Abstract
Context: Epidemiological data indicate that reduced sleep duration is associated with increased incidence of type-2 diabetes.
Objective: The aim of the study was to test the hypothesis that, when part of a Western-like lifestyle, recurrent bedtime restriction may result in decreased glucose tolerance and reduced insulin secretion and action.
Design and Setting: We conducted a randomized crossover study at a university clinical research center and sleep research laboratory.
Participants: Eleven healthy volunteers (five females and six males) with a mean (±sd) age of 39 ± 5 yr and body mass index of 26.5 ± 1.5 kg/m2 participated in the study.
Intervention: The study included two 14-d periods of controlled exposure to sedentary living with ad libitum food intake and 5.5- or 8.5-h bedtimes.
Main Outcome Measures: Oral and iv glucose challenges were used to obtain measures of glucose tolerance, glucose effectiveness, insulin secretion, and insulin sensitivity at the end of each intervention. Secondary measures included circulating concentrations of the glucose counter-regulatory hormones, cortisol, GH, epinephrine, and norepinephrine.
Results: Bedtime restriction reduced daily sleep by 122 ± 25 min. Both study periods were associated with comparable weight gain; however, recurrent sleep restriction resulted in reduced oral glucose tolerance (2-h glucose value, 144 ± 25 vs. 132 ± 36 mg/dl; P < 0.01) and insulin sensitivity [3.3 ± 1.1 vs. 4.0 ± 1.6 (mU/liter)−1 · min−1; P < 0.03], and increased glucose effectiveness (0.023 ± 0.005 vs. 0.020 ± 0.005 min−1; P < 0.04). Although 24-h cortisol and GH concentrations did not change, there was a modest increase in 24-h epinephrine and nighttime norepinephrine levels during the 5.5-h bedtime condition.
Conclusions: Experimental bedtime restriction, designed to approximate the short sleep times experienced by many individuals in Westernized societies, may facilitate the development of insulin resistance and reduced glucose tolerance.
Combining the adverse metabolic effects of a Westernized lifestyle with recurrent sleep curtailment may facilitate the development of reduced glucose tolerance.
The pathogenesis of type-2 diabetes (T2D) is influenced by genetic, environmental, and behavioral factors and involves a complex interaction between deficits in insulin secretion and action (1). The development of insulin resistance due to excessive adiposity and physical inactivity and the failure of pancreatic β-cells to maintain a compensatory increase in insulin secretion are the main forces behind the rising burden of T2D in Westernized societies (2,3). Although overeating and sedentary living have emerged as two of the most important modifiable risk factors for T2D, the need for improved preventive strategies continues to fuel the search for additional behavioral determinants of diabetes risk (4). Recently, the hypothesis that the lack of sufficient sleep may represent a risk factor for T2D and related metabolic disorders has attracted growing research interest and scientific debate (5,6).
Many Americans today sleep fewer than 6 h/night (7,8), and individuals who report such short sleep times have an increased risk of developing diabetes (9,10,11,12). Whether reduced sleep duration itself is involved in the pathogenesis of this disorder (5) or reflects the presence of other unrecognized risk factors remains unknown (8,13). Few controlled experiments have examined the effects of sleep loss on human insulin secretion and action as key processes in the development of T2D. Increased insulin resistance (14) and compensatory hyperinsulinemia (15) have been observed after one or two nights of total sleep deprivation, whereas sleep restriction to 4 h in bed for five nights was found to interfere with insulin secretion, but not sensitivity (16). Despite these differences, reduced oral carbohydrate tolerance was reported after 3–5 d of total sleep deprivation as well as six nights of partial sleep deprivation (16,17). Previous researchers have also speculated that sleep loss-induced changes in adrenocortical, somatotropic, and sympathetic nervous system activity may contribute to the development of glucose intolerance (16,18,19).
Unfortunately, our understanding of the mechanisms that link reduced sleep duration with increased risk of T2D in previous studies is clouded by a number of limitations. First, experiments that have documented the adverse effects of sleep loss on carbohydrate metabolism, so far, have restricted sleep to abnormally low levels. Sleep restriction to less than 4 h/night is difficult to tolerate for more than a few days and is unusual among chronic short sleepers (6,20), the majority of whom report sleeping 5–6 h a day (8,11,12). Second, due in part to the severity of such interventions, these studies documented only the acute effects (<7d) of sleep loss on carbohydrate metabolism. Whether exposure to less severe but more prolonged sleep restriction has any adverse impact on human insulin secretion and sensitivity remains unknown. Third, the diet and activity of the participants in prior studies with more than two nights of sleep deprivation were not documented during the entire intervention period (16,17). Finally, earlier studies included mainly young lean male volunteers (14,15,16,19), and no controlled experiments have described the impact of sleep loss on the carbohydrate metabolism of middle-aged men and women, despite the fact that these individuals have the shortest self-reported sleep times in the United States (8).
