Abstract
An important trend in current toxicology is the replacement, reduction, and refinement of the use of experimental animals (the 3R principle). We propose a model in which in vivo genotoxicity and short-term carcinogenicity assays are integrated with F344 gpt delta transgenic rats. Using this model, the genotoxicity of chemicals can be identified in target organs using a shuttle vector λ EG10 that carries reporter genes for mutations; short-term carcinogenicity is determined by the formation of glutathione S-transferase placenta form (GST-P) foci in the liver. To begin validating this system, we examined the genotoxicity and hepatotoxicity of structural isomers of 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT). Although both compounds are genotoxic in the Ames/Salmonella assay, only 2,4-DAT induces tumors in rat livers. Male F344 gpt delta rats were fed diet containing 2,4-DAT at doses of 125, 250, or 500 ppm for 13 weeks or 2,6-DAT at a dose of 500 ppm for the same period. The mutation frequencies of base substitutions, mainly at G:C base pairs, were significantly increased in the livers of 2,4-DAT–treated rats at all three doses. In contrast, virtually no induction of genotoxicity was identified in the kidneys of 2,4-DAT–treated rats or in the livers of 2,6-DAT–treated rats. GST-P–positive foci were detected in the livers of rats treated with 2,4-DAT at a dose of 500 ppm but not in those treated with 2,6-DAT. Integrated genotoxicity and short-term carcinogenicity assays may be useful for early identifying genotoxic and nongenotoxic carcinogens in a reduced number of experimental animals.
Keywords: gpt delta transgenic rat, diaminotoluenes, genotoxicity, carcinogenicity, 3R principle
Transgenic rodent models have advanced the field of in vivo genotoxicity studies (Nohmi and Masumura, 2005; Nohmi et al., 2000). In these models, λ phage DNA carrying reporter genes for mutations are integrated into the chromosomes of transgenic rodents; the phage DNA is retrieved in phage particles by in vitro packaging reactions. The rescued phages are introduced into Escherichia coli cells, and mutants that were generated in the rodents are selected. With the shuttle vector system, one can examine the mutagenicity of chemicals in any rodent organ or tissues, including germ cells (Eastmond et al., 2009; Hashimoto et al., 2009). In addition, the mutants recovered from the rodents can be characterized by DNA sequencing (Heddle et al., 2000). Transgenic genotoxicity assays are a reliable method for determining whether genotoxicity is involved in chemical carcinogenicity in the target organs of rodents (Thybaud et al., 2003).
In 1996, we developed the novel transgenic mouse gpt delta for in vivo genotoxicity assays (Nohmi et al., 1996). These mice have approximately 80 copies of λ EG10 DNA at a single site in chromosome 17 of C57 BL/6J mice (Masumura et al., 1999). A feature of this transgenic mouse is that two mutant selections can be performed instead of just one, to identify a wider spectrum of in vivo mutations: gpt selection to identify point mutations such as base substitutions and frameshift mutations and Spi− selection to identify deletion mutations. Because of their sensitivity to deletion-type mutations, gpt delta mice have been utilized for radiation biology, cancer research, and regulatory toxicology (Aoki et al., 2007; Masumura et al., 2002; Shibata et al., 2009; Xu et al., 2007). In 2003, we established gpt delta rats in a Sprague-Dawley (SD) background by introducing λ EG10 DNA into fertilized SD rat eggs (Hayashi et al., 2003). This gpt delta rat carries approximately five copies of λ EG10 DNA at a single site in chromosome 4 and is sensitive to induction of point mutations and deletions by benzo[a]pyrene and potassium bromate (Hayashi et al., 2003; Umemura et al., 2009).
Here, we report the establishment of gpt delta rat in a Fischer 344 background by backcross of SD gpt delta rats with F344 rats for 15 generations. We generated F344 gpt delta rats because this background is frequently used for 2-year cancer bioassays. In addition, glutathione S-transferase placenta form (GST-P)–positive preneoplastic hepatic foci can be analyzed in the rats (Ito et al., 2000). The results of bioassay using GST-P–positive foci show good correlation with those of 2-year cancer bioassay (Ito et al., 2000; Ogiso et al., 1985). Therefore, GST-P–positive foci formation assay was used as short-term carcinogenicity assay in this study. We hypothesized that we could integrate a genotoxicity assay with a short-term carcinogenicity assay utilizing GST-P foci in F344 gpt delta rats. This would reduce the number of animals required for both assays and would allow for examination of the relationship between genotoxicity and preneoplastic lesion formation within the same organs and tissues of chemically treated F344 gpt delta rats.
To begin validating this system, we examined the in vivo genotoxicity and hepatotoxicity of 2,4-diaminotoluene (2,4-DAT) and 2,6-diaminotoluene (2,6-DAT). The first chemical, 2,4-DAT, is used as an intermediate of the production of toluene diisocyanate, which is a monomer for the production of polyurethane, while 2,6-DAT is an intermediate of dyes, rubber chemicals, and various polymers (NTP 1979, 1980). Although both are genotoxic in vitro (Cunningham et al., 1989), only 2,4-DAT is carcinogenic in the livers of female mice and male and female rats (NTP, 1979). 2,4-DAT also induces lymphoma in female mice and mammary and subcutaneous tumors in rats. 2,6-DAT is not carcinogenic in mice and rats, regardless of their sex (NTP, 1980). Previous studies with MutaMouse (Kirkland and Beevers, 2006) and Big Blue mouse (Cunningham et al., 1996) indicate that 2,4-DAT is mutagenic in the liver, while 2,6-DAT is not. However, the transgenic mice employed for these studies were males, in which the hepatocarcinogenicity of 2,4-DAT is not observed. In addition, there are no reports on in vivo gene mutations in rats. Thus, we decided to examine the in vivo genotoxicity of both compounds in the liver, as carcinogenic target organ of 2,4-DAT, and kidney, as noncarcinogenic target, along with immunohistochemical analyses. We chose 500 ppm as the highest dose for both DATs according to the dose used in the National Toxicology Program 2-year cancer bioassay (NTP, 1979, 1980). We treated the rats with chemicals for 13 weeks because this period is customarily used to determine the appropriate doses for 2-year cancer bioassays; furthermore, shorter term treatments (e.g., treatments with potassium bromate for 5 weeks [Umemura et al., 2006]), sometimes do not induce detectable mutations in vivo.
