Abstract
The Hippo signaling pathway regulates cellular proliferation and survival, thus exerting profound effects on normal cell fate and tumorigenesis1-3. The pivotal effector of this pathway is YAP, a transcriptional co-activator amplified in mouse and human cancers, where it promotes epithelial to mesenchymal transition (EMT) and malignant transformation4-10. To date, studies of YAP target genes have focused on cell-autonomous mediators; here we show that YAP-expressing MCF10A breast epithelial cells enhance the proliferation of neighboring untransfected cells, implicating a non-cell autonomous mechanism. We identify the epidermal growth factor receptor (EGFR) ligand, amphiregulin (AREG), as a transcriptional target of YAP, whose induction contributes to YAP-mediated cell proliferation and migration, but not EMT. Knockdown of AREG or addition of an EGFR kinase inhibitor abrogates the proliferative effects of YAP expression. Suppression of the negative YAP regulators LATS1/2 is sufficient to induce AREG expression, consistent with physiological regulation of AREG by the Hippo pathway. Genetic interaction between the Drosophila YAP orthologue Yorkie and Egfr signaling components support the link between these two highly conserved signaling pathways. Thus, YAP-dependent secretion of AREG implicates activation of EGFR signaling as an important non-cell autonomous effector of the Hippo pathway, with implications for the regulation of both physiological and malignant cell proliferation.
Normal cells require mitogenic growth signals to proliferate, whereas, tumor cells often generate their own proliferative signals through the secretion of growth factors or the activation of growth factor receptors 11. We have shown that YAP transduced MCF10A cells proliferate in 3D acinar cultures in the absence of EGF 7. MCF10A are immortalized, non transformed human mammary epithelial cells, which exhibit dependence on growth factors for proliferation and survival 12, raising the possibility that YAP itself induces secretion of required growth factors or cytokines in these cells. To test this hypothesis, we performed mixing experiments with cells transduced with either GFP-labeled YAP or Red-Cherry tagged vector. MCF10A cells expressing GFP-YAP, but not Cherry-vector, formed acini in 3D cultures in the absence exogenous EGF. Remarkably, vector transduced cells did produce acini when co-cultured in a 1:1 ratio with YAP expressing cells (Fig. 1a). Thus, ectopic expression of YAP in MCF10A cells appears to result in secretion of factors that enable proliferation of neighboring untransduced cells.
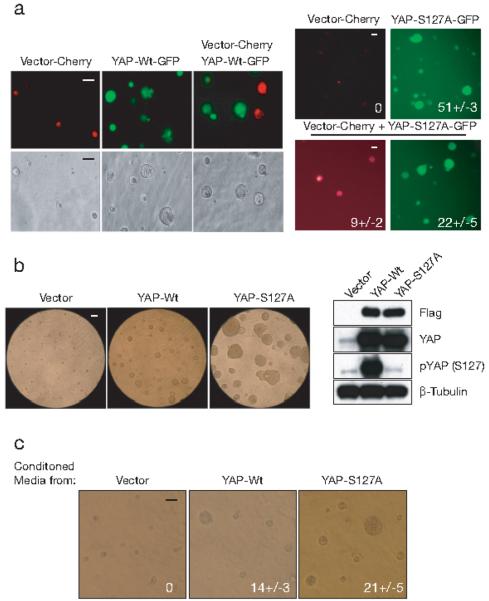
Figure 1. YAP-induced secreted factor enhances EGF-independent growth of MCF10A cells.
(a) Non-cell-autonomous effect of YAP. Vector (tagged with Cherry marker) and either YAP-Wt or YAP-S127A (tagged with GFP) transduced MCF10A cells were cultured in Matrigel either separately or as a 1:1 mixture for 25 days without EGF. Representative light and fluorescence images are shown. (Scale bars, 100μm) (b) Both YAP and YAP-S127A promote the EGF-independent growth of MCF10A cells in 3D culture. Cells transduced with vector, YAP or YAP-S127A were cultured in Matrigel for 25 days in the absence of EGF. Representative phase contrast images are shown. (Scale bars, 100μm) Immunoblotting analysis of endogenous and exogenous YAP, using antibodies to detect Flag-tag, YAP or phosphorylated S127 residue which is mutated in YAP-S127A. β-Tubulin used as loading control. (c) YAP-Wt and YAP-S127A conditioned media induce the EGF-independent growth of parental MCF10A cells in 3D culture. Equal numbers of MCF10A cells were plated in Matrigel and fed for 25 days with medium from vector, YAP or YAP-S127A transduced 3D cultures. Representative phase contrast images are shown. Numbers represent total acini in culture +/− SD (four 200X fields). (Scale bars, 100μm)
To identify mediators of this apparent YAP-induced non-cell-autonomous effect, we made use of a constitutively active YAP mutant, YAP-S127A. Mutation in the serine residue targeted for phosphorylation by the negative regulatory kinases LATS1/2 prevents cytoplasmic sequestration of YAP by 14-3-3 proteins 5, 10, thus resulting in its exclusively nuclear localization (Fig. S1a). Retroviral vectors containing wild type YAP or YAP-S127A induced similar levels of protein expression (Fig. 1b). Ectopic expression of YAP-S127A induced a strong EMT and cell migration phenotype (Fig. S1b and S1c), and robustly promoted EGF-independent acinar growth of MCF10A in 3D-cultures (Fig. 1b). The enhanced YAP phenotype displayed by YAP-S127A also led to an increased effect in co-culturing experiments, with YAP-S127A expressing MCF10A cells, dramatically stimulating acini formation by neighboring vector-transfected cells (Fig. 1a). To further test whether YAP-expressing cells release growth-inducing factors, we collected conditioned medium from these 3D cultures and applied it onto parental MCF10A cells in 3D culture assays. Conditioned media derived from cells transduced with wild type YAP (YAP-Wt), and to an even greater extent YAP-S127A but not vector, enabled MCF10A acinus formation in the absence of EGF (Fig. 1c), supporting the existence of a diffusible factor mediating this non-cell autonomous effect.
