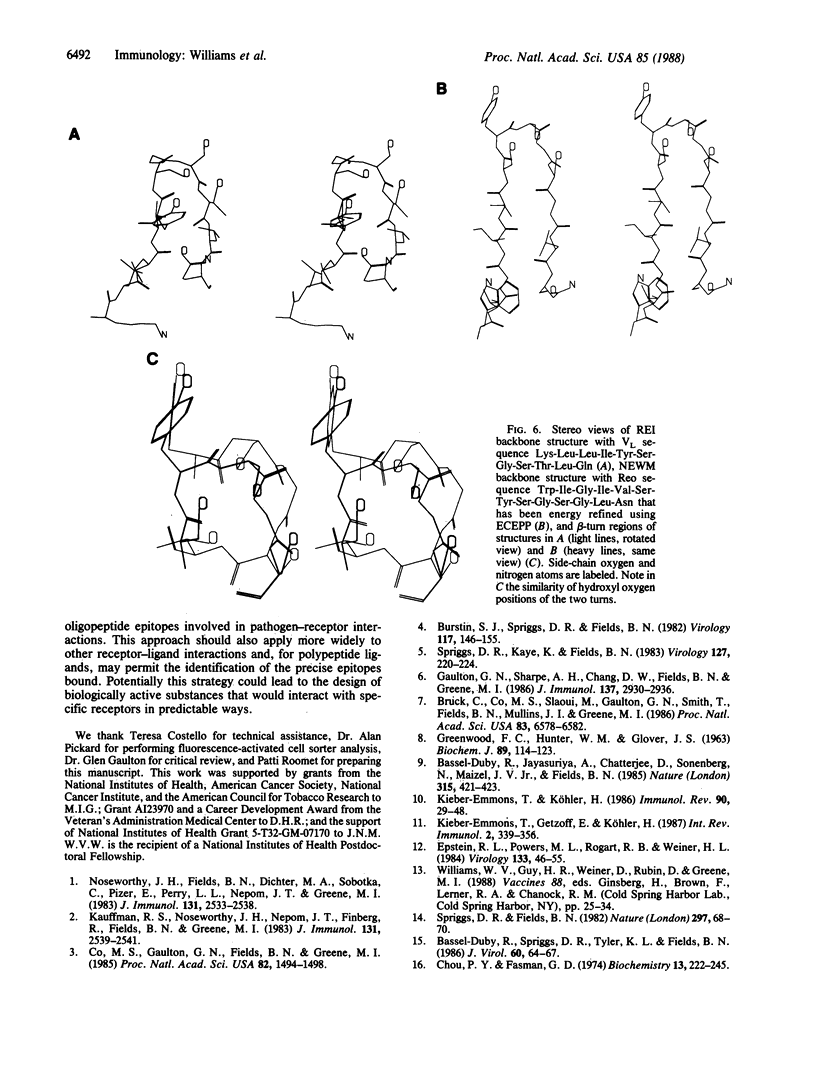
Abstract
Previous studies have identified an area of amino acid sequence similarity shared by the reovirus type 3 cell-attachment protein sigma 1 and an anti-idiotypic/antireceptor monoclonal antibody (mAb) 87.92.6 that mimics reovirus type 3 by attaching to the same cell-surface receptor. We found that synthetic peptides corresponding to this area of primary sequence similarity bind a neutralizing mAb 9BG5 against which the mAb 87.92.6 is directed. The synthetic peptides compete with mAb 87.92.6 and reovirus type 3 for binding by mAb 9BG5 and displace mAb 87.92.6 and reovirus type 3 from binding to the cell-surface reovirus type 3 receptor. Such observations show that the shared primary structure between reovirus type 3 sigma 1 polypeptide and antireceptor mAb 87.92.6 defines the oligopeptide neutralizing/cell-attachment epitope of reovirus type 3. Computer modeling of this epitope, by use of sequence similarities of known immunoglobulin hypervariable loop conformations, permits an examination of the rudimentary three-dimensional structure of this epitope.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassel-Duby R., Jayasuriya A., Chatterjee D., Sonenberg N., Maizel J. V., Jr, Fields B. N. Sequence of reovirus haemagglutinin predicts a coiled-coil structure. 1985 May 30-Jun 5Nature. 315(6018):421–423. doi: 10.1038/315421a0. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R., Spriggs D. R., Tyler K. L., Fields B. N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986 Oct;60(1):64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck C., Co M. S., Slaoui M., Gaulton G. N., Smith T., Fields B. N., Mullins J. I., Greene M. I. Nucleic acid sequence of an internal image-bearing monoclonal anti-idiotype and its comparison to the sequence of the external antigen. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6578–6582. doi: 10.1073/pnas.83.17.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstin S. J., Spriggs D. R., Fields B. N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982 Feb;117(1):146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Fields B. N., Greene M. I. Isolation and biochemical characterization of the mammalian reovirus type 3 cell-surface receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1494–1498. doi: 10.1073/pnas.82.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R. L., Powers M. L., Rogart R. B., Weiner H. L. Binding of 125I-labeled reovirus to cell surface receptors. Virology. 1984 Feb;133(1):46–55. doi: 10.1016/0042-6822(84)90424-0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulton G. N., Sharpe A. H., Chang D. W., Fields B. N., Greene M. I. Syngeneic monoclonal internal image anti-idiotopes as prophylactic vaccines. J Immunol. 1986 Nov 1;137(9):2930–2936. [PubMed] [Google Scholar]
- Kauffman R. S., Noseworthy J. H., Nepom J. T., Finberg R., Fields B. N., Greene M. I. Cell receptors for the mammalian reovirus. II. Monoclonal anti-idiotypic antibody blocks viral binding to cells. J Immunol. 1983 Nov;131(5):2539–2541. [PubMed] [Google Scholar]
- Kieber-Emmons T., Getzoff E., Köhler H. Perspectives on antigenicity and idiotypy. Int Rev Immunol. 1987 Jul;2(4):339–356. doi: 10.3109/08830188709044761. [DOI] [PubMed] [Google Scholar]
- Kieber-Emmons T., Kohler H. Towards a unified theory of immunoglobulin structure-function relations. Immunol Rev. 1986 Apr;90:29–48. doi: 10.1111/j.1600-065x.1986.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- Spriggs D. R., Fields B. N. Attenuated reovirus type 3 strains generated by selection of haemagglutinin antigenic variants. Nature. 1982 May 6;297(5861):68–70. doi: 10.1038/297068a0. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Kaye K., Fields B. N. Topological analysis of the reovirus type 3 hemagglutinin. Virology. 1983 May;127(1):220–224. doi: 10.1016/0042-6822(83)90385-9. [DOI] [PubMed] [Google Scholar]