MATERIALS AND METHODS
Establishment of F344 gpt delta rats.
All the animals were maintained at Japan SLC (Shizuoka, Japan). The F344 gpt delta transgenic rat strain was developed by backcrosses of the original SD gpt delta transgenic rat with wild-type F344 rats. In brief, male SD gpt delta transgenic rat was mated with F344 female rat to produce an F1 generation. Offspring from the F1 generation were mated with F344 rats to yield an F2 generation. All offspring from successive backcrosses were examined for the possession of the gpt gene by PCR (Hayashi et al., 2003). After 15 successive backcrosses, identity of the resulting rats to F344 recipient is more than 99.9%. Thus, they were referred to as F344 gpt delta rats.
Chemicals.
2,4-DAT (purity 95%) and 2,6-DAT (purity 98%) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Diethylnitrosamine (DEN) was obtained from Sigma-Aldrich Japan (Tokyo, Japan).
Bacterial reverse mutation test (Ames test).
The mutagenic activities of 2,4-DAT and 2,6-DAT were assayed in a bacterial reverse mutation assay using Salmonella typhimurium tester strains TA98 and YG1024, an O-acetyltransferase (OAT)–overexpressing derivative. The test was conducted by the preincubation method (Maron and Ames, 1983) in the presence or the absence of S9 mix. At least two plates were used for each dose, and the mean values of the number of revertants per plate were calculated. Chemicals were dissolved in dimethyl sulfoxide, which was used as the negative control.
Animals, diet, and housing conditions.
Male 6-week-old F344 gpt delta transgenic rats were obtained from Japan SLC and housed five animals per polycarbonate cage under specific pathogen-free standard laboratory conditions: room temperature, 23°C ± 2°C; relative humidity, 60 ± 5%; with a 12:12-h light-dark cycle; and free access to CRF-1 basal diet (Oriental Yeast Company, Tokyo, Japan) and tap water. After a 1-week acclimation period, the animals were used for the experiments.
Treatments of animals.
The protocol for this study was approved by the Animal Care and Utilization Committee of the National Institute of Health Sciences. Thirty male F344 gpt delta rats were randomized by weight into six groups. 2,4-DAT and 2,6-DAT were each mixed into Oriental CRF-1 powdered basal diet (Oriental Yeast Company) and stored at 4°C in the dark before use. Starting at 7 weeks of age, the rats were fed diets containing 0, 125, 250, or 500 ppm 2,4-DAT or 500 ppm 2,6-DAT for 13 weeks. There was also a positive control group; these rats were received a once-a-week ip injection of 20 mg/kg body weight DEN for 13 weeks. Parameters monitored included clinical signs, body weight, and food intake. The highest dose of 2,4-DAT was reduced from 500 to 400 ppm at week 9 because the dose at 500 ppm reduced the body weight of rats at week 8. All the surviving animals were killed under ether anesthesia at the end of the experiments. The liver and kidneys were isolated from each animal and were immediately excised, weighed, and cut into 2- to 3-mm-thick slices. The slices were fixed in 10% buffered formalin solution and routinely processed to paraffin blocks for histopathological examination as well as immunohistochemistry. Hematoxylin and eosin-stained tissue preparations cut from the blocks were examined by light microscopy.
Micronucleus assay.
At autopsy, 60 μl of peripheral blood was obtained from the tail veins of all animals. The samples were processed according to the instructions supplied with the MicroFlowPLUS kit (Litron, Rochester, NY), fixed in ultra-cold methanol, and stored immediately after fixation at −80°C until flow cytometry analysis was performed. Approximately 20,000 reticulocytes were counted for each sample using Becton-Dickinson FACSCalibur flow cytometer (Franslin Lakes, NJ) to detect the presence of micronuclei (MNs).
Immunohistochemical procedures.
Liver sections of 3-μm thickness were treated with rabbit anti-rat GST-P antibody (1:1000; Medical & Biological Laboratories, Nagoya, Japan) and monoclonal mouse anti-Ki67 (MIB-5) antibody (1:50; Dako, Tokyo, Japan) (1:50), respectively. Areas and numbers of GST-P–positive foci larger than 0.1 mm in diameter of the liver sections were quantitatively measured with an image processor for analytical pathology (IPAP-WIN; Sumika Technos Company, Osaka, Japan). To investigate proliferative activity, we counted at least 1000 hepatocyte nuclei in each liver; labeling indices were calculated as the percentage of cells positive for Ki67 staining. The remaining tissues were immediately frozen in liquid nitrogen and stored at −80°C for subsequent mutation assays.
DNA isolation and in vitro packaging of λ phage DNA.
High–molecular-weight genomic DNA was extracted from the liver and kidneys using the RecoverEase DNA Isolation kit (Stratagene, La Jolla, CA). λ EG10 phages were rescued using Transpack Packaging Extract (Stratagene).
gpt Mutation assay.