To identify the putative factors secreted by YAP-expressing MCF10A cells, we applied conditioned media onto a Cytokine Antibody Array (RayBiotech™) representing 41 growth factors and cytokines 13. When media was collected from cells grown under standard 3D growth conditions (including EGF supplementation, allowing acinus formation by parental MCF10A cells), no significant differences were observed between vector and YAP-S127A transduced cells (Fig. 2a). However, conditioned media collected from these cultures in the absence of EGF supplementation revealed four proteins that were highly enriched (3-fold over background) in YAP-S127A transduced cells: amphiregulin (AREG), insulin-like growth factor binding protein -6 (IGFBP-6), platelet-derived growth factor -AA (PDGF-AA) and macrophage colony-stimulating factor-receptor (M-CSF-R) (Fig. 2a). To test whether these proteins were specifically induced by YAP, and not byproducts of 3D acini formation. We analyzed vector versus YAP-S127A expressing MCF10A cells grown in 2D monolayer cultures following withdrawal of EGF (12 hrs). We observed that only AREG was dramatically induced in YAP-S127A cells as determined by immunoblotting analysis (Fig. 2b). AREG thus constitutes the primary candidate for a YAP-induced secreted factor involved in cellular proliferation.
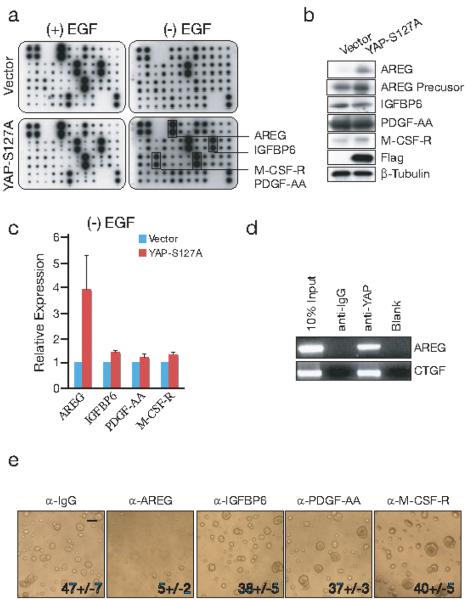
Figure 2. Amphiregulin (AREG) mediates YAP-induced EGF-independent growth.
(a) Secreted growth factor screen. Human Growth Factor Antibody Array analysis (RayBiotech, Norcross, GA) was performed using the 25th day conditioned medium from vector or YAP-S127A transduced cells grown in the presence (left) and absence (right) of EGF. The membrane is encoded with antibodies for 41 growth factors and receptors, with four positive and four negative controls in the upper left corner. Four proteins were predominantly enriched in YAP-S127A EGF-deprived conditioned medium (arrows). (b) Immunoblot of candidate YAP target genes from lysates of YAP-S127A and vector transduced MCF10A cells cultured on 2D monolayers withdraw EGF over night. (c) Induced AREG mRNA upregulation in YAP-S127A transduced cells in the absence of EGF, as detected by qRT-PCR. (d) In vivo binding of YAP to the AREG promoter by ChIP assay. The known YAP target promoter CTGF is shown as control. (e)AREG neutralizing antibody blocks YAP-S127A induced EGF-independent growth. YAP-S127A cells were cultured in Matrigel with 3D assay medium in the absence of EGF for 25 days, together with neutralizing antibody against AREG, IGFBP6, PDGF-AA or M-CSF-R, with normal goat IgG as control. Representative images are shown. (Scale bars, 100μm)
To test whether AREG is a transcriptional target of YAP, we first measured AREG mRNA in YAP transduced cells. A five-fold increase in the AREG transcript was observed in YAP-S127A transduced cells cultured in the absence of EGF (Fig. 2c). Binding of YAP to the AREG promoter in vivo was demonstrated by chromatin immunoprecipitation (ChIP) assays. Anti-YAP- immunoprecipited chromatin yielded a strong and reproducible PCR amplification for a fragment of the AREG promoter (Fig. 2d), comparable to that observed for the CTGF promoter, a known YAP target gene 9, 14.