In the present study, we tested the hypothesis that recurrent bedtime restriction to 5.5 h/night in middle-aged adults can result in reduced glucose tolerance and decreased insulin secretion and sensitivity. We also examined whether such bedtime restriction, designed to approximate the short sleep times experienced by many individuals in real life, will be accompanied by changes in the circulating levels of the glucose counter-regulatory hormones, cortisol, GH, epinephrine, and norepinephrine. Because sleep curtailment is an increasingly common aspect of the Western lifestyle, which is characterized by physical inactivity and overeating, these experiments were carried out under controlled laboratory conditions of sedentary living with ad libitum access to palatable food and were part of a broader study of the effects of sleep loss on human energy metabolism (21).
Subjects and Methods
Participants
Eleven sedentary adults (six females and five males) with mean ± sd age 39 ± 5 yr, body mass index 26.5 ± 1.5 kg/m2, and self-reported sleep duration 7.6 ± 0.7 h/d completed the study. Research volunteers gave written informed consent and were paid for their participation (see supplemental data published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Study protocol
The study protocol was approved by the University of Chicago Institutional Review Board. The experimental design has been described in detail (21). Briefly, each subject completed two 14-d study periods with sedentary activity, ad libitum food intake, and scheduled bedtimes of 5.5 or 8.5 h/night in random order at least 3 months apart (see supplemental data). Exposure to this environment resulted in a positive energy balance and similar weight gain, irrespective of the presence or absence of sleep loss (Table 1).
Table 1.
Glucose tolerance test results
| 8.5-h bedtime | 5.5-h bedtime | P value | |
|---|---|---|---|
| Initial body weight (kg) | 75.0 ± 9.0 | 77.3 ± 10.2 | 0.056 |
| Initial BMI (kg/m2) | 26.3 ± 1.7 | 27.0 ± 1.8 | 0.065 |
| Initial body fat (kg) | 24.3 ± 6.9 | 25.6 ± 5.9 | 0.120 |
| 14-d change in body weight (kg) | 2.1 ± 2.1 | 1.9 ± 1.6 | 0.676 |
| OGTT | |||
| Fasting insulin (mU/liter) | 8.4 ± 3.9 | 7.8 ± 3.2 | 0.535 |
| Fasting glucose (mg/dl) | 91 ± 5 | 92 ± 5 | 0.639 |
| 2-h glucose (mg/dl) | 132 ± 36 | 144 ± 25 | 0.006 |
| AUC-glucose 0–180 min (mg · dl−1 · min) | 23,595 ± 3,616 | 24,800 ± 3,896 | 0.042 |
| AUC-insulin 0–180 min (mU · liter−1 · min) | 11,229 ± 6,464 | 11,216 ± 6,273 | 0.819 |
| IVGTT | |||
| SI (mU/liter)−1 · min−1 | 4.0 ± 1.6 | 3.3 ± 1.1 | 0.026 |
| AIRG (mU · liter−1 · min) | 507 ± 198 | 523 ± 209 | 0.927 |
| DI | 1915 ± 910 | 1581 ± 658 | 0.174 |
| SG (min−1) | 0.020 ± 0.005 | 0.023 ± 0.005 | 0.039 |
| GEZI (min−1) | 0.018 ± 0.005 | 0.021 ± 0.005 | 0.037 |
Data are mean ± sd (n = 11). Paired t-tests were used to compare initial body weight and composition variables. The results from the glucose tolerance tests at the end of each bedtime condition were compared using a generalized estimating equation model with order of treatment and body weight as time-varying covariates. AUC, Area under curve.
Starting at 2000 h on the last day of each bedtime condition, participants were transferred to the General Clinical Research Center for 48 h of additional testing. During the last 24 h of this period, subjects remained at bed rest and blood was sampled every 30 min, except for the first hour after meals and the first 2 h of scheduled bedtime, when blood was collected every 15 min. Bedtimes were maintained at 5.5 or 8.5 h according to the assigned experimental condition. In the morning of the first day, after a 14-h overnight fast, study participants underwent a 3-h oral glucose tolerance test (OGTT) as follows: baseline blood samples were collected at −15 and 0 min for measurements of glucose and insulin, 75 g glucose was administered orally, and additional samples were collected at 30, 60, 90, 120, 150, and 180 min after the glucose challenge. An iv glucose tolerance test (IVGTT) was performed under similar settings starting at 0900 h on the second day. The IVGTT was incorporated in the ongoing sequence of 24-h blood sampling as follows: a 0.3 g/kg iv glucose bolus was given after the 0900-h blood collection, followed by a 0.03 U/kg bolus of regular insulin 20 min later; samples were taken 2, 3, 4, 6, 8, 10, 12, 14, 16, 19, 22, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 min after the glucose bolus; and the usual 24-h blood drawing sequence was resumed at 1230 h. Identical carbohydrate-rich meals were served at 1400 and 1900 h on both testing days (22).
Sleep monitoring
Sleep was recorded polygraphically and scored according to standard clinical criteria. Total sleep time was calculated as the sum of all sleep epochs (21).
