Abstract
Aims
Female sex and sex hormones contribute to cardiac remodelling. 17β-estradiol (E2) is involved in the modulation of extracellular matrix composition and function. Here, we analysed the effect of E2 on matrix metalloproteinase (MMP)-2 gene expression and studied the underlying molecular mechanisms in rat cardiac fibroblasts and in a human fibroblast cell line.
Methods and results
In adult rat cardiac fibroblasts, E2 significantly decreased MMP-2 gene expression in an estrogen receptor (ER)-dependent manner. Transient transfection experiments of human MMP-2 (hMMP-2) promoter deletion constructs in a human fibroblast cell line revealed a regulatory region between −324 and −260 bp that is involved in E2/ERα-mediated repression of hMMP-2 gene transcription. Electrophoretic mobility shift assays (EMSA) and supershift analysis demonstrated the binding of transcription factor Elk-1 within this promoter region. Elk-1 was phosphorylated by E2 via the mitogen-activated protein kinase (MAPK) signalling pathway as shown by western blotting. Treatment of cells with the MAPK inhibitor PD98059 blocked the E2-dependent repression of hMMP-2 promoter activity as well as the endogenous MMP-2 mRNA levels in both human fibroblast cells and rat cardiac fibroblasts.
Conclusion
E2 inhibits MMP-2 expression via the ER and the MAPK pathway in rat cardiac fibroblasts and in a human fibroblast cell line. These mechanisms may contribute to sex-specific differences in fibrotic processes that are observed in human heart and other diseases.
Keywords: MMP-2, Gene expression, Estrogen, Cardiac fibroblasts, ERα, Elk-1
1. Introduction
The regulation of the extracellular matrix (ECM) by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) contributes to the structural and functional integrity of the heart.1 MMPs are a family of zinc-dependent enzymes that are mainly synthesized by cardiac fibroblasts and involved in permanent matrix turnover.2 Alterations in MMP expression and activity contribute to the development of depressed cardiac function, progression of cardiac failure, and increased mortality in animal models and humans.3
MMP-2 (gelatinase A) plays a dominant role in cardiac remodelling. Increased MMP-2 expression and activity occurs after aortic stenosis, myocardial infarction (MI), during left ventricular hypertrophy, in heart failure and is associated with adverse cardiac remodelling.4–8 Constitutive cardiac-specific MMP-2 expression in transgenic mice induced sever ventricular remodelling and systolic dysfunction.9 In contrast, the inhibition or targeted deletion of MMP-2 in animal models attenuated the progression of left ventricular remodelling and improved the survival after MI or pressure overload.10–12 Thus, MMP-2 is directly involved in pathological ventricular remodelling.
Male mice subjected to transverse aortic constriction-induced pressure overload exhibit a higher MMP-2 mRNA expression than female mice.13 A higher MMP-2 activity was also observed in male mice after MI with corresponding remodelling compared with female mice.14 The sex hormone 17β-estradiol (E2) is a possible candidate that could be responsible for these sex differences.
MMP-2 expression is controlled at the transcriptional level by cytokines, hormones, and growth factors via induction or suppression of promoter activity.8,15,16 E2 regulates MMP-2 gene expression in a cell- and tissue-specific manner. E2 treatment leads to an up-regulation of MMP-2 gene expression in isolated mice mesangial cells, human vascular smooth muscle cells, and in human retinal pigment epithelium17–19 and to a down-regulation of MMP-2 expression in MCF-7 cells, isolated human pelvic floor fibroblasts, and prostate cancer cells.20–22 However, currently little is known about the molecular mechanisms regulating the MMP-2 gene expression by E2.
The effects of E2 are mainly mediated by two estrogen receptors (ER), ER alpha (α) and ER beta (β), which belong to the superfamily of nuclear receptors.23 Although the presence of both functional ER in cardiac fibroblasts obtained from male and female rats has been reported,24 it is not evident whether E2-activated ER have the same effects on the regulation of gene expression in both sexes. Therefore, in the present study, we analyse the effect of E2 on the MMP-2 gene expression in cardiac fibroblasts isolated from adult female and male rats. Furthermore, we elucidated the molecular mechanisms involved in E2-mediated regulation of the human MMP-2 gene expression in a human fibrosarcoma cell line and confirmed the regulatory pathway in rat cardiac fibroblasts. The elucidation of the effect of E2 on the regulation of MMP-2 gene expression in fibroblasts could help understand sex differences in cardiac remodelling and improve therapeutic approaches for cardiac remodelling after MI and pressure overload.
2. Methods
2.1. Cell culture
Cardiac fibroblasts from adult female and male Wistar rats (9–11 weeks) were prepared by enzymatic digestion (collagenase, dispase, and trypsin) as described earlier.25 These investigations were approved by the Landesamt für Gesundheit und Soziales (LaGeSo, Berlin, Germany) for the use of laboratory animals (O0006/05) and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of health (NIH Publication No. 85-23, revised 1996). Purity of cardiac fibroblasts was determined by morphological characterization and immunostaining [positive: with anti-Vimentin antibody (Oncogene) and negative: von Willebrand factor VIII (Dako)]. Human fibrosarcoma cell line (HT1080 cells, CCL-121), derived from a fibrosarcoma in a 35-year-old male in 1972,26 was bought from German Collection of Microorganisms and Cell Culture (DSMZ, Braunschweig, Germany).