The assay was conducted according to previously published methods (Nohmi et al., 1996). All the confirmed gpt mutants recovered from the livers were sequenced; identical mutations from the same rat were counted as one mutant. The mutant frequencies of the gpt gene (gpt MFs) in the liver and kidney were calculated by dividing the number of confirmed 6-thioguanine–resistant colonies by the number of rescued plasmids. DNA sequencing of the gpt gene was performed with the BigDye Terminater Cycle Sequencing Ready Reaction (Applied Biosystems, Foster City, CA) on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems).
Spi− assay.
The Spi− assay was conducted according to previously published methods (Masumura et al., 2002). To confirm the Spi− phenotype of the candidates, suspensions were spotted on three types of plates on which XL-1 Blue MRA, XL-1 Blue MRA P2, or WL95 P2 strains were spread with soft agar. True Spi− mutants, which made clear plaques on all of the plates, were counted. Spi− mutant lysates were obtained by infecting E. coli LE392 with the recovered Spi− mutants. The lysates were used as templates for PCR and sequencing analysis to determine the deleted regions (Masumura et al., 2002). The Spi− mutants were categorized into three classes: one base pair (bp) deletions, deletions of more than 1 bp, and complex mutations. The entire sequence of λ EG10 is available at http://dgm2alpha.nihs.go.jp/default.htm.
Statistical analysis.
The statistical significance of the difference in the value of MFs between treated groups and negative controls was analyzed by Student's t-test. A p value less than 0.05 denoted the presence of a statistically significant difference. Variances in values for body weight, organ weight, and immunohistochemical data were examined by one-way ANOVA with Dunnett's multiple test to compare the differences between control and treated groups.
RESULTS
Dietary Treatment with 2,4-DAT Induced Preneoplastic Lesions in the Livers of F344 gpt Delta Rats
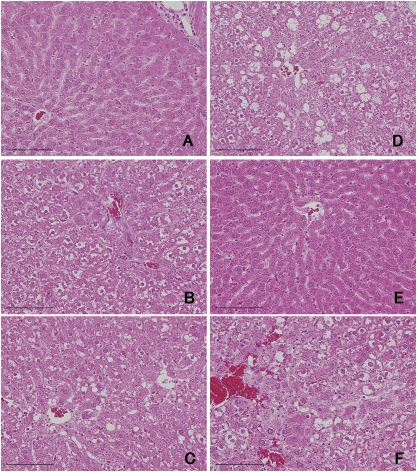
Dietary treatment with 2,4-DAT reduced body weight significantly at all three doses, while dietary treatment with 2,6-DAT did not (Supplementary table 1). Treatments with 2,4-DAT, but not 2,6-DAT, increased the relative weight of the livers and kidneys in a dose-dependent manner. Hypertrophy and vacuolar degeneration of hepatocytes was observed in the livers of rats in the 2,4-DAT treatment groups (Fig. 1). Cell proliferation was significantly enhanced by 2,4-DAT at a dose of 250 ppm but not by treatment with 2,6-DAT or other doses of 2,4-DAT (Table 1). That labeling index was twofold higher compared with that of basal diet group. GST-P–positive foci were induced by treatment with 2,4-DAT at a dose of 250 or 500 ppm and by the positive control treatment with DEN (Table 2). There were significant differences in number of foci and area of foci between rats treated with 2,4-DAT at 500 ppm and those of the basal diet group and between rats treated with DEN and the control group. No histopathological changes were observed in the kidneys of rats that were fed 2,4-DAT or 2,6-DAT. These results suggest that 2,4-DAT, but not 2,6-DAT, induced preneoplastic lesions in the livers of F344 gpt delta rats.
FIG. 1.
Histological comparison of rat livers treated with 0 ppm 2,4-DAT (A), 125 ppm 2,4-DAT (B), 250 ppm 2,4-DAT (C), 500 ppm 2,4-DAT (D), 500 ppm 2,6-DAT (E), and DEN (F). Hepatotoxicity was observed in rats administered 2,4-DAT and DEN. Bar = 100 μm.
TABLE 1.
Quantification of Hepatocyte Proliferation
| No. of Rats | No. of Total Nuclei | No. of Ki-67–Positive Nuclei | Index | |
| Basal diet | 5 | 2170.8 ± 890.9 | 27.4 ± 8.1 | 0.013 ± 0.004 |
| Basal diet (DEN) | 5 | 1749.6 ± 729.2a | 73.8 ± 19.7 | 0.042 ± 0.009* |
| 125 ppm 2,4-DAT | 5 | 1700.2 ± 700.1a | 14.0 ± 6.6 | 0.008 ± 0.005 |
| 250 ppm 2,4-DAT | 5 | 1436.4 ± 596.7a | 44.4 ± 10.5 | 0.031 ± 0.007* |
| 500 ppm 2,4-DAT | 5 | 1308.6 ± 537.6a | 20.4 ± 9.0 | 0.015 ± 0.006 |
| 500 ppm 2,6-DAT | 5 | 2048.8 ± 860.8 | 17.8 ± 7.4 | 0.014 ± 0.004 |
Total number of nuclei was significantly decreased compared to the basal diet treatment group.
Significantly different from the basal diet group (p < 0.01).
TABLE 2.
Quantification of GST-P–Positive Foci
| No. of Rats | No. of Foci (No./cm2) | Area of Foci (mm2/cm2) | |
| Basal diet | 5 | 0.00 ± 0.00 | 0.000 ± 0.000 |
| Basal diet (DEN) | 5 | 78.92 ± 17.70** | 1.924 ± 0.655** |
| 125 ppm 2,4-DAT | 5 | 0.00 ± 0.00 | 0.000 ± 0.000 |
| 250 ppm 2,4-DAT | 5 | 1.19 ± 1.21 | 0.022 ± 0.023 |
| 500 ppm 2,4-DAT | 5 | 6.05 ± 3.93* | 0.502 ± 0.476* |
| 500 ppm 2,6-DAT | 5 | 0.00 ± 0.00 | 0.000 ± 0.000 |
Significantly different from the basal diet group (p < 0.05).
