Abstract
Adrenic acid (docosatetraenoic acid), an abundant fatty acid in the adrenal gland, is identical to arachidonic acid except for two additional carbons on the carboxyl end. Adrenic acid is metabolized by cycloxygenases, cytochrome P450s, and lipoxygenases; however, little is known regarding the role of adrenic acid and its metabolites in vascular tone. Due to its abundance in the adrenal gland, we investigated the role of adrenic acid in vascular tone of bovine adrenal cortical arteries and its metabolism by bovine adrenal zona glomerulosa cells. In adrenal cortical arteries, adrenic acid caused concentration-dependent relaxations, which were inhibited by the epoxyeicosatrienoic acid antagonist 14,15-epoxyeicosa-5(Z)-enoic acid and the cytochrome 450 inhibitor SKF-525A. The large-conductance calcium-activated potassium channel channel blocker iberiotoxin or removal of the endothelium abolished these relaxations. Reverse-phase high-pressure liquid chromatography and liquid chromatography/mass spectrometry isolated and identified numerous adrenic acid metabolites from zona glomerulosa cells including dihomo-epoxyeicosatrienoic acids and dihomo-prostaglandins. In denuded adrenal cortical arteries, adrenic acid caused concentration-dependent relaxations in the presence of zona glomerulosa cells but not in their absence. These relaxations were inhibited by SKF-525A, 14,15-epoxyeicosa-5(Z)-enoic acid, and iberiotoxin. Dihomo-16,17-epoxyeicosatrienoic acid caused concentration-dependent relaxations of adrenal cortical arteries, which were inhibited by 14,15-epoxyeicosa-5(Z)-enoic acid and high potassium. Our results suggest that adrenic acid relaxations of bovine adrenal cortical arteries are mediated by endothelial and zona glomerulosa cell cytochrome P450 metabolites. Thus, adrenic acid metabolites could function as endogenous endothelium- and zona glomerulosa-derived hyperpolarizing factors in the adrenal cortex and contribute to the regulation of adrenal blood flow.
Keywords: endothelium-dependent relaxation, cytochrome P450, epoxygenase, cyclooxygenase, potassium channels, endothelium-derived hyperpolarizing factor, adrenal cortex
Introduction
Arachidonic acid (20:4, ω-6) is the precursor to eicosanoids, a group of signaling molecules with diverse physiological action, including the regulation of vascular tone. Adrenic acid (7,10,13,16-docosatetraenoic acid, 22:4, ω-6) is an abundant polyunsaturated fatty acid in the adrenal gland, brain, kidney, and vasculature that is identical to arachidonic acid except for two additional carbons on the carboxyl end (1–5). Adrenic acid is formed by arachidonic acid chain elongation or elongation and desaturation of the essential fatty acid linoleic acid (18:2, ω-6), the precursor to arachidonic acid (1, 6). Adrenic acid can also be converted to arachidonic acid by b-oxidation (1, 3, 6, 7).
As with arachidonic acid, adrenic acid is metabolized by cycloxygenases (COXs), lipoxygenases (LOs), and cytochrome P450s (CYP450s) to dihomo (DH)-eicosanoids. Adrenic acid is metabolized in the renal medulla to DH-thromboxane and DH-prostaglandins (PGs) (4) and in platelets to DH-thromboxane and DH-hydroxyeicosatetraenoic acids (HETEs) (8). Our laboratory has demonstrated that adrenic acid is metabolized in human vascular endothelial cells to DH-prostacyclin (PGI2), which inhibited thrombin-induced platelet aggregation (9). We have also demonstrated that adrenic acid induces concentration-dependent relaxations of bovine coronary arteries, which were endothelium-dependent, blocked by K+ channel inhibition, and inhibited by COX and CYP450 inhibition (10). Several adrenic acid metabolites of coronary arteries were isolated and identified, including DH-PGs and DH-epoxyeicosatrienoic acids (EETs). In particular, DH-16,17-EET, the dihomo analog of 14,15-EET, induced concentration-dependent relaxations by activation of vascular smooth muscle K+ channels and hyperpolarization (10). These observations are consistent with another study examining the effect of DH-EETs in porcine coronary arteries (11).
While adrenic acid metabolites play a potential role in the regulation of vascular tone in the coronary circulation, little is known concerning the role of adrenic acid in other vascular beds. Due to its abundance in the adrenal gland, we investigated the role of adrenic acid in the regulation of vascular tone of adrenal cortical arteries. A complex interaction occurs between the adrenal zona glomerulosa (ZG) cells and its vasculature in the regulation of adrenal arterial tone (12, 13). Therefore, we additionally investigated the metabolism of adrenic acid by ZG cells and the vascular effect of ZG cell-derived docosanoids on adrenal cortical arteries.
Methods
Vascular reactivity
Fresh bovine adrenals were obtained from a local slaughterhouse. Small adrenal cortical arteries closely attached to the adrenal surface (200–300 µm) were dissected and cleaned of connective tissues. Isolated arterial segments were threaded on two stainless steel wires (40 µm diameter) and mounted on a four-chamber wire myograph (model 610M, Danish Myo Technology) in physiological saline solution (PSS) containing 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.17 mM MgSO4, 24 mM NaHCO3, 1.18 mM KH2PO4, 26 µM EDTA, and 5.5 mM glucose, bubbled with 95% O2–5% CO2, at 37°C for 30 min. as previously described (14). Arteries were gradually stretched to a resting tension of 1 millinewton (mN) and stimulated with KCl (60 mM) plus U-46619 (100 nM) 3 times for 10 min at 10-min intervals. Arteries were then allowed to equilibrate for another 30 min prior to the initiation of experimental protocols.
The arteries were preconstricted to 50–75% of maximal KCl contraction by addition of the thromboxane A2 mimetic U-46619 (10–30 nM). Cumulative concentration-responses to adrenic acid (10−8-10−4 M) or DH-16,17-EETs (10−8-10−5 M) were measured. To examine the role of nitric oxide (NO) and PGs in the vascular responses, arteries were pretreated for 10 min with L-nitro-arginine (L-NA) (30 µM), an endothelial NO synthase (eNOS) inhibitor, or indomethacin (10 µM), a COX inhibitor, alone or in combination. To characterize the role of CYP450s and K+ channels in vascular responses, arteries were pretreated for 10 min with the CYP450 inhibitor, SKF-525A (10 µM), the EET antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) (10 µM), 60 mM KCl, or the inhibitor of large-conductance Ca2+-activated K+ (BKCa) channels, iberiotoxin (100 nM). Experiments were performed on arteries with intact endothelium.
