Summary
Rhizocticins are phosphonate oligopeptide antibiotics containing the C-terminal non-proteinogenic amino acid (Z)-l-2-amino-5-phosphono-3-pentenoic acid (APPA). Here we report the identification and characterization of the rhizocticin biosynthetic gene cluster (rhi) in Bacillus subtilis ATCC6633. Rhizocticin B was heterologously produced in the non-producer strain Bacillus subtilis 168. A biosynthetic pathway is proposed based on bioinformatics analysis of the rhi genes. One of the steps during the biosynthesis of APPA is an unusual aldol reaction between phosphonoacetaldehyde and oxaloacetate catalyzed by an aldolase homolog RhiG. Recombinant RhiG was prepared and the product of an in vitro enzymatic conversion was characterized. Access to this intermediate allows for biochemical characterization of subsequent steps in the pathway.
Introduction
Rhizocticins are phosphonate-containing oligopeptide antibiotics produced by the Gram-positive bacterium B. subtilis ATCC6633. They were originally discovered in 1949 based on their antifungal activity and collectively termed “rhizoctonia factor” (Michener and Snell, 1949). The structures of rhizocticins were determined 40 years later (Rapp et al., 1988). They are di- and tripeptide antibiotics consisting of a variable amino acid at the N-terminus followed by arginine and the non-proteinogenic amino acid (Z)-l-2-amino-5-phosphono-3-pentenoic acid (APPA, Figure 1 A). Interestingly, APPA is also the C-terminal amino acid of the tripeptide antibiotics plumbemycin A and B produced by Streptomyces plumbeus (Figure 1 B) (Park et al., 1977a; Park et al., 1977b).
Figure 1.
Chemical structures of the phosphonate antibiotics rhizocticins (A) and plumbemycins (B). C) The threonine synthase reaction inhibited by APPA. D) Chemical structures of representative phosphonate antibiotics whose biosynthetic pathways have been studied.
Rhizocticins enter the target fungal cell through the oligopeptide transport system (Kugler et al., 1990). They are then cleaved by host peptidases to release APPA, which inhibits threonine synthase, an enzyme catalyzing the pyridoxal 5′-phosphate (PLP)-dependent conversion of phosphohomoserine to l-threonine (Figure 1 C) (Kugler et al., 1990; Laber et al., 1994). Hence, APPA interferes with the biosynthesis of threonine and related metabolic pathways, ultimately affecting protein synthesis and leading to growth inhibition. The inhibitory activity of APPA is due to the structural resemblance to phosphohomoserine, but possessing a hydrolytically stable C-P bond in place of the C-O-P moiety of phosphohomoserine.
Whereas rhizocticins exhibit antifungal activity, plumbemycins are antibacterials. It has been demonstrated that plumbemycins also enter Escherichia coli K-12 via the oligopeptide transport system (Diddens et al., 1979). As in the case of rhizocticins, l-threonine reverses the growth inhibition by plumbemycins in a concentration-dependent manner (Park et al., 1977b). Therefore, similarly to rhizocticins, plumbemycins must be cleaved by peptidases of the target cell to release the active substance, APPA. The selectivity of these tripeptide antibiotics is thus not due to a difference in mode of action, but rather determined by the recognition of proteinogenic amino acids attached at the N-terminus of APPA by a specific oligopeptide transport system and/or peptidase. This feature can potentially be exploited to create APPA-containing oligopeptides with specific selectivity for a particular organism. Furthermore, the target of APPA, threonine synthase, is not present in mammals, reducing the likelihood of toxicity to humans. Thus, such phosphonate oligopeptides can be promising therapeutics.
