Abstract
In the last decade a tremendous amount has been learned about the biology and treatment of gastrointestinal stromal tumor (GIST). Imatinib mesylate has revolutionized the treatment of metastatic GIST. In addition, the role of imatinib in localized GIST has gained much interest and may improve patient outcomes. Additionally, research efforts aimed at understanding the biology and the molecular heterogeneity of GIST both at initial presentation and at the time of resistance to imatinib, has helped guide rational approaches to treatment as well as future efforts aimed at treating imatinib-resistant GIST.
Keywords: gastrointestinal stromal tumors, GIST, review, imatinib, tyrosine kinase inhibitor, TKI
Diagnosis
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumor of the gastrointestinal tract.1 As a distinct disease entity, it is estimated that GIST has an annual incidence of around 14.5 per million individuals worldwide.2 The median age of onset is ∼60 years old with a small though biologically distinct sub-population in the pediatric age group.3,4
Prior to the late 1990s, GIST was a disease poorly understood, whose pathogenesis, natural history and even the cell of origin were unclear. In addition, GISTs were frequently diagnosed as other entities, which included leiomyosarcoma, leiomyoblastoma, bizarre leiomyoma, plexosarcoma and gastrointestinal autonomic nerve tumor (GANT) amongst other names.5,6 It was not until the seminal discovery by Hirota and colleagues in 1998 that the first clear insights to this disease were gained. In this landmark publication, the group reported the finding of activating KIT mutations in a significant proportion of GISTs, with constitutive ligand-independent activation of the KIT-receptor tyrosine kinase (RTK), and a near universal expression of KIT on immunohistochemistry.7 Corroborated by Kindblom and others, it was demonstrated that GIST cells were closely related to the interstitial cells of Cajal.8 This understanding provided the platform for accurate and uniform diagnoses of this uncommon tumor and the rational development and use of tyrosine kinase inhibitors (TKI) in the management of GIST.
Prognostic factors
As it became clearer investigators could reliably identify GIST, research efforts were focused on the determination of histological and clinical prognostic factors for localized GIST. Tumors showing the usual histological criteria for malignancy did not uniformly behave aggressively. Alternatively, some tumors with typical “benign” features gave rise to metastases. Size of tumor and mitotic count gained the greatest acceptance of being predictive of outcome. Using these two indices, Fletcher and colleagues were able to stratify patients with primary GISTs into four risk groups predicting for aggressive behavior.9 More recently, work from the Armed Forces Institute of Pathology detailing their experience with a large population of GIST patients identified anatomic location being an important predictor of relapse. In this model, which is the current accepted risk model for localized GIST, the primary disease site together with tumor size and mitotic count provide a model for the risk of future recurrence following resection of localized disease.10
Imatinib
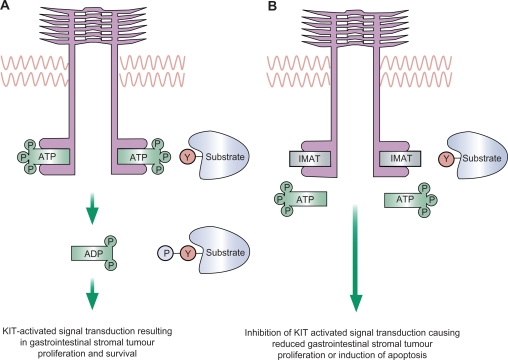
Imatinib mesylate (Gleevec®; Novartis Oncology) is an oral, small molecule tyrosine kinase inhibitor with good oral bioavailability.11 Imatinib exhibits potent inhibitory activity against KIT, platelet-derived growth factor receptor (PDGFR), ABL kinase and the chimeric BCR-ABL fusion oncoprotein of chronic myeloid leukemia. The binding of the KIT-ligand (stem cell factor) to KIT-RTK results in homodimerization and autophosphorylation of the RTK with subsequent kinase activation. Phosphorylation of specific tyrosine residues on KIT triggers a cascade of secondary signaling events and activation of downstream pathways. In GIST, tumor cells harbor gain-of-function KIT mutations leading to ligand-independent KIT activation. Imatinib occupies the ATP-binding pocket of KIT, preventing substrate phosphorylation which in turn inhibits downstream signaling, cellular proliferation and cell survival (Figure 1).
Figure 1.
Mechanism of action of imatinib. A) Under physiological conditions, ATP binds to KIT or PDGFRA, leading to phosphorylation and autoactivation of the receptor, or phosphorylation of substrate molecules resulting in activation of downstream signalling pathways. B) Imatinib occupies the ATP-binding pocket of KIT or PDGFA, preventing substrate phosphorylation which in turn inhibits downstream signaling, cellular proliferation and cell survival.
Reprinted from The Lancet, 369, Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. 1731–1741.70 Copyright © 2007, with permission from Elsevier.