Cardiac Fibroblasts and HT1080 cells were cultured in Dulbecco's modified Eagles medium (DMEM) with phenol red and 10% foetal bovine serum (FBS) supplemented with 1 mM HEPES, 2 mM l-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C under 5% CO2. For E2 treatment, cells were grown in supplemented DMEM without phenol red and 10% charcoal stripped FBS (c.s. FBS). Prior to E2 treatment, cells were starved with 2.5% c.s. FBS in DMEM without phenol red for 24 h, subsequently incubated with the physiological concentration of E2 (10−8M; Sigma) or Dextrin (Sigma) as vehicle for additional 24 h. Cells were pre-incubated with ER-antagonist ICI 182,780 (10−5M; Tocris) and inhibitor of mitogen-activated protein kinase (MAPK) cascade PD98059 (10 µM; Calbiochem) for 1 h prior to E2 treatment. Immunostaining of cardiac fibroblast treated and non-treated with E2, using anti α-smooth muscle actin antibody (Sigma), revealed that E2 has no effect on fibroblast differentiation into myofibroblasts (data not shown).
2.2. RNA isolation and real-time polymerase chain reaction
Total RNA isolation and real-time polymerase chain reaction (PCR) was performed as previously described.13 The mRNA content of target genes was normalized to the expression of hypoxanthine phosphoribosyl transferase in cardiac fibroblasts and to the expression of Glycerinaldehyde-3-phosphate-Dehydrogenase (GAPDH) in HT1080 cells. Primer sequences used for amplification are listed in Supplementary material online, Table S1).
2.3. Construction of luciferase promoter constructs
Human genomic DNA, isolated from peripheral blood samples of healthy volunteers (n = 3) by QIAamp DNA Blood kit (Qiagen), was used as a template to amplify different fragments containing the 5′-flanking region of the hMMP-2 gene by PCR. The investigation conforms with the principles outlined in the Declaration of Helsinki for investigations involving human subjects27 and has been approved by the ethics commission of the university Witten/Herdecke e.V. (04/2004). Primer sequences used for amplification are displayed in Supplementary material online, Table S2. The hMMP-2 promoter fragments (−1174 bp/+17 bp, −960 bp/+17 bp, −685 bp/+17 bp, −685 bp/−260 bp, −417 bp/+17 bp, and −324 bp/+17 bp, relative to the translational start site +1 bp) were cloned into pCR 2.1 vector using TA cloning vector kit (Invitrogen) and confirmed by sequencing. Individual promoter fragments were sub-cloned into the luciferase reporter vector pGL2-basic (Promega) using Sac I restriction sites in sense and Hind III in antisense orientation.
2.4. Transient transfection and dual luciferase reporter assays
For transient-transfection experiments, HT1080 cells were plated into 12-well tissue plates and grown to approximately 70–80% confluence before being transfected. Transfection experiments were performed using FUGENE®6 reagent (Roche Diagnostics) according to the manufacturer's protocol. HT1080 cells were transiently transfected with 1 µg of luciferase promoter construct and 10 ng of Renilla luciferase plasmid (phRL-TK, Promega) which was used as a standard reference control. In co-transfection experiments, the cells were additionally co-transfected with 500 ng human ERα (hERα)-vector (HEGO-pSG5 vector) using similar transfection procedure. Luciferase-assays were performed using the Dual-Luciferase Reporter Assay System (Promega) following the manufacturer's instructions. Firefly and Renilla luciferase activities were sequentially measured in a Wallac 409 beta-counter (Berthold-Wallac). Firefly luciferase activities were normalized based on the Renilla luciferase activity in each well. Transfection experiments were carried out in triplicates for each construct and performed independently at least three times.
2.5. Western blot analysis
HT1080 cell extract was isolated using double-detergent lysis buffer (Supplementary material online, Table S3) and separated by SDS–polyacrylamide gel electrophoresis. Western-blotting was performed using anti-p-Elk-1 antibody (sc-8406, Santa Cruz), anti-p-ERK1/2 (mAb#4370, Cell Signaling), anti-ERK1/2 antibody (mAb#4695, Cell Signaling), anti-MMP2 antibody (M4677, Sigma), and anti-GAPDH antibody (MAB374, Chemicon) for normalization. Specific bands were visualized using ECL™ detection kit (GE Healthcare). Band intensities were quantified with Alpha Ease FC™ (Version 3.1.2, Alpha Innotech Corporation).
2.6. Electrophoretic mobility shift assays (EMSA) and supershift assays
Preparation of nuclear extracts from transfected HT1080 cells, treated with E2 or vehicle, and performance of Electrophoretic mobility shift assays (EMSA) were previously reported.28 Briefly, double-stranded oligonucleotides corresponding to the hMMP-2 promoter sequence from −296 to −256 bp (5′-cctcccttgtttccgctgcatccagacttcctcaggcttcg-3′), including putative Elk-1 binding site, were end-labelled with 32P-dATP. Binding reaction mixture, including 10 µg of nuclear extracts (E2- or vehicle-treated), end-labelled Elk-1 oligonucleotide (60.000 cpm), 2 µg poly [d(IC)], and 1x binding buffer (Supplementary material online, Table S4), was incubated for 1 h at room temperature. For supershift assays, anti-Elk-1 antibody (4 µg, sc-355X, Santa Cruz) and anti-p-Elk-1 antibody (2–6 µg, sc-8406X, Santa Cruz) were incubated with nuclear extracts for 30 min at 4°C before addition of binding reaction mixture. For competition experiments, non-labelled double stranded oligonucleotides were added to the binding reaction mixture in 150-fold molar excess.
2.7. Statistical analysis
Results are expressed as mean ± SEM of at least three independent experiments. Graphic representations of the results are accomplished using the software Sigma Plot version 8. Significant differences were established by paired Student's t-test using SPSS 11.0 (SPSS Inc., Chicago, IL, USA). Probability values P ≤ 0.05 were considered statistically significant.