Significantly different from the basal diet group (p < 0.01).
Both 2,4-DAT and 2,6-DAT Induced Mutations In Vitro
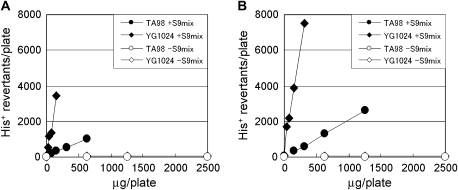
We confirmed that both 2,4-DAT and 2,6-DAT were mutagenic in S. typhimurium strain TA98 in the presence of S9 activation (Fig. 2). Treating cells with either of the DATs in the absence of S9 mix did not produce any increase in the number of revertants per plate. Similar, but less significant, results were obtained with another standard S. typhimurium strain TA100 (Supplementary table 2). These observations suggest that DAT metabolites were responsible for the mutagenic effects. To explore the metabolic activation pathways in vitro, we employed strain YG1024, which overproduces OAT, a phase II enzyme. Strain YG1024 detects frameshift mutations because it was derived from strain TA98 (Watanabe et al., 1994). As shown in Figure 2, YG1024 exhibited enhanced sensitivity to the mutagenicity of both 2,4-DAT and 2,6-DAT in the presence of S9 activation. The mutagenicity of 2,6-DAT was similar to that of 2,4-DAT in the presence of S9 activation (in strain TA98, 1036 vs. 1316 His+ revertants per plate at 625 μg of 2,4-DAT and 2,6-DAT, respectively; in strain YG1024, 3460 vs. 3896 His+ revertants per plate at 156 μg of 2,4-DAT and 2,6-DAT, respectively). These results suggest that both DATs are mutagenic in the presence of S9 activation in vitro and also that O-acetylation is important for the metabolic activation.
FIG. 2.
Mutagenic activity of 2,4-DAT (A) and 2,6-DAT (B) in Salmonella typhimurium strains TA98 (circle) and YG1024 (rhombus). Filled circle and rhombus assayed with S9 mix; open circle and rhombus assayed without S9 mix.
In Vivo Mutagenicity of 2,4-DAT
For the initial in vivo genotoxicity assay, we examined MN formation in the peripheral blood of F344 gpt delta rats treated with 2,4-DAT or 2,6-DAT. However, no significant increase in MN frequency was observed in any of the treated groups (Supplementary table 4).
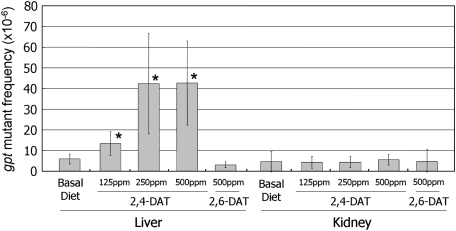
Next, we examined the mutagenicity of the DATs in the livers and kidneys of the rats. gpt MFs were significantly increased in the livers of 2,4-DAT–treated rats at all three doses and in the DEN-positive control, compared to the control group (Fig. 3, Supplementary table 3). No increases in MFs were observed in the livers of 2,6-DAT–treated rats or in the kidneys of either 2,4-DAT– or 2,6-DAT–treated rats. To characterize the gpt mutations in the liver, we performed DNA sequencing (Table 3). The predominant base substitutions were G:C-to-A:T transitions and G:C-to-T:A and G:C-to-C:G transversions in the 2,4-DAT–treated groups. In addition, base substitutions at A:T bps were also induced. In the DEN-treated positive control group, A:T-to-T:A transversions were the most predominant type of mutation. Spi− MFs in the liver were also significantly increased in 2,4-DAT treatment groups at doses of 250 and 500 ppm and in the DEN-treated group (Table 4). They were not increased by treatment with 2,6-DAT. DNA sequence analysis revealed that the specific mutant frequency (SMF) of a −1 frameshift at run sequences such as GGGG in the gam gene was increased more than fourfold after treatment with 500 ppm 2,4-DAT, while the SMF of deletions of more than two bps was not enhanced at this dose (Supplementary table 5). Thus, most of the Spi− mutations were −1 frameshift mutations at run sequences, and large deletion mutations were not significantly induced by treatment with 2,4-DAT.
FIG. 3.
MFs of gpt genes. Values represent mean SD (n = 5). Significant differences were observed in 2,4-DAT–treated livers compared to livers from rats fed negative control basal diet. *p < 0.05.
TABLE 3.
Classification of gpt Mutations in gpt Delta Rat Livers
| Basal Diet |
125 ppm 2,4-DAT |
250 ppm 2,4-DAT |
500 ppm 2,4-DAT |
500 ppm 2,6-DAT |
Basal Diet (DEN) |
|||||||
| Type of gpt Mutation | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| Base substitution | ||||||||||||
| Transition | ||||||||||||
| G:C → A:T | 4 | 22 | 15 | 26 | 18 | 38 | 12 | 35 | 4 | 27 | 13 | 22 |
| (CpG) | (1) | (6) | (6) | (3) | (1) | (3) | ||||||
| A:T → G:C | 1 | 6 | 1 | 2 | 5 | 11 | 1 | 3 | 3 | 20 | 12 | 21 |
| Transversion | ||||||||||||
| G:C → T:A | 4 | 22 | 16 | 28 | 12 | 26 | 6 | 18 | 4 | 27 | 3 | 5 |
| G:C → C:G | 1 | 6 | 7 | 12 | 4 | 9 | 7 | 21 | 0 | 0 | 0 | 0 |
| A:T → T:A | 0 | 0 | 5 | 9 | 4 | 9 | 1 | 3 | 1 | 7 | 23 | 40 |
| A:T → C:G | 1 | 6 | 4 | 7 | 3 | 6 | 1 | 3 | 0 | 0 | 7 | 12 |
| Deletion | ||||||||||||
| −1 | 4 | 22 | 3 | 5 | 1 | 2 | 2 | 6 | 1 | 7 | 0 | 0 |
| >2 | 2 | 11 | 2 | 3 | 0 | 0 | 1 | 3 | 2 | 13 | 0 | 0 |
| Insertion | 1 | 6 | 3 | 5 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 |
| Others | 0 | 0 | 2 | 3 | 0 | 0 | 2 | 6 | 0 | 0 | 0 | 0 |
| Total | 18 | 100 | 58 | 100 | 47 | 100 | 34 | 100 | 15 | 100 | 58 | 100 |
TABLE 4.