Historically, management of advanced GIST revolved around surgery with few effective systemic therapeutic options. Although the efficacy of cytotoxic chemotherapy was hard to estimate due to differences in histological classification, it is clear that despite aggressive combination chemotherapy, response rates to treatment are poor, typically less than 10%.12 Median overall survival for patients with metastatic GIST in the pre-TKI era was estimated to be between 10 and 20 months.1
Management of localized GIST in the TKI era
Standard treatment for localized GIST involves complete surgical excision. Lymph node dissection is not standard practise as tumor spread is typically hematogenous rather than through the lymphatic system. If complete surgical resection with negative margins (R0 resection) is not achieved at first attempt and can be safely accomplished by repeat surgery, this option may be considered. In cases where R0 surgery cannot be achieved due to technical reasons or entails significant morbidities, then consideration may be given to a peri-operative course of imatinib with aims for cytoreduction. This approach was demonstrated to be safe and feasible in a Radiation Therapy Oncology Group (RTOG)-led prospective non-randomized phase II study. In this study 52 analyzable patients with KIT-positive GIST, 30 with locally advanced disease (defined as tumors ≥5 cm) and 22 with potentially operable recurrent metastatic disease (defined as tumors ≥2 cm) were enrolled and treated with imatinib 600 mg/day over a period of 8 to 12 weeks prior to definitive surgery. Patients were then treated with imatinib for 2 years as postoperative adjuvant treatment.13 Results of this study are summarized in Table 1. The peri-operative use of imatinib was found to be safe and surgical complications were within expectation for this group of patients. In another separate study by Fiore and colleagues, exploring the effects of pre-operative imatinib in patients with unresectable or locally advanced primary GIST, investigators performed a single-center retrospective review of 15 patients treated with pre-operative imatinib, followed by surgery performed at the time of best response.14 Patients continued imatinib for a total of 2 years. Responses were graded per response evaluation criteria in solid tumors (RECIST) criteria.15 Median duration of imatinib pre-treatment was 9 months (range, 3 to 16 months). Median tumor size prior to imatinib was 11 cm. All patients experienced tumor shrinkage, with 1 (7%) complete radiological response, 73% partial response and 20% minor response. Three patients who were initially deemed unresectable were sufficiently cyto-reduced to allow for complete surgery, while 7 with initial indications for extensive surgery underwent conservative resection. Taken together, these studies suggest that pre-operative imatinib appears to be safe, feasible and is an option for patients with primary locally advanced GIST or gross residual disease in consideration of repeat surgery, where either a R0 resection is technically impossible or associated with significant functional morbidities. In clinical practice when faced with such a clinical situation, we would generally recommend patients be initiated on pre-operative imatinib until best response, typically at least 3 to 6 months, before proceeding to surgery.16 Monitoring with standard computed tomography (CT)/magnetic resonance imaging (MRI) is advised as up to 10% to 15% of patients may be primarily refractory to imatinib and thus a small window of opportunity for cure through aggressive surgery may be lost.18 Fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan may be useful as a rapid assessment of tumor response to aid clinical decision making.17 Mutational analysis may also be helpful in excluding GIST genotypes known to be insensitive to imatinib.
Table 1.
Response and clinical outcomes to pre-operative imatinib therapy: results of RTOG 0132/ACRIN 6665 study
| Locally advanced GIST (N = 30) | Recurrent/Metastatic GIST (N = 22) | |
|---|---|---|
| Partial response | 7% | 4.5% |
| Stable disease | 83% | 91% |
| Unknown | 10% | – |
| Progressive disease | – | 4.5% |
| 2-year progression-free survival | 83% | 77% |
Note: Response grading based on RECIST criteria.
Although surgery is undertaken with curative intent in localized GIST, 40% of patients will relapse and ultimately die of their disease.18 Following the demonstration of dramatic sustained anti-tumor activity of imatinib in advanced GIST as described below, investigators in North America led by the American College of Surgeons Oncology Group (ACOSOG) performed a randomized phase III placebo-controlled study of adjuvant treatment with imatinib following complete surgical resection of primary GIST.19 Patients had to have tumors of at least 3 cm in size and were randomly assigned to post-operative imatinib 400 mg/day or placebo for 1 year. The primary endpoint was recurrence-free survival. Median follow-up was about 20 months. The study was closed at the time of the first interim analysis after a median follow up of 15 months. At this time point, a statistically significant benefit was seen in the imatinib arm with recurrence-free survival at 1 year reported to be 98% in the imatinib arm compared to 83% in the placebo arm. A longer follow-up would be required to draw definitive conclusions with regards to the absolute delay in relapse, overall survival and time to secondary resistance, however the study was not designed to capture these endpoints. Based on the results of the ACOSOG trial, the US Food and Drug Administration (FDA) and European Medicines Agency (EMEA) approved the use of imatinib following complete resection of GIST. Two additional studies in Europe are ongoing to further evaluate the potential benefit of adjuvant imatinib. The European Organization of Research and Treatment of Cancer (EORTC) 62024 randomized patients with intermediate and high-risk GIST, based on size criteria and mitotic index as defined by Fletcher and colleagues,9 to 2 years of adjuvant imatinib versus observation, while the Scandinavian Sarcoma Group (SSG) Trial XVIII randomized patients with pathological and/or surgical (tumor spillage and microscopic margins positive) high-risk disease to 1 versus 3 years of adjuvant imatinib. Results of both studies may add valuable information to the optimal duration of adjuvant imatinib and refine patient selection criteria for such a therapeutic approach.
Role of imatinib in metastatic GIST
Prior to the use of tyrosine kinase inhibitors in GIST, the primary modality for treatment of localized and recurrent disease was surgical resection. Response rates to conventional cytotoxics were <10% and patients were typically managed with repeated surgical resections. As described above, the vast majority of GISTs are defined by mutations in KIT, which lead to constitutive ligand-independent activation of the KIT-RTK, and subsequent downstream signaling resulting in uncontrolled cell growth.
Imatinib is a selective tyrosine kinase inhibitor which binds KIT, Bcr-Abl, PDGFA/PDGFB. In vitro data demonstrated that imatinib inhibited downstream phosphorylation in GIST leading to interruption of cell proliferation.20 Because of this data, imatinib (formerly known as STI571) was evaluated clinically in GIST in a heavily pre-treated patient.21 The patient experienced a dramatic response, supporting the rationale use of imatinib in this disease. A multi-center randomized phase II trial was performed in patients with advanced GIST. In this study, 147 patients with metastatic and/or unresectable GIST participated and were randomly assigned to 400 mg or 600 mg of imatinib per day. Despite the rarity of GIST and aided by a uniform diagnosis of KIT-positivity, accrual was completed in under 9 months. Overall, 54% of patients experienced a partial response and 28% had stable disease. The median time to an objective response was 13 weeks. There was no complete response and 14% of patients demonstrated evidence of early resistance to imatinib. Treatment was well tolerated and significantly, there was no difference in either response rates or toxicity between the two doses studied.17 In a recent update of this landmark study, 68% of patients experienced objective responses, including 2 patients with complete responses. Sixteen percent had prolonged disease stability and 12% exhibited progression. Notably, the median time to response in patients who achieved at least a partial response was 2.7 months and a quarter of these patients took more than 5 months to achieve their responses. At the time of disease progression dose escalation to either 600 mg/day or 800 mg/day provided tumor control rates of 26% (16% partial response and 9% stable disease) and 15% (8% partial response and 8% stable disease) in the 400 mg/day and 600 mg/day arms respectively. The median time to progression and overall survival for the entire study cohort was 24 months and 57 months respectively. There was no difference in time to progression or overall survival between the two imatinib dose levels. Of note, overall survival was equivalent in patients who achieved stable disease or partial response and as a group was superior to patients who had initial progression to imatinib, estimated 5-year survival rate of 55% versus 9% respectively.22 This landmark study confirmed the efficacy and tolerability of imatinib in GIST and led to the approval of imatinib in patients with metastatic GIST by the FDA in 2002.
