Abstract
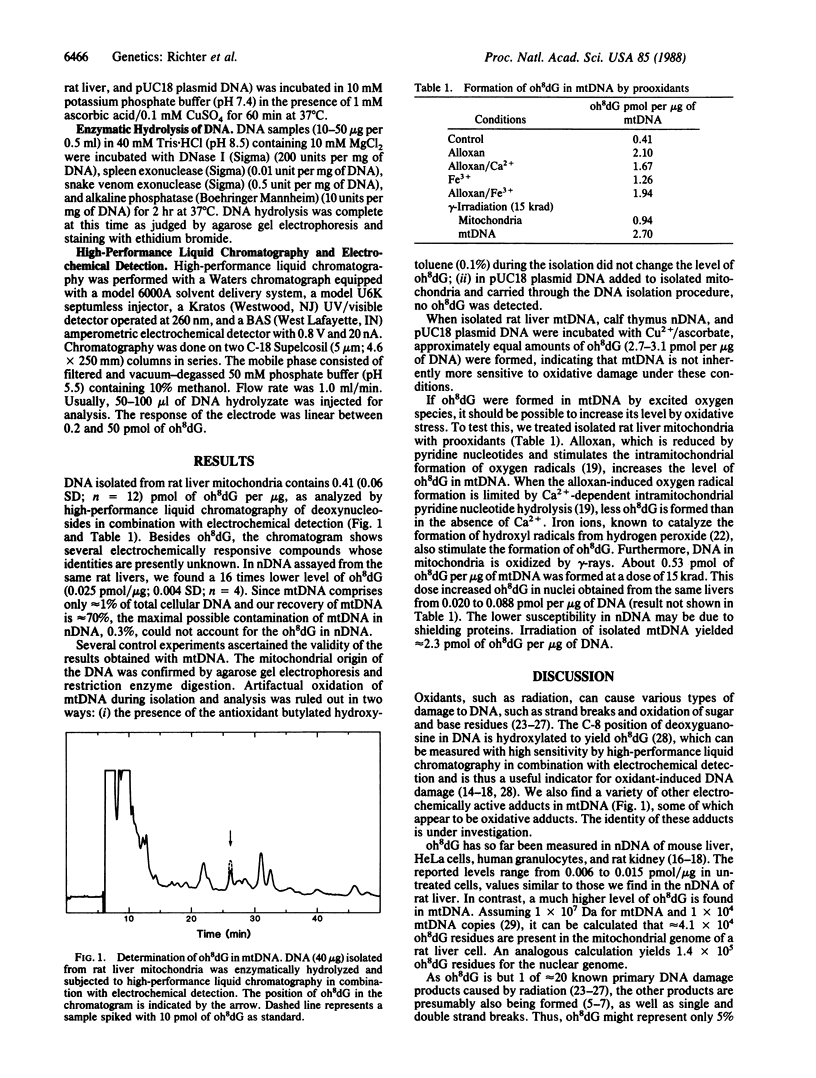
Oxidative damage to DNA can be caused by excited oxygen species, which are produced by radiation or are by-products of aerobic metabolism. The oxidized base, 8-hydroxydeoxyguanosine (oh8dG), 1 of approximately 20 known radiation damage products, has been assayed in the DNA of rat liver. oh8dG is present at a level of 1 per 130,000 bases in nuclear DNA and 1 per 8000 bases in mtDNA. Mitochondria treated with various prooxidants have an increased level of oh8dG. The high level of oh8dG in mtDNA may be caused by the immense oxygen metabolism, relatively inefficient DNA repair, and the absence of histones in mitochondria. It may be responsible for the observed high mutation rate of mtDNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman R., Saul R. L., Ames B. N. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. A., Coombs M. M. Covalent binding of polycyclic aromatic compounds to mitochondrial and nuclear DNA. Nature. 1980 Sep 18;287(5779):244–245. doi: 10.1038/287244a0. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Anderson C. T., Friedberg E. C. The presence of nuclear and mitochondrial uracil-DNA glycosylase in extracts of human KB cells. Nucleic Acids Res. 1980 Feb 25;8(4):875–888. [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Weinstein I. B. Interaction of benzo(a)pyrene and its dihydrodiol-epoxide derivative with nuclear and mitochondrial DNA in C3H10T 1/2 cell cultures. Cancer Res. 1982 Jul;42(7):2764–2769. [PubMed] [Google Scholar]
- Backer J. M., Weinstein I. B. Mitochondrial DNA is a major cellular target for a dihydrodiol-epoxide derivative of benzo[a]pyrene. Science. 1980 Jul 11;209(4453):297–299. doi: 10.1126/science.6770466. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Cadet J., Berger M. Radiation-induced decomposition of the purine bases within DNA and related model compounds. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):127–143. doi: 10.1080/09553008514550201. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Wilson A. C. Length mutations in human mitochondrial DNA. Genetics. 1983 Aug;104(4):699–711. doi: 10.1093/genetics/104.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Saul R. L., Ames B. N. Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5633–5637. doi: 10.1073/pnas.81.18.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Bergtold D. S. Characterization of free radical-induced base damage in DNA at biologically relevant levels. Anal Biochem. 1986 Jul;156(1):182–188. doi: 10.1016/0003-2697(86)90171-5. [DOI] [PubMed] [Google Scholar]
- Domena J. D., Mosbaugh D. W. Purification of nuclear and mitochondrial uracil-DNA glycosylase from rat liver. Identification of two distinct subcellular forms. Biochemistry. 1985 Dec 3;24(25):7320–7328. doi: 10.1021/bi00346a045. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Harris J., West M., Wong P. K. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986 Jun 13;137(2):841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Frei B., Winterhalter K. H., Richter C. Mechanism of alloxan-induced calcium release from rat liver mitochondria. J Biol Chem. 1985 Jun 25;260(12):7394–7401. [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Stimulation of the nuclear uracil DNA glycosylase in proliferating human fibroblasts. Cancer Res. 1981 Aug;41(8):3133–3136. [PubMed] [Google Scholar]