Assays
Plasma glucose was measured at the bedside in whole venous blood (STAT-2300 YSI Analyzer; YSI, Inc., Yellow Springs, OH). Serum insulin, total cortisol, and GH concentrations were measured using human chemiluminescent enzyme immunoassays (Immulite; Diagnostic Products Corp., Los Angeles, CA). Due to limitations in sample volume, plasma epinephrine and norepinephrine were measured in seven of the 11 study participants as previously described (22).
Data analysis
Fasting glucose and insulin concentrations were calculated as the average of the −15 and 0-min OGTT readings. The 120-min glucose concentration provided a clinically relevant measure of oral glucose tolerance. The areas under the 3-h OGTT glucose and insulin curves were calculated using the trapezoidal rule. IVGTT-based estimates of insulin sensitivity (SI), glucose effectiveness at basal insulin concentration (SG), glucose effectiveness at zero insulin (GEZI), and the acute insulin response to glucose (AIRG) were derived by minimal model analysis (MINMOD, version 5.01; BeBos Assoc., Boston, MA). To assess β-cell compensation for the degree of insulin resistance, we also calculated the disposition index, DI, as the product of AIRG and SI.
In addition to comparing the 24-h hormone concentrations during each sleep condition, we analyzed cortisol, GH, epinephrine, and norepinephrine levels during the following time intervals: 1) 0900–2300 h, daytime period; 2) 2300–0900 h, overnight period; and 3) 0900–1300 h, post-iv-glucose period. Rates of GH secretion were derived by deconvolution using a one-compartment model for GH clearance and subject-adjusted half-lives selected to minimize the number of false-negative secretory rates (18). GH profiles were interpolated at 15-min intervals, and volume of distribution was assumed to be 7% of body weight.
To characterize the diurnal rhythm of serum cortisol, we derived best-fit regression curves based on the 24-h concentrations of each subject using a robust locally weighted procedure (www.ibridgenetwork.org/uctech/chronobiological-series-analyzer-csa) with a 4-h window. The maximum and minimum of each regression curve defined the acrophase and nadir of the 24-h rhythm. Amplitude was equal to half the difference between acrophase and nadir. To allow comparisons with previously published data (16), we also determined the “quiescent period” of overnight cortisol secretion as the time when cortisol concentrations remained below 5 μg/dl.
Statistics
Measures of insulin secretion and sensitivity and glucose tolerance at the end of each study were compared using generalized estimating equation regression models with bedtime condition (5.5 vs. 8.5 h), order of treatment (initial vs. crossover study), and final body weight as time-varying factors (21). Using 14-d weight change or energy balance instead of final body weight did not alter the results of these analyses (data not shown). Paired t-tests were used for exploratory comparisons of cortisol and GH levels between the two sleep conditions. Plasma catecholamine measurements were compared using the Wilcoxon paired sign rank test. ANOVA with bedtime condition and post-iv-glucose time (four consecutive 1-h intervals between 0900 and 1300 h) as repeated measures was used to explore the hormonal changes after the iv-glucose challenge. Statistical analyses were performed using SPSS, version 16.0 (SPSS Inc., Chicago, IL). Results in the text are reported as mean ± sd.
Results
Sleep duration
As bedtime changed from 8.5 to 5.5 h, subjects went to bed later, 0031 vs. 2316 h, and got out of bed earlier, 0602 vs. 0742 h (±39 min). Mean sleep duration was reduced by 122 ± 25 min/d from 7 h 13 min (±26 min) with 8.5-h bedtimes to 5 h 11 min (±7 min) with 5.5-h bedtimes (P < 0.001).
Glucose tolerance, β-cell function, and insulin sensitivity
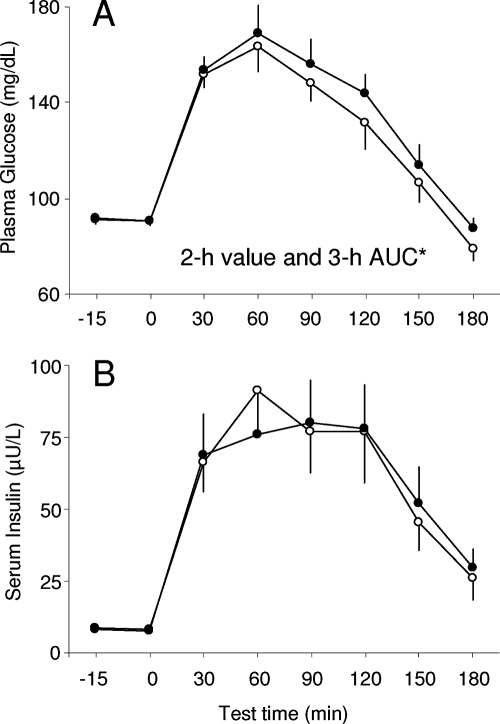
There were no differences in fasting glucose and insulin concentrations between the two sleep conditions (Table 1). In contrast, 2-h glucose values and the area under the 3-h OGTT curve for glucose, but not insulin, were significantly increased at the end of the bedtime restriction period (Fig. 1). Recurrent sleep curtailment also resulted in reduced SI and higher SG and GEZI (Table 1). There were no statistically significant differences in AIRG and DI between the two bedtime conditions (Table 1).
