Abstract
Introduction
This study evaluated long-term outcomes in patients with pulmonary arterial hypertension (PAH) undergoing treatment with ambrisentan monotherapy, a selective oral endothelin-1 receptor antagonist.
Methods
Patients who participated in the ARIES-1 clinical trial and extension phase at our institution were included. Cardiac catheterization, 6-minute walk distance (6MWD), and cardiac magnetic resonance (MRI) data were retrospectively reviewed.
Results
12 patients with PAH (11 idiopathic, 1 fenfluramine) had follow-up for 3–5.5 years from the initiation of ARIES-1. Patients received ambrisentan therapy throughout the study period and were on ambrisentan monotherapy for the first 2 years. At year 1, improvements in median mean pulmonary arterial pressure (PA), cardiac output and pulmonary vascular resistance (PVR) were seen (p=0.02, p=0.03, p<0.01), and the improvement in PVR persisted at 2 years. Six minute walk distance also improved significantly between baseline (350m) and 1 and 2 years (397 m, p<0.01 and 393 m, p=0.01). Cardiac MRI results were more varied, with an increase in RV ejection fraction from 29% at baseline to 46% at 2 years (p=0.02), but other MRI variables did not improve.
Conclusions
Ambrisentan monotherapy led to improvements in catheterization, 6MWD and RV ejection fraction, and shows promise as a long-term treatment for pulmonary arterial hypertension.
Introduction
Ambrisentan (Letairis; Gilead; Foster City, CA) is an oral endothelin-1 receptor antagonist approved in 2007 for the treatment of World Health Organization (WHO) functional class II and III pulmonary arterial hypertension (PAH). Ambrisentan is the second endothelin-1 receptor antagonist to receive approval, and unlike the dual endothelin-1 receptor antagonist, bosentan (Tracleer, Actelion, Allschwil, Switzerland), ambrisentan is a selective antagonist for the endothelin A (ETA) receptor. Endothelin-1 causes vasoconstriction and pulmonary artery smooth muscle cell growth mainly through its actions on the ETA receptor, while the ETB receptor is the predominate site for clearance of endothelin-11.
Ambrisentan has been shown to lead to improvement in functional class, walk distance and time to clinical worsening relative to placebo in two Phase 3, placebo-controlled, 12-week clinical trials (total n=394)2 and to lead to improved cardiopulmonary hemodynamics in an uncontrolled 12-week dose ranging study (n=64)3. However, the long-term effects of ambrisentan on hemodynamics and right ventricular performance have not been reported. Additionally, there are few studies evaluating serial cardiac magnetic resonance imaging (MRI) results for patients treated with any of the approved PAH medications.
In this retrospective study, we evaluate cardiac catheterization and MRI data, and 6 minute walk distance (6MWD) in twelve patients treated with first-line ambrisentan therapy over 3.5–5 years.
Methods
Patients participating in the ARIES-1 (Ambrisentan in Pulmonary Arterial Hypertension: A Phase 3, Randomized, Double-Blind, Placebo-Controlled Multicenter Efficacy Study) clinical trial and extension study at our institution were included. ARIES-1 included patients with idiopathic PAH or PAH associated with connective tissue disease, anorexigen use or human immunodeficiency virus. Entry criteria included pulmonary arterial hypertension diagnosed by cardiac catheterization with a documented mean pulmonary arterial pressure of >25 mmHg and pulmonary capillary wedge pressure or left ventricular end-diastolic pressure <15 mmHg.
Patients were randomized 1:1:1 to placebo, ambrisentan 5 mg or 10 mg once daily for 12 weeks. Patients were then given the option to enter the extension phase (ARIES-E) where those on active drug continued on the same dose and those on placebo were randomized to 5 or 10 mg ambrisentan daily. Patients could also enter the extension phase through a protocol-specified escape route: patients receiving placebo during the initial phase of the study who then developed evidence of worsening PAH could be withdrawn from the study and then receive ambrisentan in the extension study. During the extension study, add-on therapy (other approved, non-ERA, PAH therapy) was allowed at the discretion of the treating physician; after ambrisentan received FDA approval, patients were transitioned to commercially available ambrisentan. Duration of follow-up for each patient was determined from the time of the enrollment in the ARIES-1 trial to the last available follow-up.