To examine the metabolism of adrenic acid by ZG cells and their vascular effect on adrenal cortical arteries, a subset of experiments were performed in the presence of ZG cells (5 × 105 – 1 × 106) with denuded vessels. Where indicated, the endothelium was removed by gently rubbing the intimal surface with a human hair. The endothelium was considered intact if acetylcholine (1 µM) caused >80% relaxation of arteries precontracted with U-46619 and effectively removed (denuded) if acetylcholine induced <10% relaxation. Results were expressed as percentage relaxation with 100% relaxation representing basal tension.
Adrenal ZG cell isolation
ZG cells were prepared by enzymatic dissociation of adrenal cortical slices as previously described (15). Cells were 95–98% ZG cells and 2–5% zona fasiculata cells. Adrenal fibroblasts were isolated and cultured as previously described (15).
Metabolism of adrenic acid by ZG cells
ZG cells (5×106) were incubated in 5 mL 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer at 37oC with [1-14C] adrenic acid (0.8 µCi, 10−7 M). After 15 minutes, calcium ionophore A-23187 was added to final concentration of 5 µg/mL and the incubation continued for 10 min. A portion of sample (100 µL) was used for protein analysis (Bio-Rad laboratories, Hercules, CA). Incubations were stopped by adding ethanol to 15% and buffer and cells were separated by centrifugation. The buffer was removed and extracted using C18 Bond Elut solid phase extraction columns (Varian, Harbor City, CA) as previously described (15, 16). The samples were evaporated to dryness under a stream of N2 and stored at −80�C until analysis of high-pressure liquid chromatography (HPLC). For liquid chromatography-mass spectrometry (LC/MS), incubations were repeated with using adrenic acid (10−4 M) and ZG cells (5×106) under similar conditions.
Reverse-phase HPLC
Adrenic acid metabolites were resolved by reverse-phase HPLC (Nucleosil-C18 column, 5 mm, 4.6 × 250 mm) using solvent system I as previously described (15). Solvent A was water and solvent B was acetonitrile containing 0.1% glacial acetic acid. The program was a 35 min linear gradient from 50% solvent B to 94% solvent B and flow rate of 1 mL/min. Column eluates were collected in 0.2 mL fractions and radioactivity of each fraction was determined by liquid scintillation spectrometry.
For LC/MS, column eluates were collected as four major peaks corresponding to the reported major adrenic acid metabolites (10). The retention time range of the four peaks are: 3–6.8 min, 15.6–20.6 min, 21–24.6 min and 24.6–30 min. These fractions were acidified with glacial acetic acid and extracted with cyclohexane/ethyl acetate (50:50). The combined organic extracts were dried under a stream of N2 and stored at −40�C until liquid chromatography-tandem mass spectrometry (LC/MS/MS) analysis.
Liquid chromatography-electrospray ionization mass spectrometry
The chemical identity of the major metabolites isolated by reverse-phase HPLC was determined by liquid chromatography-electrospray ionization mass spectrometry (LC-ESI/MS) (17). HPLC was performed using a reverse-phase C18 column (Kromasil; 250 × 2 mm) and a Waters 2695 liquid chromatograph (Waters Corporation, Milford, MA). Samples were dissolved in acetonitrile and the volume of injection was 10 µl. The mobile phase consisted of solvent A, water containing 0.01% glacial acetic acid, and solvent B, acetonitrile containing 0.01% glacial acetic acid. The program was a 40 min linear gradient from 50% solvent B to 100% solvent B with a flow rate of 0.2 mL/min. Mass spectrometry was performed using a Micromass Quattro Micro API mass spectrometer (Waters Corporation, Milford, MA) equipped with an electrospray ionization source. The mass spectrometer was operated in the negative ion mode. Product ion spectra were generated by collision-induced decomposition of the precursor ions (m/z 381 for DH-PGF2α; m/z 379 for DH-PGE2; m/z 363 for DH-dihydroxyeicosatetraenoic acids (DH-DiHETEs); m/z 365 for dihydroxyeicosatrienoic acid (DH-DHETs); m/z 307 for 14-hydroxy-7,10,12-nonadecatrienoic acid (14-HNT); and m/z 347 for DH-HETEs and DH-EETs, respectively). Only the precursor ion was allowed to pass through the first quadrupole and the ion was activated by collision with argon in the second quadrupole. Product ion spectra were recorded for the m/z range of 50 to 420. Results were processed using Masslynx version 4.0 software (Micromass) (18).
Whole cell patch clamp
Bovine adrenal cortical artery smooth muscle cells (SMC) were freshly isolated and whole cell recordings of K+ currents were obtained as previously described (10, 19, 20).
Statistics
Vascular reactivity and patch clamp data are expressed as mean ± SEM. Significant differences between mean values were evaluated by ANOVA followed by the Student-Newman-Keuls multiple comparison test. Significance was accepted at a value of p<0.05.