Figure 1.
Mean (+se) concentrations of glucose (A) and insulin (B) in response to a 75-g oral glucose challenge at the end of the 8.5-h (open circles) and 5.5-h (solid circles) bedtime condition. Asterisk indicates that both the 2-h value and the 3-h AUC were significantly different between the two sleep conditions. AUC, Area under the curve.
Cortisol
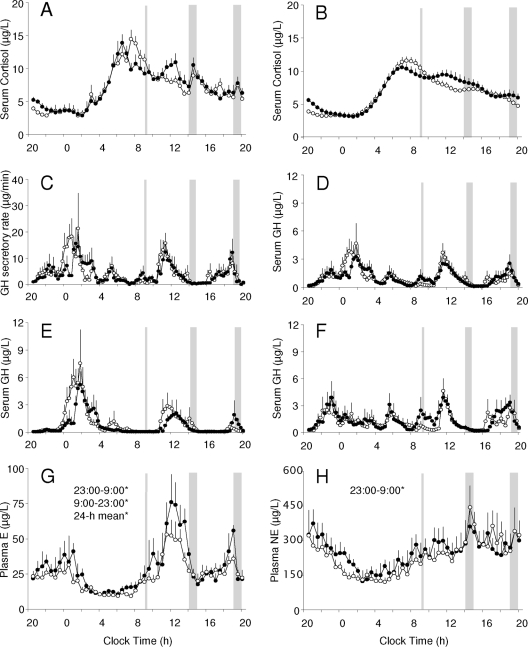
The area under the 24-h curve of serum cortisol was similar between the 5.5- and 8.5-h bedtime conditions (10,286 ± 1,850 vs. 9,993 ± 1,180 μg · dl−1 · min; P = 0.55). Measured profiles had comparable peak (18.3 ± 3.0 vs. 18.8 ± 2.2 μg/dl), trough (1.5 ± 0.9 vs. 1.7 ± 0.7 μg/dl), daytime (7.4 ± 1.6 vs. 6.8 ± 0.8 μg/dl), and nighttime (6.8 ± 2.0 vs. 7.1 ± 1.4 μg/dl) cortisol concentrations (Fig. 2A).
Figure 2.
Mean (+se) 24-h profiles of circulating counter-regulatory hormones measured at the end of the 8.5-h (open circles) and 5.5-h (solid circles) bedtime condition. Significant differences in the 24-h, daytime (0900–2300 h), and nighttime (2300–0900 h) concentrations between the two bedtime conditions are identified with an asterisk. A, Serum cortisol concentrations (n = 11); B, best-fit regression curves illustrating the 24-h cortisol rhythm (n = 11); C, GH secretory rates derived by deconvolution analysis (n = 11); D, serum GH concentrations (n = 11); E, serum GH concentrations in male study participants (n = 6); F, serum GH levels in female study participants (n = 5); G, plasma epinephrine (E) concentrations (n = 7); H, plasma norepinephrine (NE) concentrations (n = 7). The timing of the iv glucose challenge and lunch and dinner meals is marked by vertical gray lines and gray shaded bars, respectively.
Compared with the 8.5-h bedtime period, the best-fit 24-h cortisol curves at the end of the restricted sleep condition (Fig. 2B) tended to have lower acrophase (11.0 ± 2.3 vs. 12.2 ± 2.0 μg/dl; P = 0.058), similar nadir, and significantly decreased amplitude (4.3 ± 0.8 vs. 4.9 ± 0.8 μg/dl; P = 0.014). The time of nadir during the recurrent sleep restriction was delayed from 0027h (±115 min) to 0138h (±87 min) (P = 0.012) and acrophase advanced from 0749h (± 47 min) to 0705h (± 53 min) (P = 0.012). The 5.5-h bedtime condition was accompanied by a small increase in cortisol concentrations between 2000 and 2200 h (4.9 ± 1.1 vs. 3.5 ± 0.7 μg/dl; P < 0.01). This difference, although statistically significant, was very small and did not have a measurable effect on the duration of the corresponding “quiescent periods” (7 h 55 min ± 2 h 49 min vs. 8 h 02 min ± 3 h 24 min).
