Abstract
Use of cyclooxygenase (COX) inhibitors to delay preterm birth is complicated by in utero constriction of the ductus arteriosus and delayed postnatal closure. Delayed postnatal closure has been attributed to loss of vasa vasorum flow and ductus wall ischemia resulting from constriction in utero. We used the murine ductus (which does not depend on vasa vasorum flow) to determine whether delayed postnatal closure may be because of mechanisms independent of in utero constriction. Acute inhibition of both COX isoforms constricted the fetal ductus on days 18 and 19 (term) but not earlier in gestation; COX-2 inhibition constricted the fetal ductus more than COX-1 inhibition. In contrast, mice exposed to prolonged inhibition of COX-1, COX-2, or both COX isoforms (starting on day 15, when the ductus does not respond to the inhibitors) had no contractile response to the inhibitors on days 18 or 19. Newborn mice closed their ductus within 4 h of birth. Prolonged COX inhibition on days 11–14 of gestation had no effect on newborn ductal closure; however, prolonged COX inhibition on days 15–19 resulted in delayed ductus closure despite exposure to 80% oxygen after birth. Similarly, targeted deletion of COX-2 alone, or COX-1/COX-2 together, impaired postnatal ductus closure. Nitric oxide inhibition did not prevent the delay in ductus closure. These data show that impaired postnatal ductus closure is not the result of in utero ductus constriction or upregulation of nitric oxide synthesis. They are consistent with a novel role for prostaglandins in ductus arteriosus contractile development.
Keywords: patent ductus arteriosus, nonsteroidal anti-inflammatory drugs
closure of the ductus arteriosus plays a critical role in circulatory adaptation to newborn life. Delayed closure of the ductus arteriosus after birth leaves the infant at increased risk for pulmonary hemorrhage, sepsis, necrotizing enterocolitis, heart failure, and chronic lung disease (5, 7). Prostaglandins play an essential role in ductus arteriosus regulation (11). Nonsteroidal anti-inflammatory drugs (NSAIDs), like indomethacin, induce ductal constriction in infants with a patent ductus arteriosus (PDA) by inhibiting cyclooxygenase (COX)-1 and COX-2, the rate-limiting enzymes for prostaglandin synthesis.
Constriction of the ductus arteriosus can also occur in utero when NSAIDs are given to pregnant women to treat preterm labor (13, 20, 25, 30, 31, 37, 38). Surprisingly, some preterm newborn infants, who are delivered after in utero exposure to indomethacin, have an increased, rather than decreased, incidence of PDA in the newborn period; in addition, the PDA in these infants frequently fails to close with postnatal indomethacin treatment (12, 27, 34, 35). A similar finding occurs in fetal sheep that have been exposed to indomethacin for several days in utero (6, 9). The basis for this paradoxical response is not entirely clear. In the fetal sheep, indomethacin produces ductus constriction in utero, which is accompanied by cessation of ductus vasa vasorum blood flow and the development of hypoxic ischemia in the ductus muscle media. This is followed by increased production of vascular endothelial growth factor and nitric oxide and smooth muscle cell death. These in utero changes prevent the ductus from developing effective constriction after birth (6, 9). The degree of ischemia in the ductus wall is directly related to the degree of fetal ductus constriction. With advancing gestation, there is both an increase in indomethacin's ability to constrict the fetal ductus (20, 37, 38) and an increase in the thick-walled vessel's dependence on vasa vasorum flow for its oxygen delivery (14); these two observations may explain why human fetuses are more likely to have delayed postnatal ductus closure when their in utero exposure to indomethacin occurs late in gestation rather than earlier in gestation (27).
We hypothesized that prolonged in utero COX inhibition might also alter ductus contractility through mechanisms that are independent of initial ductus constriction, vasa vasorum flow, or the resultant hypoxic ischemia. In contrast to humans and sheep, the rodent ductus arteriosus has no vasa vasorum in its muscle media; the thickness of the rodent ductus is so thin that luminal blood flow is sufficient to meet all of its oxygen needs (29, 36, 40). The absence of compressible vasa vasorum in the ductus muscle media makes it less susceptible to developing hypoxia in utero during episodes of ductus constriction. In addition, although indomethacin constricts the rodent ductus arteriosus, it does so only late in gestation (23, 29). As a result, the rodent ductus is unlikely to develop hypoxic ischemia when exposed to indomethacin early in gestation. In this study, we used fetal and newborn mice after acute and chronic exposure to selective COX-1 and COX-2 inhibitors to study the nonischemic effects of prostaglandin inhibition on ductus contractility. The ductus arteriosus of mice with targeted deletions of genes encoding COX-1, COX-2, or both COX isoforms was also examined.
MATERIALS AND METHODS
Animals and tissues
Animals were housed in an American Association for the Accreditation of Laboratory Animal Care-approved facility and managed in accordance with National Institutes of Health animal care standards. All protocols were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Wild-type female CD-1 mice (7–8 wk old; Charles River, Raleigh, NC) were bred with fertile males to produce timed pregnancies (where day 1 = presence of vaginal plug). COX-1 null (Taconic, Hudson, NY) and COX-2 null (Jackson Laboratory, Bar Harbor, ME) mice were outbred on the CD-1 background to enhance reproductive vigor (28) and facilitate comparison with wild-type CD-1 results. COX-1 and COX-2 mice on this genetic background were interbred to generate COX-1(–/–)COX-2(–/+) compound heterozygote mating pairs. Cross-breeding of COX-1(–/–)COX-2(–/+) mice was performed to generate COX-1/COX-2 double null offspring (28).