Results
Vascular response to adrenic acid
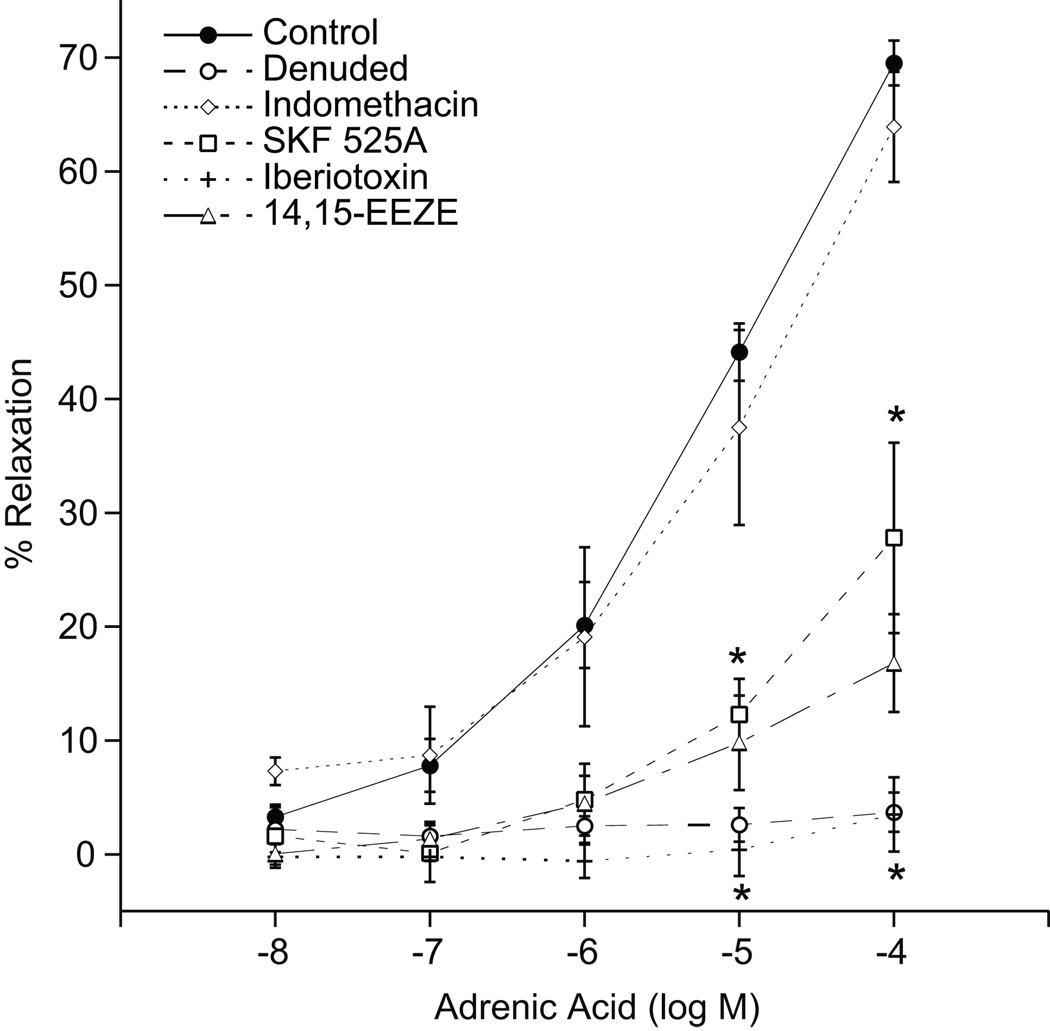
Adrenic acid caused concentration-dependent relaxations of the preconstricted adrenal cortical arteries with a maximal response of 70 ± 2% at 10−4 M (Fig 1). The CYP450 inhibitor SKF-525A (10 µM) and the EET antagonist 14,15-EEZE (10 µM) attenuated the adrenic acid relaxations (maximal relaxations of 28 ± 8% and 17 ± 4, respectively at 10−4 M). Treatment of arteries with the BKCa channel blocker, iberiotoxin (100 nM), or the removal of endothelium abolished the relaxations. Indomethacin did not alter adrenic acid relaxations. These findings suggest that endothelial CYP450 metabolites mediate the relaxations to adrenic acid and the relaxations are dependent on K+ channel activation.
Figure 1.
Adrenic acid-induced relaxations of bovine adrenal cortical arteries. Arterial rings were precontracted with U-46619 (10–30 nM) and changes in isometric tension were measured. Adrenic acid (10−8-10−4 M) induced relaxations in the absence or presence of endothelium, indomethacin (10 uM), SKF-525A (10 µM), iberiotoxin (100 nM), or 14,15-EEZE (10 µM). Each value represents the mean ± SEM. N = 4–28. *Significantly different from control, P<0.05.
Vascular response to ZG cell metabolites of adrenic acid
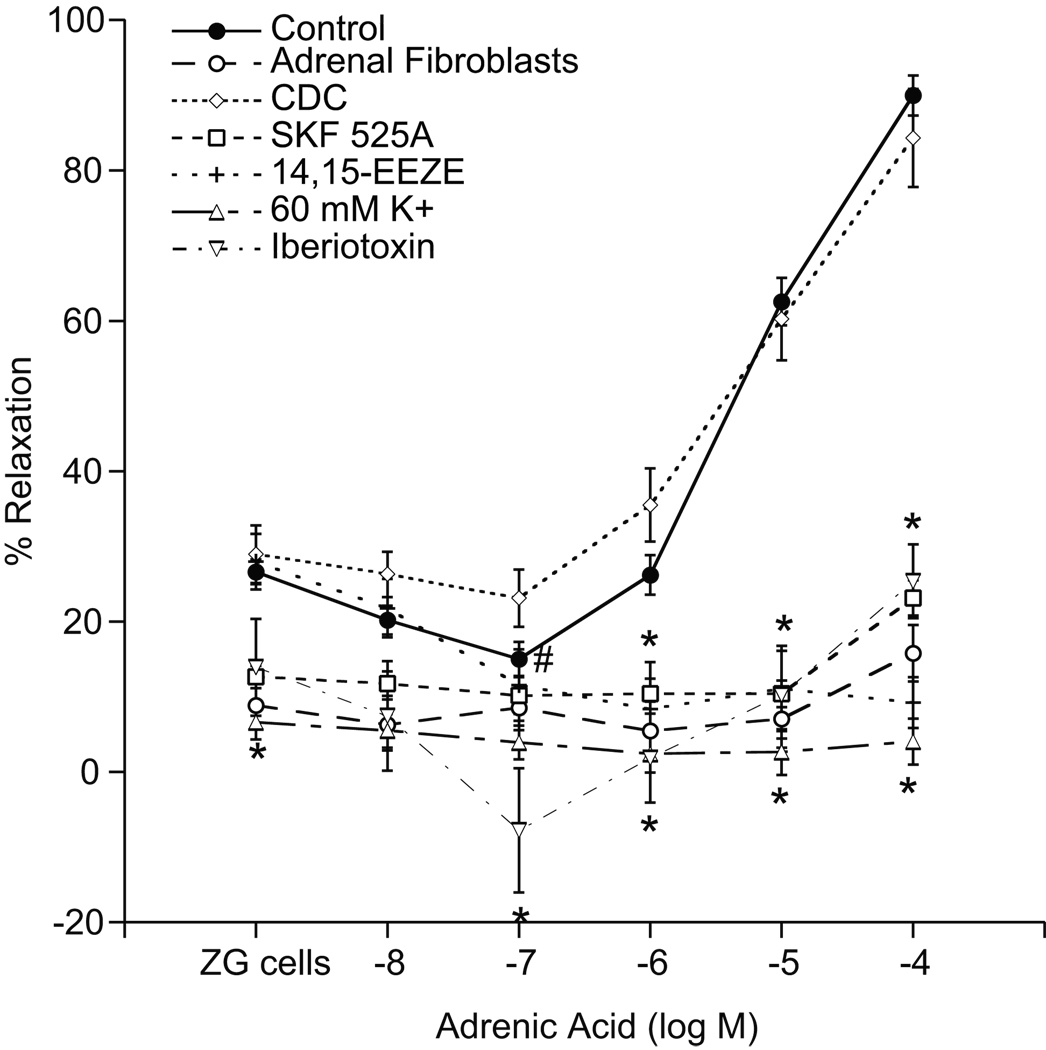
Steroid-producing cells produce diffusible vasorelaxing factors (12, 13). To examine if ZG cells metabolize adrenic acid to a vasorelaxant, adrenic acid concentration-responses were performed with denuded adrenal cortical arteries in the presence of ZG cells (Fig 2). Denuded arteries do not relax in response to adrenic acid alone; therefore, any observed relaxations of denuded vessels to adrenic acid in the presence of ZG cells can be attributed to adrenic acid metabolites produced by ZG cells. For consistency with previous experiments, all denuded vessels were pretreated with the eNOS inhibitor, L-NA (30 µM). Indomethacin (10 µM) did not alter the response to adrenic acid (data not shown) and was included in all further preparations. The addition of ZG cells (5 × 105 – 1 × 106) to preconstricted adrenal cortical arteries resulted in a slight relaxation (27 ± 2%), consistent with previous observations (13). Adrenic acid caused a slight contraction at 10−7 M (15 ± 3%) but concentration-dependent relaxations at higher concentrations (10−6-10−4 M) (maximal response of 89 ± 3% at 10−4 M). These relaxations were abolished by SKF-525A (10 µM), a CYP450 inhibitor that does not inhibit aldosterone synthesis (13). The EET antagonist 14,15-EEZE (10 µM) also abolished these relaxations, as did high K+ (60 mM) and iberiotoxin (100 nM). Inhibition of lipoxygenases with cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC) (10 µM) did not alter the relaxations to adrenic acid but inhibited the contractions observed with 10−7 M adrenic acid. When adrenal fibroblasts were substituted for ZG cells, no significant vascular responses to adrenic acid were detected. These results suggest that ZG cells produce transferrable CYP450 metabolites of adrenic acid, presumably a DH-EET that induces relaxations by activation of smooth muscle cell K+ channels.