Spi− Mutant Frequency in Rat Livers
| Treatment | No. of Rats | Mutant Frequency (× 10−6) (Mean ± SD) | p Value (t-test) |
| Basal diet | 5 | 4.43 ± 1.99 | |
| Basal diet (DEN) | 5 | 341.22 ± 180.91 | 0.002 |
| 125 ppm 2,4-DAT | 5 | 8.20 ± 4.75 | 0.07 |
| 250 ppm 2,4-DAT | 5 | 13.42 ± 4.83 | 0.003 |
| 500 ppm 2,4-DAT | 5 | 15.98 ± 4.45 | 0.0004 |
| 500 ppm 2,6-DAT | 5 | 5.49 ± 2.53 | 0.241 |
DISCUSSION
In the regulatory sciences, a default assumption is that genotoxic carcinogens have no thresholds for their activities, and thus, no acceptable daily intake can be set for these chemicals when they are used as food additives, pesticides, or veterinary medicines (Kirsch-Volders et al., 2000; Nohmi, 2008). It is thought that single molecules of genotoxic compounds can induce mutations, and thus, genotoxic carcinogens impose carcinogenic risks to humans even at very low doses. However, how the genotoxicity of chemicals should be defined is not entirely clear. Currently, more than 200 genotoxicity assays have been proposed (Preston and Hoffmann, 2007). Unsurprisingly, the results among the various genotoxicity assays are inconsistent. The aromatic amine structural isomers 2,4-DAT and 2,6-DAT are an interesting reference pair that illustrates the inconsistency between in vitro and in vivo results (Cunningham et al., 1989). Both 2,4-DAT and 2,6-DAT are mutagenic in vitro in S. typhimurium strains, but only 2,4-DAT is carcinogenic in mice and rats (NTP, 1979).
In this study, we confirmed in vitro genotoxicity with S. typhimurium TA98 and YG1024 and explored in vivo genotoxicity with F344 gpt delta rats. Both DATs were mutagenic in the S. typhimurium strains in vitro when the S9 activation system was present (Fig. 2). In contrast, only 2,4-DAT was mutagenic in the livers of rats (Fig. 3, Table 4, Supplementary table 4). Both gpt and Spi− MFs in the liver were significantly increased in 2,4-DAT–treated rats compared to those in the control group. We did not observe any increase in gpt MFs in the livers of 2,6-DAT–treated rats or in the kidneys of 2,4- or 2,6-DAT–treated rats (Fig. 3, Supplementary table 4). Kidney may not have capacity to activate 2,4-DAT as in the case of bone marrow (see below). We identified preneoplastic lesions (i.e., GST-P–positive foci) in the livers of rats treated with 250 and 500 ppm 2,4-DAT (Table 2) but not in the livers of rats treated with 2,6-DAT. Generally, proliferation is activated in cancer cells. Ki-67 is a nuclear marker of cell proliferation and detectable in cells at all phases of the cell cycles except G0 (Gerdes et al., 1983). The Ki-67 labeling index is a measure of tumor proliferation and reported the association with liver and breast cancer outcome (de Azambuja et al., 2007; Nolte et al., 1998). The increase in Ki-67 index suggested the precancerous status of liver of rats treated by 2,4-DAT (Table 1, Fig. 1). We conclude from these results that genotoxicity assays (i.e., gpt and Spi− assays) and short-term carcinogenicity assays (i.e., GST-P–positive foci formation) can be conducted with F344 gpt delta rats. Because we observed genotoxicity in the target organ of carcinogenicity, these results strongly suggest that the carcinogenicity of 2,4-DAT is due to genotoxic activities. Integration of the genotoxicity assay with the pathological assay including GST-P–positive foci formation in gpt delta rats could reduce the number of animals necessary for these assays; this would contribute to the adoption of the 3R (reduction, replacement, and refinement) principle for animal use in the life sciences (Balls, 1997). It should be mentioned, however, that GST-P–positive foci formation often needs long treatment periods, for example, 16 and 24 weeks, respectively, for 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline and 2-acetylaminofluorene (Bagnyukova et al., 2008; Tsuda et al., 2003) and such long treatments may increase the risk of false-positive results of mutations due to nongenotoxic mechanisms caused by chronic toxicity, for example, tumor induction and inflammatory responses (Thybaud et al., 2003).