Given the identification of AREG as a transcriptional target of YAP, we undertook a series of experiments to test the functional consequences of this interaction. We first tested whether neutralizing antibodies against AREG suppressed YAP-mediated 3D acini formation. Addition of 1μg/ml of anti-AREG IgG suppressed acini formation by YAP-S127A cells by 90%, whereas blocking antibodies to IGFBP6, PDGF-AA or M-CSF-R had no effect (Fig. 2e). AREG therefore contributes to YAP-mediated proliferation in this assay. To test whether AREG alone is sufficient to mediate the 3D growth of MCF10A cells, we added recombinant AREG to cultures of parental MCF10A cells. A dose-dependent effect of AREG was evident, equivalent to that of EGF in generating 3D acini (Fig. 3a).
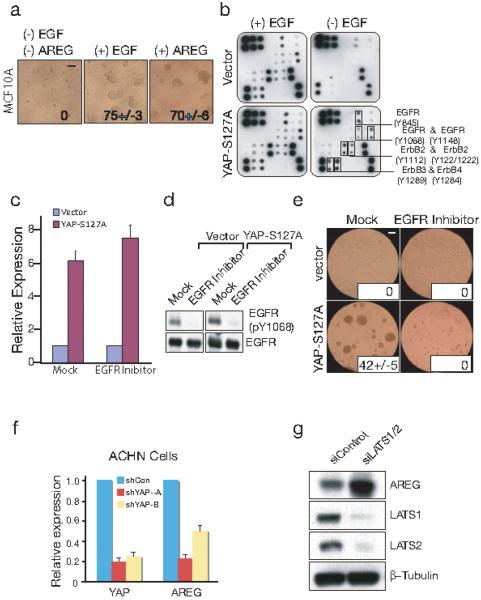
Figure 3. Regulation of AREG by the Hippo pathway.
(a) Recombinant AREG has an equivalent effect on MCF10A 3D acini growth as EGF. (Scale bars, 100μm) (b) YAP-S127A induces activation of ErbB receptor family. Human EGFR Phosphorylation Antibody Array analysis (RayBiotech, Norcross, GA) was performed using lysate from either vector or YAP-S127A transduced cells cultured in the presence and absence of EGF. Each dot presents the tyrosine phosphorylation of ErbB family members at a specific site. (c) AREG expression induced by YAP-S127A is independent from EGFR activation. qRT-PCR analysis of AREG expression in vector or YAP-S127A transduced cells in the presence and absence of EGFR inhibitor (Erlotinib). (d) Effectiveness of EGFR inhibition by erlotinib, demonstrated by abrogation of EGFR phosphorylation, shown by immunoblot. (e) EGFR inhibitor treatment abolishes YAP-induced 3D culture growth in the absence of EGF, indicating the requirement for EGFR signaling. (Scale bars, 100μm) (f) qRT-PCR analysis of AREG expression in ACHN cells infected with shRNAs targeting YAP. The Hippo pathway is activated in these cells by mutation of the upstream regulator Salvdador. (g) Knockdown of LATS1/2 induces expression of AREG. Immunoblotting analysis of AREG, LATS1 and LATS2; after treatment of MCF10A cells with control or LATS1/2 siRNA. β-Tubulin used as loading control.
To determine whether YAP-induced secretion of AREG is associated with increased ErbB receptor family signaling, we applied lysate from YAP-S127A transduced cells to an EGFR Phosphorylation Antibody Array (RayBiotech™). In the presence of exogenous EGF, the 17 ErbB phosphorylation sites interrogated by this array were comparable between vector and YAP-S127A transduced cells (Fig. 3b). However, in the absence of EGF, cellular lysates from YAP-S127A transduced cells displayed significantly increased phosphorylation of the classical EGFR target residues, EGFR-Y845, Y1068 and Y1148, as well as select residues within ErbB-2, ErbB-3 and ErbB-4 (Fig. 3b). Of note, EGFR activation by AREG in some cell types has been reported to involve an autocrine loop culminating in EGFR-dependent AREG induction 15. To exclude such a mechanism, we measured AREG levels in the presence or absence of erlotinib, a specific kinase inhibitor of EGFR. Efficient inhibition of EGFR signaling had no effect on YAP-mediated AREG induction (Fig. 3c and 3d). Thus, AREG induction by YAP appears to result from a direct transcriptional mechanism rather than from an EGFR-dependent feedback loop. Erlotinib treatment almost completely abrogated 3D acini formation by YAP-S127A transduced cells (Fig. 3e), further supporting the key role of EGFR signaling in YAP-mediated proliferation.
