Abstract
Spontaneous in vitro differentiation of mouse embryonic stem cells (mESC) is promoted by a dynamic, three-dimensional (3D), tissue-density perfusion technique with continuous medium perfusion and exchange in a novel four-compartment, interwoven capillary bioreactor. We compared ectodermal, endodermal, and mesodermal immunoreactive tissue structures formed by mESC at culture day 10 with mouse fetal tissue development at gestational day E9.5. The results show that the bioreactor cultures more closely resemble mouse fetal tissue development at gestational day E9.5 than control mESC cultured in Petri dishes.
Introduction
Embryonic stem cell (ESC) in vitro differentiation holds high potential for therapy development in regenerative medicine.1 Developing reproducible, safe and effective, in vitro differentiation protocols is important, and innovative culture methods are of interest to address the challenge of differentiating ESC into specialized cells in vitro. While current Petri dish or flask techniques offer static open two-dimensional (2D) plastic areas with discontinuous medium exchange (Fig. 1) and do not allow tissue formation similar to fetal development,2 3D culture systems offer advancements.3,4 Hollow fiber, capillary membrane bioreactor technology, with a 3D cell compartment surrounding a bundle of capillaries, enables dynamic perfusion culture.5–7 However, standard two-compartment devices provide nutrition mainly via diffusion and are limited by nonuniform mass exchange with substrate gradients over decimeters of capillary length.8
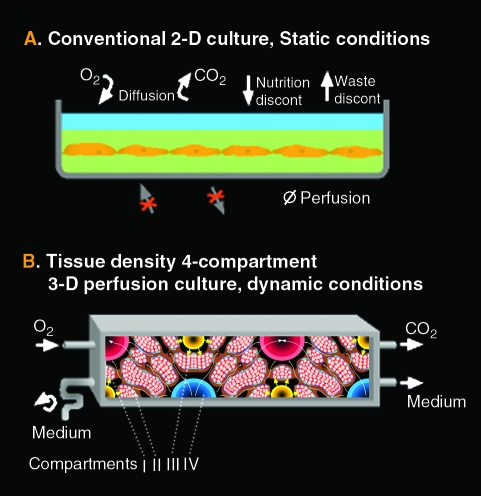
FIG. 1.
Conventional static two-dimensional (2D) culture conditions (A) and dynamic three-dimensional (3D) perfusion tissue-density conditions (B). The smallest interwoven capillary membrane unit exhibiting the compartments II to IV is shown; interweaving allows scale-up by multiplication of these units. The cell aggregate size in the cell compartment I is limited by the space between the capillaries. Each capillary membrane compartment can be perfused separately (medium perfusion compartment II, medium perfusion compartment IV, and oxygenation compartment III), addressing the reduction of substance gradient distances between the capillary units and enhancing mass exchange. Color images available online at www.liebertonline.com/ten.
Our project hypothesis is that a spontaneous ESC in vitro differentiation is qualitatively enhanced in the bioreactors described in comparison to Petri dishes and more closely resembles the development of early mid-term mouse fetal tissue.
We provide an ESC culture model and subsequently a bioreactor construction with continuous medium perfusion and exchange in a four-compartment, interwoven capillary technology. To test our project hypothesis, we compared ectodermal, endodermal, and mesodermal immunoreactive tissue structures formed by mouse ESC (mESC) at culture day 10 with mouse fetal tissue development at gestational day E9.5.
Materials and Methods
Bioreactor and perfusion periphery
The multi-compartment bioreactors were composed of three independent yet interwoven hollow fiber capillary membrane systems (compartments II to IV, see Fig. 1) that are integrated into a two-component polyurethane potting/housing (PUR 725A [isocyanat]/725B [polyol]; Room&Haas, Bremen, Germany). Two of the hydrophilic medium perfusion capillary membranes were made of microporous polyethersulphone with a molecular weight cut-off of approximately MW 500,000 (mPES; Membrana, Wuppertal, Germany; inner diameter, 300 μm ± 40 μm; wall thickness, 100 μm ±25 μm; pore size, 0.5 μm ± 0.1 μm). The third was made of hydrophobic multi-laminate hollow fiber membrane capillaries (Mitsubishi, Tokyo, Japan; inner diameter, 200 ± 10 μm; wall thickness, 42 ± 3 μm; O2-permeability >0.8 E − 5 cm3/cm2/s/cmHg) to enable gas exchange. Thus, the cells located within the extra-capillary space (cell compartment, Fig. 1, I) were exposed to decentralized medium and plasma supply with high mass exchange rates and direct membrane oxygenation via flow-enhanced diffusion. For cell injection, a flow head connected to open-ended silicone rubber capillaries (Silastic, Dow Corning, New York) was used.
