Abstract
Aims
Myocardial stunning is a contractile dysfunction that occurs after a brief ischaemic insult. Substantial evidence supports that this dysfunction is triggered by Ca2+ overload during reperfusion. The aim of the present manuscript is to define the origin of this Ca2+ increase in the intact heart.
Methods and results
To address this issue, Langendorff-perfused mouse hearts positioned on a pulsed local field fluorescence microscope and loaded with fluorescent dyes Rhod-2, Mag-fluo-4, and Di-8-ANEPPS, to assess cytosolic Ca2+, sarcoplasmic reticulum (SR) Ca2+, and transmembrane action potentials (AP), respectively, in the epicardial layer of the hearts, were submitted to 12 min of global ischaemia followed by reperfusion. Ischaemia increased cytosolic Ca2+ in association with a decrease in intracellular Ca2+ transients and a depression of Ca2+ transient kinetics, i.e. the rise time and decay time constant of Ca2+ transients were significantly prolonged. Reperfusion produced a transient increase in cytosolic Ca2+ (Ca2+ bump), which was temporally associated with a decrease in SR-Ca2+ content, as a mirror-like image. Caffeine pulses (20 mM) confirmed that SR-Ca2+ content was greatly diminished at the onset of reflow. The SR-Ca2+ decrease was associated with a decrease in Ca2+ transient amplitude and a shortening of AP duration mainly due to a decrease in phase 2.
Conclusion
To the best of our knowledge, this is the first study in which SR-Ca2+ transients are recorded in the intact heart, revealing a previously unknown participation of SR on cytosolic Ca2+ overload upon reperfusion in the intact beating heart. Additionally, the associated shortening of phase 2 of the AP may provide a clue to explain early reperfusion arrhythmias.
Keywords: Ischaemia/reperfusion, Sarcoplasmic reticulum, Calcium, Fluorescence
1. Introduction
Reperfusion after ischaemia causes a mechanical and electrical impairment of ventricular function.1–5 The ischaemic insult may lead to an injury that could be reversible (stunning) or irreversible (infarction).6 It is generally held that the two major triggers of myocardial stunning are Ca2+ overload and generation of reactive oxygen species.6 Both take place at the onset or within seconds after the onset of reperfusion, supporting experimental evidence that suggests that myocardial dysfunction/injury occurred prior to reperfusion or developed extremely rapidly during reperfusion.7 Abnormal intracellular Ca2+ cycling plays a key role in cardiac dysfunction and ventricular arrhythmias, particularly during the setting of transient cardiac ischaemia followed by reperfusion. Several reports describe an increase in cytosolic Ca2+ during ischaemia.4,8,9 However, a detailed description of intracellular Ca2+ kinetics, necessary for complete understanding of the involvement of Ca2+ in the ischaemia/reperfusion damage in the intact beating heart, has not been yet provided. At the onset of reperfusion, Ca2+ overload is thought to be further exacerbated by uncontrolled influx from the extracellular space.7,10 In a previous work, in which we studied the time course of intracellular Ca2+ during reperfusion, a noticeable episode was consistently observed immediately after reperfusion. This event was a slight but consistent transient increase in diastolic Ca2+ (Ca2+ bump), which then decreased towards pre-ischaemic values.5 This diastolic Ca2+ elevation occurred when the amplitude of Ca2+ transient was highly decreased. Although the increase in Ca2+ is brief, it might have important functional consequences because, at this time, intracellular Ca2+ attained the highest levels during reperfusion. However, its underlying mechanisms and physiological relevance have not been previously explored. Interestingly, a possible contribution of Ca2+ release from the sarcoplasmic reticulum (SR) in the genesis of Ca2+ overload at the onset of reperfusion has not been mechanistically evaluated before in intact hearts. This is particularly important in the light of new experiments revealing interactions between mitochondria and the endoplasmic/SR.11,12
The hypothesis underlying the present experiments is that the transient increase in diastolic Ca2+ at the beginning of reperfusion is due to Ca2+ release from the SR. The SR-Ca2+ depletion produced by this release of Ca2+ would diminish the amplitude of the following Ca2+ transients and shorten the action potential (AP), a condition that would in turn favour the appearance of arrhythmias. Interestingly, the epicardium, which is the outermost muscular layer of cardiac ventricle, is highly implicated in the genesis of several ventricular arrhythmias such as the Brugada syndrome and the short-QT syndrome.13,14 The duration of the AP (in particular phase 2) is critically dependent on the intracellular Ca2+ dynamics.15,16 Consequently, Ca2+ release from the SR is not only the key event triggering cardiac contraction but also could be a regulator of the electrical properties of the epicardial AP.
The effects of ischaemia/reperfusion on intracellular Ca2+ have been previously studied in cell suspensions or monolayers exposed to simulated ischaemia.17,18 Moreover, intracellular Ca2+ measured in intact organs have been reported since the late 1980s, using the visible light-emitting protein aequorin or fluorescent indicators such as Indo-1, Fura-2, Fluo-3, or the more recently introduced Rhod-2.5,9,19 To our knowledge, subcellular Ca2+ measurements in the intact heart, i.e. at the level of the organelles, have never been reported. In the present study, we used a novel technique, the pulsed local-field fluorescence (PLFF) microscopy,20 which allows for the simultaneous detection of cytosolic Ca2+ transient and/or SR-Ca2+ transient and transmembrane AP, in the intact beating heart together with the assessment of left intra-ventricular pressure with an integrated silicon piezoresistive transducer system. Evidence will be presented demonstrating that SR-Ca2+ release contributes to the transient cytosolic increase of Ca2+ at the onset of reperfusion. It will be further shown that, by depleting the SR of Ca2+, this release underlies the associated decrease in Ca2+ transient amplitude and may contribute to the shortening of AP during early reperfusion.
