-alpha induces protein glutathiolation. Biochemistry. 2000;39:11121–11128. doi: 10.1021/bi0007674. [DOI] [PubMed] [Google Scholar]
- 142.Tampo Y. Kotamraju S. Chitambar CR. Kalivendi SV. Keszler A. Joseph J. Kalyanaraman B. Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis. Circ Res. 2003;92:56–63. doi: 10.1161/01.res.0000048195.15637.ac. [DOI] [PubMed] [Google Scholar]
- 143.Tang G. Minemoto Y. Dibling B. Purcell NH. Li Z. Karin M. Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 144.Taylor ER. Hurrell F. Shannon RJ. Lin TK. Hirst J. Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 145.Thomas WD. Zhang XD. Franco AV. Nguyen T. Hersey P. TNF-related apoptosis-inducing ligand-induced apoptosis of melanoma is associated with changes in mitochondrial membrane potential and perinuclear clustering of mitochondria. J Immunol. 2000;165:5612–5620. doi: 10.4049/jimmunol.165.10.5612. [DOI] [PubMed] [Google Scholar]
- 146.Tobiume K. Matsuzawa A. Takahashi T. Nishitoh H. Morita K. Takeda K. Minowa O. Miyazono K. Noda T. Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tyurina YY. Tyurin VA. Carta G. Quinn PJ. Schor NF. Kagan VE. Direct evidence for antioxidant effect of Bcl-2 in PC12 rat pheochromocytoma cells. Arch Biochem Biophys. 1997;344:413–423. doi: 10.1006/abbi.1997.0201. [DOI] [PubMed] [Google Scholar]
- 148.Venkatraman A. Landar A. Davis AJ. Ulasova E. Page G. Murphy MP. Darley-Usmar V. Bailey SM. Oxidative modification of hepatic mitochondria protein thiols: effect of chronic alcohol consumption. Am J Physiol Gastrointest Liver Physiol. 2004;286:G521–G527. doi: 10.1152/ajpgi.00399.2003. [DOI] [PubMed] [Google Scholar]
- 149.Ventura JJ. Cogswell P. Flavell RA. Baldwin AS., Jr Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vulcano M. Meiss RP. Isturiz MA. Deferoxamine reduces tissue injury and lethality in LPS-treated mice. Int J Immunopharmacol. 2000;22:635–644. doi: 10.1016/s0192-0561(00)00026-6. [DOI] [PubMed] [Google Scholar]
- 151.Wajant H. Pfizenmaier K. Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 152.Wang T. Arifoglu P. Ronai Z. Tew KD. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem. 2001;276:20999–21003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- 153.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 154.Wheeler MD. Kono H. Yin M. Nakagami M. Uesugi T. Arteel GE. Gabele E. Rusyn I. Yamashina S. Froh M. Adachi Y. Iimuro Y. Bradford BU. Smutney OM. Connor HD. Mason RP. Goyert SM. Peters JM. Gonzalez FJ. Samulski RJ. Thurman RG. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 155.Wheeler MD. Nakagami M. Bradford BU. Uesugi T. Mason RP. Connor HD. Dikalova A. Kadiiska M. Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- 156.Winterbourn CC. Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27:322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 157.Wong GH. Elwell JH. Oberley LW. Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 158.Wu D. Cederbaum AI. Oxidative stress mediated toxicity exerted by ethanol-inducible CYP2E1. Toxicol Appl Pharmacol. 2005;207:70–76. doi: 10.1016/j.taap.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 159.Wu M. Bian Q. Liu Y. Fernandes AF. Taylor A. Pereira P. Shang F. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic Biol Med. 2009;46:62–69. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wullaert A. Heyninck K. Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090–1101. doi: 10.1016/j.bcp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 161.Xie C. Zhang N. Zhou H. Li J. Li Q. Zarubin T. Lin SC. Han J. Distinct roles of basal steady-state and induced H-ferritin in tumor necrosis factor-induced death in L929 cells. Mol Cell Biol. 2005;25:6673–6681. doi: 10.1128/MCB.25.15.6673-6681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xiong S. She H. Sung CK. Tsukamoto H. Iron-dependent activation of NF-kappaB in Kupffer cells: a priming mechanism for alcoholic liver disease. Alcohol. 2003;30:107–113. doi: 10.1016/s0741-8329(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 163.Xu Y. Jones BE. Neufeld DS. Czaja MJ. Glutathione modulates rat and mouse hepatocyte sensitivity to tumor necrosis factor toxicity. Gastroenterology. 1998;115:1229–1237. doi: 10.1016/s0016-5085(98)70095-2. [DOI] [PubMed] [Google Scholar]
- 164.Yamada Y. Kirillova I. Peschon JJ. Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang SQ. Lin HZ. Yin M. Albrecht JH. Diehl AM. Effects of chronic ethanol consumption on cytokine regulation of liver regeneration. Am J Physiol. 1998;275:G696–G704. doi: 10.1152/ajpgi.1998.275.4.G696. [DOI] [PubMed] [Google Scholar]
- 166.Yin M. Wheeler MD. Kono H. Bradford BU. Gallucci RM. Luster MI. Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 167.Zahler S. Kupatt C. Becker BF. Endothelial preconditioning by transient oxidative stress reduces inflammatory responses of cultured endothelial cells to TNF-alpha. FASEB J. 2000;14:555–564. doi: 10.1096/fasebj.14.3.555. [DOI] [PubMed] [Google Scholar]
- 168.Zhang R. Al-Lamki R. Bai L. Streb JW. Miano JM. Bradley J. Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 169.Zhao Y. Ding WX. Qian T. Watkins S. Lemasters JJ. Yin XM. Bid activates multiple mitochondrial apoptotic mechanisms in primary hepatocytes after death receptor engagement. Gastroenterology. 2003;125:854–867. doi: 10.1016/s0016-5085(03)01066-7. [DOI] [PubMed] [Google Scholar]
- 170.Zhou Q. Lam PY. Han D. Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 2008;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- 171.Zielonka J. Hardy M. Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic Biol Med. 2009;46:329–338. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zielonka J. Sarna T. Roberts JE. Wishart JF. Kalyanaraman B. Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch Biochem Biophys. 2006;456:39–47. doi: 10.1016/j.abb.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zmijewski JW. Zhao X. Xu Z. Abraham E. Exposure to hydrogen peroxide diminishes NF-kappaB activation, IkappaB-alpha degradation, and proteasome activity in neutrophils. Am J Physiol Cell Physiol. 2007;293:C255–C266. doi: 10.1152/ajpcell.00618.2006. [DOI] [PubMed] [Google Scholar]