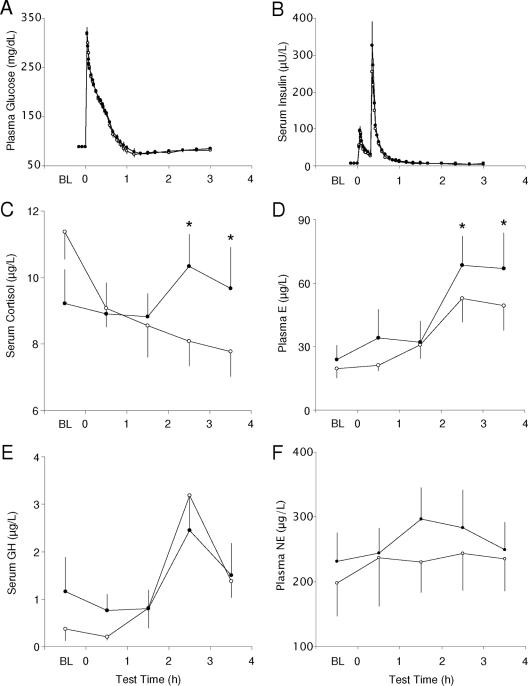
Serum cortisol concentrations declined progressively during the IVGTT period of the 8.5-h bedtime condition. The corresponding hormone levels during the 5.5-h bedtime intervention followed a different time course (P = 0.018 for sleep-condition-IVGTT-time interaction) characterized by a significant rise in cortisol concentrations during the third and fourth hours (P < 0.02) after the iv-glucose challenge (Fig. 3C).
Figure 3.
Mean (+se) profiles of circulating counter-regulatory hormones measured before (baseline, BL) and every 30 min after an iv glucose challenge at the end of the 8.5-h (open circles) and 5.5-h (solid circles) bedtime conditions. Significant differences between the two bedtime conditions are identified with an asterisk. A, Plasma glucose concentrations during the IVGTT (n = 11); B, serum insulin concentrations during the IVGTT (n = 11); C, hourly serum cortisol concentrations (n = 11); D, hourly plasma epinephrine (E) concentrations (n = 7); E, hourly serum GH concentrations (n = 11); F, hourly plasma norepinephrine (NE) concentrations (n = 7). *, P < 0.05 in post hoc comparisons between the two bedtime conditions based on repeated measures ANOVA.
GH
Study participants had similar 24-h mean (1.03 ± 0.57 vs. 1.09 ± 0.38 μg/liter), daytime (0.88 ± 0.66 vs. 0.82 ± 0.52 μg/liter), and nighttime (1.31 ± 0.63 vs. 1.57 ± 0.83 μg/liter) GH concentrations during the 5.5- and 8.5-h bedtime conditions. Comparable results were obtained when these analyses were repeated using GH secretory rates instead of serum concentrations (Fig. 2, C and D). Recurrent sleep loss was associated only with lower GH concentrations during the first 4 h of the assigned bedtime period (1.63 ± 0.88 vs. 2.35 ± 1.73 μg/liter; P = 0.04). This effect was notable mainly in male subjects, who had the highest GH concentrations during the first half of their sleep (Fig. 2E), and was not apparent in female participants, who had intervals with higher GH concentrations both at night and during the day (Fig. 2F).
There were no significant differences in GH levels during the IVGTT period between the two sleep conditions. Hormone concentrations remained low during the first 2 h of the test and increased significantly during the third (P = 0.001) and fourth hours (P = 0.021) after the iv glucose challenge (Fig. 3B).
Plasma catecholamines
Integrated daytime (36 ± 15 vs. 30 ± 11 pg/ml; P = 0.027), nighttime (20 ± 8 vs. 16 ± 5 pg/ml; P = 0.046), and 24-h epinephrine concentrations (29 ± 12 vs. 24 ± 8 pg/ml; P = 0.042) were higher during the 5.5-h compared with the 8.5-h bedtime condition (Fig. 2G). Epinephrine levels increased during the third and fourth hours of the IVGTT period during both bedtime conditions (IVGTT-time effect, P = 0.001), but reached significantly higher levels at the end of the short-sleep study (P < 0.05; Fig. 3C).
Recurrent sleep restriction was accompanied by increased overnight (198 ± 91 vs. 160 ± 74 pg/ml; P = 0.043), but not daytime (291 ± 129 vs. 284 ± 168 pg/ml) or 24-h (252 ± 113 vs. 232 ± 127 pg/ml) norepinephrine levels (Fig. 2H). There were no statistically detectable differences in plasma norepinephrine concentrations during the IVGTT period between the two bedtime conditions (Fig. 3D).
Discussion
Using a protocol of recurrent bedtime restriction, we were able to modify the amount of sleep of sedentary middle-aged men and women from over 7 h/d, which in epidemiological studies represents the sleep duration category with lowest metabolic risk, to less than 6 h/d, which corresponds to a sleep category associated with increased incidence of T2D (9,10,11,12). The key finding of our study is that recurrent bedtime restriction to 5.5 h/night in an environment that promotes overeating and physical inactivity was accompanied by higher 2-h OGTT glucose levels (Table 1) and a significantly increased area under the OGTT glucose curve (Fig. 1). Whereas prior studies have focused on the acute effects of total or severe partial sleep loss on human glucose metabolism (14,15,16,17), this is the first controlled experiment to document that recurrent bedtime restriction, which approximates the short sleep times experienced by many people in everyday life, can result in decreased oral glucose tolerance.