The bioreactor was integrated into a processor-controlled perfusion device with electronic flow/pressure controlled perfusion, medium, and waste pump operation. A heating unit provided a constant temperature of 37°C within the perfusion circuit. Flow rates of air, O2, CO2, and N2 were controlled by a gas mixing unit. For further details, see Figure 1 legend. The perfusion tubing with bubble traps was made of standard medical-grade dialysis poly vinyl chloride (PVC) (B.Braun, Melsungen, Germany). Sterilization was performed by formaldehyde gas sterilization at 60°C with alternating degassing.
Preparation of cells
129/SvEv mESC (passage 11) (#CMTI-1; Chemicon International, Billerica, MA) were cocultured with mitomycin-inactivated mouse embryonic fibroblasts (MEFs) strain circular dichroism-1 (passage 5) isolated according to protocols from the WiCell Research Institute (Madison, WI). mESC were seeded at a cell density of 3.5 × 104 cells/cm2 into culture dishes coated with 0.1% gelatin (EmbryoMax ES Cell Qualified; Millipore, Bedford, MA) and pre-seeded with inactivated MEFs which had been seeded at a density of 3.0 × 104 cells/cm2. Cells were cultured in T175 culture flasks (BD Falcon, San Jośe, CA) or in luminox multiwell plates suitable for immunofluorescence studies (TC-Quality, bio-one, Greiner). Every 2–3 days, the cells were passaged, using a 0.05% trypsin/0.02% EDTA solution (Biochrom, Berlin, Germany). Day 4–10 control cultures were seeded into 2D culture flasks or multi-well plates coated with gelatin, but lacking preseeded MEFs on the same day as the bioreactor cultures were initiated. Thus, they contained only those feeder cells that had been transferred with the mESC from the previous passage, analogous to the bioreactor cultures.
Culture medium
Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 15% fetal calf serum, penicillin/streptomycin, L-glutamine, β-mercaptoethanol, nonessential amino acids, and nucleosides (NEAA); for expansion, 1000 U/mL leukemia inhibiting factor (ESGRO) was added. All medium components were purchased from Biochrom AG, except leukemia inhibiting factor (LIF), fetal calf serum, NEAA, and β-mercaptoethanol, which were purchased from Chemicon International, CA.
Culture of cells in the bioreactors
After a conditioning phase of 24 h with recirculating culture medium, mESC/MEF cocultures were inoculated into the bioreactors without additional MEF cells. The cell compartment was supplied with 100 mL/min of a gas mixture consisting of 95% air and 5% CO2 by volume. Bioreactors were perfused at a constant recirculation rate of 40 mL/min. Fresh medium was supplied with an increasing feed rate from 6 to 12 mL/min. The bioreactors were kept at 37°C. Daily measurements of pH, pO2, pCO2, and buffer capacity were used to adjust medium perfusion and gas supply rates (I-stat [Abbot, East Windsor, NJ] and ABL 5 [Radio Meter Copenhagen, Copenhagen, Denmark]).
Metabolic parameters
Samples from the perfused medium were taken every day for biochemical analyses. The metabolic activity of the cells was determined by measuring glucose consumption and lactate production, while potential cell damage was detected by cellular lactate dehydrogenase release. Analyses were performed with assays adapted for an automated clinical analyzer (Roche/Hitachi Modular; Roche Diagnostics, Mannheim, Germany).