Phosphonate compounds are prevalent among biologically active molecules, mainly due to their ability to function as stable mimics of carboxylate and phosphate containing metabolites. At present, biosynthetic pathways of only a handful of phosphonates have been extensively studied (see (Metcalf and van der Donk, 2009) for a recent comprehensive review on the topic), including fosfomycin (Hidaka et al., 1995; Woodyer et al., 2006), dehydrophos (B. T. Circello, et al.; submitted for publication), FR-900098 (Eliot et al., 2008), and phosphinothricin-containing peptides (e.g. PTT) (Blodgett et al., 2007) (Figure 1 D). These studies have shown that the biosyntheses of this class of compounds provide a rich source of novel biochemistry as exemplified by many unique enzymatic transformations (Cicchillo et al., 2009; Higgins et al., 2005). Therefore, a better understanding of the rhizocticin pathway will be of great interest. Furthermore, biosynthetic access to the APPA warhead of the rhizocticins would be advantageous as organic synthesis of this molecule is very challenging. For instance, biosynthetic preparation of APPA would provide an avenue to combinatorially vary the N-terminal amino acids to create analogs with desired specificity.
To this end, we identified the rhizocticin biosynthetic cluster in the genome of B. subtilis ATCC6633 and confirmed its identity by heterologous expression in B. subtilis 168. Although the first two steps of the biosynthetic pathway appear to be the same as for other phosphonate metabolites, the third step – an aldol reaction between phosphonoacetaldehyde (PnAA) and oxaloacetic acid (OAA) is unprecedented. Biochemical characterization of this step is also reported here. Based on genetic and biochemical information, a pathway for the biosynthesis of rhizocticins is proposed.
Results and Discussion
Identification of the rhizocticin biosynthetic gene cluster
The first step in the biosynthetic pathways of the majority of phosphonates is the isomerization of phosphoenolpyruvate (PEP) to phosphonopyruvate (PnPy) catalyzed by phosphoenolpyruvate phosphomutase (PEP mutase) (Seidel et al., 1988). Previously, we successfully identified phosphonate biosynthetic gene clusters by screening fosmid libraries with degenerate PCR primers designed to amplify PEP mutase-encoding genes (Blodgett et al., 2005; Eliot et al., 2008). We attempted a similar approach here to identify the rhizocticin gene cluster. A fosmid library of B. subtilis ATCC6633 was constructed and screened by PCR for a PEP mutase gene fragment as described in the Supplemental Information section available online. However, none of the clones produced the desired PCR fragment, possibly due to a highly divergent sequence of the PEP mutase gene, which prevented annealing of the primers. Alternatively, a PEP mutase independent pathway might be employed by B. subtilis ATCC6633 for production of rhizocticin.
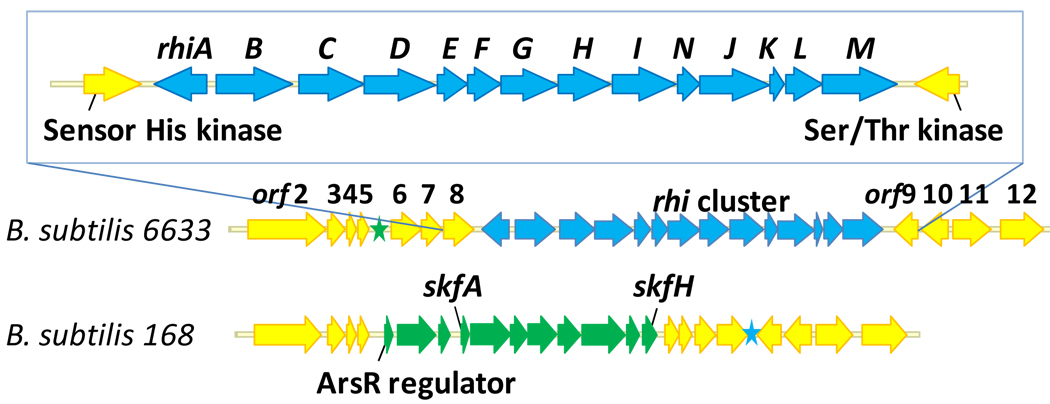
To investigate these alternatives, the genome of B. subtilis ATCC6633 was sequenced using the 454 sequencing platform. The details of the genome sequencing will be reported elsewhere. Briefly, sequencing data were assembled into 37 contigs spanning approximately 4.0 Mb. A total of 3769 open reading frames (ORFs) were determined and annotated using the RAST Server (Rapid Annotations using Subsystems Technology) (Aziz et al., 2008). For comparison, the closely related B. subtilis 168 strain has a genome of 4.2 Mb comprised of 4114 coding sequences (Kunst et al., 1997). When searched for the PEP mutase gene, the sequence of the genome of B. subtilis ATCC6633 produced a single hit. This gene appears to be a part of an operon consisting of thirteen ORFs and is preceded by a differentially transcribed additional ORF encoding a transcriptional regulator. Careful analysis of the ORFs comprising this operon led to the conclusion that these genes are likely to constitute a rhizocticin biosynthetic gene cluster (Figure 2).