Pregnant females were anesthetized with avertin (2,2,2 tribromoethanol in tert-amyl alcohol; 0.4–0.6 mg/g body wt ip) followed by isoflurane inhalation (Baxter, Deerfield, IL) to achieve deep anesthesia and allow time for fetal anesthetic effects. Caesarian section was performed to deliver fetal pups at various gestational stages. After removal from the uterus, a transverse incision was made through the abdominal wall of anesthetized nonbreathing fetuses, and the diaphragm was opened. Skin overlying the chest wall was removed to enhance tissue fixation. Fetal tissues were preserved by immersion in chilled 4% paraformaldehyde for 24 h followed by 70% ethanol. Newborn pups were delivered by caesarean section on the morning of day 19 of pregnancy (mice in this colony typically deliver on the evening of day 19). Pups were dried and stimulated until respirations were established and then placed in prewarmed microisolator cages at 37°C for 4 h. To produce hyperoxia and accelerate ductus closure, pups were placed in FiO2 = 0.8–1.0. At 4 h of age, newborn pups were killed by isoflurane overdose (~30 s exposure), and the abdomen and diaphragm were surgically exposed. Newborn tissues were preserved in 4% paraformaldehyde followed by 70% ethanol. The thorax of preserved fetal and newborn pups was excised from surrounding tissues and embedded in paraffin. The thorax was sectioned in a plane parallel to the diaphragm. Ribbons of serial 6-μm sections containing heart and outflow tracts were mounted on poly-l-lysine-coated slides and stained with hematoxylin.
Drug administration
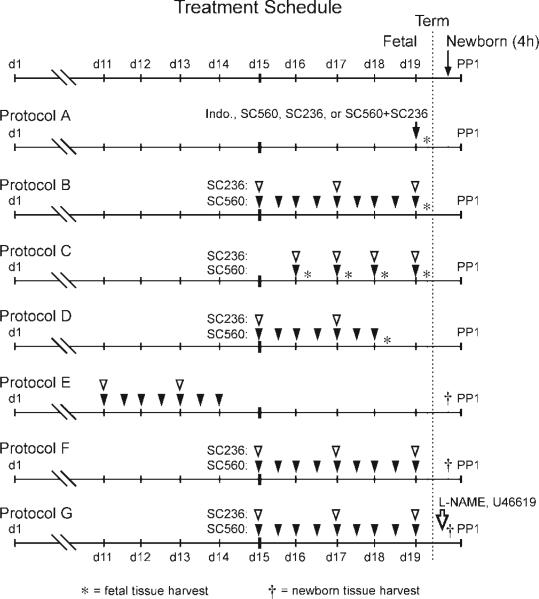
COX inhibitors were administered at different time points during mid- and late gestation according to treatment protocol (Fig. 1). All drugs have been previously shown to cross the placenta (17). Selective COX inhibitors were reconstituted in a 0.1% methylcellulose solution and continuously stirred before administration. Caesarian deliveries were performed at noon, 4 h after the final dose of any drug was administered. Protocols A-D addressed the fetal ductus arteriosus, whereas protocols E-G addressed the newborn ductus arteriosus. In protocol A, pregnant wild-type females received either a single dose of indomethacin (5.0 mg·kg–1·dose–1 ip; Merck, West Point, PA), a selective inhibitor of COX-1 (SC560, 30 mg·kg–1·dose–1, 0.2 ml gavage), or a selective COX-2 inhibitor (SC236, 15 mg·kg–1·dose–1; 0.2 ml gavage; Cayman Chemical, Ann Arbor, MI) at 0800 on day 19 of gestation. Fetal tissues were harvested 4 after the last drug dosage. In protocol B, pregnant females were chronically treated with either SC560 (30 mg·kg–1·dose–1 two times daily; 0.2 ml gavage), SC236 (15 mg·kg–1·dose–1 every other day; 0.2 ml gavage), or both compounds from day 15–19 of gestation (term = day 19). Fetal tissues were harvested 4 h after the last drug dosage. In some experiments, COX-1 null females were treated with SC236 on days 15–19 of gestation according to the schedule in protocol B. Because of signs of dehydration and lethargy in COX-1 null females treated with the COX-2 inhibitor, omeprazole (100 mg·kg–1·dose–1; Calbiochem, San Diego, CA) was given by gavage one time daily. In protocol C, pregnant wild-type females were treated with a single dose of both SC560 and SC236 at 0800 on either day 16, 17, 18, or 19 of gestation; fetal tissues were harvested 4 h after the drug dosage on each respective day. In protocol D, pregnant wild-type females were chronically treated with both SC560 and SC236 on days 15–18 of gestation. Fetal tissues were harvested 4 h after the final drug dosage on day 18. In protocol E, pregnant wild-type females were chronically treated with both SC560 and SC236 on days 11–14 of gestation. Pregnancy was allowed to continue until caesarian section at term gestation. Tissues were harvested from newborn pups after 4 h of oxygen exposure. In protocol F, pregnant females were chronically treated with either SC560, SC236, or both compounds from day 15–19 of gestation (similar to protocol B). Newborn tissues were harvested after 4 h of postnatal oxygen exposure. In protocol G, pregnant females were chronically treated with both SC560 and SC236 from day 15–19 of gestation (same drug dosage as protocol B). Newborn litters were divided into thirds, and pups were treated with one of the following: the nonselective nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 100 mg·kg–1·dose–1; Cayman Chemical), the thromboxane receptor agonist U-46619 (0.5 μg/mouse; Cayman Chemical) given at birth and 1.5 and 3 h of age, or 10 μl sterile saline. Compounds were injected through the nuchal fold to form a subcutaneous pocket along the back under direct visualization by stereomicroscopy. Pups with leaking injection sites were excluded from analysis.