Impact of imatinib dose on patient outcome
Two large phase III international studies were performed to evaluate the impact of dose of imatinib on outcome. The first was an EORTC-led international effort involving 946 patients from Europe, Australia and Asia, randomly assigned to imatinib 400 mg either once or twice daily (800 mg/day total dose). Patients randomized to the 400 mg/day arm were allowed to cross over to the 800 mg/day arm at time of disease progression. Grade 3–4 toxicities were more common on the higher dose arm (32% versus 50% respectively). In addition dose reductions and interruptions were more common on the higher dose arm. Overall objective response rates were 52% (5% complete and 47% partial), 32% had stable disease, with no significant differences between the two treatment groups when the entire cohort was analyzed. The high-dose arm did however experience a significantly longer median progression-free survival.23 In a follow-up analysis undertaken by Zalcberg and colleagues evaluating outcomes of patients who initiated imatinib at 400 mg/day who crossed over to 800 mg/day at time of disease progression, tumor control could be re-gained in 29% of patients (2% partial and 27% stable disease), with a median progression-free survival of under 3 months.24 Concurrent to the EORTC study, a North American trial randomized 746 patients with advanced GIST to standard (400 mg/day) versus high-dose (800 mg/day) imatinib. Median follow-up was 4.5 years. Tumor responses were identical in both groups and there was no statistically significant difference in median progression-free 18 months versus 20 months and overall survival, and 55 months versus 51 months. A third of patients, on the standard-dose arm, who progressed and crossed over to the high-dose arm managed to re-gain tumor control, and achieved a median progression-free survival of 5 months, closely replicating results from the EORTC study. Similar to the EORTC experience, serious adverse events were more common in the high-dose arm.25 In a pooled meta-analysis of these two studies performed by Van Glabbeke et al, a small but statistically significant progression-free survival benefit was noted in the high-dose arm, approximately 19 months versus 23 months, with results consistent across both studies but significant only in the EORTC study. Overall survival was identical in both arms.26
Response evaluation in GIST
One of the clinical challenges in the development of imatinib in GIST was the difficulty in defining best methodologies to evaluate this disease while patients are on treatment. Although PET scans demonstrated early and sustained metabolic responses in most patients, this was not always accompanied by objective responses per RECIST size criteria. Tumor shrinkage may take place many months after initiation of drug despite clinical improvement.17 Work by investigators in MD Anderson Cancer Center comparing pre- and 2-month post-imatinib treatment scans from GIST patients found that a tumor decrease of more than 10% or a decrease in tumor density of 15% on CT was both sensitive and specific in identifying patients with good metabolic response. Based on these new CT imaging criteria (termed Choi criteria), one is able to identify a cohort of patients with a longer time to progression.27 These results were validated in an independent data set demonstrating that Choi response was superior to RECIST response in predicting time to progression and disease-specific survival.28
Duration of imatinib therapy
In all the pivotal trials involving imatinib in advanced GIST, imatinib was continued until disease progression or emergence of prohibitive toxicities. Recently, the French Sarcoma Group evaluated the impact of dose interruption of imatinib in patients with advanced GIST. BFR14 is a phase III clinical trial randomizing patients with advanced GIST who achieved disease control on imatinib for at least 1 year, to either treatment interruption versus continuation of imatinib.29 Fifty-eight patients were eligible and randomized into the two study arms. Significantly more patients in the imatinib-interrupted arm (81%) experienced disease relapse as compared to those on maintenance therapy (31%). Median progression-free survival was 6 months in the imatinib-interrupted arm versus 18 months in the maintenance arm. Twenty-four (92%) of the 26 patients with documented progression in the imatinib-interrupted arm responded to imatinib re-introduction, one progressed, and one died from a cerebral infarction before evaluation. An important issue arising from this study was whether dose interruption had a negative impact on incidence of subsequent imatinib resistance. On longer follow-up, 8 (25%) of the 32 patients randomized to the imatinib-interrupted arm progressed after imatinib re-introduction, compared to 8 (31%) of the 26 patients in the maintenance arm (first evidence of progression). This difference was not statistically significant and overall survival was virtually identical in both arms.