Ectopic overexpression of YAP mimics the effect of gene amplification in cancer. However, to model physiological activation of the Hippo pathway, we made use of ACHN kidney cancer cells, in which homozygous deletion of the upstream negative regulator Salvador, leads to elevated endogenous YAP activity 5, 10, 16. Knockdown of YAP in these cells led to a significant reduction of baseline AREG expression (Fig. 3f). YAP activity is inhibited by the upstream kinases LATS1/2, which mediate the cytoplasmic retention of YAP 5, 6, 10. To test whether physiological activation of YAP through abrogation of LATS1/2 leads to induction of AREG, we knocked down LATS1/2 transcripts in MCF10A cells. Dramatic induction of AREG expression was evident following suppression of LATS1/2 in these cells (Fig. 3h). Taken together, these observations demonstrate regulation of endogenous AREG expression by YAP and its induction by disruption at multiple levels of the Hippo pathway.
To test the contribution of AREG induction to the various YAP-mediated phenotypes, we made use of lentiviral shRNA-mediated knockdown constructs targeting AREG itself. In addition to 3D acini formation, YAP-dependent phenotypes in MCF10A cells include the induction of EMT and increased cellular migration. Efficient knockdown of both baseline and YAP-S127A induced AREG expression (both precursor and cleaved AREG) was accomplished using two independent AREG-targeting constructs (Fig. 4a). YAP-induced phosphorylation of the downstream signaling molecules AKT and ERK was effectively suppressed by AREG knockdown, demonstrating disruption of an autocrine pathway in these cells (Fig. 4a). As expected, both AREG-targeting constructs also dramatically inhibited EGF-independent 3D acini formation (Fig. 4b). In addition, suppression of AREG dramatically inhibited cell migration induced by both YAP-Wt and YAP-S127A (Fig. 4c). In contrast, knockdown of AREG had no effect on expression of EMT-related markers (Fig. 4d), suggesting that AREG may not contribute to this distinct YAP-dependent effect.
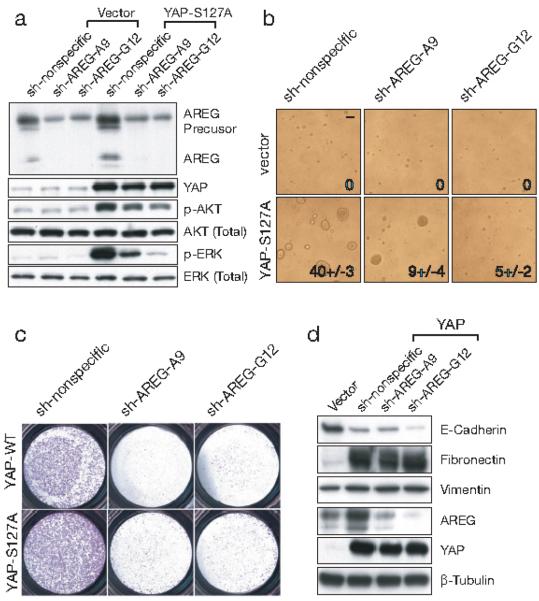
Figure 4. Requirement of AREG for YAP-mediated cell migration and 3D acini formation.
(a) Efficient knockdown of AREG with two independent lentiviral constructs, sh-AREG A9 and G12, introduced into vector or YAP-S127A transduced MCF10A cells. Knockdown of AREG inhibited the activation of AKT (p-AKT) and ERK (p-ERK) in YAP-S127A transduced cells. (b) Knockdown of AREG abrogates EGF-independent 3D acini formation induced by YAP-S127A. MCF10A cells transduced by vector or YAP-S127A were infected with lentivirus containing either control or AREG-targeting shRNAs and cultured on Matrigel for 25 days in the absence of EGF. Representative phase contrast images are shown. Insets: quantified 3D acini formation numbers as mean of four 200X fields. (Scale bars, 100μm) (c) Knockdown of AREG inhibits YAP-induced cell migration. YAP and YAP-S127A transduced MCF10A cells were infected by lentivirus containing the control or AREG-targeting shRNAs, plated onto 8-μmtranswell filters and allowed to migrate for 24 hrs in the absence of EGF. (d) Knockdown of AREG has no effect on YAP-induced EMT. Immunoblot of EMT markers (E-cadherin, Fibronectin and vimentin) in vector and YAP-S127A transduced MCF10A cells co-infected with shAREG (A9 and G12). β-Tubulin used as loading control.
Connective tissue growth factor (CTGF) has been recently identified as a transcriptional target of YAP together with the transcription factor TEAD, contributing to YAP-mediated EMT 14. To test whether CTGF plays a role in the YAP-dependent non-cell-autonomous effect studied here, we targeted CTGF expression in YAP-transduced MCF10A cells using two independent lentiviral shRNA constructs. Efficient knockdown of CTGF was accomplished, but had no effect on YAP-mediated acini formation (Fig. S 2a and 2b). Therefore, CTGF and AREG may serve as complementary effectors of YAP, mediating distinct downstream phenotypes.
The complex Hippo pathway shows a high degree of evolutionary conservation from Drosophila, where it was first uncovered to mammals. Despite the fact that components of the pathway leading to activation of mammalian YAP and Drosophila yki are highly conserved 1, 2, the downstream effectors of yki that have been identified to date in Drosophila screens do not appear to be similarly regulated in mammalian cells 5, 9. We therefore asked whether the relevant components of the EGFR pathway interact genetically with the core factors of the Drosophila Hippo pathway.