Bioreactor cell sample harvest
At the end of designated culture periods, bioreactors were shut down and tubes were disconnected. The upper bioreactor lid was opened, and the cell mass, including the capillary layers, was cut from the surrounding potting and transferred into a sterile glass vessel for dividing into sections for the microscopy methods.
Histology and immunofluorescence
Material from the bioreactor cell compartment was fixed in 4% paraformaldehyde solution before paraffin embedding and sectioning.
Antibodies and dilutions
Primary antibodies (alphabetical order): mouse anti-α smooth muscle actin (ASMA, cross-reactivity with human), 1:500, Chemicon, Billerica, MA, CBL171; rabbit anti-mammalian Neuronal Class III β-Tubulin, 1:250, Biosite, Princeton, NJ, PRB-435-P; goat-anti-mouse FoxA2 (HNF3β, cross-reactivity with human), 1:200, Santa Cruz, CA, Sc-6554; mouse anti-rat Nestin (cross-reactivity with human), 1:200, BD Transduction Laboratories, San Jośe, CA, 611658; mouse anti-human Oct ¾, 1:250, Santa Cruz, Sc-5279; mouse anti-mouse SSEA-1 (cross-reactivity with human), Developmental Studies Hybridoma Bank, University of Iowa, East Iowa City, IA, MC-480. Secondary antibodies: goat α-mouse IgG2a TRITC, 1:500, Southern Biotech, Los Angeles, CA, 1080-03; donkey α-goat IgG Cy3, 1:500, Jackson ImmunoResearch, West Groove, PA, 705-165-147; goat α-mouse IgG Alexa 488, 1:500, Molecular Probes, Eugene, OR, A-11029; donkey α-rabbit IgG Alexa 594, 1:500, Molecular Probes, A-21207; goat α-mouse IgG1 Cy3, 1:500, Jackson ImmunoResearch, 115-165-205, goat anti-mouse IgG Cy2, 1:1000, Dianova, 115-225-003.
Staining procedure
Samples were deparaffinized and rehydrated according to standard procedures using xylene as an organic solvent and a decreasing ethanol gradient to rehydrate the sections. Hematoxylin-eosin or toluidine-blue staining of sections was performed according to standard laboratory procedures. For immunofluorescence, the antigen was retrieved by boiling the sections in 0.01 M citric acid, pH6.0. After cooling the sections at room temperature for 15 min, sections were washed twice in phosphate buffered saline (PBS) and then permeabilized for 5 min in PBS supplemented with 0.5% Triton X-100 (T8532; Sigma–Aldrich, Germany). Primary antibodies diluted in staining buffer (PBS supplemented with 0.1% Triton X-100) were added and incubated with the sections overnight at 4°C. Subsequent to threefold washing with PBS, the secondary antibodies diluted in staining buffer were added to the sections and incubated at room temperature for another 2–3 h. Afterward, the sections were washed three times in PBS with addition of DAPI, 1:1000 (D9542; Sigma–Aldrich) in the last washing step. Sections were mounted with antifading fluorescent mounting medium from DakoCytomation, s3023.
Immunofluorescence microphotographs were taken using a Zeiss Axioskop 40 microscope with Zeiss Axiocam digital camera and Zeiss Axiovision software (Zeiss, Oberkochen, Germany). The brightness and contrast were uniformly enhanced using Adobe® Photoshop; color balance was used to enable specific immuno-fluorescent (IF) signals be viewed through the DAPI staining on both control and experimental cells.
Transmission electron microscopy
For transmission electron microscopy (TEM), material from the bioreactor cell compartment was fixed with 5% glutaraldehyde (Serva, Heidelberg, Germany). After immersion for 30 min in 60 mM phosphate buffer, pH 7.3, the cellular aggregates were postfixed in 2% OsO4 (Paesel + Lorei, Frankfurt, Germany) for 2 h, progressively dehydrated in ethanol, and then embedded in araldite (Serva). Ultra-thin sections were contrasted with uranyl acetate and Reynold's lead citrate (Chroma, Münster, Germany) before electron microscopic examination.