Fig. 1.
Drug treatment protocols. Pregnant dams were treated with a nonselective cyclooxygenase (COX) inhibitor [indomethacin (Indo)], a selective COX-1 inhibitor (SC560), and/or a selective COX-2 inhibitor (SC236) on the indicated days of gestation [day (d) 1 = presence of vaginal plug]. Fetal studies: protocol A examined the effects of a single dose of a COX inhibitor (either indomethacin, SC236, SC560, or the combination of SC236 with SC560) on the fetal ductus (tissue collected 4 h after treatment); protocol B examined the effects of prolonged COX-1, COX-2, or combined COX-1 and COX-2 inhibition on the fetal ductus (study drugs were administered at the indicated times, and the tissues were harvested 4 h after the last dose); protocol C examined the effects of a single treatment with both COX-1 and COX-2 inhibitors on the fetal ductus. Pregnant dams were treated at either gestation day 16, 17, 18, or 19, and the fetal tissues were harvested 4 h later; protocol D examined the effects of prolonged COX-1 and COX-2 inhibition on the fetal ductus at day 18 of gestation. Pregnant dams were treated on days 15–18 of gestation, and the fetal tissues were harvested 4 h after the last dose on day 18. Newborn studies: protocol E examined the effects of prolonged COX-1 and COX-2 inhibition (on days 11–14 of gestation) on the newborn ductus. Newborn tissues were harvested 4 h after delivery; protocol F examined the effects of prolonged in utero exposure to either COX-1, COX-2, or combined COX-1 and COX-2 inhibitors (on days 15–19 of gestation) on the newborn ductus. Newborn tissues were harvested 4 h after delivery; protocol G examined whether treatment of newborn littermates with NG-nitro-l-arginine methyl ester (l-NAME) or U-46619 (or saline) altered patency of the ductus arteriosus (after prolonged in utero exposure to both COX inhibitors on days 15–19 of gestation). PP1, postpartum day 1.
Determination of vessel caliber
Serial sections through the fetal and newborn thorax allowed quantitative assessment of ductus arteriosus diameter in relation to the size of the transverse aorta (Fig. 2). The inner diameters of the ductus arteriosus and aorta were measured with a microscope (Nikon, Tokyo, Japan) fitted with a micrometer. The size of the ductus arteriosus lumen was determined at its narrowest point. The narrowest diameter of the transverse aortic arch was measured on subsequent serial sections. Tissue sections were examined by an observer who was blind to treatment group (Clyman), and vessel caliber was recorded. Size of the ductus arteriosus was expressed as a percentage of the diameter of the transverse aortic arch (ductus arteriosus-to-aorta ratio). Comparison between treatment groups was made by Student's t-test. Results are expressed as means ± SD.
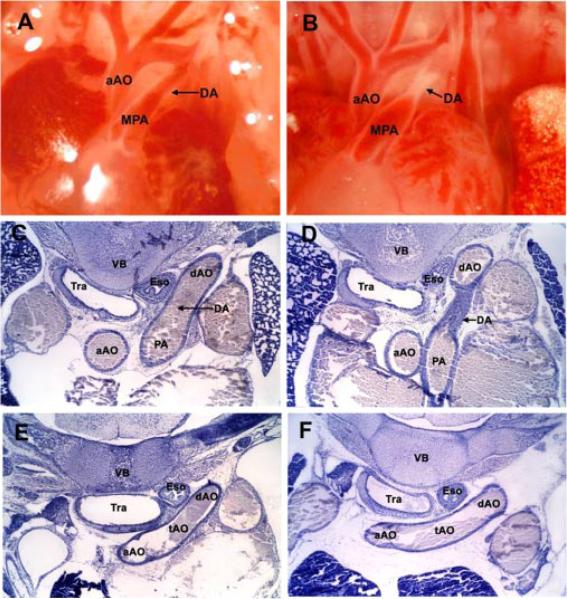
Fig. 2.
Physiological closure of the mouse ductus arteriosus. Anatomical views of the open fetal ductus arteriosus on the morning of day 19 of gestation (A) and constricted newborn ductus arteriosus at 4 h of age (B). Serial thoracic sections demonstrate histologic features and dimensions of the open fetal ductus (C) and adjacent transverse aortic arch (E) compared with the closed newborn ductus (D) and corresponding transverse aortic arch (F). aAO, ascending aorta; tAO, transverse aorta; dAO, descending aorta; PA, pulmonary artery; DA, ductus arteriosus; Tra, trachea; Eso, esophagus, VB, vertebral body.