Figure 2.
Organization of the rhizocticin gene cluster and surrounding genes on the B. subtilis ATCC6633 chromosome. The same locus of B. subtilis 168 genome is also shown for comparison. Genes with a high degree of homology between the two strains (>90% identity) are shown in yellow. The biosynthetic clusters for rhizocticin and sporulation killing factor are shown in blue and green, respectively. The corresponding location of these loci in the other genome is denoted with a star of the same color.
With the genome sequence available, the fosmid library of B. subtilis ATCC6633 was screened as described above using two sets of sequence-specific primers designed to amplify short sequences upstream of the putative rhizocticin cluster (within orf6) and within rhiM. Fosmid 2–11E was identified, and sequenced via the Sanger protocol using transposon insertions as previously described (Blodgett et al., 2005; Eliot et al., 2008). The sequence of the insert of 2–11E originating from B. subtilis ATCC6633 DNA was identical to that of the corresponding fragment obtained through 454 sequencing of the genome with the exception of a single base pair mismatch located outside of the putative rhizocticin gene cluster.
B. subtilis ATCC6633 possesses a high degree of nucleotide sequence homology to B. subtilis 168. The putative rhizocticin gene cluster appears to be a single site insertion of approximately 13 kb into the genome of B. subtilis 168. Although the genes of the rhizocticin cluster have no homologs within the B. subtilis 168 genome, the nucleotide sequences outside of the cluster are approximately 90% identical. Interestingly, B. subtilis 168 contains a gene cluster (skf) located near the “insertion site” of the rhizocticin gene cluster (blue star in Figure 2). This gene cluster is absent from B. subtilis ATCC6633 (its corresponding location is shown as a green star). The skf gene cluster is responsible for the biosynthesis and export of and the immunity to sporulation killing factor. This peptide antibiotic produced by sporulating B. subtilis 168 causes lysis of non-sporulating sibling B. subtilis 168 cells (Gonzalez-Pastor et al., 2003). Thus, the rhi and skf gene clusters occupy essentially the same locus on the genomic DNA of related species, as commonly seen for the genes involved in secondary metabolism.
Bioinformatic analysis of the rhi cluster
The genes of the rhizocticin biosynthetic cluster were first annotated using the RAST Server (Aziz et al., 2008) and further analyzed with the Basic Local Alignment Search Tool (BLAST) program at NCBI (Altschul et al., 1990) and the Phyre server (Kelley and Sternberg, 2009). The gene annotations, along with the closest and functionally confirmed homologs are shown in Table 1.
Table 1.