Figure 2.
Vasorelaxations to ZG cell metabolites of adrenic acid. Denuded arterial rings were precontracted with U-46619 (10–30 nM) in the presence of indomethacin (10 µM) and L-NA (30 µM). Changes in isometric tension were measured. Adrenic acid (10−8-10−4 M) induced relaxations in the absence or presence of adrenal fibroblasts, SKF-525A (10 µM), 14,15-EEZE (10 µM), high K+ (60 mM), or iberiotoxin (100 nM). Each value represents the mean ± SEM. N = 5–36. *Significantly different from control, P<0.05. #Significant contraction from ZG cell baseline, P<0.05.
Metabolism of adrenic acid by bovine ZG cells
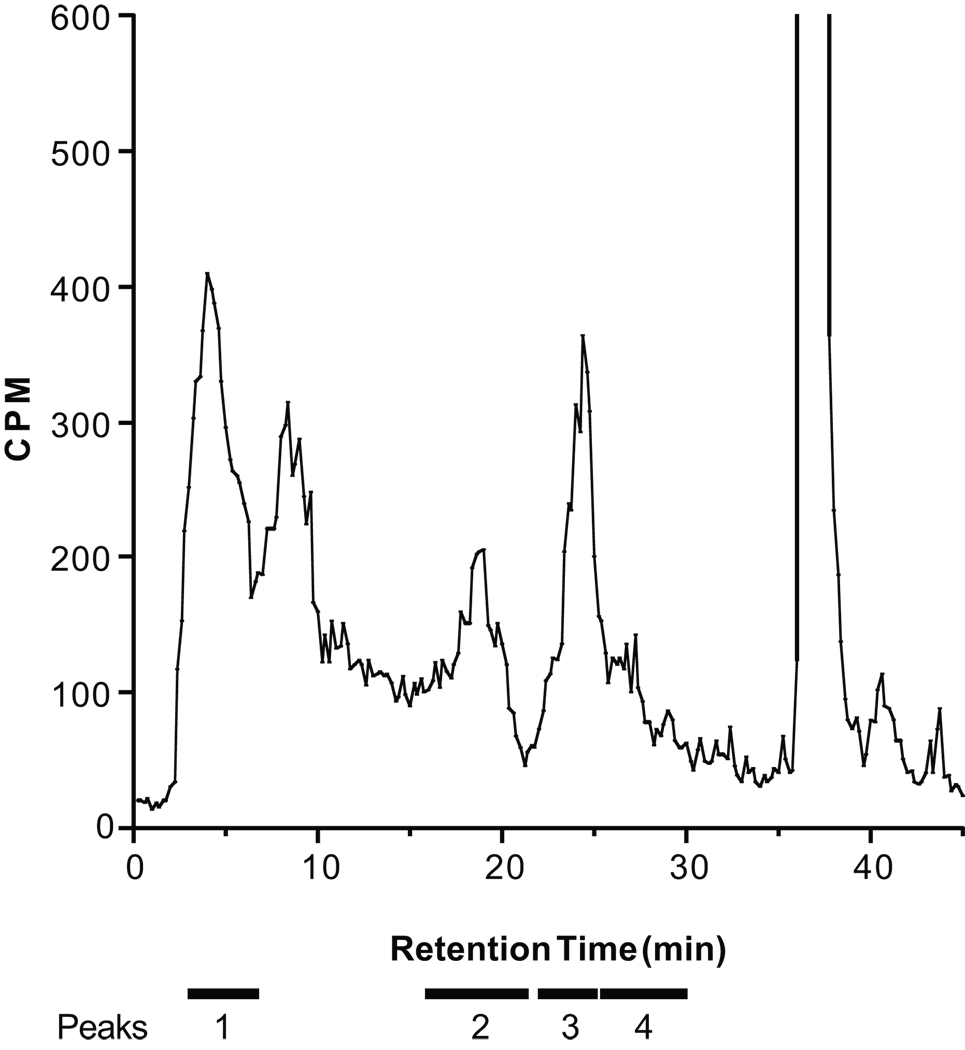
Because CYP450 metabolites appear to contribute to adrenic acid-induced relaxation, we investigated the metabolism of adrenic acid by ZG cells. Cells were incubated with 14C-labeled adrenic acid and the medium was extracted and purified by HPLC. Adrenic acid was metabolized to four major peaks (Fig 3). The four peaks were separately collected and analyzed by LC-ESI/MS (Table 1).
Figure 3.
Metabolism of [14C] adrenic acid by bovine adrenal zona glomerulosa cells. Metabolites were separated by reverse-phase HPLC and radioactivity was monitored. The location of the four peaks further analyzed by mass spectrometry and non-metabolized adrenic acid are notated above the chromatogram.