2. Methods
2.1 Heart preparation
The hearts were obtained from young Swiss Webster mice (3- to 7-week-old males). Animals used in this study were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, Revised 1996). The protocol was approved by the University of California Merced Institutional Animal Care and Use Committee (No. 2008–201). For details of this and the following methods sections, see Supplementary material online.
2.2 Electrocardiogram and ventricular pressure measurements
Transmural electrocardiogram (ECG) was recorded with a self-made DC coupled instrumentation amplifier (Burr Brown, TX, USA). Ventricular pressure was isovolumetrically measured with the aid of a plastic balloon and a differential pressure transducer (Motorola, USA).5
2.3 Fluorophores loading
Mag-fluo-4 AM, Rhod-2 AM, and Di-8-ANEPPS (Invitrogen, USA) were used to evaluate intra-SR, cytosolic Ca2+ concentrations, and transmembrane AP, respectively, in the epicardial layer of the mouse hearts.
2.4 Optical setup
A modified version of our custom-made setup for PLFF microscopy was employed to measure Ca2+ signals in the intact heart.20
2.5 Experimental protocol
After stabilization (pre-ischaemia), hearts were subjected to 12 min normothermic global ischaemia. Coronary perfusion was then restored for 30 min (reperfusion). This protocol was chosen based on the results of previous experiments which indicate that this ischaemic period produced a reversible altered Ca2+ handling and mechanical dysfunction, typical of the stunned heart.5,8 Myocardial contractility and Ca2+ transients were not altered in control experiments (n = 4), in which all conditions of the ischaemia/reperfusion protocol (dye loading, shutter opening time for acquisition), except for the interruption of coronary flow, were reproduced.
2.6 Caffeine pulses
To evaluate intracellular SR-Ca2+ content, we measured the release of Ca2+ from the SR induced by a 20 mM caffeine pulse in Rhod-2 and Mag-fluo-loaded hearts. Caffeine pulses were applied along with the Tyrode solution. Normal Tyrode was changed to Tyrode plus caffeine 20 mM, while the heart was electrically paced. After 1 min, the perfusion solution was changed back to normal Tyrode without caffeine. The highest diastolic level achieved during caffeine pulse was used to evaluate SR-Ca2+ content. Application of caffeine might not be homogeneous in the whole heart. However, in these experiments, we performed the measurements of fluorescence in the same spot (where the optic fibre was positioned), during the whole protocol, which would assure homogenous fluorescence along the complete ischaemia/reperfusion protocol.
Two successive caffeine pulses were always performed. In control experiments, two successive pulses, separated by a 6 min interval, were applied in the pre-ischaemic period. In the ischaemia/reperfusion experiments, the second pulse was applied after 6 min of reperfusion and was compared with a first pulse evoked in the pre-ischaemic period.
2.7 Data analysis
Data are expressed as mean ± SEM. Statistical significance was determined by either Student's t-test for unpaired observations or ANOVA followed by Newman Keuls test, when more than two groups were compared. The differences were considered statistically significant if P-value was less than 0.05.
3. Results
3.1 Cytosolic Ca2+ and Ca2+ kinetics during ischaemia
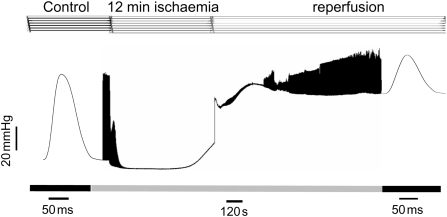
Several laboratories including ours have previously showed that ischaemia increased end-diastolic pressure (EDP), in association with a decrease in developed pressure (DP), to non-detectable levels.3,5,21 EDP was further increased at the onset of reperfusion, whereas DP was greatly diminished with respect to pre-ischaemia (Figure 1). To gain further insights into the origin of these typical alterations of the ischaemic/reperfused heart, we explored the pattern of intracellular Ca2+ changes during ischaemia/reperfusion.
Figure 1.
Time course of left ventricular pressure in a mouse heart submitted to ischaemia/reperfusion. Typical records of left ventricular DP showing that ischaemia increased EDP and reduced contractility to non-detectable levels. Reperfusion produced an immediate and long lasting increase in EDP. Contractility was depressed at the beginning of reflow and recovered during reperfusion. Similar results were obtained in six additional experiments of this type.
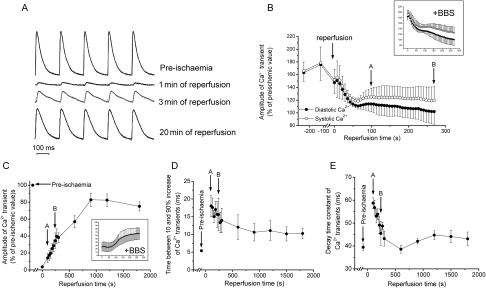
Figure 2A shows a typical example of cytosolic Ca2+ changes in a mouse heart at 3 min of global ischaemia. In agreement with the previous findings in several species and ischaemia/reperfusion models,4,8,9 ischaemia produced a substantial increase in diastolic and systolic Ca2+ which was associated with a decrease in Ca2+ transient amplitude. The inset in Figure 2A indicates that blebbistatin, a mechanical uncoupler that prevents contractions by inhibiting actin–myosin interaction,22 did not alter intracellular Ca2+ kinetics (Supplementary material online). Figure 2B and C shows overall results of the increase in diastolic and systolic Ca2+ and the associated decrease in Ca2+ transient amplitude, which reached 20% of pre-ischaemic values within the first 5 min of ischaemia. Insets show that the presence of blebbistatin did not modify the results observed in the absence of the uncoupler. Blebbistatin experiments provide evidence that no significant artifactual fluorescence changes were produced by contraction movements. Figure 2D and E shows that the kinetics of intracellular Ca2+ transients was slowed down during ischaemia. Both the rise time and decay time constant of Ca2+ transients were significantly prolonged, in agreement with the strong inhibition exerted by ischaemia/acidosis on SR-Ca2+ release and reuptake.23–25
Figure 2.