Results
To enhance mass exchange, address gradient distances, and integrate gas exchange allowing to increases to more physiological levels, we added two more compartments to the typical two-compartment devices: an additional medium capillary compartment for counter-current “arterio-venous” flow and an oxygenation capillary compartment (Fig. 2). Each capillary membrane compartment can be perfused independently. Interweaving the four-compartment capillary systems to form repetitive units allows bioreactor scalability by multiplying the capillary units, and provides decentralized medium perfusion while enhancing mass exchange and reducing gradient distances from decimeters to more physiologic lengths of <1 mm. The exterior of the resulting membrane network, the cell compartment, is used as a physically active scaffold for cell aggregation; adjusting intercapillary distances enables control of the size of cell aggregates.
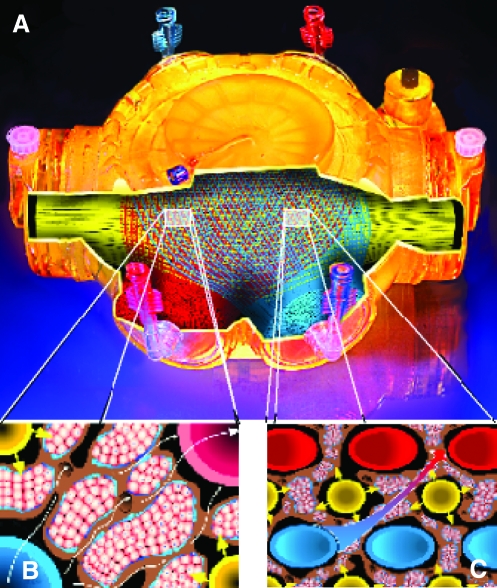
FIG. 2.
Schematic drawing of the dynamic 3D perfusion tissue-density bioreactor technology. (A) Housing with tube connectors, partially opened to view the capillary systems. All membrane compartments are interwoven, forming a tight network. Interweaving the smallest capillary units (B) allows scale-up without changing the smallest units, as shown in (C). This allows scale-up by multiplication of these units. The corresponding capillaries of each compartment are bundled to common in- and outflow heads to be connected to tube systems. Cells are inoculated via 16 (8 mL prototype) open-ended tubes (not shown), allowing cell distribution within the cell compartment. (B) The smallest interwoven capillary membrane unit exhibiting the four compartments. The cells are shown between the capillaries. The cell aggregate size in the cell compartment is limited by the space between the capillaries. (C) Each capillary membrane compartment can be perfused independently (compare Fig. 1, medium perfusion compartment II, medium perfusion compartment IV, and oxygenation compartment III) addressing the reduction of substance gradient distances between the capillary units and enhancing mass exchange. Color images available online at www.liebertonline.com/ten.
Using this four-compartment bioreactor technology for longer-term cultures, we wanted to demonstrate that a spontaneous ESC in vitro differentiation is qualitatively enhanced in comparison to Petri dishes and more closely resemble the development of early mid-term mouse fetal tissue. We compared 129/SvEv mESCs2 cultured in 3D perfusion bioreactors (3D) to those cultured in Petri dishes (2D) and also with fetal gestational day E9.5 tissue, using histology and immuno-fluorescence. We cultured 129/SvEv mESC (passage 11) with mitomycin-inactivated mouse embryonic circular dichroism-1 fibroblasts (MEFs)9,10 in 2D and then in 8 mL 3D cell compartment bioreactors for 4, 6, and 10 days. Bioreactors were inoculated with 2.5 × 107 mESC with no further MEF addition; the bioreactors contained approximately 1.5 × 107 MEFs from the previous passage. Glucose consumption and lactate production during culture continuously increased in both the dishes and the bioreactors (data not shown), indicating continuous cell growth. Histology was confirmed by repeating the day 10 experiments (n = 3).
Histology at the endpoints of 4, 6, and 10 days showed that cells in 3D culture were assembled in aggregates between the capillaries. In TEM a regular distribution of aggregates with an average estimated diameter of 490 ± 87 μm was seen; a central necrosis formation in the largest aggregates was observed.