3. Results
3.1. 17β-Estradiol inhibits MMP-2 and induces ER gene expression in rat cardiac fibroblasts
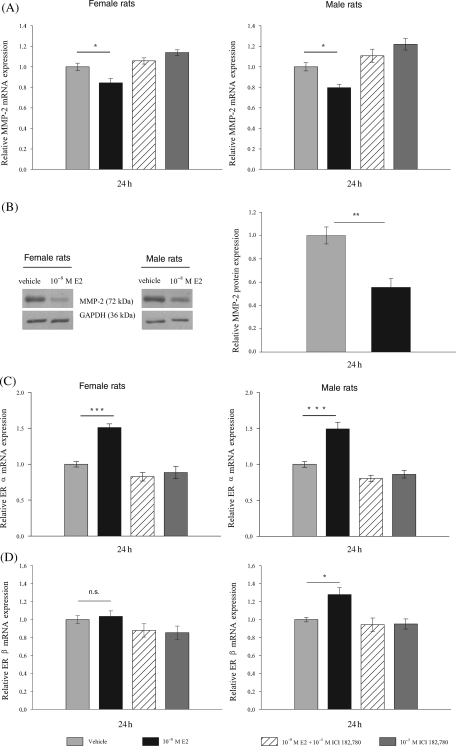
E2 treatment of isolated cardiac fibroblasts from adult female and male rats for 24 h significantly decreased the expression of MMP-2 at mRNA and protein level compared with vehicle-treated cells in both sexes (Figure 1A; P ≤ 0.05; Figure 1B; P ≤ 0.01). Pre-treatment with the specific ER-antagonist ICI 182,780 reversed the E2-mediated effect, indicating that E2 inhibits MMP-2 mRNA expression through ER. Moreover, E2 treatment of rat cardiac fibroblasts significantly increased ERα-mRNA levels compared with vehicle-treated cells in both sexes (Figure 1C; P ≤ 0.001). The ERβ-mRNA was significantly increased only in cardiac fibroblasts isolated from males, but not from female rats after 24 h of E2 treatment (Figure 1D; P ≤ 0.05). The ER-antagonist ICI 182,780 inhibited the induction of ER mRNA-levels by E2.
Figure 1.
Regulation of endogenous MMP-2, ERα, and ERβ expression in isolated cardiac fibroblasts from adult female and male rats after 17β-estradiol (10−8M) treatment for 24 h. (A) MMP-2 mRNA-expression is significantly down-regulated in cardiac fibroblasts of both sexes. (B) MMP-2 protein expression is also significantly down-regulated in cardiac fibroblasts of both sexes. Left panel shows a representative western blot for MMP-2 protein from cardiac fibroblasts of female and male rats treated with 17β-estradiol or vehicle; GAPDH was used as an internal standard. Right panel shows summarized results from cardiac cells of female and male rats. (C) ERα mRNA-expression is significantly up-regulated in both sexes, (D) whereas ERβ mRNA-expression is only significantly induced in cardiac fibroblasts from male rats. ICI 182,780 (10−5 M) inhibited the 17β-estradiol effect, indicating the involvement of ER. Relative mRNA-expression levels of 17β-estradiol treated cells are expressed as fold induction of vehicle-treated cells. Data are means ± SEM (n = 4). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
3.2. 17β-Estradiol inhibits the transcriptional activity of the human MMP-2 gene in HT1080 cells
To examine the transcriptional function of the hMMP-2 promoter, we first tested the basal promoter activity of the 5′-flanking region from −1174 to +17 bp (relative to the translational start) of the hMMP-2 gene in HT1080 cells. Luciferase reporter assays revealed an increase in promoter activity with increasing length of the hMMP-2 promoter sequence and strong enhancer regions important for basal hMMP-2 transcription, located between −417 bp/−324 bp, −685 bp/−417 bp, and −1174 bp/−960 bp of the hMMP-2 promoter (see Supplementary material online, Figure S1). Thus, the hMMP-2 promoter constructs are functionally active in HT1080 cells and can be used for further analysis.
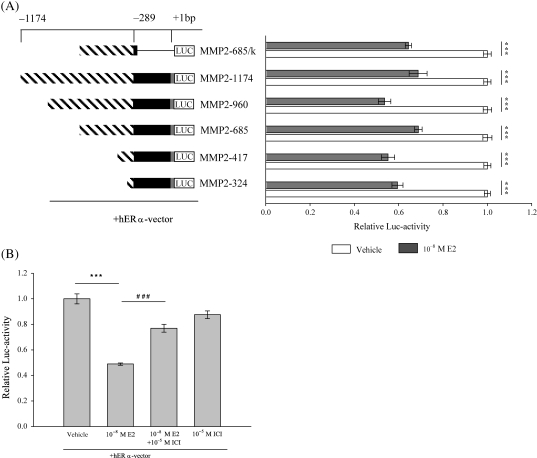
To determine the effect of E2 on the hMMP-2 promoter activity, HT1080 cells were co-transfected with the various luciferase hMMP-2 promoter constructs along with the hERα-vector and subsequently treated with E2. E2 treatment significantly reduced hMMP-2 promoter activity of about 30–40% (Figure 2A; P ≤ 0.001). The hMMP2-685/k-pGL2 construct, which lacks the 5′-UTR of the hMMP-2 promoter region, also exhibited a significant reduction of about 30% in luciferase activity after E2 treatment (P ≤ 0.001). Thus, the 5′-UTR region plays no critical role in the E2-mediated reduction of hMMP-2 promoter activity. Since the inhibitory effect of E2 is similar among the various hMMP-2 promoter constructs, and because all constructs have the proximal region between −324 and −260 bp of the hMMP-2 promoter in common, this region is likely to mediate the suppressive effect of E2 on hMMP-2 transcriptional activity (Figure 3A). Pre-treatment with ICI 182,780 significantly reversed the effect of E2 on MMP-2 promoter activity (Figure 2B; P ≤ 0.001), confirming that ERα is necessary for mediating the inhibitory effect of E2 on hMMP-2 promoter activity.
Figure 2.