RESULTS
Response of the fetal ductus arteriosus to acute COX-1 and COX-2 inhibition
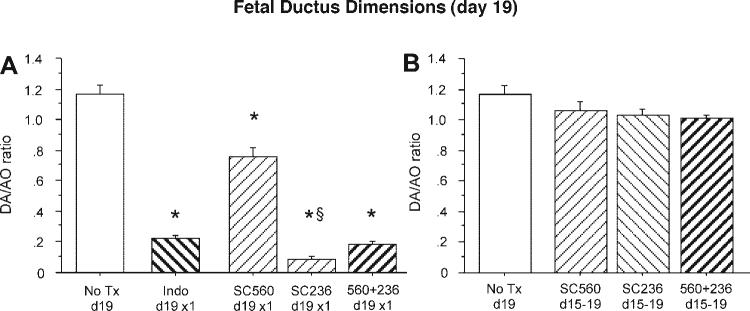
At full term (19 days gestation), a single acute dose of the nonselective COX inhibitor indomethacin (protocol A) produced marked constriction of the fetal ductus arteriosus 4 h after it was injected in the pregnant dam (Fig. 3A). Selective inhibition of either COX-1 or COX-2 appeared to produce fetal ductus constriction; however, the constriction after inhibition of COX-2 (SC236) was significantly greater than that after inhibition of COX-1 (SC560; Fig. 3A). The reduction in ductus lumen size after acute, combined treatment with both inhibitors (SC560 + SC236) was similar to the reduction found after indomethacin exposure (Fig. 3A).
Fig. 3.
Response of the fetal ductus arteriosus to acute and chronic COX-1 or COX-2 inhibition. Fetuses of wild-type dams treated with indomethacin, a selective COX-1 inhibitor (SC560), or selective COX-2 inhibitor (SC236) were compared. Vessel dimensions are expressed as a ratio of ductus arteriosus (DA) and transverse aorta (AO) diameters. A: acute pharmacological inhibition with indomethacin (n = 6, 2 litters) or combined COX inhibitors (n = 19, 7 litters) showed similar ductus constriction at term gestation compared with untreated (No Tx) controls (n = 11, 6 litters). Acute COX-1 inhibition (n = 9, 3 litters) caused less ductus constriction than COX-2 (n = 11, 4 litters). B: chronic COX-1 (n = 9, 3 litters), COX-2 (n = 35, 12 litters), or combined COX inhibition (n = 20, 6 litters) did not constrict the fetal ductus. P < 0.05 compared with control (*) and compared with SC560 (§).
The constrictive effects of acute inhibition of COX-1 and COX-2 were observed only in late gestation fetuses. There was no reduction in fetal ductus caliber when dams were treated earlier in gestation (days 16 and 17; protocol C; Fig. 4A).
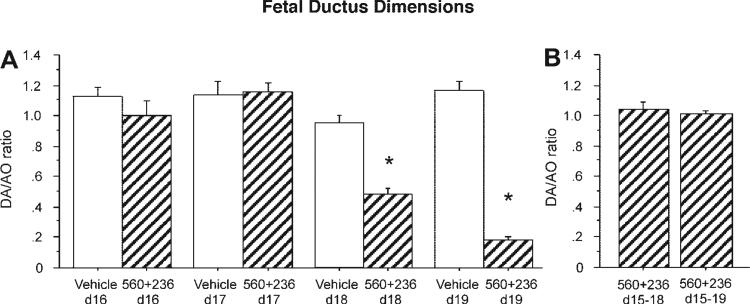
Fig. 4.
Developmental stage-specific response of the fetal ductus arteriosus to acute and chronic COX inhibition. Fetuses of wild-type dams treated with combined COX-1 (SC560) and COX-2 (SC236) inhibitors were compared. Vessel dimensions are expressed as a ratio of DA and AO diameters. A: acute inhibition of both COX isoforms did not alter the day 16 (n = 9, 3 litters) or day 17 (n = 9, 3 litters) gestation ductus but induced constriction on day 18 (n = 13, 4 litters) and day 19 (n = 19, 7 litters). B: in contrast to the acute responses, chronic exposure to both COX inhibitors, starting on day 15, was not associated with ductus constriction on either day 18 (n = 12, 3 litters) or day 19 (n = 20, 6 litters). *P < 0.05 compared with vehicle-treated dams at each gestation.
Chronic COX inhibition does not constrict the fetal ductus arteriosus
We wanted to examine the effects of chronic COX inhibition on subsequent fetal ductus contractility. Because we wanted to examine the effects that were independent of the initial acute constriction, we exposed fetal mice to prolonged COX inhibition and started the treatment at a point in gestation when the inhibitors had no acute contractile effect on the fetal ductus (day 15, see Fig. 4A). Chronic exposure to a combination of COX-1 and COX-2 inhibitors significantly altered fetal ductus contractility in vivo. In contrast to the marked ductus constriction that occurs when dams are treated acutely on days 18 or 19 of gestation (Fig. 4A), the fetuses of dams that received chronic treatment (from days 15–18 or 15–19 of gestation; protocols B and D) had ductus that remained patent and were unresponsive to treatment on days 18 and 19 (Fig. 4B). Prolonged administration of either COX inhibitor alone (from days 15 to 19) also was associated with failure of the fetal ductus to constrict after treatment with the inhibitor on day 19 (Fig. 3B).