Time course of intracellular Ca2+ transient and kinetics during ischaemia. (A) Typical recordings of intracellular Ca2+ transients in an intact beating heart before and at 3 min of ischaemia. (B–E) Overall results showing that ischaemia increased diastolic and systolic Ca2+ (B), decreased the amplitude of Ca2+ transient (C), and slowed Ca2+ kinetics of Ca2+ transients (D–E), n = 8. Decay time constant was calculated as time between 10 and 90% decline divided by 2.2. Similar results were obtained in the presence of 10 µM of the mechanical uncoupler blebbistatin (BBS) (Insets in B–C. Axis legends in the insets are the same as the corresponding figure), n = 3.
3.2 Cytosolic Ca2+ and Ca2+ kinetics during reperfusion
Figure 3A shows typical records of intracellular Ca2+ transients at different times during reperfusion. Intracellular Ca2+ transients were completely depressed at 1 min of reperfusion and slowly recovered towards pre-ischaemic levels during reperfusion. Figure 3B shows overall results of the time course of diastolic and systolic Ca2+ at the end of ischaemia and during the first minutes of reperfusion. Consistent with Ca2+ measurements during ischaemia, diastolic and systolic Ca2+ emerged from ischaemia at a high level with respect to pre-ischaemia. At the onset of reperfusion, there was a slight but consistent transient increase in cytosolic Ca2+ (Ca2+ bump) which was associated with diminished Ca2+ transient amplitude. Figure 3C shows that Ca2+ transient amplitude increased with reperfusion. Similar results were obtained in the presence of blebbistatin (Insets). Figure 3D and E shows overall results of Ca2+ transients kinetics during reperfusion. Whereas the decay time constant of Ca2+ transient fully recovered (Figure 3E), the time from 10 to 90% rise did not reach pre-ischaemic levels within the 30 min period of reperfusion (Figure 3D).
Figure 3.
Time course of intracellular Ca2+ transient and dynamics during reperfusion. (A) Typical recordings of intracellular Ca2+ transients before ischaemia and during reperfusion. (B) Overall results of the time course of diastolic and systolic Ca2+ at two different times during ischaemia (8 and 10 min) and the first minutes of reperfusion. The increase in cytosolic Ca2+ at the beginning of ischaemia (Figure 2) still persisted at the end of ischaemia. Cytosolic Ca2+ transiently increased at the onset of reperfusion and then decreased towards pre-ischaemic values. (C) Amplitude of Ca2+ transients. (D and E) Rising time and decay time constant of Ca2+ transient, n = 8. Decay time constant was calculated as time between 10 and 90% decline divided by 2.2. (B–D) Arrows indicate pre-ischaemic values and the same time points during reperfusion. Insets indicate that similar changes in Rhod-2 fluorescence were obtained in the presence of 10 µM of blebbistatin (BBS) (Insets in B and C. Axis legends in the insets are the same as the corresponding figure), n = 3.
3.3 The increase in cytosolic Ca2+ at the onset of reperfusion is due to SR-Ca2+ release
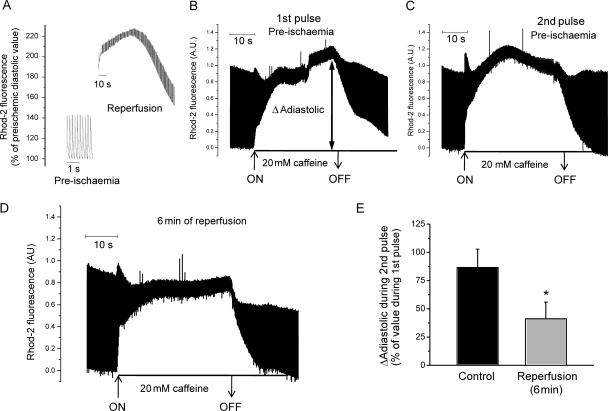
Figure 4A shows a typical example of cytosolic Ca2+ increase at the beginning of reperfusion in an expanded time scale. Since the mechanism of this transient increase is not obvious, we explored the possibility that the SR was involved. To test this hypothesis, we evaluated the intra SR-Ca2+ content, by estimating the change in cytosolic Ca2+ produced when Ca2+ was released from the SR following an application of a 20 mM caffeine pulse through the coronary system in Rhod-2 loaded hearts. Figure 4B and C shows that two successive caffeine pulses separated by a 6 min interval induced a reproducible increase in diastolic Ca2+. In contrast, a caffeine pulse applied at the beginning of reperfusion produced a smaller diastolic Ca2+ increase compared with that produced in pre-ischaemia (Figure 4D), suggesting that SR-Ca2+ content was lower at the beginning of post-ischaemic recovery than before ischaemia. Overall results are shown in Figure 4E. The decrease in SR-Ca2+ content observed might be explained by either one of the following possibilities: (i) the SR-Ca2+ content diminishes during ischaemia and is lower at the beginning of reperfusion than before ischaemia; (ii) the onset of reperfusion is associated with a release of Ca2+ from the SR, which causes SR-Ca2+ depletion, so that after 6 min of reperfusion, SR-Ca2+ content was lower than before ischaemia. To directly explore these possibilities, hearts loaded with Mag-fluo-4 were challenged with the ischaemia/reperfusion protocol, whereas the intraluminal SR-Ca2+ was continuously monitored. The procedure for dye loading and intra SR-Ca2+ measurement with Mag-fluo-4 is illustrated in Figure 5A. The dye was loaded at room temperature using coronary retroperfusion. After 30 