17β-Estradiol inhibits human MMP-2 promoter activity in HT1080 cells. Lengths of human MMP-2 promoter fragments are displayed by numbers (bp), referring to the translation start site (+1 bp). Streaked bars present different promoter fragments, black bars present the 5′-UTR and grey bars present part of Exon 1 of the human MMP-2 gene. The ‘k’ marks the truncated hMMP2-promoter construct without 5′-UTR. Luciferase activities were assayed as described in Material and Methods. (A) 17β-Estradiol (10−8 M) significantly inhibits luciferase activity of human MMP-2 promoter constructs compared with vehicle-treated cells. Results represent luciferase activities as mean ± SEM (n = 4). (B) 17β-Estradiol significantly inhibited luciferase-activity of hMMP2-417-pGL2 construct. ICI 182,780 (10−5M) significantly reversed the 17β-estradiol mediated inhibition of luciferase activity. Results represent luciferase activities as mean ± SEM (n = 3). Relative luciferase levels of 17β-estradiol treated cells are expressed as fold induction of vehicle-treated cells. ***P ≤ 0.001, ###P ≤ 0.001.
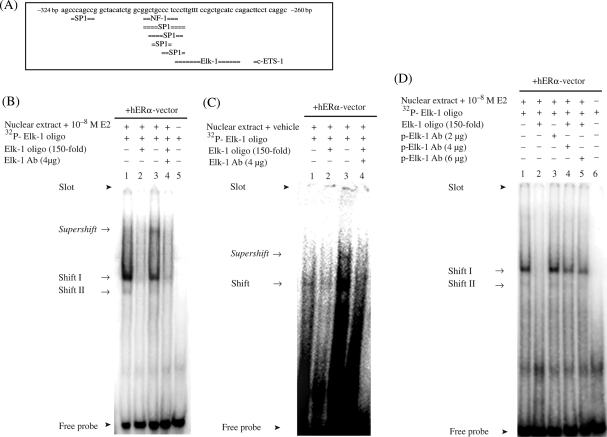
Figure 3.
Inhibitory effect of 17β-estradiol on the human MMP-2 promoter is mediated through the region between −324 and −260 bp. (A) Regulatory region of the human MMP-2 promoter contains putative binding sites for different transcription factors. (B) EMSA and supershift analysis with the specific Elk-1 antibody revealed the binding of the transcription factor Elk-1 to the human MMP-2 promoter region with and (C) without 17β-estradiol-treatment (10−8M). (D) Incubation of nuclear extracts from HT1080 cells with the specific antibody against p-Elk-1 did not reveal a supershift, but intensities of specific shift bands decreased gradually with increasing amount of p-Elk-1 antibody. Specific shifts and supershifts are marked with an arrow.
3.3. Transcription factor Elk-1 binds to the human MMP-2 promoter
We hypothesized that the region between −324 and −260 bp was responsible for the inhibitory effect of E2 on the hMMP-2 promoter activity. This region contains putative binding sites for Elk-1, c-ETS-1, NF-1, and Sp1 (Figure 3A). All four transcription factors either interact with ERα or can be activated by E2.29–32 No estrogen responsive element (ERE) is present within this region. The incubation of the oligonucleotide spanning the putative Elk-1 binding site (−296 to −256 bp) with nuclear extract from E2-treated HT1080 cells resulted in a retarded DNA–protein complex (Figure 3B). Complex formation was efficiently competed by a 150-fold molar excess of non-labelled Elk-1 oligonucleotide (Figure 3B), suggesting the presence of Elk-1 within this complex. The addition of a specific anti-Elk-1 antibody resulted in a supershifted band, which was efficiently inhibited by an excess of non-labelled Elk-1 oligonucleotide (Figure 3B). These findings confirmed the presence of Elk-1 in the DNA–protein complexes. However, the incubation of nuclear extract from vehicle-treated HT1080 cells with the oligonucleotide carrying the putative Elk-1 binding site also resulted into a retarded DNA–protein complex (Figure 3C). Addition of specific anti-Elk-1 antibody demonstrated the presence of Elk-1 within the formed complex, similar to E2 treated cells (Figure 3C). These findings suggest a constitutive binding of Elk-1 to the hMMP-2 promoter in HT1080 cells.
To understand how E2 modulates the hMMP-2 promoter activity via Elk-1, we investigated whether or not the phosphorylated Elk-1 (p-Elk-1) is present in the DNA–protein complex. Since E2 induces the phosphorylation of Elk-1 in different cell lines and phosphorylation of Elk-1 modulates its transcriptional activity, we performed supershift assays using anti p-Elk-1 antibody. No supershifted band was observed. However, the intensity of both shift bands gradually decreased with an increasing amount of anti p-Elk-1 antibody (Figure 3D). These data suggest that p-Elk-1 is present in the DNA–protein complexes at the hMMP-2 promoter.