IF marker staining in the 2D group showed mostly individual positive cells up to day 10. No extended organized tissue-like structures were formed, except for Nestin11-positive neural rosettes observed in multi-layer parts of the dishes.
IF positivity in the 3D group, demonstrated for marker of all three germ layer, shows a time-dependent development of tissue-like structures partly resembling structures of the embryonic day E9.5 mouse embryo (Figs. 3 and 4). FoxA212 and Nestin-positive cells were observed already after 2 days, while ASMA13 and class III β-Tubulin14–positive filaments appeared after 6 and 10 days, respectively. After 10 days in the 3D cultures, complex epithelial-like structures positive for the endodermal marker FoxA2, resembling the primitive gut tube, were observed (Fig. 3), and structures positive for the mesodermal marker ASMA resembled the primitive cardium (Fig. 3). In addition, neural tube–like structures were positive for the ectodermal marker Nestin (Fig. 3), and some cells exhibited protrusions that were positive for the post-mitotic neuronal marker class III β-Tubulin (Fig. 3). Simultaneously, mESC characterized by Oct-415 staining diminished time dependently in both groups (Fig. 4).
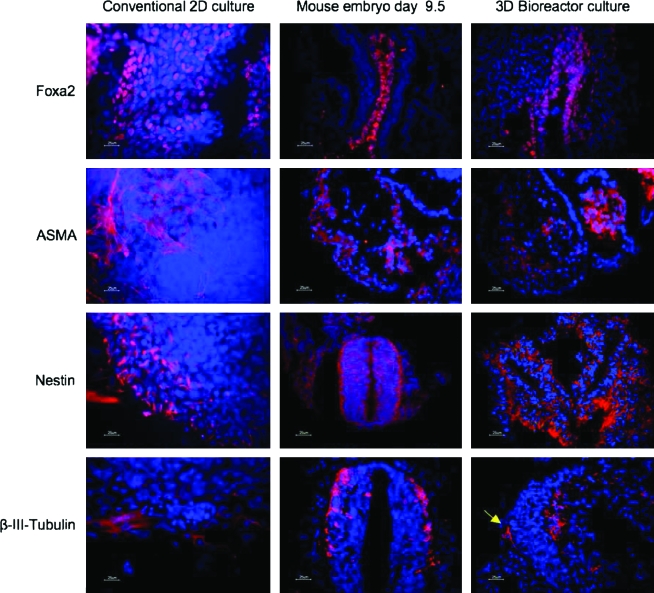
FIG. 3.
Tissue-like structure formation of mouse embryonic stem cell (mESC) and lineage-specific immunofluorescence in conventional 2D cultures versus 3D bioreactor cultures, compared to mouse embryos. DAPI staining (blue) was used as a nuclear counter stain. Center column: histology of the day E9.5 mouse embryo; primitive gut tube (endoderm/Foxa2), cardiac region (mesoderm/anti-α smooth muscle actin [ASMA]), neural tube (ectoderm/Nestin), and post-mitotic neurons in the neuronal tissue (ectoderm/class III β-Tubulin). Right column: mESC after 10 days in 3D culture are partly differentiated into structures resembling primitive gut (endodermal lineage FoxA2), structures resembling primitive cardium (mesodermal lineage, ASMA), and structures resembling neural tube as well as neuronal-like cells (arrow; ectodermal lineage, Nestin and class III β-Tubulin, respectively), similar in part to that in E9.5 mouse embryo (center column). Left column: differentiated cells in 2D cultures at day 10 are unorganized, and most tissue-like structures found in 3D were absent. Scale bars: 25 μm. Color images available online at www.liebertonline.com/ten.
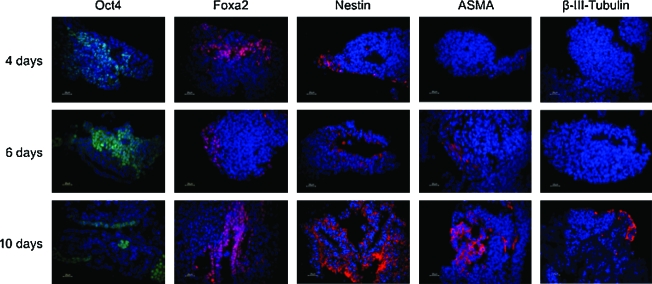
FIG. 4.