Why do both 2,6-DAT and 2,4-DAT exhibit mutagenicity in vitro? The inconsistency between in vitro and in vivo results could be due to the different metabolic pathways of 2,6-DAT in vitro and in vivo. It is plausible that a DAT amino group is first oxidized by a specific cytochrome P450 (e.g., CYP1A2), and the resulting N-hydroxy group is further activated by OAT, which leads to the generation of nitrenium ions that can bind to DNA in vitro (Watanabe et al., 1994). In vitro, both DATs were mutagenic only in the presence of S9 activation, and strain YG1024, which overexpresses OAT, exhibited greater sensitivity to the DATs than did strain TA98 (Fig. 2). Both S. typhimurium strains possess GC repetitive sequences in the hisD gene that serve as target sites for mutations. We speculate, therefore, that 2,4-DAT could be activated in vivo via the pathway described above and induce mostly guanine adducts in DNA. In fact, it was reported that 2,4-DAT induces DNA adducts in the livers of rats over 6000 times more efficiently than does 2,6-DAT (Taningher et al., 1995). The sequence analysis that we conducted indicates that most of the mutations induced by 2,4-DAT at 500 ppm were guanine base substitutions; that is, G:C-to-A:T, G:C-to-T:A, and G:C-to-C:G (Table 3). 2,6-DAT might be more efficiently detoxicated than 2,4-DAT in vivo because its para site at position 4 can be oxidized and subsequently conjugated by phase II enzymes (Cunningham et al., 1989). Detoxication of 2,6-DAT by phase II enzymes may be ineffective in vitro compared to in vivo. Appropriate cofactors, for example, uridine 5′-diphosphoric acid P2-β-D-glucopyranuronosyl ester for glucuronidation, may be needed to effectively detoxify the active metabolites of 2,6-DAT in vitro. At present, however, we cannot rule out the possibility that other factors, such as DNA repair, cell proliferation, translesion DNA synthesis, or apoptosis, might be involved in the differences in mutagenicity of 2,6-DAT in vitro and in vivo.
In addition to the discrepancy between in vitro and in vivo mutagenicity, neither 2,4-DAT nor 2,6-DAT was genotoxic in the bone marrows of rats according to the MN assay (Supplementary table 3). 2,4-DAT is also not mutagenic when applied to MutaMouse skin (Kirkland and Beevers, 2006). Thus, we suggest that the negative results of the MN assay may be due to inefficient metabolic activation of 2,4-DAT at extrahepatic sites. Alternatively, the active metabolites generated in the liver may not reach the bone marrow. The poor metabolic activation in extrahepatic sites and/or short half-lives of the active metabolites may also account for the negative results of the MN assay with DEN in the bone marrow (Supplementary table 3). The MN assay is usually the first choice for in vivo genotoxicity assays in the development of pharmaceuticals; our results indicate that rather than relying on the MN assay in the bone marrow, genotoxicity should be evaluated in multiple organs, including the target organs of carcinogenicity.
The Spi− assay is unique to gpt delta mice and rats and identifies deletion-type mutations (Nohmi et al., 2000). Previous studies with gpt delta mice suggested that genotoxic compounds and physical factors (e.g., radiation) induce different types of deletion mutations in vivo (Nohmi and Masumura, 2005). For example, heavy-ion radiation, ultraviolet B radiation, and mitomycin C induce large deletions in the liver, epidermis, and bone marrow, respectively, at molecular sizes of >1 kbp (Horiguchi et al., 2001; Masumura et al., 2002; Takeiri et al., 2003). In contrast, aromatic amines such as 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and aminophenylnorharman (APNH) induce −1 frameshift mutations in runs of guanine bases in the colon and liver, respectively (Masumura et al., 2000, 2003). We characterized Spi− mutants obtained from the livers of rats treated with 500-ppm 2,4-DAT and concluded that, like PhIP and APNH, 2,4-DAT induces mostly −1 frameshift mutations (Supplementary table 5). These results suggest that Spi− assay, as well as the gpt assay, is useful for characterizing mutations, which may constitute the molecular basis of chemically induced carcinogenesis.
At the 2006 International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use meeting held in Yokohama, Japan, revisions of the guidelines for a basic test battery of in vitro and in vivo genotoxicity tests were discussed (Hayashi, 2008). The current guidelines recommend two in vitro assays (Ames test and either a mammalian chromosome aberration test or a mammalian gene mutation test) plus one in vivo assay (usually MN test). Because of the high rate of false positives with in vitro mammalian cell assays (Kirkland et al., 2005), however, an alternative test battery was proposed at the meeting (Hayashi, 2008). The new battery is composed of one in vitro assay (Ames test) plus two in vivo assays (MN test plus a second in vivo test such as a transgenic assay or in vivo comet assay). It is possible to choose the classical battery of two in vitro assays plus one in vivo assay instead of the alternative new battery. With the 3R principle in mind, integration of in vivo genotoxicity assays and a 28-day repeated dose toxicity assay was also discussed. In vivo mutagenicity assays and an in vivo MN assay can be integrated into a 28-day repeated dose toxicity study when transgenic rodents are used (Thybaud et al., 2003). In this study, we found that an in vivo genotoxicity assay and a short-term bioassay for liver carcinogenesis using GST-P–positive foci as an end point of preneoplastic lesions can be conducted with F344 gpt delta rats. Integration of the two assays using transgenic rats may further facilitate adoption of the 3R principle in regulatory toxicology.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Ministry of Education, Culture, Sports, Science and Technology, Japan (18201010); the Ministry of Health, Labour and Welfare, Japan (MHLW; H21-Food-General-009); the Japan Health Science Foundation (KHB1007); MHLW (20 designated-8).