Summary of the open reading frames of the rhizocticin gene cluster
| ORF | No. of aaa |
Protein homology (NCBI No.) | % aa identityb |
|---|---|---|---|
| orf6 | 325 | B. subtilis 168 putative hydrolase/transferase (CAB11993) (325 aa) | 94 |
| orf7 | 223 | B. subtilis 168 two-component response regulator YbdJ (BAA33098) (223 aa) | 95 |
| orf8 | 322 | B. subtilis 168 sensor histidine kinase YbdK (BAA33099) (320 aa) | 87 |
| rhiA | 296 | B. licheniformis transcriptional activator of the cysJI operon (AAU21843) (298 aa) | 65 |
| Salmonella enterica Typhimurium transcriptional regulator CysB (NP_460672) (324 aa) | 18 | ||
| rhiB | 433 | Sphaerobacter thermophilus threonine synthase (ZP_04494878) (420 aa) | 46 |
| Mycobacterium tuberculosis threonine synthase (2D1F_B) (360 aa) | 28 | ||
| B. subtilis ATCC6633 threonine synthase ThrC (this study) (352 aa) | 27 | ||
| rhiC | 408 | B. licheniformis hypothetical protein, related to NikS (YP_077482) (405 aa) | 62 |
|
Streptomyces ansochromogenes nikkomycin biosynthesis protein SanS, d-Ala-d-Ala ligase homolog (AAK53061) (424 aa) |
30 | ||
| rhiD | 407 | B. licheniformis MFS transporter (YP_077483) (408 aa) | 68 |
| rhiE | 167 | Sorangium cellulosum sulfopyruvate decarboxylase α-subunit (YP_001617955) (170 aa) | 40 |
| rhiF | 186 | S. hygroscopicus phosphonopyruvate decarboxylase (Q54271) (401 aa) | 40 |
| rhiG | 337 | Legionella pneumophila 4-hydroxy-2-oxovalerate aldolase (YP_096686) (295 aa) | 34 |
| Pseudomonas sp. bifunctional aldolase-dehydrogenase DmpG (1NVM_A) (345 aa) | 25 | ||
| rhiH | 296 | Paenibacillus larvae putative PEP phosphomutase (ZP_02329666) (297 aa) | 56 |
| S. viridochromogenes PEP phosphomutase of PTT biosynthesis (AAU00071) (313 aa) | 42 | ||
| rhiI | 362 | Pseudomonas syringae hypothetical protein (BAF32889) (354 aa) | 36 |
| Mycoplasma pneumonia HPr kinase/phosphatase (1KNX_A) (312 aa) | 14 | ||
| rhiN | 132 |
Chloroherpeton thalassium protein of unknown function UPF0047 (YP_001997537) (138 aa) |
35 |
| E. coli conserved hypothetical protein YjbQ (ZP_03048862) (138 aa) | 22 | ||
| rhiJ | 393 | Thermotoga lettingae aminotransferase class V (YP_001471385) (381 aa) | 42 |
|
Methanocaldococcus jannaschii broad-specificity class V aspartate aminotransferase (NP_247954) (385 aa) |
38 | ||
| rhiK | 85 | Natronomonas pharaonis glutaredoxin (CAI48716) (82 aa) | 35 |
| E. coli glutaredoxin 3 (1FOV_A) (82 aa) | 23 | ||
| rhiL | 215 | Frankia sp. EAN1pec putative metallophosphoesterase (YP_001510901) (243 aa) | 32 |
| E. coli metal-dependent phosphodiesterase YfcE (P67095) (184 aa) | 19 | ||
| rhiM | 413 | B. licheniformis hypothetical, related to NikS (YP_077482) (405 aa) | 25 |
|
S. ansochromogenes nikkomycin biosynthesis protein SanS, d-Ala-d-Ala ligase homolog (AAK53061) (424 aa) |
26 | ||
| orf9 | 256 | B. subtilis 168 putative serine/threonine protein kinase YbdM (O31435) (256 aa) | 90 |
| orf10 | 284 | B. subtilis 168 putative phage protein YbdN (CAB11998) (285 aa) | 94 |
| orf11 | 394 | B. subtilis 168 putative phage protein YbdO (CAB11999) (394 aa) | 89 |
aa, amino acids.
Percent identity and one of the closest homologs were based on NCBI searches conducted October 8th 2009. The homolog whose biochemical function was experimentally supported is shown for proteins of particular interest.
As mentioned above, the genes surrounding the putative rhizocticin gene cluster (rhiA-rhiM), (e.g., immediately adjacent orf6–8 and orf9–11) have nearly identical counterparts in B. subtilis 168. Therefore, they are not likely to be involved in rhizocticin biosynthesis.
The rhiA gene encodes a putative transcriptional regulator of the LysR family (Schell, 1993). The helix-turn-helix DNA-binding motif, typical of many LysR regulators (Maddocks and Oyston, 2008), is predicted by the Phyre server to be located within the N-terminal residues 30–85. A putative ligand-binding domain is also present at the C-terminus of RhiA. The rhiA gene is located upstream and in the opposite direction of the other genes in the rhi operon as commonly seen for LysR-regulated operons (Maddocks and Oyston, 2008).