Differentiation pattern of mESCs inoculated into an 8 mL bioreactor at days 4, 6, and 10. Oct-4 staining was used to reveal undifferentiated mESCs. Differentiation markers used were Foxa2 for endoderm, Nestin for ectoderm, ASMA for cardiac and vascular mesoderm, and class III β-Tubulin for postmitotic neurons. Nuclei were costained with DAPI (blue). Scale bars: 25 μm. Color images available online at www.liebertonline.com/ten.
Histology and IF marker suggest that the spontaneous mouse ESC differentiation after day 10 in 3D perfusion is qualitatively enhanced in comparison to 2D static culture, and closer resembles the histology of E9.5 mouse fetal tissue.
Discussion
Because of their unique characteristics, ESCs, initially generated from mice16,17 and later from humans,18 hold great potential as a cell source for applications in basic science, pharmacological drug screening, toxicity testing, and cell-based therapies in regenerative medicine.
The embryoid body (EB) formation, originally developed as hanging drop, is a well-established ESC culture method,19–23 already considering 3D techniques, but under static conditions. EB culture is performed to enable the onset of a spontaneous ESC differentiation, traditionally to take the cells into further 2D culture or animal models. EB culture can be performed until the EBs start showing central necrosis, due to the limited mass exchange in the body. Providing dynamic mass exchange in a larger cell mass was goal of our culture model development. Our TEM analysis shows that a central necrosis formation in the largest aggregates could not be avoided, but was not seen in the regular aggregates, as displayed in Figures 3 and 4. This may be addressed by changing the membrane weaving patterns and thus reducing the interfiber distances to mechanically further limit the available space for ESC aggregate growth.
The types of bioreactors described for ESC culture and differentiation include stirred bioreactors (e.g., Spinner flasks), rotary systems like slow-turning lateral vessels,24,25 and fibrous bed bioreactors.26,27 Because embryonic stem cells grow adherent in colonies and are dependent on tight cell–cell contacts, they do not grow as single cells in suspension; to culture the cells in stirred tank bioreactors, they are either maintained as aggregates or adhered to microcarriers. Undifferentiated expansion of mESCs has been shown using microcarriers28,29 as well as in carrier-free suspension.30 The culture of ESC as aggregates in suspension is limited by the size of the cell clusters. If the clusters become too large in size, mass transport into their center is reduced, for example, oxygen and nutrients, leading to cell necrosis. Employing microcarriers reduces the shear stress on the cells, but only low cell expansions and seeding efficiencies could be achieved.31,32 The use of microcarriers is limited by their available surface area, thereby requiring frequent cell dissociation and passaging, which leads to cell loss due to low seeding efficiencies and the fragility of the cells. To solve these problems novel techniques are needed. Perfusion culture techniques are of interest, since it has been shown that medium perfusion is beneficial even in 2D cultures,33 and perfusion also facilitates continuous nutrient and oxygen supply34 in larger cell masses.
The four-compartment capillary membrane technology described offers dynamic, perfusion-based culture conditions with continuous medium exchange and decentralized mass exchange, including oxygenation at controllable gas tensions in larger cell masses. It also will allow the application of differentiation regimes in a closed system, suitable for good manufacturing practice conditions, which can be easily scaled from laboratory to clinical translation requirements. Controllable medium and system parameters include gas factor application, medium factor gradients, and physical stimuli such as flow and pressure. Dynamic perfusion also enables the combination of twin bioreactors in one circuit, where factors or soluble mediators of the first bioreactor can stimulate the second bioreactor, while the cells remain compartmentalized in each bioreactor. This would be of interest if soluble factors of a coculture are to be used, but cell–cell contaminations between the cultures avoided. Thus, we believe that this technology provides unique opportunities to further ESC research by facilitating customized, well-controlled differentiation and tissue induction protocols.