Follow-up testing included 6MWD testing every 3 months. In addition and separate from ARIES-1 study procedures, patients at our institution underwent right-sided heart catheterization and cardiac MRI on an approximately annual basis. The cardiac MRI imaging was performed on a GE 1.5T scanner (GE Healthcare, Milwaukee, USA) using Fiesta cine short axis images; left and right heart volume and mass measurements were performed using MASS software (MEDIS, Leiden, the Netherlands).
Plasma brain natriuretic peptide (BNP) levels (pg/ml) were not routinely checked during the earlier years of the study. The most recent results are reported in the Patient Details when available.
Statistical Analysis
Changes in hemodynamics, 6MWD and cardiac MRI results between baseline, 1, and 2 years were analyzed using Wilcoxon rank sum test. Comparison of baseline values with normal values for cardiac MRI4 was performed using a z conversion in order to account for the difference between the male and female normal ranges. SPSS 16.0 (SPSS Inc., Chicago, IL) was used for the statistical analysis, and p values <0.05 were considered statistically significant. Six minute walk distance, right sided heart catheterization and cardiac MRI data are reported for the 12 patients who entered the extension study and thus had serial evaluations available. All results are reported as medians. For the Kaplan-Meier curves of survival, all 14 of the originally enrolled patients were included. This study was approved by the UT Southwestern Institutional Review Board.
Results
Fourteen patients at our institution (mean age 46 years, range 18 – 67 years) were enrolled in the ARIES-1 clinical trial. One patient died (described further below) and 1 patient withdrew consent (both randomized to the placebo arm) during the initial 12 week randomized portion of the study. The remaining 12 patients entered the long-term, extension study (Figure 1), where all received ambrisentan treatment. Eleven patients had idiopathic PAH, and 1 patient had prior fenfluramine exposure. No patients were diagnosed with familial PAH. At baseline of ARIES-1, all patients had severe PAH as evidenced by the hemodynamic data, WHO classification, and exercise capacity (Table 1 and 2).
Figure 1. Flow chart.
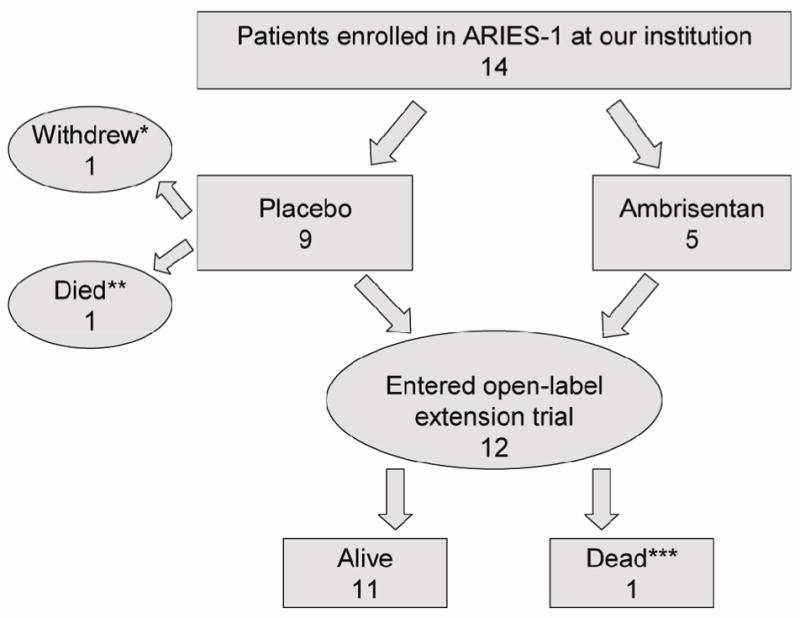
Fourteen patients at our institution enrolled in ARIES-1 trial. Twelve were included in the long-term open label trial
Table 1.
Demographics
| Gender (% female) | 75% |
| Age (mean, range) | 46 (18–67) |
| Baseline walk (meters) | 336±77 |
| WHO Class III | 100% |
| Etiology | Idiopathic: N=11 Fenfluramine: N=1 |
Table 2.