Failure of newborn ductus arteriosus closure after exposure to chronic COX inhibition in utero
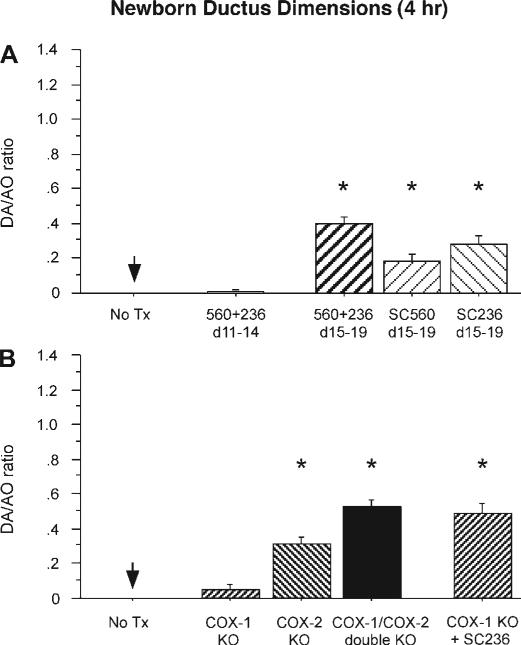
We examined the effects of chronic in utero COX inhibition on the ability of the newborn ductus to constrict after birth. Newborns were delivered by caesarean section and exposed to 80% oxygen for 4 h. Control pups, born to dams that did not receive COX inhibitors during pregnancy, closed their ductus completely by 4 h after delivery (Fig. 5A, compare with fetal dimensions in Fig. 2B). Newborn pups, born to dams that received both COX-1 and COX-2 inhibitors in midpregnancy, on days 11–14 of gestation (protocol E) also had complete ductus closure by 4 h after delivery. In contrast, newborn pups exposed to both COX inhibitors on days 15–19 of gestation (protocol F) had incomplete constriction of their ductus (Fig. 5A). There was no significant difference between the treated (SC560 + SC236) and control groups in mixed arterial-venous blood Po2 samples obtained by cervical transection after 4 h of postnatal oxygen exposure (Po2: 101.5 ± 28, n = 5 vs. 89.1 ± 14, n = 8, respectively). Prolonged exposure to either COX-1 or COX-2 inhibitor alone also resulted in incomplete closure of the newborn ductus (Fig. 5A).
Fig. 5.
Ductus contractility in the newborn mouse after genetic and/or prolonged pharmacological COX inhibition in utero. A: newborn pups born to dams with chronic COX-1 (SC560), COX-2 (SC236), or combined COX inhibitors were placed in 80% oxygen for 4 h after birth. Vessel dimensions are expressed as a ratio of DA and AO diameters. Chronic COX inhibition on days 11–14 of gestation (n = 20, 7 litters) did not alter ductus closure after birth, whereas pups with COX-1 (n = 12, 4 litters), COX-2 (n = 23, 13 litters), or combined COX inhibition (n = 29, 11 litters) on days 15–19 of gestation had delayed closure of the ductus compared with controls (n = 12, 4 litters). B: targeted deletion of COX-1 (n = 11, 4 litters) had minimal effect on ductus closure after birth, whereas COX-2 deletion (n = 20, 8 litters) and COX-1/COX-2 double-null pups (n = 12, 6 litters) had a significant delay in ductal closure compared with controls (n = 12, 4 litters). Closure of the ductus of COX-1 null pups chronically exposed to COX-2 inhibitor in utero (n = 16, 6 litters) was also significantly delayed. KO, knockout. *P < 0.05 compared with untreated newborns at 4 h of age.
Patency of the ductus arteriosus in newborn mice with targeted deletion of COX isoforms
We also examined the effects of COX inhibition on postnatal ductus closure by using targeted deletion of the COX-1 and COX-2 isoforms. Deletion of the COX isoforms resulted in incomplete closure of the newborn ductus (Fig. 5B). Targeted deletion of the individual COX isoforms produced a pattern of closure that was similar to that found after treatment of pregnant dams with selective COX inhibitors (between days 15 and 19 of gestation; Fig. 5B). Newborn mice with combined COX-1 and COX-2 deletion showed the highest degree of ductus patency, and COX-2 null mice had a much greater degree of ductus patency than COX-1 null mice (Fig. 5B). In addition, COX-1 null fetuses exposed to the selective COX-2 inhibitor SC236 on days 15–19 of gestation had the same degree of postnatal ductus patency as the COX double-null mice. These data demonstrate that combined inhibition or deletion of COX-1 and COX-2 has a greater effect than inhibition or deletion of a single COX isoform alone.
Impaired response of newborn PDAs to vasoconstrictive stimuli
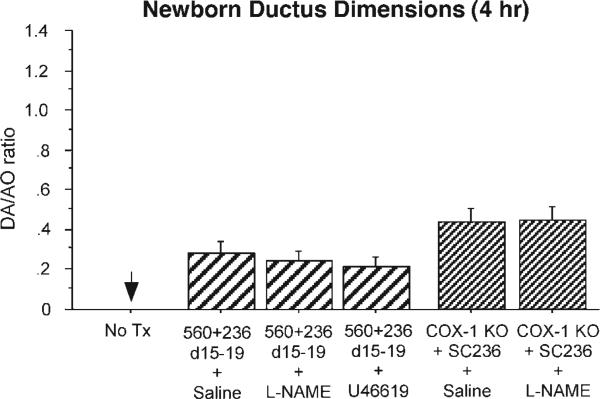
We examined whether the persistent postnatal PDA that follows chronic in utero COX inhibition might be due, in part, to increased nitric oxide production (3). Fetuses that were exposed to both COX-1 plus COX-2 inhibitors in utero (on days 15–19 of gestation) were delivered by caesarean section and either treated with saline or the nonspecific NOS inhibitor l-NAME (protocol G). The l-NAME-treated newborns had the same degree of ductus patency as the saline-treated controls (Fig. 6). The effect of NOS inhibition was also examined in COX-1 null mice that were chronically treated with COX-2 inhibitors on days 15–19 of gestation. Newborns from these dams showed no difference in ductus caliber between saline-treated pups and pups that were treated with l-NAME (Fig. 6).