Table 1.
Mass spectral analysis of adrenic acid metabolites from bovine adrenal ZG cells.
| Peak | Retention time (min) | MS (m/z) [M-H]− | MS/MS (m/z) | Name |
|---|---|---|---|---|
| 1 A | 6.20 | 381 | 381,363,345,337,291,275,193 | DH-PGF2a |
| B | 8.4 | 379 | 379,361,343,299,261,217 | DH-PGE2 |
| 2 A | 20.60 | 363 | 363,345,263,183 | DH-10,17-DiHETE |
| B | 21.80 | 363,345,237,221 | DH-13,15-DiHETE | |
| C | 22.25 | 363,345,263,221 | DH-13,17-DiHETE | |
| D | 23.15 | 363,345,327,223,181,153 | DH-10,14-DiHETE | |
| E | 23.89 | 363,345,223,143 | DH-7,14-DiHETE | |
| F | 20.1 | 365 | 365,347,235 | DH-16,17-DHET |
| G | 21.25 | 365,347,253,195 | DH-13,14-DHET | |
| H | 21.9 | 365,347,213,155 | DH-10,11-DHET | |
| I | 22.57 | 365,347,173 | DH-7,8-DHET | |
| J | 21.6 | 307 | 307,289,207 | 14-HNT |
| 3 A | 27.7 | 347 | 347,329,247 | DH-17-HETE |
| B | 28.6 | 347,329,195 | DH-13-HETE | |
| C | 29.2 | 347,329,207 | DH-14-HETE | |
| D | 29.5 | 347,329,203,143 | DH-7-HETE | |
| E | 29.87 | 347,329,195,167,151 | DH-11-HETE | |
| 4 A | 33.34 | 347 | 347,329,247 | DH-16,17-EET |
| B | 34.60 | 347,329,207,195 | DH-13,14-EET | |
| C | 34.85 | 347,329,285,195,163,155 | DH-10,11-EET | |
| D | 35.51 | 347,329,143,127 | DH-7,8-EET |
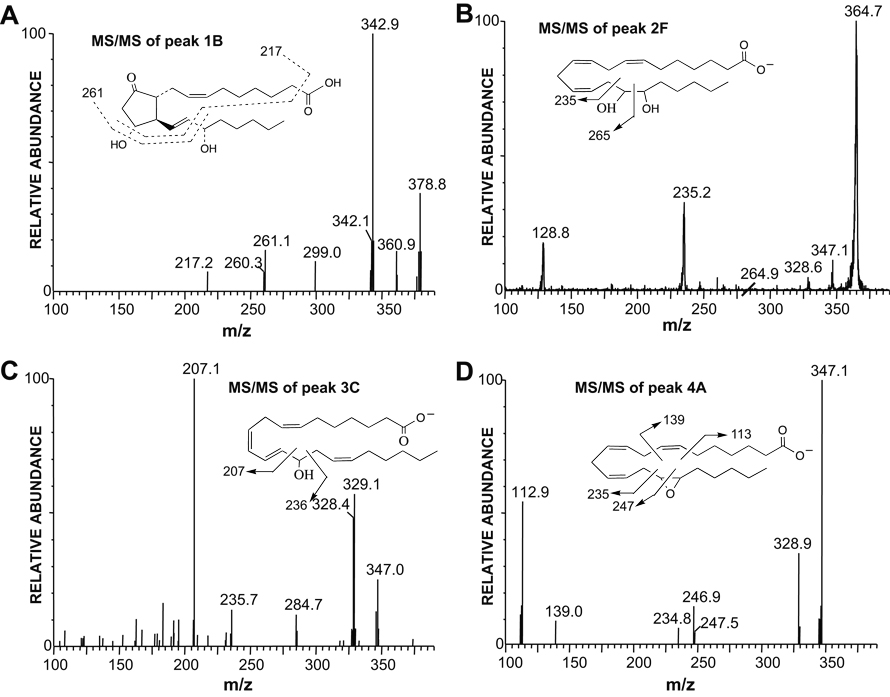
Peak 1 (fractions 3–6.8 min) metabolites comigrated with the DH-PGs. The metabolite eluting at 6.20 min (peak 1A) comigrated with the DH-PGF2α standard. Mass spectral analysis indicated a molecular weight of 382 and collisional disassociation produced a fragmentation pattern corresponding to DH-PGF2α. Peak 1B eluted at 8.4 min and mass spectral analysis indicated a molecular weight of 379 (Table 1, Fig 4A). Major MS/MS ions were m/z 379 [M-H]−, 361 [M-H-H2O]−, 343 [M-H-2H2O]− and 299 [M-H-2H2O-CO2]−. Dissociation of the m/z 261 and 217 ions follows the fragmentation pattern of PGE2 (10, 21). Based on these results, we conclude that peak 1 (Fig 3) contains DH-PGF2α and DH-PGE2.
Figure 4.
Negative ion electrospray tandem mass spectra of metabolites eluting from the HPLC column in Peak 1B, 2F, 3C and 4A (Table 1). A. MS/MS spectrum of m/z 379 [M-H]− of peak 1B, DH-PGE2. B. MS/MS spectrum of m/z 365 [M-H]− of peak 2F, DH-16,17-DHET. C. MS/MS spectrum of m/z 347 [M-H]− of peak 3C, DH-14-HETE. D. MS/MS spectrum of m/z 347 [M-H]− of peak 4A, DH-16,17-EET.
Metabolites in peak 2 (fractions 15.6–20.6 min) eluted LC-ESI/MS between 20–24 min (Table 1). There were three groups of metabolites in peak 2. The molecular weight of the first group (2A–2E) was 364, which exceeds the molecular weight of DiHETEs (molecular weight = 336) by 28 (2CH2) (10). The metabolites eluted at 20.60, 21.80, 22.25, 23.15 and 23.89 min. The fragmentation patterns of these five metabolites correspond to DH-DiHETEs (10) and were identified as DH-10,17-DiHETE, DH-13,15-DiHETE, DH-13,17-DiHETE, DH-10,14-DiHETE, and DH-7,14-DiHETE, respectively. The molecular weight of the second group of metabolites from peak 2 (2F–2I) was 366, which exceeds the molecular weight of DHETs (molecular weight = 338) by 28 (2CH2) (10). They eluted at 20.10, 21.25, 21.9 and 22.57 min. For the peak with a retention time of 20.10 min (peak 2F), major ions were 365 [M-H]−, 347 [M-H-H2O]− and 235 [cleavage between C15 and C16] indicating DH-16,17-DHET (Table 1, Fig 4B) (10). The spectra of the 21.25, 21.9, and 22.57 min peaks (2G, 2H and 2I) showed similar ions at 365 [M-H]− and 347 [M-H-H2O]− with fragmentation patterns indicating DH-13,14-DHET, DH-10,11-DHET, and DH-7,8-DHET, respectively (10). A third metabolite in peak 2 (2J) eluted at 21.6 min. It had a molecular weight of 308 and its major ions corresponded to 14-HNT (10).