Impact of genotype on therapeutics
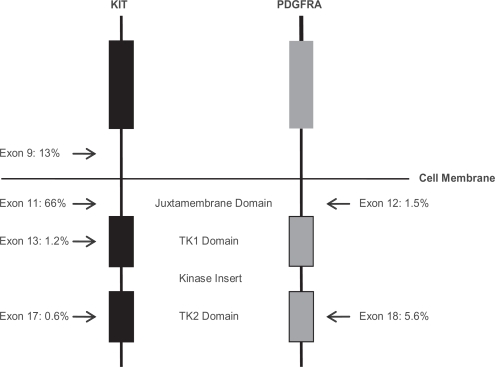
Shortly after the demonstration of imatinib efficacy in metastatic GIST, research efforts were focused on the molecular heterogeneity and impact of genotypic variations on clinical outcomes. It is now appreciated that 85% to 90% of all GISTs harbor activating mutations in either KIT or PDGFRA. As shown in Figure 2, the majority of these mutations occur in KIT; exon 11 (66%) being most commonly affected followed by exon 9 (13%), with low incidences in exon 13 and 17 (about 1% each). Mutations in PDGFRA represent less than 10% of all mutations with exon 18 mutations (about 6%) being more common than exon 12 mutations (about 2%). The remainder of GISTs (12%) are wild-type for both KIT and PDGFRA.30 Tumor genotype has a major influence on clinical outcomes in the setting of imatinib therapy. In one of the earliest studies correlating genotype to clinical response, using tumor tissue available from 127 of 147 patients enrolled on the North American phase II trial of imatinib in patients with advanced GIST, partial response rate in patients with tumors that harbor exon 11 KIT mutation was significantly superior to those patients whose tumors harbor exon 9 KIT mutation and wild-type GIST, 84% versus 48% respectively. In addition, progression-free and overall survival was superior in patients with exon 11 mutation.31 Similar findings were reported in the larger phase III studies evaluating standard and high dose imatinib. In the North American study, the presence of tumor KIT exon 11 mutation correlated with an improved objective response rate to imatinib (72%, 44%, 45% respectively), time to progression (median 25 months, 17 months and 13 months respectively) and overall survival (median 60 months, 38 months and 49 months respectively) when compared to patients with KIT exon 9 mutation and wild-type GIST. Additionally no significant differences were detected between KIT exon 9 mutants and wild-type GIST.32 Likewise in the EORTC-led study, of 946 patients randomized to treatment, 377 had adequate tumor material for mutational analysis. When compared with patients whose tumors harbor exon 11 mutants, presence of exon 9 mutations and wild-type GIST were the strongest adverse prognostic factor for objective response, risk of progression and death.33 Having identified a “high-risk” cohort of patients, investigators next questioned if imatinib dose-escalation could overcome this adverse prognostic feature. In the meta-analysis of the two large phase III imatinib dose-efficacy studies undertaken by Van Glabbeke and colleagues, a statistically significantly progression-free survival benefit in patients who received imatinib 800 mg/day was demonstrated in the subset of patients with KIT exon 9 mutations from EORTC dataset. This finding was not confirmed in the North American dataset. However, the benefit of imatinib 800 mg/day remained significant in the pooled dataset, median progression-free survival of 6 months versus 19 months, in the standard-dose and high-dose imatinib arms respectively, for patients whose tumors harbor exon 9 mutations.26,32,33 This finding may account for the improved overall progression-free survival seen in the EORTC study attributed to high-dose imatinib, where a larger proportion of KIT exon 9 mutants were enrolled (15% of analyzed patients in the EORTC study versus 8% in the North American study).
Figure 2.
Structure of KIT and PDGFRA. The location and relative frequencies of GIST-associated kinase mutations are depicted in relation to the structural features of KIT and PDGFRA. The remainder of GIST (about 12% in this series) do not harbor detectable KIT or PDGFRA mutations.
Adapted with permission. © 2004 American Society of Clinical Oncology. All right reserved. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22(18):3813–3825.30 Adapted with permission. © 2003 American Society of Clinical Oncology. All right reserved. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–4349.31
Role of imatinib trough levels
More recently plasma imatinib trough levels have been found to correlate with improved outcomes in patients with chronic myeloid leukemia (CML), another imatinib-sensitive disease, suggesting a trough threshold value of about 1000 ng/mL.34,35 Imatinib is orally administered with good oral bioavailability. It is extensively bound to plasma proteins and is thought to be predominantly metabolized by CYP3A4 to an active metabolite CGP74588.11 Imatinib pharmacokinetic exposure demonstrates wide inter-patient variability34–36 and it is well documented that treatment-related toxicities are frequently dose-related.23,25 Recently, imatinib trough levels taken from patients enrolled in the pivotal phase II GIST trial were correlated with patient outcome. In this study by Demetri and colleagues, 73 patients had imatinib trough samples collected for pharmacokinetic analysis. Pharmacokinetic exposure showed wide inter-patient variability, consistent with previous reports. Interestingly progression-free survival appeared to be associated with imatinib trough levels. Although the median imatinib trough levels of responding patients (a composite end-point defined as objective response plus stable disease) was numerically superior to non-responding patients, 1446 ng/mL (range, 414 to 3336 ng/mL) versus 1155 ng/ml (range, 545 to 4182 ng/mL), the drug exposure ranges were wide with considerable overlap and were not statistically significant. However, patients in the lowest imatinib trough quartile, corresponding to a threshold value of 1110 ng/mL, was significantly correlated with a shortened time to disease progression 11.3 months versus 30 months, when compared to those in quartiles 2 to 4.36 Interestingly, there was no significant difference between quartiles 2, 3, and 4. Additional research is needed to prospectively confirm these exploratory results, as well as evaluate clinical factors which may impact trough level as well as outcome.
Therapeutic options following progression on imatinib
Median time to progression on first-line imatinib is approximately 2½ years. Fifteen percent of patients are primarily refractory to imatinib while the majority of patients who fail imatinib do so after a period of disease control (secondary failure). Patterns of failure can be classified into progression in a limited fashion, involving a small number of new tumor nodules and/or progressive lesion in some of the existing tumors, or a more generalized pattern of failure. Studies have consistently shown secondary kinase mutations to be the most common mechanism of imatinib resistance.37–39 A unique pattern of GIST progression has been reported whereby a resistant clonal nodule develops within a pre-existing tumor mass. In this setting, of note, molecular studies have demonstrated the presence of new kinase mutations in 80% of cases.40
Metastacectomy in the TKI era
In the pre-TKI era, metastacectomy was routinely recommended in patients with metastatic GIST especially those with limited metastases or where complete surgical resection was possible. Whether this remains to be true in the TKI era is an area of active investigation. Current literature on this topic is retrospective in nature and prospective randomized clinical trials are under development to address this clinical question. Raut and colleagues retrospectively evaluated the outcome of 69 consecutive patients with primary unresectable or metastatic GIST, who underwent surgery while receiving TKI.41 Complete surgical resection was attempted whenever possible. Patients were categorized based on response to TKI at the time of surgery (stable disease which included patients initially with unresectable or metastatic GIST who achieved a drug response to render all disease resectable and whose tumors were not growing at the time of surgery, limited disease progression or generalized disease progression) and surgical result (no evidence of disease, minimal residual disease or bulky residual disease). Results of this single center study suggested that response to TKI was significantly associated with surgical outcome. Seventy-eight percent of patients with stable disease at the time of surgery were rendered radiographically disease-free post-operatively, as compared to 25% and 7% of patients with limited progression and generalized progression respectively. Conversely bulky residual disease remained after surgery in 4%, 16%, and 43% of these patients respectively. One-year progression-free survival in patients with stable disease, limited progression and generalized progression was 80%, 33%, and 0%. The median time to progression for patients with limited and generalized disease progression was 7.7 months and 2.9 months respectively, while the median time to progression for patients with stable disease has not been reached after a median follow-up of 14.6 months (range, 0.5 to 36.4 months). One-year overall survival was 95%, 86%, 0% respectively. The authors concluded that patients with advanced GISTs exhibiting stable disease or limited progression on TKI have prolonged survival after debulking procedures while surgery has little to offer in the setting of generalized progression. While it is clear that patients with generalized disease progression are unlikely to benefit from surgery, it is unclear from this study whether the improved progression-free and overall survival seen in patients with stable disease and limited disease progression was a result of surgical intervention or inherent tumor biology and response to TKI.