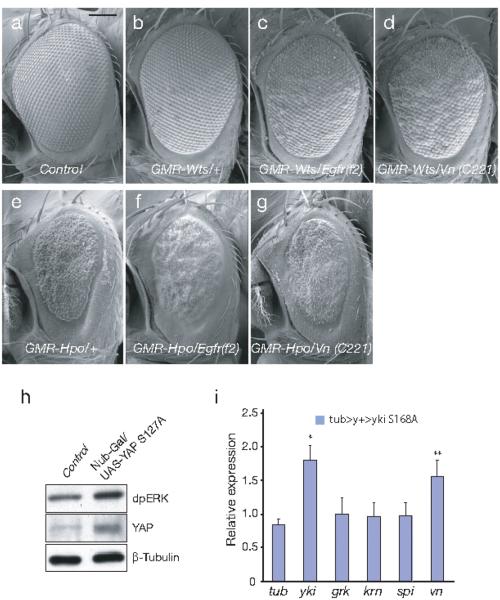
EGFR signaling in Drosophila involves one receptor (Egfr) and four Egfr ligands: Spitz, Keren, Gurken and Vein 17. To test for genetic interactions, we first crossed flies carrying mutations in EGFR, EGFR ligands, or EGFR processing proteins 17 with flies harboring mutations in the Hippo pathway, including warts (wts)and Hippo (Hpo), the negative regulators of yki, which were over-expressed under the control of an eye-specific promoter (GMR). Consistent with a previous report 16, GMR-wts eyes are normal in appearance (Fig. 5b) as compared to the wild-type (Fig 5a). A rough eye phenotype is evident when GMR-wts is combined with a heterozygous loss-of-function mutation of Egfr which itself does not have a phenotype (Fig. 5c). A similar synthetic interaction was also observed when GMR-wts was combined with several other loss-of-function alleles of Egfr (Table S1), or with a mutant allele of vein, an EGFR ligand (Fig. 5d). In contrast, mutant alleles of other EGFR ligands, such as spitz, keren,and gurken, produced no synergistic effect (Table S1). We carried out a second set of genetic tests combining GMR-hpo with EGFR pathway components. Unlike GMR-wts, the eye-specific overexpression of Hippo (hpo) generates a small, rough eye phenotype, in which the regular ommatidial organization is disrupted but individual ommatidia can still be distinguished 18 (Fig. 5e). When combined with heterozygous mutations of Egfr (Fig. 5f) or vein (Fig. 5g), the ommatidial organization was more severely disrupted: the eyes had a glassy appearance and fused ommatidia were apparent (Fig. 5f, g), suggesting that mutant alleles of Egfr and vein dominantly enhanced the GMR-hpo rough-eye phenotype. In contrast, mutant alleles of spitz, keren, or gurken hadnosucheffect (Table S1). In a third set of genetic analyses, we used the previously reported GMR-yki phenotype 19 (Fig. S 3b), which causes a bulgy, rough eye phenotype (GMR-yki). This yki overgrowth phenotype was partially suppressed by heterozygous loss-of-function alleles in Egfr (Fig. S 3c). Again, partial suppression of this phenotype was observed with mutant alleles of vein, but not with the other three EGFR ligands, i.e., spitz, keren, or gurken (Table S1). Taken together, these genetic crosses indicate that increased activity of the Drosophila YAP ortholog yki can be partially suppressed by Egfr pathway mutants, while reduced activity of the yki pathway resulting from overexpression of the negative regulators wts and hpo is enhanced by loss of function of the Egfr pathway.
Figure 5. Evolutionary conservation of interactions between EGFR and Hippo pathways in Drosophila.
(a) Scanning electron micrograph (SEM; 200X) of adult eye from wild type (GMR-Gal4/+). (Scale bars, 100μm) (b) Over-expression of wts in the eye (GMR-wts/+) displays normal morphology. (c, d) Eyes from GMR-wts/Egfrf2 and GMR-wts/+; vnC221/+ display a rough eye phenotype. (e) Over-expression of hpo in the eye (GMR-hpo/+) leads to a smaller and rough eye phenotype (f, g) GMR-hpo/Egfrf2; GMR-hpo/+; vnC221/+ lead to a glassy appearance and fused ommatidia were apparent. (h) YAPS127A induces dpERK activation. Immunoblot of dpERK was performed using wing imaginal discs from either control (w1118) or wing discs with YAPS127A overexpression (genotype: nubbin-Gal4/UAS-YAPS127A). β-Tubulin used as a loading control. (i) qRT-PCR analysis of Egfr ligands using eye discs either from control (w1118)or YkiS168A expressing flies (genotype: yw eyFlp/+; tub>w+>ykiSA/+). Ct volume normalized by ribosomal protein 49; β-Tubulin expression was used as internal negative control. (*p<0.001; **p<0.003 based on T-test)
To investigate the molecular mechanisms by which the Hpo and Egfr signaling pathways interact in Drosophila, we measured phospho-ERK activity by immunoblotting in wing imaginal discs ectopically expressing constitutively active form of human YAPS127A (genotype: w; nubbin-Gal4/UAS-YAPS127A; +). Increased phospho-ERK levels were observed in YAPS127A wing discs (Fig. 5h), consistent with activation of Egfr signaling.