min, temperature was increased to 37°C. As illustrated in Figure 5A, fluorescent transients recorded under these conditions show both a positive deflection due to Ca2+ binding to the dye in the myoplasm and a negative component due to the unbinding of Ca2+ from the dye in the SR. The increase in heart temperature induced the extrusion of the free dye from the cytosol.26 The dye loaded into the SR stays trapped inside the organelle and reports the SR-Ca2+ transient movements. Figure 5B shows a typical recording of luminal SR-Ca2+ at 1 min of ischaemia. Whereas SR diastolic fluorescence increased, the amplitude of SR-Ca2+ transient decreased after 1 min of ischaemia compared with pre-ischaemic level. Figure 5C depicts overall results of diastolic and systolic Ca2+ and SR-Ca2+ transient amplitude during the first 5 min of ischaemia, where the increase in SR diastolic Ca2+ associated with a continuous decrease towards non-detectable values of SR-Ca2+ transients is evident.
Figure 4.
Changes in Rhod-2 fluorescence during reperfusion and in the response to caffeine pulses applied before ischaemia and during post-ischaemic recovery. (A) Typical recording of the time course of diastolic Ca2+ during the first 90 s of reperfusion, in an expanded time scale. As already shown (Figure 3), the onset of reperfusion produced a rapid and transient increase in diastolic Ca2+. (B and C) Two successive caffeine pulses during pre-ischaemia induced a similar increase (amplitude) in diastolic Ca2+ (ΔAdiastolic). ΔAdiastolic: difference between the maximum diastolic fluorescence attained during the caffeine pulse and the diastolic fluorescence before caffeine. ON and OFF: moment of caffeine application and washout, respectively. (D) After 6 min of reperfusion, caffeine provoked a smaller increase in diastolic Ca2+ in the same heart. (E) Overall results of the experiments. Control indicates mean ΔAdiastolic values of the second of two successive pulses during pre-ischaemia, as shown in (B and C), expressed as % of the first one (B). Reperfusion (6 min) indicates mean ΔAdiastolic values of the caffeine pulse after 6 min of reperfusion expressed as % of the first caffeine pulse in pre-ischaemia [i.e. (D) as % of (B)]. n = 6. Asterisks (*) indicate statistically significant differences (P < 0.05) with respect to pre-ischaemic value.
Figure 5.
Reperfusion induces a rapid Ca2+ release from the SR. (A) Typical recordings showing the procedure for Mag-fluo-4 loading. (B and C) Typical recordings and overall results of intraluminal SR-Ca2+ during ischaemia. (D) Decrease in SR diastolic Ca2+ during the first minutes of reperfusion and typical recordings of SR-Ca2+ transients obtained in an intact beating heart before ischaemia and during reperfusion. There is an initial decrease in the amplitude of SR-Ca2+ transient compared with pre-ischaemia. The SR-Ca2+ gradually recovered with time of reperfusion. (E) Two successive caffeine pulses during pre-ischaemia induced a similar decrease in SR-Ca2+. Inset: typical SR-Ca2+ transients in an expanded scale. Last record: after 6 min of reperfusion caffeine provoked a smaller decrease in intraluminal SR-Ca2+.
Figure 5D shows typical recordings of luminal SR-Ca2+ transients in the pre-ischaemic period and at 2 and 5 min of reperfusion. As evident, reperfusion produced an abrupt decrease in luminal SR-Ca2+ content. These results clearly indicate that the transient increase in cytosolic Ca2+ during early reperfusion was primarily due to the release of Ca2+ from the SR. To further confirm this finding, caffeine pulses were applied to mice hearts loaded with Mag-fluo-4. Figure 5E represents a typical experiment (n = 3) in which the first caffeine pulse was fully reproduced by a second one applied 5 min later. At the onset of reperfusion (last trace to the right), caffeine evoked a small change in luminal diastolic SR-Ca2+ in the same heart, a result that should be expected from a reduced SR-Ca2+ content.
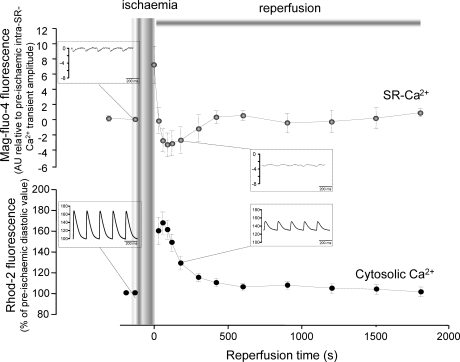
Figure 6 depicts overall results of the experiments in hearts loaded with Mag-fluo-4, showing the time course of intraluminal diastolic SR-Ca2+ during reperfusion. Noteworthy, resting diastolic Ca2+ level inside the SR just before reperfusion is significantly higher than that prior to ischaemia, as shown in Figure 5B and C. Results obtained with Rhod-2 in the ischaemia/reperfusion protocol are shown for comparison. The increase in diastolic Ca2+ in the cytosol (Rhod-2 fluorescence) is the mirror image of the decrease in diastolic SR-Ca2+ (Mag-fluo-4 fluorescence). Insets in the figure depict typical records of Mag-Fluo-4 and Rhod-2 fluorescence in pre-ischaemia and during reperfusion. Taken together, the results imply that the beginning of reperfusion is associated with a release of Ca2+ from the SR.