- Gupta R. C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Wilkins S. Copper-dependent hydroxyl radical damage to ascorbic acid: formation of a thiobarbituric acid-reactive product. FEBS Lett. 1982 Jan 25;137(2):327–330. doi: 10.1016/0014-5793(82)80377-3. [DOI] [PubMed] [Google Scholar]
- Kasai H., Crain P. F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986 Nov;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of guanine in nucleosides and DNA at the C-8 position by heated glucose and oxygen radical-forming agents. Environ Health Perspect. 1986 Aug;67:111–116. doi: 10.1289/ehp.8667111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nishimura S., Kurokawa Y., Hayashi Y. Oral administration of the renal carcinogen, potassium bromate, specifically produces 8-hydroxydeoxyguanosine in rat target organ DNA. Carcinogenesis. 1987 Dec;8(12):1959–1961. doi: 10.1093/carcin/8.12.1959. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Levin C. J., Zimmerman S. B. A DNA ligase from mitochondria of rat liver. Biochem Biophys Res Commun. 1976 Mar 22;69(2):514–520. doi: 10.1016/0006-291x(76)90551-9. [DOI] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Myers K. A., Saffhill R., O'Connor P. J. Repair of alkylated purines in the hepatic DNA of mitochondria and nuclei in the rat. Carcinogenesis. 1988 Feb;9(2):285–292. doi: 10.1093/carcin/9.2.285. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in nuclear and mitochondrial DNA of yeast irradiated with low doses of ultraviolet light. J Mol Biol. 1975 Nov 15;98(4):781–795. doi: 10.1016/s0022-2836(75)80010-6. [DOI] [PubMed] [Google Scholar]
- Romslo I., Flatmark T. Energy-dependent accumulation of iron by isolated rat liver mitochondria. I. General features. Biochim Biophys Acta. 1973 Apr 27;305(1):29–40. doi: 10.1016/0005-2728(73)90228-4. [DOI] [PubMed] [Google Scholar]
- Totter J. R. Spontaneous cancer and its possible relationship to oxygen metabolism. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1763–1767. doi: 10.1073/pnas.77.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troll W., Wiesner R. The role of oxygen radicals as a possible mechanism of tumor promotion. Annu Rev Pharmacol Toxicol. 1985;25:509–528. doi: 10.1146/annurev.pa.25.040185.002453. [DOI] [PubMed] [Google Scholar]
- Téoule R. Radiation-induced DNA damage and its repair. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Apr;51(4):573–589. doi: 10.1080/09553008414552111. [DOI] [PubMed] [Google Scholar]
- Wunderlich V., Schütt M., Böttger M., Graffi A. Preferential alkylation of mitochondrial deoxyribonucleic acid by N-methyl-N-nitrosourea. Biochem J. 1970 Jun;118(1):99–109. doi: 10.1042/bj1180099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich V., Tetzlaff I., Graffi A. Studies on nitrosodimethylamine: preferential methylation of mitochondrial DNA in rats and hamsters. Chem Biol Interact. 1972 Jan;4(2):81–89. doi: 10.1016/0009-2797(72)90001-4. [DOI] [PubMed] [Google Scholar]