The rhiB gene encodes a putative threonine synthase. Interestingly, the genome of B. subtilis ATCC6633 contains another copy of a threonine synthase gene, thrC, located in an operon with genes involved in the biosynthesis of threonine that is present at the same site as the threonine synthase gene in the B. subtilis 168 genome. Unlike RhiB, ThrC is highly homologous to threonine synthases of Gram-positive bacteria, predominantly Bacillus species (98% identical to threonine synthase of B. subtilis 168), suggesting ThrC is a bona fide threonine synthase involved in primary metabolism. RhiB and ThrC appear to be evolutionary distinct as they share only 27% amino acid identity. It is conceivable that rhiB is involved in rhizocticin self-resistance by encoding a threonine synthase homolog that is not inhibited by APPA.
The translated products of rhiC and rhiM are homologs (26% identity). The proteins with the closest homology are NikS and SanS, participating in the biosynthesis of the peptidyl nucleoside antibiotic nikkomycin (Lauer et al., 2001; Li et al., 2004). They belong to the carboxylate-amine/thiol ligase superfamily of enzymes possessing a signature ATP-grasp structural motif (Galperin and Koonin, 1997). The enzymes of this superfamily catalyze the ATP-dependent formation of peptide or thioester bonds via a reactive acylphosphate intermediate. Examples include d-Ala-d-Ala ligase of peptidoglycan biosynthesis (Fan et al., 1994; Zawadzke et al., 1991), glutathione synthetase (Fan et al., 1995; Yamaguchi et al., 1993), and biotin carboxylase (Artymiuk et al., 1996; Waldrop et al., 1994). Despite a variety of reactions catalyzed by the members of the ATP-grasp superfamily, enzymes catalyzing the formation of a “conventional” proteinogenic α-peptide bond between l-amino acids have been identified only recently (Kino et al., 2009; Kino et al., 2008a; Kino et al., 2008b; Tabata et al., 2005). During the course of our study, the identification and substrate specificity of RhiM was reported (named RizA by the authors) (Kino et al., 2009). RhiM (RizA) is capable of ligating l-arginine to 19 other amino acids, including a saturated analog of l-APPA, 2-amino-5-phosphonopentanoic acid (Kino et al., 2009). A sequence of RhiC has also been deposited into GenBank (accession number BAH56723) by Kino and co-workers; its activity has not been reported.
RhiD is a putative transporter of the major facilitator superfamily (MFS). Between 8 and 10 transmembrane helixes are predicted by different topology prediction tools (ca.expasy.org). RhiD is likely responsible for the export of rhizocticins from the cell.
The genes rhiE and rhiF encode two subunits of a putative PnPy decarboxylase. PnPy decarboxylases catalyze the irreversible thiamin pyrophosphate (TPP)-dependent decarboxylation of PnPy to PnAA. This step is present in the majority of biosynthetic pathways of known phosphonates, including fosfomycin and phosphinothricin tripeptide (Metcalf and van der Donk, 2009). Unlike RhiE/RhiF these enzymes usually consist of a single polypeptide chain. On the other hand, the functionally similar sulfopyruvate decarboxylase of coenzyme M biosynthesis is usually a two-subunit enzyme, e.g. ComD and ComE in Methanococcus jannaschii (Graupner et al., 2000). ComD (α -subunit) is homologous to the N-terminal portion of single chain PnPy decarboxylases, whereas ComE (β-subunit) has homology to the C-terminus. In a similar manner, RhiE and RhiF are homologs of the α- and β-subunits of sulfopyruvate decarboxylase, respectively, and the N- and C-terminal portions of single chain PnPy decarboxylases. RhiF (like ComE) contains a canonical TPP-binding motif. To the extent of our knowledge, RhiE/RhiF is the first example of a PnPy decarboxylase consisting of two subunits.