Figure 6.
The increase in diastolic Ca2+ is the mirror-like image of the decrease in SR-Ca2+ content. Overall results of Mag-fluo-4 fluorescence (upper panel) in pre-ischaemia and reperfusion (n = 9). Changes in diastolic level of Mag-fluo-4 fluorescence during reperfusion are presented in arbitrary units and normalized to the amplitude of the intra-SR-Ca2+ transient before ischaemia. Each data point was calculated as the difference between current diastolic value during reperfusion and the pre-ischaemic diastolic value and then divided by the amplitude of the pre-ischaemic intra-SR-Ca2+ transient. Results of Rhod-2 obtained in experiments from the same group (lower panel) are shown for comparison (n = 13). Changes in diastolic level of Rhod-2 fluorescence are expressed as percentage of the pre-ischaemic diastolic value. As shown, the recovery profile of SR-Ca2+ (Mag-fluo-4) was a mirror-like image of cytosolic Ca2+ (Rhod-2). Insets depict typical records of Mag-fluo-4 and Rhod-2 fluorescence in pre-ischaemia and during reperfusion (Y-axis titles in the insets are the same as the figure).
3.4 Electrical changes occurring during ischaemia/reperfusion
Ischaemic insults followed by reperfusion may also induce alterations in cardiac excitability and dromotropism. To test whether mouse hearts displayed electrical alterations under our experimental conditions, we evaluated the electrophysiological function. Figure 7A illustrates that ischaemia produced dramatic alterations in the transmural ECG. The most notable features were an inversion of the T-wave and a decrease in excitability illustrated by the inability of the stimulus to elicit an AP. The excitability completely recovered after 15 min of reperfusion. Similar results were obtained in other five from six experiments of this type.
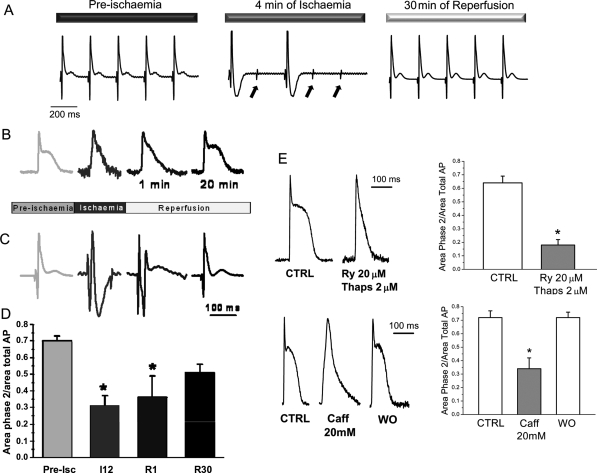
Figure 7.
(A–D) Ischaemia impairs electrical excitability and shortens AP. (A) Typical recordings of transmural electrocardiograms during pre-ischaemia, ischaemia and reperfusion. Arrows indicate the moments when the electrical pacing was applied. (B and C) Typical recordings of intracellular AP and ECG measured simultaneously. Ischaemia significantly shortened phase 2 of AP. (D) Time course of the contribution of phase 2 to the AP, during ischaemia/reperfusion (Pre-Isc, I12, R1, and R30 indicate pre-ischaemia, 12 min of ischaemia, 1 and 30 min of reperfusion, respectively). Asterisk indicates the results with P < 0.05 when compared with pre-ischaemia, n = 4–6 experiments in each group. (E) Depletion of SR is associated with a loss of phase 2 of AP. Two interventions that deplete the SR of Ca2+, like the combination of ryanodine/thapsigargin (Ry/Thaps) or caffeine (Caff), were associated with a loss of phase 2 of the AP, n = 6. *P < 0.05 with respect to control (without drug, CTRL) (WO indicates Washout).
Experiments, in which we determined the epicardial transmembrane AP by optically measuring AP in hearts loaded with Di-8-ANEPPS, showed that another typical electrophysiological change during ischaemia is the shortening of the AP due to the dramatic reduction in phase 2. Typical recordings obtained at different times during the ischaemia/reperfusion are shown in Figure 7B. The AP shortening induced by ischaemia was maintained at the onset of reperfusion. Interestingly, pre-ischaemic AP waveshape was recovered after 15 min reperfusion. Furthermore, the decrease in AP duration, both in ischaemia and at the onset of reperfusion, was associated with a triangulation of the AP waveshape. These AP changes tightly correlate with the time course of transmural ECG recorded simultaneously (Figure 7C). Overall results of the contribution of the phase 2 to the AP at different times during ischaemia/reperfusion are presented in Figure 7D.