Baseline, One and Two Year Results for Ambrisentan Treated PAH Patients
| Gender | Age | Last Rx | RA (mmHg) | PA (mmHg) | PVR (Wood U) | CI (L/m2) | RVEF (ml) | RVEDVi (ml) | RVmass (gm/m2) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | ||||
| 1 | F | 40 | A,T | 14 | 7 | 7 | 64 | 47 | 72 | 12.8 | 5.2 | 8.4 | 1.7 | 3.1 | 3.3 | 56 | 47 | 63 | 76 | 67 | 69 | 33 | 26 | |
| 2 | F | 60 | A, E iv | 11 | 1 | 7 | 44 | 28 | 37 | 6.3 | 3.9 | 5.8 | 2.7 | 3.7 | 2.8 | 55 | 61 | 59 | 52 | 41 | 55 | 18 | 24 | 19 |
| 3 | F | 60 | A | 2 | 7 | 7 | 32 | 26 | 27 | 3.2 | 2.0 | 3.0 | 4.6 | 3.7 | 3.0 | 68 | 71 | 70 | 80 | 63 | 67 | 23 | 21 | 23 |
| 4 | F | 40 | A, E iv | 3 | 14 | 6 | 58 | 58 | 59 | 16.8 | 12.8 | 15.8 | 1.8 | 2.2 | 2.1 | 26 | 33 | 28 | 94 | 117 | 102 | 44 | 36 | 68 |
| 5 | M | 20 | A, T iv | 11 | 9 | 6 | 65 | 52 | 51 | 11.1 | 6.9 | 7.9 | 2.1 | 2.1 | 2.4 | 23 | 16 | 18 | 242 | 193 | 229 | 109 | 127 | 139 |
| 6 | F | 50 | A | 54 | 16.4 | 1.6 | 45 | 92 | ||||||||||||||||
| 7 | M | 30 | A,T,S | 21 | 18 | 20 | 73 | 56 | 45 | 19.9 | 8.6 | 10.1 | 1.3 | 2.9 | 1.7 | 27 | 22 | 25 | 162 | 183 | 214 | 68 | 93 | 108 |
| 8 | F | 60 | A,T | 4 | 8 | 9 | 88 | 47 | 51 | 16.3 | 9.5 | 9.5 | 1.0 | 2.4 | 2.1 | 36 | 63 | 68 | 64 | 64 | 61 | 52 | 36 | 36 |
| 9 | M | 40 | A | 17 | 15 | 18 | 67 | 65 | 61 | 9.8 | 8.6 | 8.1 | 2.1 | 2.4 | 2.3 | 24 | 30 | 30 | 98 | 83 | 99 | 69 | 66 | 71 |
| 10 | F | 50 | A,T iv | 4 | 10 | 8 | 75 | 75 | 68 | 17.1 | 18.9 | 17.5 | 2.4 | 2.2 | 2.2 | 29 | 36 | 41 | 92 | 118 | 123 | 63 | 72 | 77 |
| 11 | F | 50 | A,T | 7 | 9 | 11 | 60 | 65 | 60 | 18.3 | 15 | 12.6 | 1.7 | 2.1 | 2.2 | 17 | 47 | 52 | 78 | 69 | 82 | 41 | 42 | 51 |
| 12 | F | 20 | A,T | 8 | 4 | 4 | 41 | 39 | 40 | 7.0 | 3.9 | 5.4 | 2.6 | 3.4 | 2.5 | 43 | 56 | 52 | 67 | 56 | 49 | 38 | 19 | 19 |
| Median | 8 | 9 | 7 | 62 | 52 | 51 | 14.6 | 8.6 | 8.4 | 2 | 2.4 | 2.3 | 32.5 | 47 | 52 | 86 | 69 | 82 | 44 | 36 | 60 | |||
| P value | 0.96 | 0.96 | 0.02 | 0.05 | <0.01 | <0.01 | 0.03 | 0.18 | 0.09 | 0.04 | 0.5 | 0.53 | 0.93 | 0.17 | ||||||||||
Patient numbers are in order by date of enrollment, with all patients enrolled between 2004 and 2005. P values are for comparison of median baseline (0) to year 1 and year 2, respectively. Age is rounded to the nearest decade. Abbreviations: PA: pulmonary artery, PVR: pulmonary vascular resistance, CI: cardiac index, RVEF: right ventricular ejection fraction, RVEDVi: right ventricular end diastolic volume index, 6MWD: 6 minute walk distance. A: ambrisentan. T: oral treprostinil. S: sildenafil. E iv: epoprostenol. T iv: intravenous treprostinil.