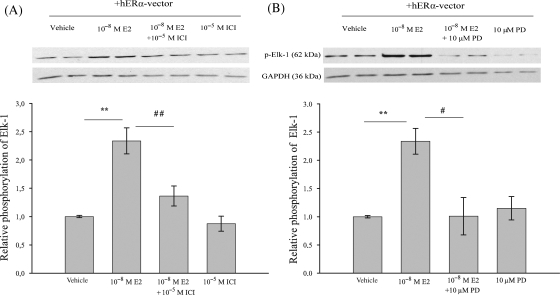
3.4. 17β-Estradiol phosphorylates Elk-1 via ERK1/2 signalling
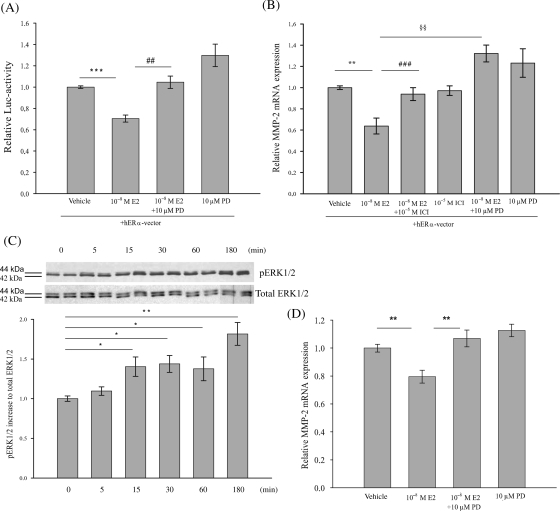
Next, we investigated whether or not the phosphorylation level of Elk-1 in HT1080 cells is affected by E2. Treatment of HT1080 cells with E2 showed a significant increase in Elk-1 phosphorylation in whole cell extract compared with the vehicle-treated cells (Figure 4A; P ≤ 0.01). Pre-treatment with ICI 182,780 significantly reversed (P ≤ 0.01) the effect of E2-mediated phosphorylation of Elk-1, indicating that Elk-1 phosphorylation by E2 in HT1080 cells is ER-dependent. The phosphorylation of Elk-1 is mainly mediated by the activation of MAPK-ERK1/2 signalling pathway.33 To elucidate whether E2 mediates its effect via this signalling pathway, HT1080 cells were pre-treated with PD98059 to inhibit activation of MAPK-ERK1/2. PD98059 significantly reduced the E2-mediated phosphorylation of Elk-1 (Figure 4B; P ≤ 0.05). In summary, E2 treatment elevates phosphorylation level of Elk-1 in HT1080 cells via activation of ER and MAPK pathway.
Figure 4.
17β-Estradiol mediates Elk-1 phosphorylation in HT1080 cells via ER and activated MAP Kinase-ERK1/2 signalling pathway. HT1080 cells were transiently transfected with hERα-vector and analysed after 24 h of treatment with Dextrin (vehicle), 17β-estradiol (10−8 M), ICI 182,780 (10−5 M) and PD98059 (10 µM) by immunoblotting using anti p-Elk-1 antibody. (A and B) 17β-Estradiol significantly increased phosphorylation-status of Elk-1 in HT1080 cells, whereas ICI 182,780 and PD98059 significantly inhibited E2-mediated Elk-1 phosphorylation. Membranes were subsequently re-probed with a specific GAPDH antibody as internal standard. Protein-expression levels of cells treated with 17β-estradiol, ICI 182,780 and PD98059 are expressed as fold induction of vehicle-treated cells. Bars represent means ± SEM (n = 3). **P ≤ 0.01, ##P ≤ 0.01, #P ≤ 0.05.
3.5. 17β-Estradiol inhibits transcriptional activity of the human MMP-2 promoter via ERK1/2 signalling
To investigate whether or not E2-mediated down-regulation of the hMMP-2 transcriptional activity is mediated by MAPK pathway, HT1080 cells, co-transfected with hMMP2-417-pGL2 construct and hERα-vector, were incubated with PD98059 prior to E2 treatment. The inhibition of MAPK-ERK1/2 signalling pathway by PD98059 significantly inhibited the suppressive effect of E2 (Figure 5A; P ≤ 0.01). This experiment demonstrates that E2 mediates its inhibitory effects on the transcriptional activity of hMMP-2 promoter through activation of MAPK-ERK1/2 pathway.
Figure 5.
Inhibitory effect of 17β-estradiol on MMP-2 transcriptional activity is mediated via ER and MAP Kinase-ERK1/2 signalling pathway in HT1080 cells and rat cardiac fibroblasts. (A) HT1080 cells were transiently co-transfected with the hMMP2-417-promoter construct and hERα vector. PD98059 (10 µM) significantly reversed the 17β-estradiol-dependent (10−8 M) repression of human MMP-2 promoter activity. Relative luciferase activity of 17β-estradiol and PD98059 treated cells are expressed as fold induction of vehicle-treated cells. Luciferase activities are means ± SEM (n = 4). (B) Significant down-regulation of endogenous human MMP-2 mRNA expression by 17β-estradiol in HT1080 cells was significantly inhibited by ICI 182,780 (10−5M) and PD98059 (10 µM) (n = 3). (C) 17β-Estradiol induced phosphorylation of ERK1/2 in HT1080 cells. Membranes were subsequently re-probed with a specific ERK1/2 antibody. Western blots are representatives of three independent experiments (n = 3). (D) Significant reduction of endogenous MMP-2 mRNA-levels in cardiac fibroblasts of both sexes was significantly inhibited by PD98059 (n = 4). The mRNA-expression level of 17β-estradiol, ICI 182,780 and PD98059 treated cells are expressed as fold induction of vehicle-treated cells. Data are means ± SEM. ***P ≤ 0.001, ###P ≤ 0.001 and **P ≤ 0.01, ##P ≤ 0.01, §§P ≤ 0.01.
3.6. 17β-Estradiol inhibits the human MMP-2 mRNA-expression via ER and ERK1/2
Next we analysed whether or not the endogenous hMMP-2 gene expression in HT1080 cells is modulated by E2 and if E2 mediates its effect by activating ER and MAPK-ERK1/2 signalling pathway. E2 treatment of HT1080 cells led to a significantly reduced endogenous hMMP-2 mRNA expression (Figure 5B; P ≤ 0.01). Pre-treatment of the cells with ICI 182,780 and PD98059 significantly reversed the E2-mediated reduction of hMMP-2 mRNA expression (P ≤ 0.001 and P ≤ 0.01, respectively). Additionally, we showed that E2 treatment increased the phosphorylation of ERK1/2 time-dependently (Figure 5C). Taken together, these data indicate that E2 exerts its inhibitory effect on the endogenous hMMP-2 mRNA expression by the activation of ER and MAPK-ERK1/2 pathway. Theses results support our presented data reporting the inhibitory effect of E2 on hMMP-2 promoter activity.