Since the AP shortening occurred simultaneously with a decrease in cytosolic Ca2+ transients, we hypothesized that both phenomena may be related: a decrease in Ca2+ release from the SR would slow the forward NCX mode and diminish the influx of Na+, a major component of late repolarization (i.e. phase 2) of the AP in the mouse hearts.27 Figure 7E shows that interventions that deplete the SR of Ca2+, like ryanodine/thapsigargin or caffeine, were associated with a loss of phase 2 and triangulation of the AP. Taken together, these results suggest that the release of Ca2+ from SR at the onset of reperfusion may be responsible for two concomitant phenomena, namely, the decrease in Ca2+ transient amplitude and the disappearance of the plateau phase of the AP.
4. Discussion
The main findings of the present experiments are: (i) the transient increase in cytosolic Ca2+ at the onset of reperfusion is due to SR-Ca2+ release; (ii) the SR-Ca2+ release produces a decrease in SR-Ca2+ content associated with a diminished Ca2+ transient amplitude and shortening of the phase 2 of AP. To the best of our knowledge, this is the first study in which SR-Ca2+ transients have been recorded in the intact heart. This approach clearly reveals the role of the SR in the increase in cytosolic Ca2+ at the beginning of reperfusion. The results further suggest that the decrease in SR-Ca2+ content produced by Ca2+ release from this compartment may contribute to maintaining the diminished duration of the AP at the onset of reflow. This would create a scenario particularly prone to arrhythmias.
4.1 Intracellular and subcellular Ca2+ measurements in the intact beating heart
Measurements of intracellular Ca2+ handling during whole organ ischaemia/reperfusion have been reported since the late 1980s using different fluorescent indicators or the visible light-emitting protein aequorin.5,8,9 More recently, longer wavelength Ca2+ indicators, like Rhod-2, were introduced.19 Rhod-2 has spectral properties that allow it to be used with optical mapping (OM) systems designed for potentiometric dyes.28 In the present study, we used PLFF microscopy, a novel technique previously described by us,20 that allows for simultaneous or alternative detection of intraventricular pressure and cytosolic Ca2+, SR-Ca2+ transients, and transmembrane AP in the intact beating heart. This method improved the signal-to-noise ratio by combining the PLFF illumination with an integrating current-to-voltage conversion and with a digital evaluation of the integrated photocurrent. One limitation of our study is that myoplasmic Ca2+ was only assessed at the epicardium and differences in Ca2+ transients may arise from the ventricular transmural dispersion of the mechanisms underlying excitation–contraction coupling.29 Moreover, since myoglobin (Mb) strongly absorbs light depending on tissue oxygenation, at 540 and 580 nm (oxy-Mb) and 550 nm (deoxy-Mb),30 it could be argued that this may affect Ca2+ measurements with Rhod-2. However, this possibility seems unlikely because similar results were obtained in ischaemia/reperfusion experiments, in which hearts were simultaneously loaded with Rhod-2 and Fluo-4 (excited at 532 and 473 nm, respectively), supporting the idea that the changes of Rhod-2 signals observed reflect actual variations of Ca2+ during ischaemia/reperfusion.
The use of Mag-fluo-4 in the present study allowed us to assess, for the first time, SR-Ca2+ transients in the intact heart. This approach clearly shows a decrease in Ca2+ content at the onset of reperfusion that was further corroborated with the diminished caffeine-induced Ca2+ release. A major advantage of the PLFF microscopy is its much higher spatial resolution (up to 8 µm) than other standard OM methods (usually 950 µm). Moreover, avalanche photodiodes are 250 times more sensitive than PIN photodiodes generally used in OM.
4.2 Intracellular Ca2+ and Ca2+ dynamics during ischaemia/reperfusion
In agreement with the previous findings,4,8,9 the experiments described in this paper confirmed that there is an increase in cytosolic Ca2+ during ischaemia. Several mechanisms have been considered to explain this cytosolic Ca2+ increase. Most of these mechanisms have been related to the acidosis associated with the ischaemic period like a decrease in Ca2+ myofilament responsiveness, a depression of SERCA2a activity and/or the mechanisms that extrude Ca2+, and an inhibition of RyR2.6,23,24 The dissociation between the decrease in DP and the increase in cytosolic Ca2+ during ischaemia would support a decrease in Ca2+ myofilament responsiveness as a main factor responsible for the increase in cytosolic Ca2+ during ischaemia. Moreover, the prolongation of both the rising and relaxation kinetics of the Ca2+ transients would suggest an inhibition of RyR2 and a depression of SERCA2a activity. Indeed, previous experiments clearly showed that acidosis reversibly inhibits RyR2.24,25
The data presented in Figures 5C and 6 imply that intra-SR-Ca2+ level was increased during ischaemia. This experimental observation supports our hypothesis that SR is one of the main sources of Ca2+ that is being released into cytosol during reperfusion. Indeed, in order to return to pre-ischaemic Ca2+ concentrations, SR should extrude the extra Ca2+ ions accumulated during ischaemia.