Results of the cardiac catheterization, cardiac MRI data, and 6MWD for these 12 patients were collected over a period of 2 years, while survival and add-on therapy data were collected over 3.5 to 5 years; 1 patient had limited testing due to financial constraints. All patients remained on ambrisentan monotherapy for the initial 2 years. Afterwards, add on therapy was required, as detailed below. Add-on therapy (oral or intravenous) was not needed prior to the 2 year follow-up period, and thus, the 2 year cardiac MRI, catheterization, and 6MWD data represent ambrisentan monotherapy.
Cardiac Catheterizations
Baseline catheterizations were performed within the 4 months prior to the initiation of the ARIES-1 trial. Baseline hemodynamic data were abnormal in all patients with a median pulmonary arterial pressure of 62 mmHg (mean pressure) and pulmonary vascular resistance of 14.6 Wood units. Evidence of cardiac compromise was seen with the depressed median cardiac index of 2.0 L/min/m2. After one year of therapy, significant improvements in pulmonary arterial pressure, cardiac output and pulmonary vascular resistance were seen (Table 2). This improvement was maintained in most patients at two years, although 2 patients showed a significant increase (>10%) in pulmonary artery pressures. Pulmonary vascular resistance at year 2 was similar to year 1, and remained significantly lower than baseline, and there was a trend in improvement of mean pulmonary artery wedge pressure (0.05) and cardiac output (0.09).
Exercise Capacity
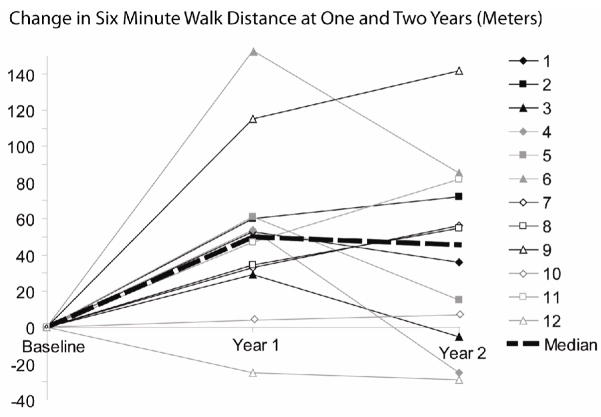
Baseline 6MWD was performed between 1 and 6 days prior to the start of the ARIES-1 trial. Study patients showed impairment of exercise capacity with a median baseline distance of 350 m. At one year follow-up, the median 6MWD had improved to 397 m (p<0.01). At year 2, the 6MWD was unchanged at 393 m, but remained a significant improvement from baseline (p= 0.01) (Figure 3).
Figure 3.
Cardiac Magnetic Resonance Imaging
Baseline cardiac MRI studies were performed prior to initiation of ambrisentan in 5 patients, within the first month of ambrisentan treatment in 3 patients, and during the first 4 months of therapy in 4 patients, dated from initiation of ambrisentan after adjustment for the placebo period (all dates in this paper outside of this section include the placebo-controlled period). Unlike cardiac catheterization which is required in all patients at our center prior to the initiation of a PAH specific medication, a pretreatment cardiac MRI is preferred but not required. Thus some “baseline” cardiac MRI measurements were performed after the initiation of therapy. Study patients showed markedly abnormal ventricles compared to normals 4 (Table 2 and Figure 2) on baseline MRI measurements. The median right ventricular (RV) end-diastolic volume indexed was elevated at 80 ml/m2 and the median RV ejection fraction was depressed at 29% (normal 61%)4. Follow-up MRI data was missing altogether for one patient and RV mass values were unavailable for a second patient. At 1 year follow-up, there was no significant change from baseline in right and left ventricular volumes and function. By two years, however, there was statistically significant improvement in right ventricular ejection fraction compared with baseline (p=0.02) but no significant change in the indexed right ventricular mass or end-diastolic volume.