Metabolites eluting in peak 3 (fractions 21–24.6 min) were resolved by LC-ESI/MS and had elution times of 27.7, 28.6, 29.2, 29.5 and 29.87 min (Table 1). These five compounds had identical molecular weights of 348, which exceeds the molecular weight of the HETE (molecular weight = 320) by 28 (2CH2) suggesting DH-HETE (10). Analysis of the peak with a retention time of 27.7 min (3A) indicated DH-17-HETE (10). The other four compounds in peak 3 (3B-3E), showed similar ions at 347 [M-H]− and 329 [M-H-H2O]−. Collisional disassociation produced fragmentation patterns indicating DH-13-HETE, DH-14-HETE (Fig 4C), DH-7-HETE, and DH-11-HETE, respectively (10).
Metabolites eluting in peak 4 (fractions 24.6–30 min) comigrated on LC-ESI/MS with authentic DH-EET standards. Elution times were 33.34, 34.6, 34.85 and 35.51 min. Their molecular weight of 348 was identical to DH-EET (10). The MS/MS spectra of the four metabolites (peaks 4A-4D) were identical to the DH-16,17-, DH-13,14-, DH-10,11- and DH-7,8-EET standards, respectively. For the peak with the retention time of 33.34 min (4A), major ions were 347 [M-H]−, 329 [M-H-H2O]− and 247 [cleavage between C16 and C17, the epoxide bond] indicating DH-16,17-EET (Table 1, Fig 4D) (10). Collisional disassociation of peaks 4B-4D produced fragmentation patterns corresponding to DH-13,14-EET, DH-10,11-EET, and DH-7,8-EET, respectively (10).
Vascular response to DH-16,17-EET
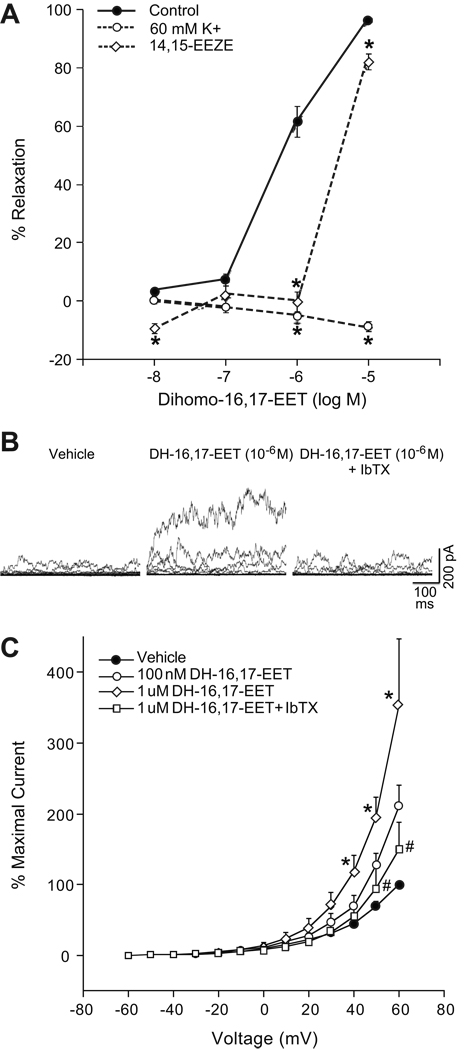
In precontracted coronary arteries, DH-16,17-EET causes concentration-related relaxations with a maximal relaxation at 10−5 M (96 ± 1%) (Fig 5A). 14,15-EEZE (10 µM) inhibited the relaxation to DH-16,17-EET at lower concentrations but only attenuated the relaxation at 10−5 M. Relaxations to DH-16,17-EET were abolished by pretreatment with high K+ (60 mM). Thus, DH-16,17-EET-induced relaxations are dependent on K+ channel activation and is attenuated by 14,15-EEZE.
Figure 5.
A: DH-16,17-EET-induced relaxations of bovine adrenal cortical arteries. Arterial rings were precontracted with U-46619 (10–30 nM) and changes in isomeric tension were measured. DH-16,17-EET (10−8-10−5 M) induced relaxations in the absence or presence of high K+ (60 mM) or 14,15-EEZE (10 µM). Each value represents the mean ± SEM. N = 8–18. *Significantly different from control, P<0.05. B–C: Effect of DH-16,17-EET on whole cell outward K+ currents of isolated bovine adrenal cortical artery SMCs. B: Tracings of outward K+ currents from a single SMC (capacitance = 50.6 pF) with vehicle control, DH-16,17-EET (10−6 M), or DH-16,17-EET (10−6 M) plus iberiotoxin (100 nM). C: Averaged whole cell current density. Each value represents the mean ± SEM. N = 4. *Significantly different from control, P<0.05. #Significantly different from DH-16,17-EET (10−6 M).
Activation of SMC K+ currents by DH-16,17-EET
Macroscopic, whole cell, outward K+ currents were generated by 10 mV depolarizing steps from −60 mV to 60 mV in isolated bovine adrenal cortical artery SMCs (Fig 5B–C). DH-16,17-EET (10−7-10−6 M) activated outward K+ currents in a concentration dependent manner. At 60 mV, DH-16,17-EET (10−6 M) increased current density by 353%. A subsequent addition of iberiotoxin reduced current density to 149% of control current density. Membrane capacitance averaged 51.4 ± 7.8 pF. These results demonstrate that DH-16,17-EET activates iberiotoxin-sensitive K+ channels of isolate bovine adrenal cortical artery SMCs.
Discussion
This is the first study to examine the role of adrenic acid in the regulation of vascular tone in arteries of the adrenal cortex, a tissue with an abundance of adrenic acid. Adrenic acid relaxations of bovine adrenal cortical arteries are mediated by the production of endothelial and ZG cell CYP450 metabolites. These findings further indicate that a functional and intimate interaction exists between the vasculature and the closely associated steroidogenic cells of the adrenal gland.