Sunitinib
Sunitinib malate (Sutent®; Pfizer) is a multi-targeted small molecule TKI with activity active against KIT, PDGFR, all 3 isoforms of vascular endothelial growth factor receptors (VEGFR), FLT3, and RET. Due to this spectrum of inhibition, it has both antiproliferative and antiangiogenic properties and was felt to be a rational choice for evaluation in patients with imatinib-resistant GIST. Following promising results from a phase I/II trial, a large, international, phase III, randomized, placebo-controlled trial was undertaken in patients with imatinib-resistant or imatinib-intolerant GIST. Three hundred and twelve patients were randomized in a 2:1 ratio to either sunitinib 50 mg daily, in a 4-weeks-on and 2-weeks-off regimen, or placebo.42 The primary end-point was time to progression in an intention-to-treat analysis. The study was unblinded early when an interim analysis revealed significantly longer time to progression in the sunitinib arm, approximately 6.8 months versus 1.6 months in the placebo arm. Treatment was fairly well tolerated with serious treatment-related toxicities reported in 20% and 5% sunitinib- and placebo-treated patients respectively. Common adverse events include fatigue, diarrhea, hand-foot syndrome, hypertension, and skin discoloration. Based on the results of this study, sunitinib was approved by the FDA for treatment of imatinib-resistant or intolerant advanced GIST.
Although sunitinib, given in the intermittent dosing schedule, clearly has benefit in this patient population, earlier clinical trials demonstrated a metabolic “flare” as defined by an increase in activity of 18FDG-PET, during the 2-week rest period. When patients were followed by 18FDG-PET, metabolic response was noted as early as 7 days post-initiation of therapy, but this suppression was followed by a rebound during the 2-week-off period, suggesting a flare in disease activity, consistent with lack of TK inhibition during the wash-out period.43 In an attempt to provide consistent TK inhibition, and to enhance convenience of dosing, an international, multicenter phase II study using continuous daily dosing of sunitinib, at 37.5 mg/day, was undertaken to examine this issue.44 In this study, sixty-one patients with advanced GIST following imatinib failure were enrolled. Clinical benefit was observed in 53% of patients (defined as RECIST complete or partial response or stable disease lasting 24 weeks or longer), including a 13% partial response rate. The median progression-free survival was 8.5 months. Toxicity assessment yielded no new safety concerns and was similar to intermittent dosing schedule, which included diarrhea, abdominal pain and asthenia. Pharmacokinetic evaluations demonstrated sunitinib continuous daily dosing achieved constant drug exposure with no unexpected accumulation.
Mechanisms of resistance to tyrosine kinase inhibitors
Imatinib resistance can be divided into primary resistance (defined as progressive disease as best response) and secondary resistance (disease progression after a period of objective response or stable disease). Preclinical data demonstrate that KIT kinase is inhibited in patients with imatinib-responsive GIST but reactivation of KIT and subsequent downstream phosphorylation occurs at the time of secondary resistance. In contrast, KIT signaling in primary imatinib-resistant GIST shows no evidence of inhibition to imatinib and is similar to that seen in untreated GIST, indicating that KIT primary resistance is associated with persistent KIT phosphorylation and activation of downstream pathways.37 The molecular mechanisms responsible for primary-imatinib resistance differ from those of secondary resistance. KIT exon 9 and PDGFRA mutations more commonly demonstrate primary-imatinib resistance when compared to KIT exon 11 mutations. The underlying mechanisms responsible for this resistance may be secondary to differences in the structure of the ATP-binding loop and the relative affinity of imatinib. Phase III studies demonstrating higher dose of imatinib correlating with a progression-free survival in the subset of KIT exon 9 mutants would suggest an inherent decreased drug sensitivity, which may potentially be overcome by imatinib dose escalation.26 The predominant mechanism by which GIST cells develop secondary-imatinib resistance is through the acquisition of secondary kinase mutations. This phenomenon is more commonly observed in primary KIT exon 11 mutant GISTs.37,39,45 In contrast, patients with primary imatinib-resistant GIST rarely harbor secondary kinase mutations, 10% versus 67% in those with primary versus secondary resistance respectively.37 These secondary mutations are non-random in distribution and cluster around KIT exon 13/14 (which encodes the ATP-drug binding pocket) and exon 17/18 (which encodes kinase activation loop) and are associated with decreased imatinib sensitivity, confirmed in in vitro models.37,39,45 Additionally these secondary kinase mutations also impact on clinical outcomes in second-line sunitinib treatment. In a report by Heinrich and colleagues, examining a large cohort of imatinib-refractory patients uniformly treated with sunitinib, patients with secondary KIT exon 13/14 mutations had significantly improved clinical benefit (objective response and stable disease ≥ 6 months) and survival outcomes than those with secondary KIT exon 17/18 mutations.45 Structural and enzymologic reasons could in part explain the resistance to sunitinib consequent to secondary mutations in the kinase activation loop.46
Pediatric GIST