Figure 2. Cardiac MRI.
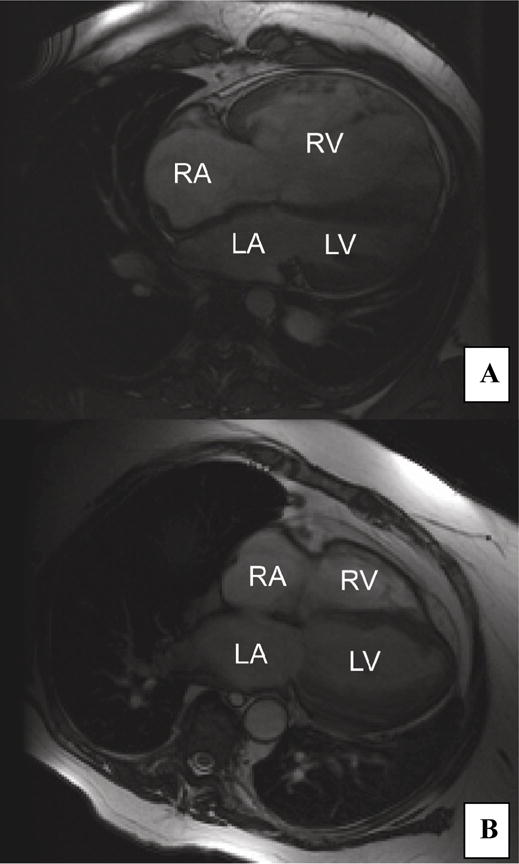
Four-chamber views of the heart by cardiac MRI. A) Dilated right ventricle (RV) in patient with RV end-diastolic volume of 600ml (patient 5) and B) Normal right ventricle in patient with RV end-diastolic volume of 150 ml (patient 3)
Survival and Requirement for Add-on PAH Therapy
Fourteen patients were initially randomized to placebo or ambrisentan under the ARIES-1 protocol. One patient randomized to placebo worsened unexpectedly during the second month of the study, and through the protocol-specific escape route was enrolled into the extension study and began ambrisentan treatment. She continued to decline rapidly due to what was subsequently diagnosed as severe lupus flare and died a few weeks later. A second patient randomized to placebo withdrew from the trial, but continued to be followed in our clinic for several years. To date, she has not received ambrisentan.
All twelve patients who enrolled in the extension study after the 12 week randomization remained on ambrisentan monotherapy during the first 2 years of follow-up, and most remained stable during this initial 2 year period. However, a total of four patients developed worsening symptoms requiring initiation of intravenous therapy: 2 towards the latter part of the second year (patient 4 and 5), and 2 in later clinical follow-up.
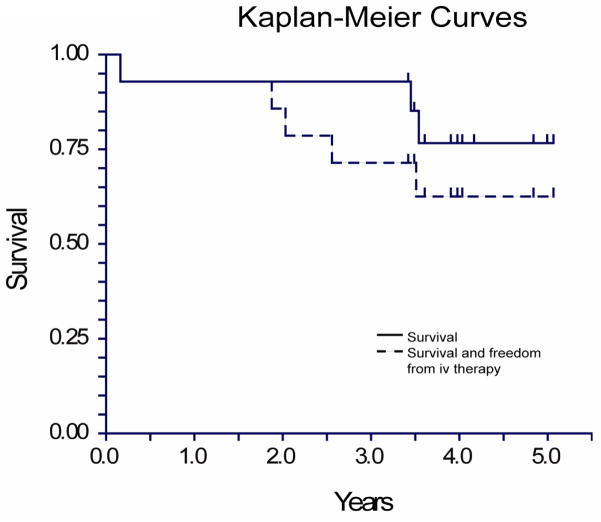
Add-on combination oral therapy has subsequently became more readily available and more widely used, and most (7 of 8) of the remaining patients received at least a trial of combination oral therapy during their third or fourth year of follow-up. Of the surviving patients, five remain on combinations of oral therapies: 3 are on ambrisentan monotherapy, and 2 are on ambrisentan plus an intravenous prostacyclin (Figure 4).