3.7. 17β-Estradiol inhibits MMP-2 mRNA-expression via ERK1/2 signalling in rat cardiac fibroblasts
Finally, we confirmed that the inhibiting effect of E2 on MMP-2 mRNA in adult rat cardiac fibroblasts is also mediated via MAPK-ERK1/2 signalling. Pre-treatment of cardiac fibroblasts with PD98059 significantly abolished the E2-mediated reduction of endogenous MMP-2 mRNA-expression in both sexes (Figure 5D; P ≤ 0.01). Taken together, these data strongly indicate that E2 regulates MMP-2 gene expression in human fibrosarcoma cells and rat cardiac fibroblasts via the same regulatory pathways.
4. Discussion
Female rodents exhibit less pro-fibrotic remodelling than males when exposed to MI or pressure overload; however, the mechanisms are not yet understood. This study is the first to show the inhibitory effect of E2 on MMP-2 gene expression via ER and MAPK-ERK1/2 signalling in adult rat cardiac fibroblasts. Furthermore, we show that E2 mediates its effect through a defined region within the hMMP-2 promoter, which binds the transcription factor Elk-1. Phosphorylation of Elk-1 by E2 was also mediated via ER and MAPK pathway, suggesting Elk-1 as a mediator of E2-induced down-regulation of MMP-2 gene expression in fibroblast cells.
To understand sex differences in cardiac remodelling, we analysed the regulation of MMP-2 gene expression by E2. Sex differences in response to E2 have been observed in different cell types. In human adenocarcinoma cell lines from women and men, E2 stimulated proliferation and transcription of an E2 response element-driven reporter gene only in cells from women, but not men.34 This was due to the differential sex-specific activation of ER without differences in the protein expression level of both receptors. In rat cardiac fibroblasts from male and female hearts, E2 differentially regulated the proliferative response to hypoxia via ER-dependent pathways.35 The E2 effect on MMP-2 gene expression has not yet been directly compared in cardiac fibroblasts from male and females. We found that E2 has the same inhibitory effect on the MMP-2 gene expression in cardiac fibroblasts from adult female and male rats. Using the specific ER-antagonist ICI 182,780, we obtained evidence that the E2 effect was mediated by ER in both sexes.
Both, ERα and ERβ, are expressed in female and male hearts of humans and rodents.24,36,37 Gene expression profiling and cluster analysis in the mouse aorta revealed that ERα, in contrast to ERβ, plays a critical role in the E2-induced regulation of ECM associated genes.38 Furthermore, E2-induced transcriptional activity of MMP-13 and MMP-1 promoter is regulated by ERα,39,40 supporting that ERα acts as a key driver for estrogenic effects on MMP expression. In this study, parallel to the E2-mediated down-regulation of MMP-2 gene expression in cardiac fibroblasts, we show an up-regulation of ERα-mRNA, but not ERβ-mRNA expression in cells of both sexes. These findings suggest that ERα, rather than ERβ is involved in MMP-2 gene regulation in cardiac fibroblasts and we therefore focused our experiments on the effects of ERα.
E2 treatment of cultured human pelvic floor fibroblasts, breast cancer, and prostate cancer cells led to a down-regulation of MMP-2 expression.21,22,41 These studies, however, did not identify any regulatory cis- and trans-acting elements required for mediating the inhibitory effect of E2 on the MMP-2 expression. In the current study, we characterized an E2-responsive region within the hMMP-2 promoter, from −324 to −260 bp, with putative binding sites for several transcription factors including Elk-1. In line with previous reports,30,42 E2 increased the phosphorylation of Elk-1 through activation of ERα in HT1080 cells. Since in our experiments Elk-1 bound, with and without E2 treatment, within the DNA–protein complex at the hMMP-2 promoter, the phosphorylation of Elk-1 by E2 was considered to mediate the E2-induced down-regulation of the hMMP-2 promoter activity. Similar findings, whereas phosphorylation of Elk-1 down-regulated c-fos promoter activity in a human embryonic kidney cell line and primary rat embryo fibroblasts, were previously reported.43,44 The gradual decrease of the specific nuclear protein binding to the promoter-region, in the presence of p-Elk-1 antibody, suggests the presence of p-Elk-1 in the DNA–protein complexes on the hMMP-2 promoter. The failure to detect the binding of p-Elk-1 to the hMMP-2 promoter after E2 treatment may be caused by a low amount of phosphorylation or instability of p-Elk-1. This may lead to p-Elk-1 protein concentrations, which are insufficient to obtain a super-shift signal.
Elk-1 can be phosphorylated by E2 activated MAPK-ERK1/2 signalling pathway in MCF-7 cells.30 E2-activated ERK1/2 in HT1080 cells also mediated Elk-1 phosphorylation in our study. Additionally, the inhibition of ERK1/2 pathway abolished the inhibitory effect of E2 on hMMP-2 promoter activity, indicating that MAPK-ERK1/2 pathway mediates the E2-dependent transcriptional repression of the hMMP-2 promoter in HT1080 cells. This regulatory mechanism is not only limited to the transfected hMMP-2 promoter, but also applies to the E2-mediated reduction of endogenous MMP-2 mRNA-expression in HT1080 cells as well as in adult rat cardiac fibroblasts of both sexes.