Supplementary Material
References
- Aoki Y, Hashimoto AH, Amanuma K, Matsumoto M, Hiyoshi K, Takano H, Masumura K, Itoh K, Nohmi T, Yamamoto M. Enhanced spontaneous and benzo(a)pyrene-induced mutations in the lung of Nrf2-deficient gpt delta mice. Cancer Res. 2007;67:5643–5648. doi: 10.1158/0008-5472.CAN-06-3355. [DOI] [PubMed] [Google Scholar]
- Bagnyukova TV, Tryndyak VP, Montgomery B, Churchwell MI, Karpf AR, James SR, Muskhelishveli L, Beland FA, Pogribny IP. Genetic and epigenetic changes in rat preneoplastic liver tissue induced by 2-acetylaminofluorene. Carcinogenesis. 2008;29:638–646. doi: 10.1093/carcin/bgm303. [DOI] [PubMed] [Google Scholar]
- Balls M. The three Rs concept of alternatives to animal experimentation. In: van Zutphen LFM, Balls M, editors. Animal Alternatives. Amsterdam: Elsevier; 1997. pp. 27–41. [Google Scholar]
- Cunningham ML, Burka LT, Matthews HB. Metabolism, disposition, and mutagenicity of 2,6-diaminotoluene, a mutagenic noncarcinogen. Drug Metab. Dispos. 1989;17:612–617. [PubMed] [Google Scholar]
- Cunningham ML, Hayward JJ, Shane BS, Tindall KR. Distinction of mutagenic carcinogens from a mutagenic noncarcinogen in the big blue transgenic mouse. Environ. Health Perspect. 1996;104(Suppl. 3):683–686. doi: 10.1289/ehp.96104s3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azambuja E, Cardoso F, de Castro G, Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast caner: a meta-analysis of published studies involving 12,155 patients. Br. J. Cancer. 2007;96:1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond DA, Hartwig A, Anderson D, Anwar WA, Cimino MC, Dobrev I, Douglas GR, Nohmi T, Phillips DH, Vickers C. Mutagenicity testing for chemical risk assessment: update of the WHO/IPCS Harmonized Scheme. Mutagenesis. 2009;24:341–349. doi: 10.1093/mutage/gep014. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Hashimoto AH, Amanuma K, Masumura K, Nohmi T, Aoki Y. In vivo mutagenesis caused by diesel exhaust in the testis of gpt delta mouse. Genes Environ. 2009;31:1–8. [Google Scholar]
- Hayashi H, Kondo H, Masumura K, Shindo Y, Nohmi T. Novel transgenic rat for in vivo genotoxicity assays using 6-thioguanine and Spi-selection. Environ. Mol. Mutagen. 2003;41:253–259. doi: 10.1002/em.10152. [DOI] [PubMed] [Google Scholar]
- Hayashi M. Update on the maintenance of the ICH S2 genetic toxicology. Pharm. Regul. Sci. 2008;39:515–521. [Google Scholar]
- Heddle JA, Dean S, Nohmi T, Boerrigter M, Casciano D, Douglas GR, Glickman BW, Gorelick NJ, Mirsalis JC, Martus HJ, et al. In vivo transgenic mutation assays. Environ. Mol. Mutagen. 2000;35:253–259. doi: 10.1002/(sici)1098-2280(2000)35:3<253::aid-em11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Masumura KI, Ikehata H, Ono T, Kanke Y, Nohmi T. Molecular nature of ultraviolet B light-induced deletions in the murine epidermis. Cancer Res. 2001;61:3913–3918. [PubMed] [Google Scholar]
- Ito N, Imaida K, Asamoto M, Shirai T. Early detection of carcinogenic substances and modifiers in rats. Mutat. Res. 2000;462:209–217. doi: 10.1016/s1383-5742(00)00038-7. [DOI] [PubMed] [Google Scholar]
- Kirkland D, Aardema M, Henderson L, Muller L. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity, specificity and relative predictivity. Mutat. Res. 2005;584:1–256. doi: 10.1016/j.mrgentox.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Kirkland D, Beevers C. Induction of LacZ mutations in Muta Mouse can distinguish carcinogenic from non-carcinogenic analogues of diaminotoluenes and nitronaphthalenes. Mutat. Res. 2006;608:88–96. doi: 10.1016/j.mrgentox.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Kirsch-Volders M, Aardema M, Elhajouji A. Concepts of threshold in mutagenesis and carcinogenesis. Mutat. Res. 2000;464:3–11. doi: 10.1016/s1383-5718(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Maron DM, Ames BN. Revised methods for the salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Masumura K, Matsui M, Katoh M, Horiya N, Ueda O, Tanabe H, Yamada M, Suzuki H, Sofuni T, Nohmi T. Spectra of gpt mutations in ethylnitrosourea-treated and untreated transgenic mice. Environ. Mol. Mutagen. 1999;34:1–8. doi: 10.1002/(sici)1098-2280(1999)34:1<1::aid-em1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Masumura K, Matsui K, Yamada M, Horiguchi M, Ishida K, Watanabe M, Wakabayashi K, Nohmi T. Characterization of mutations induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in the colon of gpt delta transgenic mouse: novel G: C deletions beside runs of identical bases. Carcinogenesis. 2000;21:2049–2056. doi: 10.1093/carcin/21.11.2049. [DOI] [PubMed] [Google Scholar]
- Masumura K, Kuniya K, Kurobe T, Fukuoka M, Yatagai F, Nohmi T. Heavy-ion-induced mutations in the gpt delta transgenic mouse: comparison of mutation spectra induced by heavy-ion, X-ray, and gamma-ray radiation. Environ. Mol. Mutagen. 2002;40:207–215. doi: 10.1002/em.10108. [DOI] [PubMed] [Google Scholar]