Acknowledgments
This study was supported by grants from the National Institutes of Health (NIH R21 EB005739-01, to J.G. and G.S.), the European Commission (EU STREP-CT-2005-018940, to J.G.), and the Federal Ministry of Education and Research, Germany (BMBF, 01GG0731/0313911, to K.Z.). We are grateful for excellent help with mESC cultures from Dr. Chris Navara (Pittsburgh Development Center), perfusion systems from Matt Baun, TEM from Petra Schrade (Department of Anatomy, Charité Berlin, Germany), and graphics from Wolfgang Mudra (Charité).
Author Contributions
J.G. and K.Z. designed, coordinated, and performed the research and bioreactor development; analyzed data; and wrote the article.
M.H. designed, coordinated, and performed the mESC bioreactor runs; developed methods for cell characterization; analyzed data; and provided useful suggestions.
J.E. performed IF studies of mESC cultures, analyzed data, and provided useful suggestions.
T.M., E.S., H.S., and G.S. evaluated experimental data and provided useful suggestions.
M.L. performed 2D mESC cultures and data analysis.
P.Bj. provided IF methods and useful suggestions.
Disclosure Statement
The biorector prototypes were produced by StemCell Systems, Berlin, Germany. They were purchased. J.G. licensed the technology. No author has shares or financial interests in the company.
References
- 1.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human embryonic blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Wiles M.V. Embryonic stem cell differentiation in vitro. Methods Enzymol. 1993;225:900. doi: 10.1016/0076-6879(93)25057-9. [DOI] [PubMed] [Google Scholar]
- 3.Cukierman E. Pankov R. Yamada K.M. Embryonic stem cell differentiation in vitro. Curr Opin Cell Biol. 2002;14:633. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 4.Abbott A. Cell culture: biology's new dimension. Nature. 2003;424:870. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 5.Knazek R.A. Gullino P.M. Kohler P.O. Dedrick R.L. Cell culture on artificial capillaries: an approach to tissue growth in vitro. Science. 1972;178:65. doi: 10.1126/science.178.4056.65. [DOI] [PubMed] [Google Scholar]
- 6.Gerlach J.C. Mutig K. Zeilinger K. Sauer I.M. Mieder T. Naumann G. Gruenwald A. Pleß G. Schrade P. Efimova E. Goetz M. Mas A. Vienken J. Bachmann S. Neuhaus P. The use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors—a morphological study. Transplantation. 2003;76:781. doi: 10.1097/01.TP.0000083319.36931.32. [DOI] [PubMed] [Google Scholar]
- 7.Monga P.S. Hout M.S. Baun M.J. Micsenyi A. Muller P. Tummalapalli L. Ranade A.R. Strom S.C. Gerlach J.C. Mouse fetal liver cells in artificial capillary beds in three-dimensional four-compartment bioreactors. Am J Pathol. 2005;167:1279. doi: 10.1016/S0002-9440(10)61215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu A.S.L. Luntz T.L. Macdonalds J.M. Kubota H. Hsu E. London R.E. Reid L.M. Lineage and biology and liver. In: Lanza R.P., editor; Langer R., editor; Vacanti J., editor. Principles of Tissue Engineering. 2nd. Vol. 41. Academic Press; San Diego, CA: 1999. pp. 559–598. [Google Scholar]
- 9.Smith A.G. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;78:435. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 10.Williams R.L. Hilton D.J. Pease S. Wilson T.A. Stewart C.L. Gearing D.P. Wagner E.F. Metcalf D. Nicola N.A. Gough N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 11.Dahlstrand J. Lardelli M. Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 12.Ang S.L. Wierda A. Wong D. Stevens K.A. Cascio S. Rossant J. Zaret K.S. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- 13.Clement S. Stouffs M. Bettiol E. Kampf S. Krause K. Chaponnier C. Jaconi M. Expression and function of α-smooth muscle actin during embryonic-stem-cell-derived cardiomyocyte differentiation. J Cell Sci. 2007;120:229. doi: 10.1242/jcs.03340. [DOI] [PubMed] [Google Scholar]