Figure 4.
Survival
Patient Details
Patient 1: ambrisentan + oral treprostinil
Female, 40s, alive. Oral treprostinil was added during year 3. With combination therapy symptoms remain stable and walk distance remains in the low 400 meters, and the last BNP was 37 pg/ml.
Patient 2: ambrisentan + attempt of iv epoprostenol
Female, 60s, deceased. Ambrisentan led to improvement in symptoms, and walk distance also improved to a peak distance of 413 meters. However, after 3 years treatment, walk distance fell precipitously to 69 meters, and repeat catheterization and MRI showed marked worsening PAH (right atrial pressure 11 mmHg, mean PA pressure 65 mmHg, cardiac index of 2.3 L/min/m2, RV ejection fraction of 36%). Epoprostenol was attempted, but she became profoundly hypoxic even on 15L oxygen, and it was discontinued; she died 2 weeks later. She had known mild COPD on study entry in 2004 (FEV1 70% predicted); CT angiogram at the time epoprostenol was attempted showed evidence of emphysematous changes, no new findings, no pulmonary emboli. The last BNP was 387 pg/ml which had increased from 37 pg/ml approximately 8 months prior.
Patient 3: ambrisentan monotherapy
Female, 60s, alive. After 4 years of ambrisentan, sildenafil was added for persistent exertional symptoms. However, she felt worse (short of breath, sinus congestion) and it was discontinued. She has stable, mildly abnormal hemodynamics (mean PA pressure <30 mmHg) and normal RV function. The BNP has always been less than 10 pg/ml.
Patient 4: ambrisentan + iv epoprostenol
Female, 40s, alive. After improvement between years 0 and year 1 on ambrisentan, symptoms and other tests worsened at 2 years, and intravenous epoprostenol was initiated. PA pressures remain in the mid-50s, but cardiac index is now normal and RV ejection fraction has improved. Exercise capacity remains reduced (last walk distance 341 meters). She has stable class III symptoms, and the last BNP was 354 pg/ml.
Patient 5: ambrisentan + iv treprostinil
Male, older teenager, alive. Ambrisentan led to considerable improvement in symptoms and hemodynamics, but the markedly dilated RV improved only modestly and then began dilating again, RV function remained poor, and symptoms were (slightly) increasing. Intravenous treprostinil was begun with improvement in mean PA pressure to the high 40s, and walk distance increased to 491 meters (he is 6′ tall), and a stable BNP in the 70s pg/ml. However, RV ejection fraction remains in the high teens and the right heart has continued to dilate.
Patient 6: ambrisentan monotherapy
Female, 50s, alive. Symptoms persist but are better than baseline, and walk distance remains above baseline at 289 meters.
Patient 7: ambrisentan + sildenafil + oral treprostinil
Male, 30s, alive. Sildenafil was added during year 3 due to persistent symptoms and hemodynamic abnormalities, and later that year oral treprostinil was added. Symptoms improved, but other results are mixed with a persistent low cardiac index and high right atrial pressure, but improved RV ejection fraction by MRI and a walk distance of 623 meters. The BNP level peaked at 199 pg/ml, but has been < 100 pg/ml for the last year.
Patient 8: ambrisentan + oral treprostinil
Female, 60s, alive. Oral treprostinil was added during year 3, and symptoms are stable. Catheterization and MRI remain improved from baseline, and last walk distance was 370 meters.
Patient 9: ambrisentan + sildenafil
Male, 40s, alive. Following 2 years of ambrisentan monotherapy, oral treprostinil was attempted but discontinued due to side effects and he was begun on sildenafil. Symptoms have improved and catheterization/MRI results have not worsened, but given the severity of the abnormalities, intravenous therapy was recommended; this was declined. The last BNP was 79 pg/ml, and he remains clinically stable.
Patient 10: ambrisentan + iv treprostinil
Female, 50s, deceased. Ambrisentan led to initial improvement in symptoms, walk distance and hemodynamics, but due to persistent symptoms and catheterization abnormalities, oral treprostinil was begun during year 4. Several months later she developed a sepsis syndrome with fever and hypotension leading to an intensive care unit admission. Antibiotics were begun, and intravenous epoprostenol as well as pressors were required to treat decompensated right heart failure. She recovered, and epoprostenol was changed to intravenous treprostinil due to patient preference. One year later she developed pneumonia along with progressive right heart failure, and she died several weeks later; multiple BNP levels were > 1000 pg/ml.