GIST in the pediatric age group is rare, accounting for less than 1% to 2% of all GIST cases.47 Although pediatric GISTs express KIT and display a high level of KIT activation, unique differences exist that distinguish them from adult GISTs, notably, the relative absence of KIT or PDGFRA mutations, with only less than 15% of cases harboring these mutations.3–4 In contrast, activating mutations in KIT or PDGFRA are found in more than 85% of adult GISTs.30 Recent reports suggest pediatric GISTs exhibit distinct gene-expression signatures4 and genetic progression mechanisms from adult GISTs.3 Consistent with the molecular profile of wild-type GISTs, most pediatric GIST cases respond less favorably to imatinib than adult KIT exon 11 mutant GISTs. In vitro studies suggest sunitinib and other TKIs including nilotinib (Tasigna®; Novartis Oncology), dasatinib (Sprycel®; Bristol-Myers Squibb) and sorafenib (Nexavar®; Bayer HealthCare Pharmaceuticals–Onyx Pharmaceuticals) may be more active than imatinib against wild-type GIST.4 A series published by Janeway and colleagues reported 7 imatinib-refractory pediatric patients (age range 10 to 17) with advanced GIST treated with sunitinib. Five patients had sufficient tumor for genotyping, and were KIT or PDGFRA wild-type. Prior to sunitinib, three of the 6 patients treated with imatinib (one received adjuvant imatinib following tumor resection and experienced disease recurrence while on therapy) had progressive disease as best response while the remaining 3 had stable disease lasting between 12 to 16 months. On sunitinib, 1 patient achieved a partial response with time to progression lasting more than 21 months, 5 patients had stable disease, and 1 had progressive disease. The duration of disease stabilization ranged from 7 to more than 21 months with a mean of 15 months, suggesting that sunitinib may have activity in this unique subset of GISTs.48
More recently reports have shown insulin-like growth factor-1 receptor (IGF-1R) to be strongly over-expressed in both adult and pediatric wild-type GIST.49,50 IGF-1R amplification is also detected at a higher frequency in wild-type as compared to kinase-mutant GIST49 and aberrant IGF-1R expression may be associated with pathogenesis of wild-type GISTs. Further studies are needed to determine if inhibition of the IGF-1R pathway is relevant to clinical outcomes in these patients. Also, recent reports have suggested that a small percentage of patients with KIT and PDGFR wildtype GIST may harbor an activating mutation in BRAF.51 These findings highlight the heterogeneity of these tumors on a molecular level which may ultimately lead to more personalized treatment strategies.
Familial GIST
Heritable mutations in KIT and PDGFRA, likely of autosomal dominant inheritance pattern, have been reported in the literature.52–54 Affected kindreds with familial GIST may present with multi-focal disease, and in some cases associated with cutaneous and mucous membrane hyperpigmentation, urticaria pigmentosa, mast cell disease and diffuse spindle cell hyperplasia in the myenteric plexus of the gastrointestinal tract. Carney’s triad is a rare and possibly familial tumor syndrome.55 It predominantly affects young women and comprises of gastric stromal sarcoma (GIST), pulmonary chrondroma and extra-adrenal paraganglioma. Neurofibromatosis type 1 (NF-1) has also been associated with development of GIST. In a population-based study of 70 patients with NF-1 conducted in Sweden, 7% of patients were diagnosed with GIST.56 These NF-1 associated GISTs are frequently multi-focal, often affecting the small bowel, and are typically KIT/PDGFRA mutation negative.57
Strategies in imatinib- and sunitinib-resistant GIST
As mentioned above, imatinib has revolutionized the management of advanced GIST. Resistance however does develop in the majority of patients and the management of imatinib-resistant GIST remains a challenge. Dose escalation of imatinib may be supported for patients treated at 400 mg/day. However, responses to dose escalation tend to be relatively short-lived. Because of this there has been tremendous interest in strategies to manage imatinib-resistant GIST. Sunitinib, is currently the only FDA-approved therapy for patients with imatinib-resistant GIST. However, as with imatinib, resistance to sunitinib ultimately develops in most patients. Because of this, there remains intense interest in additional approaches to this disease.
A wide range of newer and more potent small molecule TKI that target KIT and/or PDGRA are in development. An agent with interesting single agent activity is sorafenib. Sorafenib is a multi-targeted small molecule TKI with potent activity against B-RAF tyrosine kinase, VEGFR, PDGFR, KIT, and FLT3 recently approved for use in patients with renal cell and hepatocellar carcinoma. In a phase II efficacy study carried out by the University of Chicago consortium, 26 patients with imatinib (6 patients) and sunitinib-resistant GIST (20 patients) were enrolled and treated with sorafenib 400 mg twice daily. Three (13%) and 14 (58%) out of 24 patients evaluable for response exhibited partial response and stable disease respectively, for a disease control rate of 71%. The median progression-free survival was 5.3 months.58 These results were supported by the recently reported European experience with sorafenib in this same patient population. In this retrospective study, thirty-two heavily pre-treated patients who failed imatinib, sunitinib and nilotinib were treated with sorafenib in the 4th-line setting. Nineteen percent of patients achieved a partial remission and 44% had disease stabilization. Median progression-free survival was 20 weeks and median overall survival was 42 weeks.59 These findings were corroborated in cell line models studying the in vitro activity of sorafenib against imatinib and/sunitinib-resistant kinases. The predominant mechanism of imatinib resistance is through the acquisition of secondary kinases as described. Sorafenib demonstrated significant activity in imatinib-resistant KIT secondary mutations involving the ATP-binding pocket and activation loop. And notably, sorafenib unlike sunitinib is active against most imatinib-resistant secondary mutations involving the KIT activation loop.60 As the majority of patients in these studies had failed both imatinib and sunitinib, these results suggest that sorafenib may have promising activity in the treatment of GIST following imatinib and sunitinib failure.
Nilotinib is a second generation small molecule TKI with good activity against receptors of KIT and PDGFR. In a dose-finding phase I study, 53 GIST patients resistant to imatinib and other TKIs, were enrolled and treated with nilotinib alone (18 patients) or in combination with imatinib (35 patients). Although not designed as an efficacy study, one patient on single agent nilotinib had a partial response while 13 others had stable disease for a disease control rate of 78% a median progression-free survival of 5.6 months.61 Clinical trials are currently ongoing evaluating the benefit of nilotinib in the third line setting.