Fig. 6.
Responsiveness of the newborn patent ductus arteriosus. Newborn pups born to dams with chronic COX-1 (SC560) plus COX-2 (SC236) inhibition on days 15–19 of gestation were placed in 80% oxygen for 4 h after birth. Vessel dimensions are expressed as a ratio of DA and AO diameters. Ductus closure rates of pups treated with saline (n = 24, 12 litters), l-NAME (n = 21, 6 litters), or U-46619 (n = 24, 7 litters) were significantly delayed compared with untreated controls (n = 12, 4 litters; P < 0.05) but did not differ between the treatment groups. Similarly, there was no difference in ductus caliber between saline-treated (n = 15, 5 litters) and l-NAME-treated (n = 14, 4 litters) COX-1 null pups chronically exposed to COX-2 inhibitor in utero.
To examine the effects of chronic in utero COX inhibition on the ability of the newborn ductus to respond to other vasoconstrictive stimuli, newborn pups (that had been pre-treated with COX-1 + COX-2 inhibitors in utero on days 15–19 of gestation) were treated with either saline or the thromboxane mimetic U-46619 immediately after delivery (protocol G). U-46619 had no significant effect on the degree of ductus patency after chronic in utero COX inhibition (Fig. 6).
DISCUSSION
We found that inhibition of prostaglandin production has different effects on ductus patency depending on developmental stage and duration of treatment. Acute inhibition of either COX isoform led to fetal ductus constriction, with the inhibition of COX-2 having greater effects than those of COX-1. The constrictive effects of acute COX inhibition were not apparent until day 18 of gestation (last 10% of gestation). Our findings are consistent with previous studies in mice (29), rats (22), and humans (20, 37, 38).
In contrast, prolonged COX inhibition during the last 25% of gestation did not induce fetal ductus constriction; rather, it led to impaired contractile responses in the near-term fetus and a persistent PDA in the newborn mouse. Combined COX gene deletion had similar effects in delaying ductus closure as prolonged pharmacological COX inhibition. This finding is similar to previous reports (17). In contrast to the ovine ductus (6, 9), the inhibitory effects of prolonged COX inhibition in the full-term mouse did not appear to be because of prior vasa vasorum hypoperfusion secondary to in utero ductus constriction. First, the muscle media of the fetal mouse ductus has no vasa vasorum (29, 36, 40), and, second, no change in ductus diameter was observed throughout gestation (Fig. 4) since the prolonged COX inhibition was started at a time when COX inhibition does not contract the fetal ductus (day 15, see Fig. 4). One possible explanation for the delayed closure after prolonged COX inhibition is that prolonged COX inhibition results in the upregulation of other vasodilators. Functional coupling of COX and NOS systems is a well-recognized phenomenon (10, 18, 21). In the mouse ductus arteriosus, endothelial NOS is the predominant isoform for nitric oxide production, and its expression is stable in mid- to late gestation (29). Baragatti et al. (3) recently hypothesized that absence of COX-derived prostaglandins in utero may lead to an increase in nitric oxide production in the ductus; they found that the NOS inhibitor l-NAME produced a stronger contractile response in isolated ductus from COX-1 null or COX-2 null fetal mice than from wild-type mice. Although functional coupling of COX and NOS systems may occur locally within the ductus, it does not appear to explain the decreased contractility of the postnatal ductus after prolonged COX inhibition in vivo; we observed no increase in postnatal ductus constriction when fetuses that were chronically exposed to COX inhibition in utero were treated with l-NAME after birth (Fig. 6).
Our studies suggest that prostaglandins may have a unique and previously unappreciated role in the development of pathways that control ductus contractility to any vasoactive stimulus. For example, after chronic in utero COX inhibition, the thromboxane mimetic U-46619 has no effect on the degree of ductus patency even when used at doses that previously have been shown to constrict the neonatal ductus (Fig. 6 and Refs. 17 and 33). Muscular development of the ductus is marked by precocious differentiation of specific myosin isoforms (15) and cytoskeletal proteins (4, 8, 32) that appear to play a role in its postnatal closure and remodeling. Interruption of smooth muscle differentiation leads to delayed postnatal ductus closure (24). Prostaglandins play an important role in vascular smooth muscle migration and differentiation (1, 16, 19). We hypothesize that prolonged COX inhibition on days 15–19 of gestation alters the development of the contractile apparatus that is essential for rapid constriction after birth. Our results also suggest that days 15–19 of gestation span the critical period of contractile development since ductus contractility was unaffected when mice were exposed to combined COX inhibition at an earlier time in gestation (days 11–15 of gestation; Fig. 5).
Although both COX-1 and COX-2 affect the development of ductus contractility, they do so to different degrees. Our results are similar to the observations of Loftin et al. (17) where chronic COX-2 inhibition on days 16–19 of gestation resulted in a postnatal PDA, whereas chronic COX-1 inhibition over the same period did not. We observed a small but significant PDA after chronic COX-1 inhibition on days 15–19 of pregnancy (Fig. 5). This discrepancy may be explained by a longer duration of COX-1 inhibition in our studies, strain differences in susceptibility to COX inhibition, or different environmental conditions that alter COX functions (2, 39). Nevertheless, our data clearly show that prolonged inhibition of either COX-1 or COX-2 alone impairs ductal constriction after birth and is not the result of in utero constriction of the fetal ductus. Mice with targeted deletion of the PGE2 receptor EP4 have a similar PDA phenotype (26), suggesting that a specific prostaglandin receptor pathway may regulate the development of ductus arteriosus contractility.