Patient 11: ambrisentan + oral treprostinil
Female, 50s, alive. Oral treprostinil was added during year 3. The BNP level fell from 743 pg/ml pre-treatment to most recently 108 pg/ml. Symptoms have remained stable, catheterization has modestly improved, and last walk distance was 475 meters.
Patients 12: ambrisentan + sildenafil
Female, 20s, alive. During year 4, oral treprostinil was added due to stable but persistent symptoms, but it was subsequently discontinued due to significant side effects and sildenafil was begun. Despite combination therapy, catheterization results have worsened slightly; walk distance and BNP level at the visit was 349 meters and 36 pg/ml, respectively.
Discussion
In this study, we describe significant improvements in cardiac catheterization hemodynamics, six minute walk distance and cardiac MRI results for 12 PAH patients receiving open-label ambrisentan monotherapy for a period of 2 years. This study on its own is not large enough to make widely generalizable predictions on how other PAH patients will respond to treatment with ambrisentan, but it does add significantly to the existing literature. Prior studies have shown that ambrisentan, at current FDA-approved doses (5–10 mg), leads to 31–59 meter improvements in 6MWD compared with placebo over 12 weeks, and that these improvements in walk distance are maintained at 48 weeks (ARIES-1 and 2). Additionally, ambrisentan has also been shown to lead to improvements in hemodynamics relative to baseline at 12 weeks (N=64 total, 29 received the now approved 5 or 10 mg dose)3.
This descriptive study is the first to suggest that both the 6MWD and hemodynamic improvements with ambrisentan treatment may be maintained at 1 to 2 years, and it is also the first study to report on cardiac MRI results in ambrisentan patients. Statistically significant improvements were seen in 6MWD, cardiac index, pulmonary arterial pressure and pulmonary vascular resistance at one year, and improvements in exercise capacity and pulmonary vascular resistance remained significant at 2 years. Looking at individual patients, almost all patients showed at least some improvement between baseline and one year follow-up in most of these measures. Exercise capacity and hemodynamics are strong predictors of outcome in PAH, and the sustainability of these results is encouraging5–11
Cardiac MRI results were mixed, with improvement in RV ejection fraction over two years but no statistical significant improvement in RV end-diastolic volume index or RV mass. This lack of improvement in RV end-diastolic volume index and mass index was seen despite a mean 10 mmHg drop in mean pulmonary arterial pressures, a mean 1.1L/min increase in cardiac output, and a mean 31% reduction in pulmonary vascular resistance by right heart catheterization. The lack of a true baseline for some patients may have lead to an underestimation of the effects, but right ventricular function clearly remained very abnormal in many patients at both 1 and 2 years of therapy.
Failure to see improvement in RV size, despite hemodynamic improvement, has been seen in other studies of PAH, including studies of bosentan and epoprostenol, with assessment of RV size and function by either echocardiogram or cardiac MRI12–15 This is in contrast to the marked improvement in RV size and RV ejection fraction seen after lung transplantation in PAH or successful pulmonary thromboendarterectomy in chronic thromboembolic pulmonary hypertension. It is likely, therefore, that the failure to improve is related to persistant hemodynamic abnormalities rather than irreversible cardiac injury16, 17, 18. Interestingly, most patients in our cohort did not have worsening RV end diastolic volume index or RV ejection fraction. In a disease whose natural history is progressive decline in RV function, halting this decline may, in some cases, be considered at least a partial success.
Including the 2 patients who did not enter the long-term extension phase, survival in this group was 93% at 3 years follow-up, and 79% overall at 3.5–5 years follow-up (Figure 4). This compares favorably with reported survival rates with other PAH therapies11. Although the small sample size makes comparisons between actual and expected survival of limited utility, these results do at least suggest that a strategy of initial treatment with ambrisentan monotherapy could lead to very good overall survival rates, and larger studies should be conducted to assess this.