To examine the effects of recurrent bedtime restriction on insulin secretion and sensitivity as key factors in the pathogenesis of T2D, we obtained measures of AIRG, SI, and DI at the end of each sleep condition. The finding of decreased insulin sensitivity at the end of our restricted bedtime condition is important because in previous experiments only total sleep deprivation has been associated with increased insulin resistance (14) and compensatory hyperinsulinemia (15), whereas partial sleep loss did not have a significant effect (16). Our data now indicate that, when part of a Western-like lifestyle, recurrent sleep restriction can also result in reduced insulin sensitivity.
As SI declines, AIRG increases and individual DI remains stable to maintain normal glucose tolerance (23). Despite the decrease in SI at the end of the short-sleep study period, there was no significant difference in AIRG between the two bedtime conditions (Table 1), which raises the possibility that recurrent sleep restriction might interfere with β-cell compensation. The lack of hyperinsulinemia, despite the increase in the area under the OGTT glucose curve at the end of the 5.5-h bedtime intervention (Fig. 1), is also consistent with this hypothesis. However, compared with the considerable decrease in insulin secretion after more acute and severe sleep restriction when AIRG was reduced by 30% (16), the more moderate and prolonged sleep restriction in our study had no such impact on β-cell function. Indeed, analysis of DI as a measure of β-cell compensation for the degree of insulin resistance showed no significant differences in this variable at the end of the 5.5-h bedtime condition (Table 1). Because DI is a derivative measure with very large variability, it is possible that our analysis did not have sufficient power to detect a smaller than expected change in this endpoint. Alternatively, recurrent exposure to more moderate sleep restriction may have allowed the β-cell to adapt, at least partially, to the increase in systemic insulin resistance. Even when such β-cell compensation is entirely normal, this can result in higher OGTT glucose levels and increased long-term risk of T2D (23).
The increase in SG and GEZI at the end of the bedtime restriction period (Table 1) illustrates another previously unknown and potentially significant effect of recurrent sleep restriction on human glucose metabolism. Increased glucose effectiveness in association with reduced insulin sensitivity has been reported in healthy offspring of patients with T2D and is thought to represent an important compensatory mechanism for the maintenance of normal glucose tolerance (24). The rise in SG and GEZI in our experiment may, therefore, occur in response to the development of insulin resistance caused, e.g. by proinflammatory, oxidative, and endoplasmic reticulum stress pathways during the period of recurrent sleep restriction (25,26,27). The putative role of mammalian sleep in maintaining the energy stores of the brain has led others to propose that sleep deprivation results in increased metabolic demands to support neuronal activity during extended wakefulness (28,29), depletion of glycogen in some areas of the brain (27), and activation of neuroendocrine mechanisms to enhance the supply of glucose to the central nervous system (30). According to this hypothesis, the rise in SG and GEZI during our 5.5-h bedtime condition may reflect a state of increased brain glucose utilization, which triggers secondary changes in autonomic nervous system activity, release of counter-regulatory hormones, and reductions in peripheral insulin secretion and sensitivity. There have been conflicting reports in support of this hypothesis: reduced insulin secretion or sensitivity was associated with markers of enhanced sympathetic activity and changes in plasma cortisol and GH levels during short-term sleep restriction (16,18,19), but not necessarily in studies of total sleep deprivation (14,28,30). Similarly, increased levels of brain glucose metabolism were found in chronic insomnia sufferers (31), whereas more acute sleep loss has been associated with decreased SG (16) and cerebral glucose metabolism (32).
We also measured circulating cortisol, GH, epinephrine, and norepinephrine concentrations to examine whether 24-h changes in these counter-regulatory hormones may have contributed to the decline in glucose tolerance during the period of recurrent sleep restriction. Unlike the effects of more acute and severe bedtime restriction (16), our short-sleep intervention did not result in significantly increased exposure to high levels of GH or cortisol. The small increase in cortisol concentrations between 2000 and 2200 h (Fig. 2A) did not exceed the level of concentrations seen during the “quiescent period” of cortisol secretion and, by itself, was unlikely to have a significant impact on glucose tolerance and SI (33). Similarly, the rise in late-evening GH levels observed in lean young men during more acute and severe sleep restriction (18) was not present in our study. Instead, recurrent sleep restriction was accompanied by lower GH concentrations during the first 4 bedtime hours in our male subjects (Fig. 2E). The potential for chronic sleep loss to decrease the secretion of this and other anabolic hormones in men (34), and thus to modify their long-term risk of T2D (11), may warrant additional investigation.