4.3 The depletion of SR-Ca2+ content as the cause of the increase in diastolic Ca2+ at the beginning of reperfusion
The present results also confirmed an increase in cytosolic Ca2+ at the onset of reperfusion.4,5 Although the mechanism of this increase in Ca2+ is not clear, it is generally accepted that it results from Ca2+ influx from the extracellular space.6,7,10 Surprisingly, a possible role of the SR on cytosolic Ca2+ overload at the onset of reperfusion has not been directly evaluated in intact beating hearts. The present results clearly showed that the SR directly contributes to the cytosolic Ca2+ increase upon reperfusion. This contribution was confirmed by the measurements of cytosolic as well as intra-SR-Ca2+ after caffeine application. This manoeuvre revealed a decrease in SR-Ca2+ content at this time of reperfusion (Figures 4 and 5). More important, the decrease in SR-Ca2+ content was directly detected by the estimation of SR-Ca2+ with the fluorescent dye Mag-fluo-4 (Figure 5D). A small increase in cytosolic Ca2+ during ischaemia and a further increase upon reperfusion were observed in Rhod-loaded rabbit hearts.4 It was found, however, that when the Kd for Rhod-2 and Ca2+ were corrected for the temperature and pH to calculate cytosolic Ca2+, the increase in Ca2+ during ischaemia was greater than before correction and there was no further increase in Ca2+ upon reperfusion. The present experiments showed that ischaemia increased cytosolic Ca2+, in agreement with these previous findings, and a transient but consistent increase in Ca2+ (Ca2+ bump) was still detected during early reperfusion. Since this increase was temporarily associated with a decrease in SR-Ca2+ content, assessed with a different dye, the possibility that it was an artefact seems unlikely. Moreover, control experiments demonstrated that a decrease in pH similar to that produced by acidosis, failed to affect diastolic fluorescence (data not shown). In any case, the main point of the present experiments is to show that SR depletion occurs at the onset of reperfusion. This Ca2+ release may be actually underestimated when measuring the bulk cytosolic Ca2+, in which the Ca2+ contribution of a smaller compartment like the SR, may be diluted. Experimental evidence indicated that a fraction of the total SR was physically coupled and transferred Ca2+ locally to the mitochondria in cardiac muscle.12 Moreover, de Brito and Scorrano11 recently showed that mitofusin-2, a mitochondria dynamin-related protein, tethers ER to mitochondria, a juxtaposition required for efficient mitochondrial Ca2+ uptake. In this scenario, it cannot be excluded that mitofusin may be also involved in destiny of Ca2+ released by the SR. A mitofusin-mediated process leading to direct and rapid uptake by the mitochondria of the released SR-Ca2+ might be at least co-responsible for the fact that the relatively strong SR-Ca2+ depletion observed at the onset of reperfusion becomes hardly reflected in elevation of the basic concentration of free Ca2+ in the cell. In any case, the Ca2+ release from the SR described here is responsible for at least part of the cytosolic Ca2+ overload at the onset of reflow. Although the role of the SR at the onset of simulated reperfusion has been previously emphasized in isolated quiescent ventricular cardiomyocytes, the cytosolic Ca2+ pattern observed in these quiescent cells differed from the ones observed in the intact beating hearts.18 The reason for these differences is not apparent to us. However, this may arise from the different ischaemia/reperfusion models and preparations described in the literature.
The trigger for SR-Ca2+ release at the beginning of reperfusion was not explored in the present experiments, but different mechanisms may contribute to this release. In the first place, the present results revealed that the SR emerged full of Ca2+ from ischaemia. Either the influx of Ca2+ from the extracellular space and/or the relief of RyR2 from the acidosis-induced RyR2 inhibition upon returning to normal pH may act independently or in combination to produce the release of Ca2+ from an overloaded SR. Indeed, recent experiments from our laboratory demonstrated that the return to normal pH after acidosis evoked a release of SR-Ca2+ produced by the rapid relief of the RyR2 from the previous acidosis-induced inhibition.25
4.4 The shortening of the AP at the beginning of reperfusion
Ischaemia evoked a shortening of the AP which still persists in early reperfusion and is mainly due to a decrease in phase 2 (Figure 7). The decrease in AP duration was associated with a triangulation of the AP waveshape. This AP shortening is also associated with a decrease in the amplitude of intracellular Ca2+ transients, which by slowing the direct NCX mode would decrease the Na+ influx, a main component of phase 2 of the AP in the mouse heart.27 The fact that SR-Ca2+ depletion induced by different interventions like thapsigargin/ryanodine or caffeine was systematically associated with a decrease in phase 2 and AP triangulation is in agreement with this hypothesis. It is known that early reperfusion is prone to arrhythmias. The present results support the idea that the described shortening of the AP may be a main contributor in the genesis of early reperfusion arrhythmias. Interestingly, the presence of triangulation, independently of the length of AP, constitutes a proarrhythmic event.31
In summary, the present findings revealed for the first time that the increase in diastolic Ca2+ at the onset of reperfusion is associated with a release of Ca2+ from the SR. They also provided evidence indicating that the SR-Ca2+ depletion at the beginning of reperfusion underlies two related phenomena: a decrease in Ca2+ transient amplitude and in AP duration. This would provide an adequate scenario for the appearance of early reperfusion arrhythmias.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institute of Health (NIH R01-HL-084487 to A.L.E. and FIRCA # R03 TW007713-01 to A.M.); FONCYT & CONICET PICT # 05-26117; PIP # 5300 and 02139 to A.M.