In summary, we suggest that prostaglandins may play a novel role in ductus arteriosus development that is distinct from their function as a mediator of vascular tone. A better understanding of this process will be important for the development of new strategies to treat preterm labor without affecting fetal vascular development.
GRANTS
This work was supported by National Institutes of Health Grants HL-77395 (to J. Reese), HL-46691, and HL-56061 (to R. I. Clyman), American Heart Association Grant AHA455360 (to J. Reese), and by gifts from the Philip S. Astrowe Trust (to J. Reese) and the B and J Gates Foundation (to R. I. Clyman).
REFERENCES
- 1.Abe M, Hasegawa K, Wada H, Morimoto T, Yanazume T, Kawamura T, Hirai M, Furukawa Y, Kita T. GATA-6 is involved in PPAR-gamma-mediated activation of differentiated phenotype in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:404–410. doi: 10.1161/01.ATV.0000059405.51042.A0. [DOI] [PubMed] [Google Scholar]
- 2.Badawi AF, El-Sohemy A, Stephen LL, Ghoshal AK, Archer MC. The effect of dietary n-3 and n-6 polyunsaturated fatty acids on the expression of cyclooxygenase 1 and 2 and levels of p21ras in rat mammary glands. Carcinogenesis. 1998;19:905–910. doi: 10.1093/carcin/19.5.905. [DOI] [PubMed] [Google Scholar]
- 3.Baragatti B, Brizzi F, Ackerley C, Barogi S, Ballou LR, Coceani F. Cyclooxygenase-1 and cyclooxygenase-2 in the mouse ductus arteriosus: individual activity and functional coupling with nitric oxide synthase. Br J Pharmacol. 2003;139:1505–1515. doi: 10.1038/sj.bjp.0705391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergwerff M, DeRuiter MC, Poelmann RE, Gittenbergerde Groot AC. Onset of elastogenesis and downregulation of smooth muscle actin as distinguishing phenomena in artery differentiation in the chick embryo. Anat Embryol (Berl) 1996;194:545–557. doi: 10.1007/BF00187468. [DOI] [PubMed] [Google Scholar]
- 5.Brook MM, Heymann MA. Patent ductus arteriosus. In: Emmanouilides GC, Riemenscneider TA, Allen HD, Gutgesell HP, editors. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult. 5th ed. Williams & Wilkins; Baltimore, MD: 1995. pp. 746–764. [Google Scholar]
- 6.Clyman RI, Chen YQ, Chemtob S, Mauray F, Kohl T, Varma DR, Roman C. In utero remodeling of the fetal lamb ductus arteriosus: the role of antenatal indomethacin and avascular zone thickness on vasa vasorum proliferation, neointima formation, and cell death. Circulation. 2001;103:1806–1812. doi: 10.1161/01.cir.103.13.1806. [DOI] [PubMed] [Google Scholar]
- 7.Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriosus. J Pediatr. 1978;92:467–473. doi: 10.1016/s0022-3476(78)80451-x. [DOI] [PubMed] [Google Scholar]
- 8.de Reeder EG, Poelmann RE, van Munsteren JC, Patterson DF, Gittenberger-de Groot AC. Ultrastructural and immunohistochemical changes of the extracellular matrix during intimal cushion formation in the ductus arteriosus of the dog. Atherosclerosis. 1989;79:29–40. doi: 10.1016/0021-9150(89)90030-0. [DOI] [PubMed] [Google Scholar]
- 9.Goldbarg SH, Takahashi Y, Cruz C, Kajino H, Roman C, Liu BM, Chen YQ, Mauray F, Clyman RI. In utero indomethacin alters O2 delivery to the fetal ductus arteriosus: implications for postnatal patency. Am J Physiol Regul Integr Comp Physiol. 2002;282:R184–R190. doi: 10.1152/ajpregu.2002.282.1.R184. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. FASEB J. 1999;13:1121–1136. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- 11.Hammerman C. Patent ductus arteriosus. Clinical relevance of prostaglandins and prostaglandin inhibitors in PDA pathophysiology and treatment. Clin Perinatol. 1995;22:457–479. [PubMed] [Google Scholar]
- 12.Hammerman C, Glaser J, Kaplan M, Schimmel MS, Ferber B, Eidelman AI. Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics. 1998;102:E56. doi: 10.1542/peds.102.5.e56. (Abstract) [DOI] [PubMed] [Google Scholar]
- 13.Huhta JC, Moise KJ, Fisher DJ, Sharif DS, Wasserstrum N, Martin C. Detection and quantitation of constriction of the fetal ductus arteriosus by Doppler echocardiography. Circulation. 1987;75:406–412. doi: 10.1161/01.cir.75.2.406. [DOI] [PubMed] [Google Scholar]
- 14.Kajino H, Goldbarg S, Roman C, Liu BM, Mauray F, Chen YQ, Takahashi Y, Koch CJ, Clyman RI. Vasa vasorum hypoperfusion is responsible for medial hypoxia and anatomic remodeling in the newborn lamb ductus arteriosus. Pediatr Res. 2002;51:228–235. doi: 10.1203/00006450-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Aikawa M, Kimura K, Kuro-o M, Nakahara K, Suzuki T, Katoh H, Okamoto E, Yazaki Y, Nagai R. Ductus arteriosus. dvanced differentiation of smooth muscle cells demonstrated by myosin heavy chain isoform expression in rabbits. Circulation. 1993;88:1804–1810. doi: 10.1161/01.cir.88.4.1804. [DOI] [PubMed] [Google Scholar]