The most consistent effect of sleep restriction on the counter-regulatory hormones in this study was the 20–25% increase in overnight catecholamine levels (35). Although exposure to higher levels of epinephrine and norepinephrine can result in reduced insulin sensitivity and impaired glucose tolerance, it is not known whether the increase of plasma catecholamine levels within the human physiological range during the 5.5-h bedtime condition can have similar direct consequences (36,37). These biomarkers of increased sympathetic and adrenomedullary release, however, suggest that sleep curtailment may be accompanied by widespread changes in autonomic nervous system activity that affect insulin secretion and sensitivity (38).
A closer look at the hormonal profiles during the restricted sleep condition also indicates that epinephrine levels tended to increase when the supply of exogenous glucose dwindled 3–4 h after the iv glucose challenge and during the late postprandial period (Fig. 2G). The combination of increased SG and GEZI with these changes in plasma epinephrine levels raises the possibility that the lack of sufficient sleep may amplify the neuroendocrine defense of humans against declines in exogenous glucose delivery (26,30). This is perhaps best illustrated by the increased release of counter-regulatory hormones during the post-IVGTT period of the restricted sleep condition (Fig. 3, C and D); however, the impact of recurrent sleep restriction on the human neuroendocrine response to interruptions in the supply of exogenous glucose remains unexplored.
Our study has several limitations. First, we could not define the target tissues that were responsible for the effects of recurrent sleep restriction on whole-body insulin sensitivity using routine oral and iv glucose tolerance tests. Second, counter-regulatory responses to hypoglycemia during the insulin-assisted IVGTT may result in lower estimates of SI (39). Although there were no significant differences in cortisol, GH, and catecholamine levels during the first 2 h of the IVGTT (Fig. 3), methods that avoid the relative hypoglycemia of the test may be particularly informative when assessing insulin sensitivity in the presence of recurrent sleep restriction. Similarly, the accuracy of IVGTT-based estimates of glucose effectiveness in sleep-deprived subjects has not been established, and our novel SG and GEZI findings will require confirmation using alternative model-independent approaches. Finally, although the overall intake of energy and macronutrients was comparable between the two sleep conditions, recurrent bedtime restriction modified the amount, composition, and timing of snack consumption (21). Although incorporating statistical control for the intake of energy from snacks did not change the results of our study (data not shown), the long-term effects of differences in snacking behavior on insulin secretion and sensitivity in individuals with self-reported short sleep are not known (40).
In summary, experimental exposure to recurrent bedtime restriction in an environment that promotes overeating and physical inactivity was accompanied by reduced glucose tolerance, lower SI, increased SG and GEZI, and no statistically significant changes in AIRG and DI. These findings suggest that combining the adverse metabolic effects of Westernized lifestyles with chronically reduced sleep duration may increase the long-term risk of developing impaired glucose tolerance and T2D. Although this hypothesis is consistent with current epidemiological data, our conclusions are based on the detailed evaluation of a small group of subjects over a limited period of time under carefully controlled laboratory conditions. Additional intervention studies will be needed to examine the impact of habitual sleep curtailment on human glucose metabolism under free-living conditions.
Supplementary Material
Acknowledgments
This study involved more than 300 inpatient days in the University of Chicago Sleep Research Laboratory directed by Dr. Eve Van Cauter. The contributions of Dr. Van Cauter and Dr. Dale Schoeller (University of Wisconsin at Madison) to the study design are gratefully acknowledged. Dr. Schoeller performed all total energy expenditure measurements and commented on the initial draft of this manuscript. We also thank our volunteers for their participation and the staff of the University of Chicago General Clinical Research Center, Sleep Research Laboratory, and Diabetes Research and Training Center for their skilled technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants PO1-AG11412, RO1-HL089637, MO1-RR00055, and P60-DK020595.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 30, 2009
Abbreviations: AIRG, Acute insulin response to glucose; DI, disposition index; GEZI, glucose effectiveness at zero insulin; IVGTT, iv glucose tolerance test; OGTT, oral glucose tolerance test; SG, glucose effectiveness at basal insulin concentration; SI, insulin sensitivity; T2D, type-2 diabetes.