Adrenic acid caused concentration-dependent relaxations of preconstricted adrenal cortical arteries, which was endothelium-dependent. COX inhibition had no affect on these relaxations; however, these relaxations were attenuated by CYP450 inhibition or EET antagonism and abolished by BKCa channel blockade. These data suggest that adrenic acid mediates relaxation of adrenal cortical arteries by an endothelium-derived CYP450 metabolite, presumably an EET or DH-EET, which activates the BKCa channels of vascular smooth muscle cells. In bovine coronary arteries, exogenous adrenic acid also caused relaxation, which was mediated by endothelial COX and CYP450 metabolites that active K+ channels with resulting vascular smooth muscle cell hyperpolarization (10). These vasoactive metabolites were identified as DH-PGI2 and DH-EETs. These similarities suggest that endothelial metabolism of adrenic acid may contribute factors involved in the regulation of vascular tone in multiple vascular beds. Interestingly, COX metabolites contribute to adrenic acid relaxations in the coronary vasculature but not in the adrenal vasculature. With respect to adrenic acid, this difference between vascular beds suggests a greater role of COX metabolism in the bovine coronary vasculature and of CYP450 metabolism in the bovine adrenal vasculature.
Several lines of evidence indicate that ZG cells metabolize adrenic acid into vasorelaxing factors. Adrenic acid caused concentration-dependent relaxations of denuded adrenal cortical arteries when incubated with ZG cells. These relaxations were abolished by CYP450 inhibition, EET antagonism, high K+, and BKCa channel blockade. COX inhibition had no effect on these relaxations. As with endothelium-dependent relaxation in response to adrenic acid, the relaxant factor produced by ZG cells is likely a CYP450 metabolite that causes hyperpolarization of vascular smooth muscle cells by activation of BKCa channels. Upon stimulation by ACTH, ZG cells produce EETs that diffuse to adjacent arteries and cause relaxation (13). Adrenic acid-derived DH-EETs may represent another possible vasorelaxing factor from ZG cells.
ZG cells metabolize adrenic acid by COX to the DH-PGs, by LO to DH-HETEs and DH-DiHETEs, and by CYP450 to DH-EETs and DH-DHETs. Identification of these metabolites was based upon reverse-phase HPLC co-migration with known DH-PG and DH-EET standards and analysis of MS/MS spectra. Thus, adrenic acid is metabolized by ZG cells to a myriad of docosanoids by COX, LO, and CYP450 enzymatic pathways. Bovine coronary vessels produce a similar array of COX, LO, and CYP450 metabolites of adrenic acid (10). Moreover, adrenic acid is metabolized to DH-thromboxane by COX and DH-14-HETE by 12-LO in human platelets (8), DH-PGs and DH-thromboxane by the renal medulla (4), and DH-PGI2 by COX in human endothelial cells (9).
Since both the endothelium- and ZG cell-mediated relaxations to adrenic acid involve CYP450 metabolism and require K+ channel activation, a DH-EET is a likely metabolite contributing to these relaxations. DH-16,17-EET caused concentration-dependent relaxations of preconstricted adrenal cortical arteries. These relaxations were attenuated by EET antagonism and abolished by high K+. Moreover, DH-16,17-EET activated iberiotoxin-sensitve outward K+ currents in isolated bovine adrenal cortical SMCs. These results are similar to results concerning DH-16,17-EET in bovine coronary arteries from a previous study (10). Thus, the mechanism of relaxation induced by DH-16,17-EET is similar to EETs, which diffuse to the vascular smooth muscle and activate membrane K+ channels, resulting in hyperpolarization and vascular relaxation (19, 22, 23).
Perspectives
Endothelium- and ZG cell-derived adrenic acid metabolites may play a role in the regulation of adrenal vascular tone. Adrenic acid relaxations of adrenal cortical arteries are mediated by endothelial and ZG cell CYP450 metabolites. Moreover, the closely associated ZG cells produce COX, LO, and CYP450 metabolites with potential vasoactivity, including DH-PGs, DH-HETEs, DH-DiHETEs, and DH-EETs. The CYP450-derived DH-16,17-EET induces vasorelaxtion and represents a potential endogenous adrenic acid metabolite that functions as an endothelium- and ZG cell-derived hyperpolarizing factor in the adrenal vasculature. While the role of these metabolites in the regulation and maintenance of vascular tone is unknown, adrenic acid and its metabolites should be considered in future studies examining the regulation of vascular tone; especially in tissues with known abundance of adrenic acid.
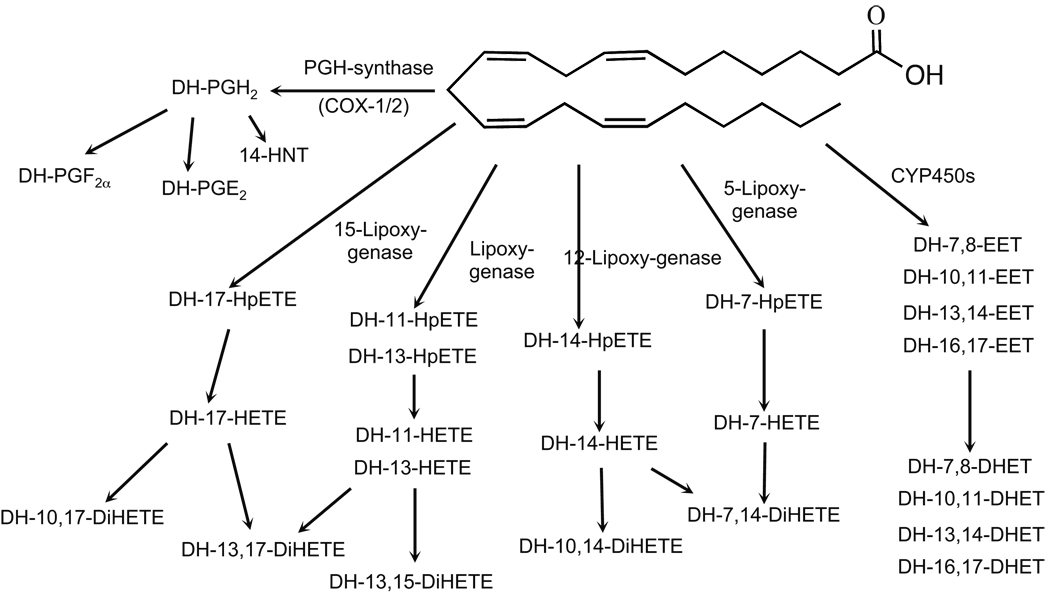
Figure 6.
Schematic of adrenic acid metabolism by bovine ZG cells. Adrenic acid is metabolized by the COX, LO and CYP450 pathways.