To determine whether any of the Egfr ligands are induced by Drosophila Yki, we tested their expression using qRT-PCR in eye discs ectopically expressing the constitutively active form of Yki (YkiS168A, equivalent to YAPS127A, Huang et al., 2005) (genotype: yw eyFlp/+; tub>y+>ykiS168A/+). Among Egfr ligands, only vein displayed moderate, but reproducible increased expression in yki activated clones (Fig. 5i). The selective effect on this Egfr ligand is particularly interesting in that it is consistent with the genetic interactions between yki and Egfr pathways described above. These genetic studies also implicated vein, but not other ligands, as a modifier of the Hippo phenotypes (Table S1). Taken together, our results suggest interactions between the Hippo and Egfr pathways in Drosophila that are compatible with the functional relationship identified in mammalian cells. Since genetic interactions are often complex, further studies will be necessary to determine whether vein expression is directly regulated by Yki, or altered indirectly. Nevertheless, these results suggest that the selective induction of an Egfr ligand by Yki may contribute to the interplay between Egfr and Hippo pathways in Drosophila. Unlike mammalian cells, where YAP effectors have been implicated in cell autonomous pathways, other secreted ligands have been linked to the Drosophila Hippo pathway. Yki activation leads to activation of Wingless, a ligand for the Wnt pathway and Serrate, a ligand for the Notch pathway 20, 21. Loss of the upstream Hippo component fat also results in activation of Egfr signaling 22. Activation of Egfr signaling through AREG secretion in mammalian cells may thus represent an evolutionarily conserved feature of the Hippo pathway.
In summary, the EGFR ligand AREG appears to be a downstream effector of the Hippo pathway and a direct target of YAP. Although the role of YAP in mammalian systems was first established through tumorigenesis studies, it has been recently linked to the regulation of progenitor cell pools and organ size during liver regeneration 4, 5. As such, YAP target genes may play important roles in both physiological and malignant proliferation signals. YAP target genes have been investigated by gene expression profiling in MCF10A cells and in the mouse 5, 6, 9, 14. Interestingly, AREG is among potential targets listed in these screens in MCF10A cells. YAP mediated transcriptional induction is thought to involve its binding to known transcription factors, including p73, Runx2 and TEAD family members 1, 2, 23. Our Chip experiments were unable to identify binding sites for these transcription factors in the AREG promoter (data not shown), pointing to potential additional transcriptional partners for YAP in mediating AREG induction.
Like YAP, AREG overexpression has been implicated in both normal organ proliferation and malignancy. In addition to its secretion by many cancer cell lines 24-26, AREG mediates protection against liver injury in a mouse model 27, and in the breast, it is a key mediator of estrogen-induced pubertal epithelial morphogenesis. Mouse knockouts of estrogen receptor alpha, AREG and EGFR all display similar defects in mammary gland development 28. Thus, in the breast and potentially other tissues, AREG may mediate both developmentally regulated proliferation and malignant transformation, consistent with its contribution to the broad role of YAP and the Hippo pathway in the regulation of cellular homeostasis. The fact that YAP may mediate some of its key functions through a secreted growth factor implicates a non-cell autonomous mechanism that may contribute to the expansion of both normal and malignant progenitors, raising the possibility of eventual therapeutic applications.
METHODS
Cell Culture and Transfections
MCF10A cell culture and 3D culture were performed as described 29. ACHN cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50 U/ml penicillin/streptomycin and incubated at 5% CO2 at 37 °C. Transwell cell migration assay was performed as previously described 7.
For knockdown studies, shRNA hairpins targeting human YAP and AREG were obtained from the RNAi Consortium (Broad Institute). Forward oligo sequences are listed (in the 5′-3′ direction): shYAP-A: CCGGCCCAGTTAAATGTTCACCAATCTCGAGATTGGTGAACATTTAACTGGGTTTTTG; shYAP-B: CCGGGCCACCAAGCTAGATAAAGAACTCGAGTTCTTTATCTAGCTTGGTGGCTTTTTG. shAREG-A9: CCGGCACTGCCAAGTCATAGCCATACTCGAGTATGGCTATGACTTGGCAGTGTTTTTG; shAREG-G12:CCGGGAACGAAAGAAACTTCGACAACTCGAGTTGTCGAAGTTTCTTTCGTTCTTTTTG. Control shRNA: CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT. shCTGF-1 and shCTGF-2 are as previously reported 14. Lentivirus packaging, MCF10A cell transduction and drug selection were performed following standard protocols. Human YAP expression construct was described previously 7; YAP-S127A expression construct was synthesized by PCR-based mutagenesis. siRNA experiments were performed as described 9. siRNA duplex (ON-TARGETplus-SMARTpool) targeting human LATS1(L-004632-00), LATS2 (L-003865-00) and non-targeting control (D-001810-10) were purchased from Dharmacon RNAi Technologies.