Similarly masitinib with reportedly greater affinity and selectivity for both the wild-type and mutated KIT than imatinib was investigated in a phase I dose-escalation study in patients with advanced and/or metastatic cancer. Half of the enrolled cohort had GIST. Treatment was generally well tolerated and the maximally tolerated dose was not determined in this study. One of 2 imatinib-intolerant patients demonstrated a partial response and about 29% of imatinib-resistant patients had stable disease.62 Building on these results, a multi-center phase II study of masitinib in treatment-naïve GIST patients led by the French Sarcoma Group was initiated. In a preliminary report 50% of patients demonstrated objective partial response (6.7% complete and 43.3% partial response), 47% had stable disease and 3% were primarily refractory to masitinib, yielding a overall disease control rate of 97%. The median progression-free survival was 27 months, comparable with imatinib.63 Phase III studies comparing nilotinib and masitinib as single agents with imatinib in the first line setting are now underway.
As discussed above there is substantial heterogeneity in the secondary mutations which render GIST resistant to TKIs. In addition, there may be pathways and strategies other than direct KIT inhibition, which are relevant to the biology of these tumors. Because of this, research efforts are focused on strategies which may be relevant in this disease other than direct KIT inhibition.
Heat shock protein-90 (HSP90) is an ATP-dependent protein chaperone involved in the regulation of cellular protein homeostasis. It regulates the stability of key proteins, including the KIT oncoproteins, important in oncogenesis, cancer cell proliferation, and cancer cell survival and plays a central role in protein folding in response to various environmental stresses.64 Pre-clinical work involving cell line models using 17-allylamino-18-demethoxy-geldanamycin (17-AAG), an inhibitor of the HSP90 chaperone protein, demonstrated significant reduction of both phospho- and total KIT expression, inactivation of downstream signaling pathways and inhibition of cellular proliferation and survival in both imatinib-sensitive and imatinib-resistant KIT-positive cell lines. Similar activity could not be demonstrated in a KIT-negative cell line suggesting that HSP90 inhibitor exerts its therapeutic function through its actions on KIT oncoprotein.65 Based on this preclinical rationale, a phase I/II study was conducted using IPI-504 (Retaspimycin Hydrochloride; Infinity Pharmaceuticals) in patients with metastatic, TKI-resistant GIST or metastatic soft tissue sarcoma.66 In the subset of 38 GIST patients, treatment was well tolerated; dose-limiting toxicities were headache and myalgia. Of the 18 GIST patients assessed by PET, 22% had a partial response and an additional 66% had stable disease according to the EORTC PET response criteria.67 Although no RECIST-defined responses were observed, approximately three-quarters of evaluable patients had stable disease as best response. This led to the initiation of an international phase III study in GIST. However the trial was closed early at the recommendation of an independent data monitoring committee due to safety concerns. Trials are now underway evaluating other HSP90 inhibitors in GIST. Additionally, in preclinical studies, PI3-kinase/mTOR pathways appear to be important in cell signaling and proliferation not only in imatinib-resistant cell lines but also in imatinib-sensitive and KIT-negative GIST.68 A number of PI3-kinase inhibitors and dual PI3-kinase/mTOR inhibitors are now in development and may prove to be effective in TKI-refractory GIST.
Combining agents that possess non-lapping toxicities targeting different aspects of the GIST cancer pathway in a synergistic fashion is another rationale approach to development of novel therapeutics. Based on in vitro synergism demonstrated between imatinib and everolimus (previously known as RADOO1; Novartis Oncology) in human imatinib-resistant GIST cell lines, phase I/II clinical studies combining the 2 agents were performed. Primary study end point, defined as 4-month progression-free survival, was achieved in 17% (imatinib-refractory) and 37% (imatinib plus additional therapy) of patients prompting further studies into the various strategies of combined tyrosine kinase and mTOR inhibition.69
Conclusions
In the decade following the landmark discovery of activating KIT mutation in GIST, much has been learned about this disease. A further understanding of the biology and molecular genetics of GIST, has translated into rationale approaches to clinical care. Through extensive international collaborations, large studies in this uncommon disease have first confirmed activity of imatinib in treatment-naïve patients and then sunitinib in the second line setting. Significant inroads have also been made into understanding the mechanisms of TKI resistance and molecular heterogeneity at disease progression which should lead to effective therapeutic strategies for patients in the future.
Footnotes
Diclosures
Dr Suzanne George has served on advisory boards for Pfizer and Novartis.
References
- 1.Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumors. Lancet Oncol. 2002;3(11):655–664. doi: 10.1016/s1470-2045(02)00899-9. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era- a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 3.Janeway KA, Liegl B, Harlow A, et al. Pediatric KIT wild-type and platelet-derived growth factor receptor alpha-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res. 2007;67(19):9084–9088. doi: 10.1158/0008-5472.CAN-07-1938. [DOI] [PubMed] [Google Scholar]
- 4.Agaram NP, Laquaglia MP, Ustun B, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14(10):3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrera GA, Cerezo L, Jones JE, et al. Gastrointestinal autonomic nerve tumors. ‘Plexosarcomas’. Arch Pathol Lab Med. 1989;113(8):846–853. [PubMed] [Google Scholar]
- 6.Lauwers GY, Erlandson RA, Casper ES, Brennan MF, Woodruff JM. Gastrointestinal autonomic nerve tumors. A clinicopathological, immunohistochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1993;17(9):887–897. doi: 10.1097/00000478-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 8.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152(5):1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44(9):879–894. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- 12.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33(5):466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99(1):42–47. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiore M, Palassini E, Fumagalli E, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST) Eur J Surg Oncol. 2009;35(7):739–745. doi: 10.1016/j.ejso.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, Benjamin RS, Blanke CD, et al. for NCCN Task Force. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)-update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5(Suppl 2):S1–29. [PubMed] [Google Scholar]