A search of the NCBI database for protein sequences homologous to the translated product of rhiG yielded a number of putative 4-hydroxy-2-oxovalerate aldolases with modest homology to RhiG (identity of 35% and lower). The closest homologs of RhiG that have been biochemically characterized are the 4-hydroxy-2-oxovalerate aldolases NahM and DmpG (25% identity) of Pseudomonas putida strains (Manjasetty et al., 2003; Platt et al., 1995). They belong to the class II family of aldolases that are dependent on divalent metal ions for catalysis. DmpG (and NahM) catalyzes the penultimate step of the meta-cleavage pathway from catechol to pyruvate and acetyl-CoA during the catabolism of aromatic compounds by Pseudomonas strains. DmpG is a part of a bifunctional enzyme complex as it physically associates with the enzyme of the following step, acetaldehyde dehydrogenase (acylating) DmpF, to ensure efficient transfer of the reactive intermediate acetaldehyde (Figure S1).
The rhiH gene encodes a putative PEP mutase that presumably would catalyze the first step in the biosynthetic pathway, the conversion of PEP to PnPy.
The translated product of rhiI has no significant end-to-end homology to any of the entries in the NCBI database. However, the C-terminus of RhiI (approximately 213 amino acids) shows low homology to the C-terminal domain of the histidine containing phospho carrier protein (HPr) kinase/phosphorylase from several species. In low GC Gram-positive bacteria, HPr is involved in the regulation of carbon catabolism (Martin-Verstraete et al., 1999). HPr kinase/phosphorylase is a bi-functional protein that modifies Ser-46 of HPr and accepts ATP or pyrophosphate (PPi) as a phosphate group donor. RhiI contains an easily identifiable canonical nucleotide binding P-loop (GSKGKGKS). It is conceivable that RhiI catalyzes an ATP-dependent phosphorylation of a small molecule or plays a regulatory role similar to HPr kinase/phosphorylase.
The translated gene product of rhiN shows homology to a number of hypothetical proteins belonging to an uncharacterized protein family UPF0047 (ExPASy, Prosite). The members of this family are small proteins of 14–16 kDa and are widely distributed among the three domains of life. Although several crystal structures were solved (submitted to the Protein Data Bank but not published) for the UPF0047 family, no function has been established for any of the homologs. One of the UPF0047 proteins, YjbQ of E. coli, has a low promiscuous activity as thiamine phosphate synthase (Morett et al., 2008), catalyzing the coupling of the thiazole and pyrimidine portions of thiamine phosphate via a nucleophilic substitution reaction proceeding through a carbocation intermediate (Peapus et al., 2001). However, the biological function of YjbQ and its homologs remains to be elucidated.
The gene rhiJ encodes a putative aminotransferase belonging to a family of Fold Type I PLP-dependent enzymes (Eliot and Kirsch, 2004). It can be further classified into phylogenetic class V of aminotransferases (also referred to as subgroup IV) (Mehta et al., 1993). One of the closest homologs (38% identical) of RhiJ, MJ0959 of Methanocaldococcus jannaschii, displayed the highest specific activity for transamination of aspartate and α-ketoglutarate when assayed in vitro (Helgadottir et al., 2007). However, MJ0959 is thought to be a part of l-phosphoserine biosynthesis, converting phosphohydroxypyruvate to l-phosphoserine (Helgadottir et al., 2007).
BLAST analysis revealed that RhiK is a homolog of glutaredoxins, small proteins related to thioredoxins and involved in the maintenance of the reducing environment of the cytoplasm. RhiK contains a CPYC motif conserved among glutaredoxins and is predicted to have a typical βαβαββα thioredoxin fold (Pan and Bardwell, 2006). RhiK also shows homology to the N-terminal domain of glutathione S-transferase, another member of the thioredoxin-like superfamily.
The translated sequence of rhiL belongs to the calcineurin-like superfamily (PF00149) that includes metal-dependent phosphomonoesterases and phosphodiesterases catalyzing the hydrolysis of diverse substrates, from phosphorylated proteins to nucleic acids (Koonin, 1994). Several conserved amino acid residues are present in RhiL, most notably all those comprising the binuclear metal center, suggesting it may have a phosphodiesterase activity as well.