- Masumura K, Totsuka Y, Wakabayashi K, Nohmi T. Potent genotoxicity of aminophenylnorharman, formed from non-mutagenic norharman and aniline, in the liver of gpt delta transgenic mouse. Carcinogenesis. 2003;24:1985–1993. doi: 10.1093/carcin/bgg170. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. Bioassay of 2,4-diaminotoluene for possible carcinogenicity. Natl. Cancer Inst. Carcinog. Tech. Rep. Ser. 1979;162:1–139. [PubMed] [Google Scholar]
- National Toxicology Program. Bioassay of 2,6-toluenediamine dihydrochloride for possible carcinogenicity (CAS No. 15481-70-6) Natl. Toxicol. Program Tech. Rep. Ser. 1980;200:1–123. [PubMed] [Google Scholar]
- Nohmi T. Possible mechanisms of practical thresholds for genotoxicity. Genes Environ. 2008;30:108–113. [Google Scholar]
- Nohmi T, Katoh M, Suzuki H, Matsui M, Yamada M, Watanabe M, Suzuki M, Horiya N, Ueda O, Shibuya T, et al. A new transgenic mouse mutagenesis test system using Spi-and 6-thioguanine selections. Environ. Mol. Mutagen. 1996;28:465–470. doi: 10.1002/(SICI)1098-2280(1996)28:4<465::AID-EM24>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Nohmi T, Masumura K. Molecular nature of intrachromosomal deletions and base substitutions induced by environmental mutagens. Environ. Mol. Mutagen. 2005;45:150–161. doi: 10.1002/em.20110. [DOI] [PubMed] [Google Scholar]
- Nohmi T, Suzuki T, Masumura K. Recent advances in the protocols of transgenic mouse mutation assays. Mutat. Res. 2000;455:191–215. doi: 10.1016/s0027-5107(00)00077-4. [DOI] [PubMed] [Google Scholar]
- Nolte M, Werner M, Nasarek A, Bektas H, von Wasielewski R, Klempnauer J, Georgii A. Expression of proliferation associated antigens and detection of numerical chromosome aberrations in primary human liver tumors: relevance to tumor characteristic and prognosis. J. Clin. Pathol. 1998;51:47–51. doi: 10.1136/jcp.51.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso T, Tatematsu M, Tamano S, Tsuda H, Ito N. Comparative effects of carcinogens on the induction of placental glutathione S-transferase-positive liver nodules in a short-term assay and of hepatocellular carcinomas in a long-term assay. Toxicol. Pathol. 1985;13:257–265. doi: 10.1177/019262338501300402. [DOI] [PubMed] [Google Scholar]
- Preston RJ, Hoffmann GR. Genetic toxicology. In: Klaassen CD, editor. Casarett and Doull’s Toxicology: The Basic Science of Poisons. New York: The McGraw-Hill Companies, Inc.; 2007. pp. 381–413. [Google Scholar]
- Shibata A, Maeda D, Ogino H, Tsutsumi M, Nohmi T, Nakagama H, Sugimura T, Teraoka H, Masutani M. Role of Parp-1 in suppressing spontaneous deletion mutation in the liver and brain of mice at adolescence and advanced age. Mutat. Res. 2009;664:20–27. doi: 10.1016/j.mrfmmm.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Takeiri A, Mishima M, Tanaka K, Shioda A, Ueda O, Suzuki H, Inoue M, Masumura K, Nohmi T. Molecular characterization of mitomycin C-induced large deletions and tandem-base substitutions in the bone marrow of gpt delta transgenic mice. Chem. Res. Toxicol. 2003;16:171–179. doi: 10.1021/tx0255673. [DOI] [PubMed] [Google Scholar]
- Taningher M, Peluso M, Parodi S, Ledda-Columbano GM, Columbano A. Genotoxic and non-genotoxic activities of 2,4- and 2,6-diaminotoluene, as evaluated in Fischer-344 rat liver. Toxicology. 1995;99:1–10. doi: 10.1016/0300-483x(95)02976-f. [DOI] [PubMed] [Google Scholar]
- Thybaud V, Dean S, Nohmi T, de Boer J, Douglas GR, Glickman BW, Gorelick NJ, Heddle JA, Heflich RH, Lambert I, et al. In vivo transgenic mutation assays. Mutat. Res. 2003;540:141–151. doi: 10.1016/j.mrgentox.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Fukushima S, Wanibuchi H, Morimura K, Nakae D, Imaida K, Tatematsu M, Hirose M, Wakabayashi K, Moore MA. Value of GST-P positive preneoplastic hepatic foci in dose-response studies of hepatocarcinogenesis: evidence for practical thresholds with both genotoxic and nongenotoxic carcinogens. A review of recent work. Toxicol. Pathol. 2003;31:80–86. doi: 10.1080/01926230390173879. [DOI] [PubMed] [Google Scholar]
- Umemura T, Kanki K, Kuroiwa Y, Ishii Y, Okano K, Nohmi T, Nishikawa A, Hirose M. In vivo mutagenicity and initiation following oxidative DNA lesion in the kidneys of rats given potassium bromate. Cancer Sci. 2006;97:829–835. doi: 10.1111/j.1349-7006.2006.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura T, Tasaki M, Kijima A, Okamura T, Inoue T, Ishii Y, Suzuki Y, Masui N, Nohmi T, Nishikawa A. Possible participation of oxidative stress in causation of cell proliferation and in vivo mutagenicity in kidneys of gpt delta rats treated with potassium bromate. Toxicology. 2009;257:46–52. doi: 10.1016/j.tox.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Igarashi T, Kaminuma T, Sofuni T, Nohmi T. N-hydroxyarylamine O-acetyltransferase of Salmonella typhimurium: proposal for a common catalytic mechanism of arylamine acetyltransferase enzymes. Environ. Health Perspect. 1994;102(Suppl. 6):83–89. doi: 10.1289/ehp.94102s683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Smilenov LB, He P, Masumura K, Nohmi T, Yu Z, Hei TK. New insight into intrachromosomal deletions induced by chrysotile in the gpt delta transgenic mutation assay. Environ. Health Perspect. 2007;115:87–92. doi: 10.1289/ehp.9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.