- 14.Katsetos C.D. Herman M.M. Mork S.J. Class III β-tubulin in human development and cancer. Cell Motil Cytoskeleton. 2003;55:77. doi: 10.1002/cm.10116. [DOI] [PubMed] [Google Scholar]
- 15.Nichols J. Zevnik B. Anastassiadis K. Niwa H. Klewe-Nebenius D. Chambers I. Schöler H. Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 16.Evans M.J. Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 17.Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 19.Itskovitz-Eldor J. Schuldiner M. Karsenti D. Eden A. Yanuka O. Amit M. Soreq H. Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies comprising the three embryonic germ layers. Mol Med. 2000;6:88. [PMC free article] [PubMed] [Google Scholar]
- 20.Desbailltes I. Ziegler U. Groscurth P. Gassmann M. Embryoid bodies: in an in vitro model of mouse embryogenesis. Exp Physiol. 2000;85:645. [PubMed] [Google Scholar]
- 21.Cameron C.M. Harding F. Hu W. Kaufman D.S. Activation of hypoxic response in human embryonic stem cell-derived embryonic bodies. Exp Biol Med online June 5, 2005. doi:10.3181/0709-RM-263. [DOI] [PubMed]
- 22.Vallier L. Pederson R.A. An in vitro model to study mechanisms controlling pluripotency in early mammalian development. Hum Embryonic Stem Cells Stem Cell Rev. 2005;1:119. doi: 10.1385/SCR:1:2:119. [DOI] [PubMed] [Google Scholar]
- 23.Kurosawa H. Methods of inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng Rev. 2007;103:389. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 24.Come J. Nissan X. Aubry L. Tournois J. Girard M. Perrier A.L. Peschanski M. Cailleret M. Improvement of culture conditions of human embryoid bodies using a controlled perfused and dialyzed bioreactor system. Tissue Eng C Methods. 2008;14:289. doi: 10.1089/ten.tec.2008.0029. [DOI] [PubMed] [Google Scholar]
- 25.Hwang Y.S. Cho J. Tay F. Heng J.Y.Y. Ho R. Kazarian S.G. Williams D.R. Boccaccini A.R. Polak J.M. Mantalaris A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30:499. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Li Y. Kniss D.A. Lasky L.C. Yang S.T. Culturing and differentiation of murine embryonic stem cells in a three-dimensional fibrous matrix. Cytotechnology. 2003;41:23. doi: 10.1023/A:1024283521966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouyang A. Yang S.T. A two-stage perfusion fibrous bed bioreactor system for mass production of embryonic stem cells. Expert Opin Biol Ther. 2008;8:895. doi: 10.1517/14712598.8.7.895. [DOI] [PubMed] [Google Scholar]
- 28.Abranches E. Bekman E. Henrique D. Cabral J.M.S. Expansion of mouse embryonic stem cells on microcarriers. Biotechnol Bioeng. 2007;96:1211. doi: 10.1002/bit.21191. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes A.M. Fernandes T.G. Diogo M.M. da Silva C.L. Henrique D. Cabral J.M.S. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. 2007;132:227. doi: 10.1016/j.jbiotec.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 30.zur Nieden N.I. Cormier J.T. Rancourt D.E. Kallos M.S. Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J Biotechnol. 2007;129:421. doi: 10.1016/j.jbiotec.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Phillips B.W. Horne R. Lay T.S. Rust W.L. Teck T.T. Crook J.M. Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol. 2008;138:24. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- 32.Nie Y. Bergendahl V. Hei D.L. Jones M.J. Palecek S.P. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog. 2009;25:20. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fong W.J. Tan H.L. Choo A. Oh S.K. Perfusion cultures of human embryonic stem cells. Bioprocess Biosyst Eng. 2005;27:381. doi: 10.1007/s00449-005-0421-5. [DOI] [PubMed] [Google Scholar]
- 34.Ouyang A. Yang S.T. A two-stage perfusion fibrous bed bioreactor system for mass production of embryonic stem cells. Expert Opin Biol Ther. 2008;8:895. doi: 10.1517/14712598.8.7.895. [DOI] [PubMed] [Google Scholar]