Heterologous production of rhizocticin B
To confirm that the identified gene cluster is responsible for the biosynthesis of rhizocticins in B. subtilis ATCC6633, we introduced the rhi cluster in the B. subtilis 168 genome through homologous recombination (see the Experimental Procedures and Figure S2 for details). To do this, a spectinomycin resistance cassette (Spec) was introduced into fosmid 2–11E downstream of the rhi cluster using λ Red recombinase-mediated recombination (Datsenko and Wanner, 2000). The resulting fosmid 2–11E+Spec was linearized by restriction digestion and used for the transformation of B. subtilis 168. We anticipated that the level of homology between the DNA sequence immediately outside of the rhi cluster in B. subtilis ATCC6633 and the corresponding sequence of B. subtilis 168 (over 90% identity on the nucleotide level) was sufficiently high for the homologous recombination to occur. This proved correct, as the recombinant B. subtilis 168 colonies selected on spectinomycin-containing medium contained the rhi cluster as verified by PCR amplification of rhiC and rhiM genes.
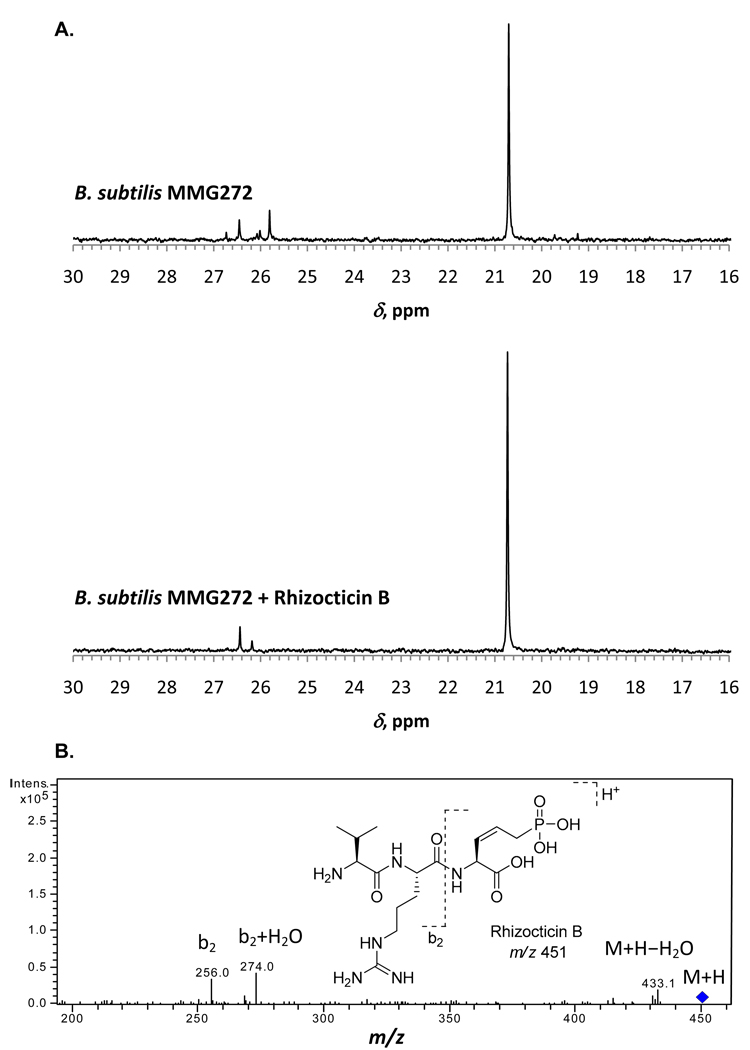
One of the recombinant strains, B. subtilis MMG272, was grown for the production of rhizocticins, and its clarified spent medium was partially purified and fractionated as described in the Experimental Procedures. Samples were analyzed by phosphorus (31P) NMR spectroscopy for the presence of phosphonates. One of the fractions produced a major phosphonate peak with a characteristic chemical shift (δ) of 20.7 ppm in the 31P NMR spectrum (Figure 3). Addition of purified rhizocticin B to the sample resulted in an increase in intensity of the 20.7 ppm peak and no new peaks in the 31P NMR spectrum (Figure 3 A), suggesting that the major phosphonate product is rhizocticin B. Analysis of the sample by liquid chromatography-mass spectrometry (LC-MS) further supported the presence of rhizocticin B (see Figure 3 B and the Experimental Procedures for details). No phosphonates were produced in a control experiment with the parent B. subtilis 168 strain (data not shown). Taken together, these results confirm that B. subtilis MMG272 produces rhizocticin B and that the rhi gene cluster is responsible for its biosynthesis.