For conditioned medium treatment, vector, YAP-Wt or YAP-S127A transduced MCF10A cells were grown in 3D culture in the absence of EGF. Media were collected and added to MCF10A parental cells in 3D culture every 4 days.
Antibodies and Molecular Analyses
Phospho-AKT (Ser-473), phospho-p44/42 MAP kinase (Thr202/Tyr204), total AKT, ERK, and YAP (phospho-S127) antibodies were purchased from Cell Signaling Technology (Beverly, MA); Phospho-EGFR (Y1068) antibody from Biosource (Camarillo, CA); YAP, EGFR antibodies from Santa Cruz biotechnology (Santa Cruz, CA); LATS1 and LATS2 antibodies from Bethyl (Montgomery TX); β-Tubulin antibody from upstate (Lake Placid, NY); Flag (M2) antibody from Sigma (St. Louis, MO); hAREG, hIGFBP-6, hPDGF-AA, hM-CSF R and control IgG neutralizing antibodies were purchased from R & D systems (Minneapolis, MN). For Western blot analysis, cells were washed with phosphate-buffered saline and collected with IP buffer: 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 20% glycerol, 0.5% NP-40, 1x protease inhibitor cocktail (Complete™ EDTA-free, Roche). Cell lysate was cleared by centrifugation at 14,000 rpm for 20 min at 4 °C. Lysate was loaded onto 4-15% SDS-PAGE gel (ReadyGel, Bio-Rad) with 2X SDS sample buffer. For immunoblotting analysis, proteins were transferred onto Immobilon PVDF (Millipore), detected by various antibodies and visualized with Western Lightning Plus Chemiluminescence Kit (Perkin Elmer). The Human Cytokine Antibody Array and Human EGFR Phosphorylation Antibody Array analyses were performed according to the user manual (RayBiotech, Norcross, GA).
For RNA preparation and qRT-PCR detection, RNA was extracted using the RNeasy Mini Kit (Qiagen). cDNA synthesis was performed using First-Strand cDNA Synthesis Kit (GE Healthcare) and quantitative real-time PCR was performed using Power SYBR Green PCR Master Mix (Applied Biosystems).
Sequences of the qPCR primer pairs (in the 5′-3′ direction) are as follows: GAPDH-F: GGTGAAGGTCGGAGTCAACGG; GAPDH-R: GAGGTCAATGAAGGGGTCATTG; YAP-F: CCTTCTTCAAGCCGCCGGAG; YAP-R: CAGTGTCCCAGGAGAAACAGC; AREG-F: CGAACCACAAATACCTGGCTA; AREG-R: TCCATTTTTGCCTCCCTTTT; IGFBP6-F: CACTGCCCGCCCACAGGATGTG; IGFBP6-R: CTCGGTAGACCTCAGTCTGGAG; PDGF-AA-F: GGGGTCCATGCCACTAAGCATGTGC; PDGF-AA-R: GGAATCTCGTAAATGACCGTCC; M-CSF1-R-F: CAGGAAGGTGATGTCCATCAGC; M-CSF1-R-R: CCAGCTCTGCAGGCACCAG. All measurements were performed in triplicate and standardized to the levels of GAPDH. Sequences of the qPCR primer pairs (in the 5′-3′ direction) for flies are as follows: Rp49-F: ACAGGCCCAAGATCGTGAAGA; Rp49-R: CGCACTCTGTTGTCGATACCCT; Tubulin-F: AGACCTACTGCATCGACAAC; Tubulin-R: GACAAGATGGTTCAGGTCAC; Yki-F: GCGCCTTGCCGCCGGGATG; Yki-R: GCTGGCGATATTGGATTCTG; Grk-F: CTGTTGCGACGCCAAATTG; Grk-R: CCGATTGTCCACCACTAGGAAA; Krn-F: CTGCATTGCCACATTCCCA; Krn-R: CGGTAGGTAAGCGCCGATTAA; Spi-F: AGGAACTGCAGCAGGAATACGA; Spi-R: AGCTTGCGCTCCAGAATGACT; Vn-F: ATCAAGCATGGAAAGAAGCTGC; Vn-R: CGCTTGCGATTGATGGACTT
Chromatin Immunoprecipitation (ChIP)
Assays were performed as previously described 30. Briefly, MCF10A cells transduced with YAP-S127A retrovirus were cross-linked, lysed, and sonicated to generate DNA fragments with an average size of 0.5 kb. The immunoprecipitation was performed using 1 μg antibody to IgG and YAP (Santa Cruz, CA), respectively. Sequences of ChIP PCR primers (in the 5′-3′ direction) are: AREG-F: CCAAAAGAATCTTTTGAAATCTTTGGGC; AREG-R: GTGAGGGTATGACCAAGTATGGGAAATAAG. Sequences of the CTGF PCR primers were as previously reported 14.