Supplementary Material
References
- 1.Braunwald E, Kloner RA. The stunned myocardium: prolonged postischemic ventricular dysfunction. Circulation. 1982;66:1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- 2.Himori N, Walls AP, Burkman AM. Ischaemically induced alterations in electrical activity and mechanical performance of isolated blood perfused canine myocardial preparations. Cardiovasc Res. 1990;24:786–792. doi: 10.1093/cvr/24.10.786. [DOI] [PubMed] [Google Scholar]
- 3.Vittone L, Mundiña-Weilenmann C, Said M, Ferrero P, Mattiazzi A. Time course and mechanisms of phosphorylation of phospholamban residues in ischemia-reperfused rat hearts. Dissociation of phospholamban phosphorylation pathways. J Mol Cell Cardiol. 2002;34:39–50. doi: 10.1006/jmcc.2001.1488. [DOI] [PubMed] [Google Scholar]
- 4.Stamm C, Friehs I, Choi YH, Zurakowski D, McGowan FX, del Nido PJ. Cytosolic calcium in the ischemic rabbit heart: assessment by pH- and temperature-adjusted rhod-2 spectrofluorometry. Cardiovasc Res. 2003;59:695–704. doi: 10.1016/s0008-6363(03)00467-x. [DOI] [PubMed] [Google Scholar]
- 5.Valverde CA, Mundiña-Weilenmann C, Reyes M, Kranias EG, Escobar AL, Mattiazzi A. Phospholamban phosphorylation sites enhance the recovery of intracellular Ca2+ after perfusion arrest in isolated, perfused mouse heart. Cardiovasc Res. 2006;70:335–345. doi: 10.1016/j.cardiores.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R, Marbán E. Molecular and cellular mechanism of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 7.Piper HM, Abdallah Y, Schäfer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004;61:365–371. doi: 10.1016/j.cardiores.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Steenbergen C, Murphy E, Levy L, London RE. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987;60:700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- 9.Kihara Y, Grossman W, Morgan JP. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res. 1989;65:1029–1044. doi: 10.1161/01.res.65.4.1029. [DOI] [PubMed] [Google Scholar]
- 10.Kusuoka H, Porterfield JK, Weisman HF, Weisfeldt ML, Marban E. Pathophysiology and pathogenesis of stunned myocardium. Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest. 1987;90:609–623. doi: 10.1172/JCI112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 12.García-Pérez C, Hajnóczky G, Csordás G. Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. J Biol Chem. 2008;283:32771–32780. doi: 10.1074/jbc.M803385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:268–272. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 14.Antzelevich C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm. 2007;4:964–972. doi: 10.1016/j.hrthm.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988;62:116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- 16.Guo D, Zhou J, Zhao X, Gupta P, Kowey PR, Martin J, et al. L-type calcium current recovery versus ventricular repolarization: preserved membrane-stabilizing mechanism for different QT intervals across species. Heart Rhythm. 2008;5:271–279. doi: 10.1016/j.hrthm.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Louch WE, Ferrier GR, Howlett SE. Changes in excitation–contraction coupling in an isolated ventricular myocyte model of cardiac stunning. Am J Physiol Heart Circ Physiol. 2002;283:H800–H810. doi: 10.1152/ajpheart.00020.2002. [DOI] [PubMed] [Google Scholar]
- 18.Siegmund B, Ladilov YV, Piper HM. Importance of sodium for recovery of calcium control in reoxygenated cardiomyocytes. Am J Physiol. 1994;267:H506–H513. doi: 10.1152/ajpheart.1994.267.2.H506. [DOI] [PubMed] [Google Scholar]
- 19.Qian YW, Clusin WT, Lin SF, Han J, Sung RJ. Spatial heterogeneity of calcium transient alternans during the early phase of myocardial ischemia in the blood-perfused rabbit heart. Circulation. 2001;104:2082–2087. doi: 10.1161/hc4201.097136. [DOI] [PubMed] [Google Scholar]
- 20.Mejía-Alvarez R, Manno C, Villalba-Galea CA, del Valle Fernández L, Costa RR, Fill M, et al. Pulsed local-field fluorescence microscopy: a new approach for measuring cellular signals in the beating heart. Pflugers Arch. 2003;445:747–758. doi: 10.1007/s00424-002-0963-1. [DOI] [PubMed] [Google Scholar]
- 21.Varma N, Morgan JP, Apstein CS. Mechanisms underlying ischemic diastolic dysfunction: relation between rigor, calcium homeostasis, and relaxation rate. Am J Physiol. 2003;284:H758–H771. doi: 10.1152/ajpheart.00286.2002. [DOI] [PubMed] [Google Scholar]
- 22.Dou Y, Arlock P, Arner A. Blebbistatin specifically inhibits actin–myosin interaction in mouse cardiac muscle. Am J Physiol. 2007;293:C1148–C1153. doi: 10.1152/ajpcell.00551.2006. [DOI] [PubMed] [Google Scholar]
- 23.Rapundalo ST, Briggs FN, Feher JJ. Effects of ischemia on the isolation and function of canine cardiac sarcoplasmic reticulum. J Mol Cell Cardiol. 1986;18:837–851. doi: 10.1016/s0022-2828(86)80958-0. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Mann G, Meissner G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ Res. 1996;79:1100–1109. doi: 10.1161/01.res.79.6.1100. [DOI] [PubMed] [Google Scholar]
- 25.Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, et al. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II. Am J Physiol. 2008;295:H1669–H1683. doi: 10.1152/ajpheart.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valverde CA, Kornyeyev D, Escobar A, Mattiazzi A. Reperfusion causes cytosolic calcium overload due to rapid calcium release from the sarcoplasmic reticulum. Circulation. 2008;118:5407. (Abstract) [Google Scholar]
- 27.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, et al. Functional adult myocardium in the absence of Na+–Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res. 2004;95:604–611. doi: 10.1161/01.RES.0000142316.08250.68. [DOI] [PubMed] [Google Scholar]
- 28.Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol. 2000;529:171–188. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–675. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 30.Du C, MacGowan GA, Farkas DL, Koretsky AP. Calcium measurements in perfused mouse heart: quantitating fluorescence and absorbance of Rhod-2 by application of photon migration theory. Biophys J. 2001;80:549–561. doi: 10.1016/S0006-3495(01)76037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential serious proarrhythmia, but the action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.