- 16.Koppel R, Rabinovitch M. Regulation of fetal lamb ductus arteriosus smooth muscle cell migration by indomethacin and dexamethasone. Pediatr Res. 1993;33:352–358. doi: 10.1203/00006450-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Loftin CD, Trivedi DB, Langenbach R. Cyclooxygenase-1-selective inhibition prolongs gestation in mice without adverse effects on the ductus arteriosus. J Clin Invest. 2002;110:549–557. doi: 10.1172/JCI14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minghetti L, Nicolini A, Polazzi E, Creminon C, Maclouf J, Levi G. Inducible nitric oxide synthase expression in activated rat microglial cultures is downregulated by exogenous prostaglandin E2 and by cyclooxygenase inhibitors. Glia. 1997;19:152–160. [PubMed] [Google Scholar]
- 19.Miwa Y, Takahashi-Yanaga F, Morimoto S, Sasaguri T. Involvement of clusterin in 15-deoxy-delta12,14-prostaglandin J2-induced vascular smooth muscle cell differentiation. Biochem Biophys Res Commun. 2004;319:163–168. doi: 10.1016/j.bbrc.2004.04.163. [DOI] [PubMed] [Google Scholar]
- 20.Moise KJ., Jr Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol. 1993;168:1350–1353. doi: 10.1016/s0002-9378(11)90763-7. [DOI] [PubMed] [Google Scholar]
- 21.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 22.Momma K, Takao A. In vivo constriction of the ductus arteriosus by nonsteroidal antiinflammatory drugs in near-term and preterm fetal rats. Pediatr Res. 1987;22:567–572. doi: 10.1203/00006450-198711000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Momma K, Toyono M. The role of nitric oxide in dilating the fetal ductus arteriosus in rats. Pediatr Res. 1999;46:311–315. doi: 10.1203/00006450-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, Kott M, Haase H, Bader M. Smooth-muscle contraction without smooth-muscle myosin. Nat Cell Biol. 2000;2:371–375. doi: 10.1038/35014065. [DOI] [PubMed] [Google Scholar]
- 25.Mushiake K, Motoyoshi F, Kinoshita Y, Nakagawa A, Ito M. Severe heart failure due to ductal constriction caused by maternal indomethacin. Pediatr Int. 2002;44:174–176. doi: 10.1046/j.1328-8067.2001.01512.x. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PA, Malouf NN, Koller BH. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 27.Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 28.Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci USA. 2000;97:9759–9764. doi: 10.1073/pnas.97.17.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard C, Gao J, LaFleur B, Christman BW, Anderson J, Brown N, Reese J. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol Regul Integr Comp Physiol. 2004;287:R652–R660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph AM, Heymann MA. Effects of prostaglandins and inhibitors of prostaglandin synthesis in the fetus and newborn infant. J Perinat Med. 1981;9(Suppl 1):91–92. doi: 10.1515/jpme.1981.9.s1.91. [DOI] [PubMed] [Google Scholar]
- 31.Schoenfeld A, Bar Y, Merlob P, Ovadia Y. NSAIDs: maternal and fetal considerations. Am J Reprod Immunol. 1992;28:141–147. doi: 10.1111/j.1600-0897.1992.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 32.Slomp J, Gittenberger-de Groot AC, Glukhova MA, Conny van Munsteren J, Kockx MM, Schwartz SM, Koteliansky VE. Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler Thromb Vasc Biol. 1997;17:1003–1009. doi: 10.1161/01.atv.17.5.1003. [DOI] [PubMed] [Google Scholar]
- 33.Smith GC, McGrath JC. Contractile effects of prostanoids on fetal rabbit ductus arteriosus. J Cardiovasc Pharmacol. 1995;25:113–118. doi: 10.1097/00005344-199501000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Souter D, Harding J, McCowan L, O'Donnell C, McLeay E, Baxendale H. Antenatal indomethacin–adverse fetal effects confirmed. Aust N Z J Obstet Gynaecol. 1998;38:11–16. doi: 10.1111/j.1479-828x.1998.tb02949.x. [DOI] [PubMed] [Google Scholar]
- 35.Suarez VR, Thompson LL, Jain V, Olson GL, Hankins GD, Belfort MA, Saade GR. The effect of in utero exposure to indomethacin on the need for surgical closure of a patent ductus arteriosus in the neonate. Am J Obstet Gynecol. 2002;187:886–888. doi: 10.1067/mob.2002.127464. [DOI] [PubMed] [Google Scholar]
- 36.Tada T, Kishimoto H. Ultrastructural and histological studies on closure of the mouse ductus arteriosus. Acta Anat (Basel) 1990;139:326–334. doi: 10.1159/000147020. [DOI] [PubMed] [Google Scholar]
- 37.Van den Veyver IB, Moise KJ, Jr, Ou CN, Carpenter RJ., Jr The effect of gestational age and fetal indomethacin levels on the incidence of constriction of the fetal ductus arteriosus. Obstet Gynecol. 1993;82:500–503. [PubMed] [Google Scholar]
- 38.Vermillion ST, Scardo JA, Lashus AG, Wiles HB. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol. 1997;177:256–261. doi: 10.1016/s0002-9378(97)70184-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA. 2005;102:9960–9965. doi: 10.1073/pnas.0501632102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967;20:409–421. doi: 10.1161/01.res.20.4.409. [DOI] [PubMed] [Google Scholar]