References
- Kahn SE 2003 The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19 [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Shaw J 2001 Global and societal implications of the diabetes epidemic. Nature 414:782–787 [DOI] [PubMed] [Google Scholar]
- Leahy JL 2005 Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209 [DOI] [PubMed] [Google Scholar]
- Sherwin RS, Anderson RM, Buse JB, Chin MH, Eddy D, Fradkin J, Ganiats TG, Ginsberg HN, Kahn R, Nwankwo R, Rewers M, Schlessinger L, Stern M, Vinicor F, Zinman B, American Diabetes Association, National Institute of Diabetes and Digestive and Kidney Diseases 2004 Prevention or delay of type 2 diabetes. Diabetes Care 27(Suppl 1):S47–S54 [DOI] [PubMed] [Google Scholar]
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E 2005 Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol 99:2008–2019 [DOI] [PubMed] [Google Scholar]
- Horne J 2008 Short sleep is a questionable risk factor for obesity and related disorders: statistical versus clinical significance. Biol Psychol 77:266–276 [DOI] [PubMed] [Google Scholar]
- 2005 “Sleep in America” poll. Washington, DC: National Sleep Foundation. http://www.sleepfoundation.org/product/nsf-2005-sleep-america-poll [Google Scholar]
- Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, Dinges DF 2007 American time use survey: sleep time and its relationship to waking activities. Sleep 30:1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB 2003 A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 26:380–384 [DOI] [PubMed] [Google Scholar]
- Mallon L, Broman JE, Hetta J 2005 High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care 28:2762–2767 [DOI] [PubMed] [Google Scholar]
- Yaggi HK, Araujo AB, McKinlay JB 2006 Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 29:657–661 [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D 2007 Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 30:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, Bixler EO 2008 Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 32:801–809 [DOI] [PubMed] [Google Scholar]
- González-Ortiz M, Martínez-Abundis E, Balcázar-Muñoz BR, Pascoe-González S 2000 Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes Nutr Metab 13:80–83 [PubMed] [Google Scholar]
- VanHelder T, Symons JD, Radomski MW 1993 Effects of sleep deprivation and exercise on glucose tolerance. Aviat Space Environ Med 64:487–492 [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E 1999 Impact of sleep debt on metabolic and endocrine function. Lancet 354:1435–1439 [DOI] [PubMed] [Google Scholar]
- Kuhn E, Brodan V, Brodanová M, Rysánek K 1969 Metabolic reflection of sleep deprivation. Act Nerv Super Praha 11:165–174 [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Colecchia EF, L'Hermite-Balériaux M, Nie Z, Copinschi G, Van Cauter E 2000 Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol 279:R874–R883 [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L'hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E 2004 Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 89:5762–5771 [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR 2002 Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 59:131–136 [DOI] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD 2009 Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 89:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penev P, Spiegel K, Marcinkowski T, Van Cauter E 2005 Impact of carbohydrate-rich meals on plasma epinephrine levels: dysregulation with aging. J Clin Endocrinol Metab 90:6198–6206 [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Tataranni PA, Stefan N, Vozarova B, Bogardus C 2003 Glucose allostasis. Diabetes 52:903–909 [DOI] [PubMed] [Google Scholar]
- Henriksen JE, Alford F, Handberg A, Vaag A, Ward GM, Kalfas A, Beck-Nielsen H 1994 Increased glucose effectiveness in normoglycemic but insulin-resistant relatives of patients with non-insulin-dependent diabetes mellitus. A novel compensatory mechanism. J Clin Invest 94:1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK 2009 Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis 51:294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penev PD 2007 Sleep deprivation and energy metabolism: to sleep, perchance to eat? Curr Opin Endocrinol Diabetes Obes 14:374–381 [DOI] [PubMed] [Google Scholar]
- Scharf MT, Naidoo N, Zimmerman JE, Pack AI 2008 The energy hypothesis of sleep revisited. Prog Neurobiol 86:264–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore JN, Nestler JE, Blackard WG 1989 Sleep-associated fall in glucose disposal and hepatic glucose output in normal humans. Putative signaling mechanism linking peripheral and hepatic events. Diabetes 38:285–290 [DOI] [PubMed] [Google Scholar]
- Maquet P, Dive D, Salmon E, Sadzot B, Franco G, Poirrier R, von Frenckell R, Franck G 1990 Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res 513:136–143 [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Bandorf N, Born J, Schultes B 2007 Sleep loss alters basal metabolic hormone secretion and modulates the dynamic counterregulatory response to hypoglycemia. J Clin Endocrinol Metab 92:3044–3051 [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ 2004 Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 161:2126–2128 [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D 2000 Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 9:335–352 [DOI] [PubMed] [Google Scholar]
- Dinneen S, Alzaid A, Miles J, Rizza R 1993 Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J Clin Invest 92:2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penev PD 2007 Association between sleep duration and morning testosterone levels in older men. Sleep 30:427–432 [DOI] [PubMed] [Google Scholar]
- Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M 1999 Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab 84:1979–1985 [DOI] [PubMed] [Google Scholar]
- Cryer PE 1993 Adrenaline: a physiological metabolic regulatory hormone in humans? Int J Obes Relat Metab Disord 17(Suppl 3):S43–S46; discussion S68 [PubMed] [Google Scholar]
- Marangou AG, Alford FP, Ward G, Liskaser F, Aitken PM, Weber KM, Boston RC, Best JD 1988 Hormonal effects of norepinephrine on acute glucose disposal in humans: a minimal model analysis. Metabolism 37:885–891 [DOI] [PubMed] [Google Scholar]
- Nonogaki K 2000 New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43:533–549 [DOI] [PubMed] [Google Scholar]
- Brehm A, Thomaseth K, Bernroider E, Nowotny P, Waldhäusl W, Pacini G, Roden M 2006 The role of endocrine counterregulation for estimating insulin sensitivity from intravenous glucose tolerance tests. J Clin Endocrinol Metab 91:2272–2278 [DOI] [PubMed] [Google Scholar]
- Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S 2002 An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci 21:115–120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.