Acknowledgments
Sources of Funding
These studies were supported by grants from the National Institute of Health (HL-83297 and GM-31278) and the Robert A. Welch Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosure Statement
None
Contributor Information
Phillip G. Kopf, Department of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226
David X. Zhang, Department of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226
Kathryn M. Gauthier, Department of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226
Kasem Nithipatikom, Department of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226.
John R. Falck, Department of Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390
William B. Campbell, Department of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226
References
- 1.Mann CJ, Kaduce TL, Figard PH, Spector AA. Docosatetraenoic acid in endothelial cells: formation, retroconversion to arachidonic acid, and effect on prostacyclin production. Arch Biochem Biophys. 1986;244:813–823. doi: 10.1016/0003-9861(86)90650-8. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal MD, Hill JR. Human vascular endothelial cells synthesize and release 24- and 26-carbon polyunsaturated fatty acids. Biochim Biophys Acta. 1984;795:171–178. doi: 10.1016/0005-2760(84)90063-8. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal MD, Hill JR. Elongation of arachidonic and eicosapentaenoic acids limits their availability for thrombin-stimulated release from the glycerolipids of vascular endothelial cells. Biochim Biophys Acta. 1986;875:382–391. doi: 10.1016/0005-2760(86)90189-x. [DOI] [PubMed] [Google Scholar]
- 4.Sprecher H, VanRollins M, Sun F, Wyche A, Needleman P. Dihomo-prostaglandins and -thromboxane. A prostaglandin family from adrenic acid that may be preferentially synthesized in the kidney. J Biol Chem. 1982;257:3912–3918. [PubMed] [Google Scholar]
- 5.Wijendran V, Lawrence P, Diau GY, Boehm G, Nathanielsz PW, Brenna JT. Significant utilization of dietary arachidonic acid is for brain adrenic acid in baboon neonates. J Lipid Res. 2002;43:762–767. [PubMed] [Google Scholar]
- 6.Rosenthal MD, Whitehurst MC. Fatty acyl delta 6 desaturation activity of cultured humanendothelial cells. Modulation by fetal bovine serum. Biochim Biophys Acta. 1983;750:490–496. doi: 10.1016/0005-2760(83)90189-3. [DOI] [PubMed] [Google Scholar]
- 7.Danon A, Heimberg M, Oates JA. Enrichment of rat tissue lipids with fatty acids that are prostaglandin precursors. Biochim Biophys Acta. 1975;388:318–330. doi: 10.1016/0005-2760(75)90090-9. [DOI] [PubMed] [Google Scholar]
- 8.VanRollins M, Horrocks L, Sprecher H. Metabolism of 7,10,13,16-docosatetraenoic acid to dihomo-thromboxane, 14-hydroxy-7,10,12-nonadecatrienoic acid and hydroxy fatty acids by human platelets. Biochim Biophys Acta. 1985;833:272–280. doi: 10.1016/0005-2760(85)90199-7. [DOI] [PubMed] [Google Scholar]
- 9.Campbell WB, Falck JR, Okita JR, Johnson AR, Callahan KS. Synthesis of dihomoprostaglandins from adrenic acid (7,10,13,16-docosatetraenoic acid) by human endothelial cells. Biochim Biophys Acta. 1985;837:67–76. doi: 10.1016/0005-2760(85)90086-4. [DOI] [PubMed] [Google Scholar]
- 10.Yi XY, Gauthier KM, Cui L, Nithipatikom K, Falck JR, Campbell WB. Metabolism of adrenic acid to vasodilatory 1alpha,1beta-dihomo-epoxyeicosatrienoic acids by bovine coronary arteries. Am J Physiol Heart Circ Physiol. 2007;292:H2265–H2274. doi: 10.1152/ajpheart.00947.2006. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
- 12.Campbell WB, Brady MT, Rosolowsky LJ, Falck JR. Metabolism of arachidonic acid by rat adrenal glomerulosa cells: synthesis of hydroxyeicosatetraenoic acids and epoxyeicosatrienoic acids. Endocrinology. 1991;128:2183–2194. doi: 10.1210/endo-128-4-2183. [DOI] [PubMed] [Google Scholar]
- 13.Zhang DX, Gauthier KM, Falck JR, Siddam A, Campbell WB. Steroid-producing cells regulate arterial tone of adrenal cortical arteries. Endocrinology. 2007;148:3569–3576. doi: 10.1210/en.2007-0169. [DOI] [PubMed] [Google Scholar]
- 14.Zhang DX, Gauthier KM, Campbell WB. Characterization of vasoconstrictor responses in small bovine adrenal cortical arteries in vitro. Endocrinology. 2004;145:1571–1578. doi: 10.1210/en.2003-1448. [DOI] [PubMed] [Google Scholar]
- 15.Rosolowsky LJ, Campbell WB. Endothelin enhances adrenocorticotropin-stimulated aldosterone release from cultured bovine adrenal cells. Endocrinology. 1990;126:1860–1866. doi: 10.1210/endo-126-4-1860. [DOI] [PubMed] [Google Scholar]
- 16.Pfister SL, Schmitz JM, Willerson JT, Campbell WB. Characterization of arachidonic acid metabolism in Watanabe heritable hyperlipidemic (WHHL) and New Zealand white (NZW) rabbit aortas. Prostaglandins. 1988;36:515–532. doi: 10.1016/0090-6980(88)90047-0. [DOI] [PubMed] [Google Scholar]
- 17.Nithipatikom K, Grall AJ, Holmes BB, Harder DR, Falck JR, Campbell WB. Liquid chromatographic-electrospray ionization-mass spectrometric analysis of cytochrome P450 metabolites of arachidonic acid. Anal Biochem. 2001;298:327–336. doi: 10.1006/abio.2001.5395. [DOI] [PubMed] [Google Scholar]
- 18.Davies SS, Zackert W, Luo Y, Cunningham CC, Frisard M, Roberts LJ., 2nd Quantification of dinor, dihydro metabolites of F2-isoprostanes in urine by liquid chromatography/tandem mass spectrometry. Anal Biochem. 2006;348:185–191. doi: 10.1016/j.ab.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-epoxyeicosa-5(Z)-enoicmSI: a 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003;42:555–561. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- 21.Murphy RC, Barkley RM, Zemski Berry K, Hankin J, Harrison K, Johnson C, Krank J, McAnoy A, Uhlson C, Zarini S. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346:1–42. doi: 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Campbell WB, Falck JR, Gauthier K. Role of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factor in bovine coronary arteries. Med Sci Monit. 2001;7:578–584. [PubMed] [Google Scholar]
- 23.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]