Drosophila Genetic Analyses
All flies were maintained on standard cornmeal-agar-molasses medium, and w1118 flies were used as the control. To test the genetic modification of the yki+ over-expression phenotype, we first established the w−; GMR-Gal4/CyO; UAS-yki+/TM6B stock by combining the GMR-Gal4 chromosome with UAS-yki+. The female virgins (w−;GMR-Gal4/CyO; UAS-yki+/TM6B) were then crossed with males of different EGFR signaling mutant alleles (obtained from the Bloomington Drosophila Stock Center, Table S1). All of the crosses were cultured at 25°C and the resulting female progenies without balancer chromosomes (with genotypes of either w−; GMR-Gal4/mutant; UAS-yki+/+ or w−;GMR-Gal4/+; UAS-yki+/mutant) were analyzed and presented in Figure S3 and Table S1. To test potential interactions with hpo and wts, we performed similar genetic crosses using GMR-hpo+/CyO or GMR-wts+/CyO stocks in Figure 5 and Table S1..
Immunoblotting of dpERK was performed by dissecting the wing imaginal discs from third instar larvae either control (w1118)or nubbin-Gal4/UAS-YAPS127A. qRT-PCR analysis of Egfr ligands was carried out using eye discs from either control (w1118)or YkiS168A over-expression larvae (genotype: yw eyFlp/+; tub>w+>ykiSA/+). Following standard protocols for both western blot and qRT-PCR. Primers for qRT-PCR were listed in the previous section.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs Duojia Pan, Max Frolov, G Halder, and Zhichun Lai for generously sharing Drosophila stocks used in this work. We appreciate discussions with Drs Julie Wells, Fajun Yang and Tianyi Zhang and thank Bill Fowle for assistance with electron microscopy. This work was supported by the National Institutes of Health (NIH) grant R01 95281, the Doris Duke Foundation Distinguished Clinical Investigator Award, the National Foundation for Cancer Research grant and the Howard Hughes Medical Institute (to D.A.H.); NIH grants CA080111 and CA089393 and the Breast Cancer Research Foundation (to J.S.B.); NIH T32 training grants CA09361-27 (to J.Z.) and CA09361 (to M.O.); NIH grants F32 CA117737 (to G.A.S.); NIH grants GM81607 and GM053203 and the Saltonstall Foundation (to N.J.D.); and the Tosteson postdoctoral Fellowship (to J.Y.J.).
REFERENCES
- 1.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–91. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 2.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–97. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 3.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–21. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 4.Camargo FD, et al. YAP1 Increases Organ Size and Expands Undifferentiated Progenitor Cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 7.Overholtzer M, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–10. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zender L, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008;68:2789–94. doi: 10.1158/0008-5472.CAN-07-6205. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Soule HD, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–86. [PubMed] [Google Scholar]
- 13.McAllister SS, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008 doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willmarth NE, Ethier SP. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J Biol Chem. 2006;281:37728–37. doi: 10.1074/jbc.M606532200. [DOI] [PubMed] [Google Scholar]
- 16.Tapon N, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–78. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 17.Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–27. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- 18.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–56. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–50. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 21.Mao Y, et al. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–51. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 22.Garoia F, et al. The tumor suppressor gene fat modulates the EGFR-mediated proliferation control in the imaginal tissues of Drosophila melanogaster. Mech Dev. 2005;122:175–87. doi: 10.1016/j.mod.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–46. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berquin IM, Dziubinski ML, Nolan GP, Ethier SP. A functional screen for genes inducing epidermal growth factor autonomy of human mammary epithelial cells confirms the role of amphiregulin. Oncogene. 2001;20:4019–28. doi: 10.1038/sj.onc.1204537. [DOI] [PubMed] [Google Scholar]
- 25.Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007;117:337–45. doi: 10.1172/JCI29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streicher KL, et al. Activation of a nuclear factor kappaB/interleukin-1 positive feedback loop by amphiregulin in human breast cancer cells. Mol Cancer Res. 2007;5:847–61. doi: 10.1158/1541-7786.MCR-06-0427. [DOI] [PubMed] [Google Scholar]
- 27.Berasain C, et al. Novel role for amphiregulin in protection from liver injury. J Biol Chem. 2005;280:19012–20. doi: 10.1074/jbc.M413344200. [DOI] [PubMed] [Google Scholar]
- 28.McBryan J, Howlin J, Napoletano S, Martin F. Amphiregulin: role in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:159–69. doi: 10.1007/s10911-008-9075-7. [DOI] [PubMed] [Google Scholar]
- 29.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 30.Wells J, Farnham PJ. Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods. 2002;26:48–56. doi: 10.1016/S1046-2023(02)00007-5. [DOI] [PubMed] [Google Scholar]
- 31.Gabay L, Seger R, Shilo BZ. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277:1103–6. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.