In addition to the small number of study patients, our data was limited by the lack of placebo controls for the long term data, the retrospective data acquisition, and the failure to obtain all MRI studies prior to the initiation of therapy. Nevertheless, descriptive studies such as this may provide valuable information in areas that are unlikely to be addressed in experimental studies.
Conclusion
Mortality in pulmonary arterial hypertension has been shown to relate to right ventricular failure19. Ambrisentan monotherapy at our institution led to improvements in both hemodynamics from cardiac catheterization as well as right ventricular performance measured directly by cardiac MRI and indirectly by 6MWD. The results with the use of this oral, selective endothelin receptor antagonist show clinical promise for long term therapy, although the modest improvements underline the need for continued therapeutic advances for pulmonary arterial hypertension.
Acknowledgments
Grant support: This publication was supported by Grant Number UL1RR024982 and KL2RR024983, titled, “North and Central Texas Clinical and Translational Science Initiative” (Milton Packer, M.D., PI) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Abbreviations
- 6MWD
six minute walk distance
- ETA/ET B
endothelin A/endothelin B
- MRI
magnetic resonance imaging
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PVR
pulmonary vascular resistance
- RV
right ventricle/right ventricular
- WHO
world health organization
- BNP
brain natriuretic peptide
Footnotes
Disclosures: Dr. Torres has served as a speaker/consultant and has received research grants from Gilead, which makes ambrisentan. Dr. Chin has received research grants from Gilead and has served as a speaker/consultant for Actelion and United Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):62S–67S. doi: 10.1016/j.jacc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117(23):3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46(3):529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP., Jr Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1(1):7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 5.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28(10):1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 6.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 7.Sitbon O, Badesch DB, Channick RN, Frost A, Robbins IM, Simonneau G, et al. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary arterial hypertension: a 1-year follow-up study. Chest. 2003;124(1):247–254. doi: 10.1378/chest.124.1.247. [DOI] [PubMed] [Google Scholar]
- 8.Provencher S, Sitbon O, Humbert M, Cabrol S, Jais X, Simonneau G. Long-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertension. Eur Heart J. 2006;27(5):589–595. doi: 10.1093/eurheartj/ehi728. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin VV, Sitbon O, Badesch DB, Barst RJ, Black C, Galie N, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005;25(2):244–249. doi: 10.1183/09031936.05.00054804. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 11.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 12.Roeleveld RJ, Vonk-Noordegraaf A, Marcus JT, Bronzwaer JG, Marques KM, Postmus PE, et al. Effects of epoprostenol on right ventricular hypertrophy and dilatation in pulmonary hypertension. Chest. 2004;125(2):572–579. doi: 10.1378/chest.125.2.572. [DOI] [PubMed] [Google Scholar]
- 13.Hinderliter AL, Willis PWt, Barst RJ, Rich S, Rubin LJ, Badesch DB, et al. Effects of long-term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Primary Pulmonary Hypertension Study Group. Circulation. 1997;95(6):1479–1486. doi: 10.1161/01.cir.95.6.1479. [DOI] [PubMed] [Google Scholar]
- 14.Galie N, Hinderliter AL, Torbicki A, Fourme T, Simonneau G, Pulido T, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41(8):1380–1386. doi: 10.1016/s0735-1097(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 15.Chin KM, Kingman M, de Lemos JA, Warner JJ, Reimold S, Peshock R, et al. Changes in right ventricular structure and function assessed using cardiac magnetic resonance imaging in bosentan-treated patients with pulmonary arterial hypertension. The American journal of cardiology. 2008;101(11):1669–1672. doi: 10.1016/j.amjcard.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 16.Reesink HJ, Marcus JT, Tulevski II, Jamieson S, Kloek JJ, Vonk Noordegraaf A, et al. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. The Journal of thoracic and cardiovascular surgery. 2007;133(1):58–64. doi: 10.1016/j.jtcvs.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Kasimir MT, Seebacher G, Jaksch P, Winkler G, Schmid K, Marta GM, et al. Reverse cardiac remodelling in patients with primary pulmonary hypertension after isolated lung transplantation. Eur J Cardiothorac Surg. 2004;26(4):776–781. doi: 10.1016/j.ejcts.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 18.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. American heart journal. 2004;147(2):218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114(17):1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]