- 17.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 18.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dematteo RP, Ballman KV, Antonescu CR, et al. for American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuveson DA, Willis NA, Jacks T, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20(36):5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 21.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14):1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 22.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26(4):620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 23.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 24.Zalcberg JR, Verweij J, Casali PG, et al. for EORTC Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group; Australasian Gastrointestinal Trials Group. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41(12):1751–1757. doi: 10.1016/j.ejca.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 26.Van Glabbeke MM, Owzar K, Rankin C, Simes J, Crowley J for GIST Meta-analysis Group (MetaGIST) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors (GIST): A meta-analysis based on 1,640 patients (pts) J Clin Oncol (Meeting Abstracts) 2007;25:10004. [Google Scholar]
- 27.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25(13):1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 29.Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25(9):1107–1113. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- 30.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22(18):3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 31.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26(33):5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debiec-Rychter M, Sciot R, Le Cesne A, et al. for EORTC Soft Tissue and Bone Sarcoma Group; Italian Sarcoma Group; Australasian Gastrointestinal Trials Group. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Picard S, Titier K, Etienne G, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109(8):3496–3499. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 35.Larson RA, Druker BJ, Guilhot F, et al. for IRIS (International Randomized Interferon vs STI571) Study Group. Imatinib pharmaco-kinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111(8):4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 36.Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27(19):3141–3147. doi: 10.1200/JCO.2008.20.4818. [DOI] [PubMed] [Google Scholar]
- 37.Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24(29):4764–4774. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 38.Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216(1):64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11(11):4182–4190. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 40.Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18):5398–5405. doi: 10.1158/1078-0432.CCR-06-0858. [DOI] [PubMed] [Google Scholar]
- 41.Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006;24(15):2325–23231. doi: 10.1200/JCO.2005.05.3439. [DOI] [PubMed] [Google Scholar]
- 42.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 43.Demetri GD, Heinrich MC, Fletcher JA, et al. Molecular target modulation, imaging, and clinical evaluation of gastrointestinal stromal tumor patients treated with sunitinib malate after imatinib failure. Clin Cancer Res. 2009;15(18):5902–5909. doi: 10.1158/1078-0432.CCR-09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959–1968. doi: 10.1016/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26(33):5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gajiwala KS, Wu JC, Christensen J, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009;106(5):1542–1547. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29(10):1373–1381. doi: 10.1097/01.pas.0000172190.79552.8b. [DOI] [PubMed] [Google Scholar]
- 48.Janeway KA, Albritton KH, Van Den Abbeele AD, et al. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52(7):767–771. doi: 10.1002/pbc.21909. [DOI] [PubMed] [Google Scholar]
- 49.Tarn C, Rink L, Merkel E, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2008;105(24):8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corless CL, Beadling C, Justusson E, Heinrich MC. Evaluation of the presence of IGF1R overexpression in wild-type and kinase mutant GI stromal tumors. J Clin Oncol (Meeting Abstracts) 2009;27:10506. [Google Scholar]
- 51.Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47(10):853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleinbaum EP, Lazar AJ, Tamborini E, et al. Clinical, histopathologic, molecular and therapeutic findings in a large kindred with gastrointestinal stromal tumor. Int J Cancer. 2008;122(3):711–718. doi: 10.1002/ijc.23137. [DOI] [PubMed] [Google Scholar]
- 53.Li FP, Fletcher JA, Heinrich MC, et al. Familial gastrointestinal stromal tumor syndrome: phenotypic and molecular features in a kindred. J Clin Oncol. 2005;23(12):2735–2743. doi: 10.1200/JCO.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Chompret A, Kannengiesser C, Barrois M, et al. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004;126(1):318–321. doi: 10.1053/j.gastro.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 55.Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74(6):543–552. doi: 10.4065/74.6.543. [DOI] [PubMed] [Google Scholar]
- 56.Zöller ME, Rembeck B, Odén A, et al. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer. 1997;79(11):2125–2131. [PubMed] [Google Scholar]
- 57.Miettinen M, Fetsch JF, Sobin LH, et al. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30(1):90–96. doi: 10.1097/01.pas.0000176433.81079.bd. [DOI] [PubMed] [Google Scholar]
- 58.Wiebe L, Kasza KE, Maki RG, et al. Activity of sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): A phase II trial of the University of Chicago Phase II Consortium. J Clin Oncol (Meeting Abstracts) 2008;26:10502. [Google Scholar]
- 59.Reichardt P, Montemurro M, Gelderblom H, et al. Sorafenib fourth-line treatment in imatinib-, sunitinib-, and nilotinib-resistant metastatic GIST: A retrospective analysis. J Clin Oncol (Meeting Abstracts) 2009;27:10564. [Google Scholar]
- 60.Heinrich MC, Carden R, Griffith D, et al. In vitro activity of sorafenib against imatinib- and sunitinib-resistant kinase mutations associated with drug-resistant GI stromal tumors. J Clin Oncol (Meeting Abstracts) 2009;27:10500. [Google Scholar]
- 61.Blay JY, Casali PG, Reichardt P, et al. A phase I study of nilotinib alone and in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors (GIST): Study update. J Clin Oncol (Meeting Abstracts) 2008;26:10553. [Google Scholar]
- 62.Soria JC, Massard C, Magné N, et al. Phase 1 dose-escalation study of oral tyrosine kinase inhibitor masitinib in advanced and/or metastatic solid cancers. Eur J Cancer. 2009;45(13):2333–2341. doi: 10.1016/j.ejca.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Le Cesne A, Blay J, Bui NB, et al. Masatinib mesylate in imatinib-naive locally advanced or metastatic gastrointestinal stromal tumor (GIST): Results of the French Sarcoma Group phase II trial. J Clin Oncol (Meeting Abstracts) 2009;27:10507. [Google Scholar]
- 64.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 65.Bauer S, Yu LK, Demetri GD, Fletcher JA. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66(18):9153–9161. doi: 10.1158/0008-5472.CAN-06-0165. [DOI] [PubMed] [Google Scholar]
- 66.Wagner AJ, Morgan JA, Chugh R, et al. Inhibition of heat shock protein 90 (Hsp90) with the novel agent IPI-504 in metastatic GIST following failure of tyrosine kinase inhibitors (TKIs) or other sarcomas: Clinical results from phase I trial. J Clin Oncol (Meeting Abstracts) 2008;26:10503. [Google Scholar]
- 67.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 68.Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26(54):7560–7568. doi: 10.1038/sj.onc.1210558. [DOI] [PubMed] [Google Scholar]
- 69.Dumez H, Reichard P, Blay JY, et al. for CRAD001C2206 Study Group. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients (pts) with imatinib-resistant gastrointestinal stromal tumors (GIST) J Clin Oncol (Meeting Abstracts) 2008;26:10519. [Google Scholar]
- 70.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369(9574):1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]