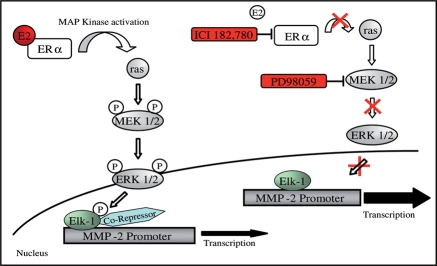
Based on our results, we propose that upon E2 treatment, E2 activates the cytosolic ERα, which in turn activates the cytosolic signal transduction cascades ras/raf, MEK1/2, and ERK1/2 (Figure 6). Activated ERK1/2 translocates into the nucleus45 where it phosphorylates the transcription factor Elk-1.43,44 The binding of phosphorylated Elk-1 promotes the recruitment of co-repressors.46 Yang et al.44 and Kukushkin et al.43 showed that the down-regulation of the c-fos promoter activity occurs through Elk-1 phosphorylation and subsequent recruitment of co-suppressor complex Sin3A/HDAC-1. Conceivably, a similar mechanism could be responsible for the inhibition of hMMP-2 promoter activity after E2 treatment in fibroblasts.
Figure 6.
Proposed model for 17β-estradiol effect on human MMP-2 promoter activity mediated by ERα and MAP Kinase-ERK1/2 signalling pathway. Activation of ERα and MAP Kinase-ERK1/2 signalling pathway by 17β-estradiol mediates phosphorylation of the transcription factor Elk-1. The binding of phosphorylated Elk-1 leads to recruitment of co-repressor(s) and results into down-regulation of MMP-2 transcriptional activity. ICI 182,780 and PD98059 inhibit the 17β-estradiol mediated phosphorylation of Elk-1 by blocking estrogen receptors and MAPK-ERK1/2 pathway, resulting in normal MMP2-promoter activity.
In summary, we report the significant down-regulation of MMP-2 by E2 in adult cardiac fibroblasts of both sexes. Moreover, we present a novel mechanism for the E2-mediated inhibition of MMP-2 gene expression that is active in a human fibroblast cell line and in rat cardiac fibroblasts. These findings may explain how E2 could account for an attenuated adverse remodelling by down-regulating MMP-2 expression. This may contribute to the understanding of the more beneficial remodelling in female hearts vs. male hearts under stress conditions.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (GRK 754, RE 662/6-1, and Re 662/9-1 FOR1054) and Eugene heart (EUIP 018833). Funding to pay the open access charges was provided by the Deutsche Forschungsgemeinschaft (DFG Re 662/9-1 FOR 1054).
Supplementary Material
Acknowledgements
We thank Prof. Dr P. Chambon for providing the human ERα-vector (HEGO-pSG5 vector).
Conflict of interest: none declared.
References
- 1.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;3:604–613. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;31:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 3.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;4:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 4.Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res. 1998;4:482–495. doi: 10.1161/01.res.82.4.482. [DOI] [PubMed] [Google Scholar]
- 5.Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res. 2000;2:307–315. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 6.Fielitz J, Leuschner M, Zurbrugg HR, Hannack B, Pregla R, Hetzer R, et al. Regulation of matrix metalloproteinases and their inhibitors in the left ventricular myocardium of patients with aortic stenosis. J Mol Med. 2004;12:809–820. doi: 10.1007/s00109-004-0606-4. [DOI] [PubMed] [Google Scholar]
- 7.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;17:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee R, Mingoia JT, Bruce JA, Austin JS, Stroud RE, Escobar GP, et al. Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;5:H2216–H2228. doi: 10.1152/ajpheart.01343.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, et al. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol. 2007;4:H1847–H1860. doi: 10.1152/ajpheart.00434.2006. [DOI] [PubMed] [Google Scholar]
- 10.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, et al. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;3:H1229–H1235. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;3:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, et al. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension. 2006;4:711–717. doi: 10.1161/01.HYP.0000208840.30778.00. [DOI] [PubMed] [Google Scholar]
- 13.Witt H, Schubert C, Jaekel J, Fliegner D, Penkalla A, Tiemann K, et al. Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med. 2008;9:1013–1024. doi: 10.1007/s00109-008-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;18:2181–2192. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Tyagi SC, Kumar S, Katwa L. Differential regulation of extracellular matrix metalloproteinase and tissue inhibitor by heparin and cholesterol in fibroblast cells. J Mol Cell Cardiol. 1997;1:391–404. doi: 10.1006/jmcc.1996.0283. [DOI] [PubMed] [Google Scholar]
- 16.Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;3:889–894. doi: 10.1095/biolreprod.102.005116. [DOI] [PubMed] [Google Scholar]
- 17.Guccione M, Silbiger S, Lei J, Neugarten J. Estradiol upregulates mesangial cell MMP-2 activity via the transcription factor AP-2. Am J Physiol Renal Physiol. 2002;1:F164–F169. doi: 10.1152/ajprenal.0318.2000. [DOI] [PubMed] [Google Scholar]
- 18.Wingrove CS, Garr E, Godsland IF, Stevenson JC. 7beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophys Acta. 1998;2:169–174. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 19.Marin-Castano ME, Elliot SJ, Potier M, Karl M, Striker LJ, Striker GE, et al. Regulation of estrogen receptors and MMP-2 expression by estrogens in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;1:50–59. doi: 10.1167/iovs.01-1276. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson UW, Garvin S, Dabrosin C. MMP-2 and MMP-9 activity is regulated by estradiol and tamoxifen in cultured human breast cancer cells. Breast Cancer Res Treat. 2007;3:253–261. doi: 10.1007/s10549-006-9335-4. [DOI] [PubMed] [Google Scholar]
- 21.Moalli PA, Klingensmith WL, Meyn LA, Zyczynski HM. Regulation of matrix metalloproteinase expression by estrogen in fibroblasts that are derived from the pelvic floor. Am J Obstet Gynecol. 2002;1:72–79. doi: 10.1067/mob.2002.124845. [DOI] [PubMed] [Google Scholar]
- 22.Kanagaraj P, Vijayababu MR, Ilangovan R, Senthilkumar K, Venkataraman P, Aruldhas MM, et al. Effect of 17beta-estradiol on apoptosis, IGF system components and gelatinases A and B in prostate cancer cells (PC-3) Clin Chim Acta. 2007;1–2:70–78. doi: 10.1016/j.cca.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;3:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 24.Grohe C, Kahlert S, Lobbert K, Stimpel M, Karas RH, Vetter H, et al. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1997;1:107–112. doi: 10.1016/s0014-5793(97)01179-4. [DOI] [PubMed] [Google Scholar]
- 25.Neuss M, Regitz-Zagrosek V, Hildebrandt A, Fleck E. Isolation and characterisation of human cardiac fibroblasts from explanted adult hearts. Cell Tissue Res. 1996;1:145–153. doi: 10.1007/s004410050683. [DOI] [PubMed] [Google Scholar]
- 26.Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P, Gardner MB. Characterization of a newly derived human sarcoma cell line (HT-1080) Cancer. 1974;4:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res. 1997;35:2–3. [PubMed] [Google Scholar]
- 28.Mahmoodzadeh S, Fritschka S, Dworatzek E, Pham TH, Becher E, Kuehne A, et al. Nuclear factor-Kappa B regulates estrogen receptor-alpha transcription in the human heart. J Biol Chem. 2009;284:24705–24714. doi: 10.1074/jbc.M109.000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- 30.Duan R, Xie W, Burghardt RC, Safe S. Estrogen receptor-mediated activation of the serum response element in MCF-7 cells through MAPK-dependent phosphorylation of Elk-1. J Biol Chem. 2001;15:11590–11598. doi: 10.1074/jbc.M005492200. [DOI] [PubMed] [Google Scholar]
- 31.Ricci MS, Toscano DG, Mattingly CJ, Toscano WA., Jr Estrogen receptor reduces CYP1A1 induction in cultured human endometrial cells. J Biol Chem. 1999;6:3430–3438. doi: 10.1074/jbc.274.6.3430. [DOI] [PubMed] [Google Scholar]
- 32.Lincoln DW, 2nd, Phillips PG, Bove K. Estrogen-induced Ets-1 promotes capillary formation in an in vitro tumor angiogenesis model. Breast Cancer Res Treat. 2003;2:167–178. doi: 10.1023/a:1022904624054. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki Y, Kubota H, Nozaki M, Nagata K. Transcriptional regulation of the cytosolic chaperonin theta subunit gene, Cctq, by Ets domain transcription factors Elk-1, Sap-1a, and Net in the absence of serum response factor. J Biol Chem. 2003;33:30642–30651. doi: 10.1074/jbc.M212242200. [DOI] [PubMed] [Google Scholar]
- 34.Dougherty SM, Mazhawidza W, Bohn AR, Robinson KA, Mattingly KA, Blankenship KA, et al. Gender difference in the activity but not expression of estrogen receptors alpha and beta in human lung adenocarcinoma cells. Endocr Relat Cancer. 2006;1:113–134. doi: 10.1677/erc.1.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin M, Lee HW, Zhao L, Eghbali-Webb M. Gender-related differences in proliferative response of cardiac fibroblasts to hypoxia: effects of estrogen. Mol Cell Biochem. 2000;1–2:21–30. doi: 10.1023/a:1026585420021. [DOI] [PubMed] [Google Scholar]
- 36.Nordmeyer J, Eder S, Mahmoodzadeh S, Martus P, Fielitz J, Bass J, et al. Upregulation of myocardial estrogen receptors in human aortic stenosis. Circulation. 2004;20:3270–3275. doi: 10.1161/01.CIR.0000147610.41984.E8. [DOI] [PubMed] [Google Scholar]
- 37.Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, et al. Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J. 2006;7:926–934. doi: 10.1096/fj.05-5148com. [DOI] [PubMed] [Google Scholar]
- 38.O'Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, et al. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;6:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- 39.Lu T, Achari Y, Sciore P, Hart DA. Estrogen receptor alpha regulates matrix metalloproteinase-13 promoter activity primarily through the AP-1 transcriptional regulatory site. Biochim Biophys Acta. 2006;8:719–731. doi: 10.1016/j.bbadis.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Scafonas A, Reszka AA, Kimmel DB, Hou XS, Su Q, Birzin ET, et al. Agonist-like SERM effects on ERalpha-mediated repression of MMP1 promoter activity predict in vivo effects on bone and uterus. J Steroid Biochem Mol Biol. 2008;3–5:197–206. doi: 10.1016/j.jsbmb.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson UW, Dabrosin C. Estradiol and tamoxifen regulate endostatin generation via matrix metalloproteinase activity in breast cancer in vivo. Cancer Res. 2006;9:4789–4794. doi: 10.1158/0008-5472.CAN-05-4012. [DOI] [PubMed] [Google Scholar]
- 42.Chen CC, Lee WR, Safe S. Egr-1 is activated by 17beta-estradiol in MCF-7 cells by mitogen-activated protein kinase-dependent phosphorylation of ELK-1. J Cell Biochem. 2004;5:1063–1074. doi: 10.1002/jcb.20257. [DOI] [PubMed] [Google Scholar]
- 43.Kukushkin AN, Abramova MV, Svetlikova SB, Darieva ZA, Pospelova TV, Pospelov VA. Downregulation of c-fos gene transcription in cells transformed by E1A and cHa-ras oncogenes: a role of sustained activation of MAP/ERK kinase cascade and of inactive chromatin structure at c-fos promoter. Oncogene. 2002;5:719–730. doi: 10.1038/sj.onc.1205118. [DOI] [PubMed] [Google Scholar]
- 44.Yang SH, Vickers E, Brehm A, Kouzarides T, Sharrocks AD. Temporal recruitment of the mSin3A-histone deacetylase corepressor complex to the ETS domain transcription factor Elk-1. Mol Cell Biol. 2001;8:2802–2814. doi: 10.1128/MCB.21.8.2802-2814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;11:1395–1413. doi: 10.1038/sj.onc.1202174. (Reviews) [DOI] [PubMed] [Google Scholar]
- 46.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;11:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.