Figure 3.
Analysis of rhizocticin B production by B. subtilis MMG272. A) 31P NMR spectra of partially purified spent medium of B. subtilis MMG272 and of the same sample supplemented with rhizocticin B authentic standard. The concentrations of components in the sample B. subtilis MMG272 + rhizocticin B are the same as in the individual sample of B. subtilis MMG272. Both spectra were collected for 400 transients and adjusted to the same absolute vertical scale. B) LC-MS analysis of partially purified spent medium of B. subtilis MMG272. The fragmentation of the rhizocticin B parent ion is shown and the peaks corresponding to the characteristic fragments are labeled.
Proposed rhizocticin biosynthetic pathway
Based on the amino acid sequence homology of Rhi proteins to enzymes with known activities and previous knowledge of phosphonate biosynthetic pathways, a proposed biosynthetic pathway for rhizocticins is shown in Figure 4. First, PEP is converted to PnPy by the action of the PEP mutase RhiH. PnPy then undergoes decarboxylation catalyzed by PnPy decarboxylase RhiE/RhiF to yield PnAA. These steps are common to the vast majority of known phosphonate antibiotic biosyntheses (Metcalf and van der Donk, 2009). The subsequent step is a novel transformation, an aldol reaction between PnAA and pyruvate (Py) catalyzed by the aldolase homolog RhiG. An alternative possibility is that the enolate of pyruvate is generated by decarboxylation of OAA (see below).
Figure 4.
Proposed pathways for the biosynthesis of rhizocticins.
A minimum of two steps, dehydration and aminotransfer, are required to convert the putative RhiG product I to APPA. The aminotransferase RhiJ is likely responsible for the introduction of the amino group at C-2. It is less obvious how dehydration happens. It is possible that the aminotransferase RhiJ is also capable of catalyzing a PLP-dependent γ-elimination of water in tandem with aminotransfer, single-handedly converting I to APPA. Another possibility is activation of the hydroxyl leaving group via phosphorylation by the action of the kinase homolog RhiI. Elimination could then be achieved by a yet unknown activity of RhiI (e.g. via acid-base catalysis) or by the action of RhiJ. Alternatively, RhiG could be responsible for aldol addition followed by dehydration. Regardless of the order in which dehydration and aminotransfer happen (path a vs. b), the APPA product can then be decorated at its N-terminus with Arg and Val (or Leu/Ile) by the action of carboxylate-amine ligases. Two of these proteins, RhiC and RhiM, are encoded by the rhi cluster, suggesting that each of the peptide bonds is formed by the action of a dedicated ligase.
The timing of dehydration could also be later in the pathway. Namely, once intermediate I is converted by RhiJ to amino acid III, III may be incorporated by RhiC and RhiM into di- or tri-peptide precursor(s) IV of rhizocticins. In this case, the dehydration would commence on a peptide intermediate(s) IV. In this scenario, no α-amino group would be available for RhiJ-catalyzed PLP-dependent chemistry and at least one another enzyme must be involved. This path (c) is particularly appealing as it avoids the presence of toxic APPA as an intermediate.
The functions of the proteins RhiK, RhiL, and RhiN are unclear. Although unusual for secondary metabolite biosynthesis, the glutaredoxin homolog RhiK may be involved in maintaining a reduced active state for specific proteins of the pathway, and RhiL and/or RhiN may be involved in a dehydration sequence. Further studies are needed to clarify the functions of these proteins.
Catalytic activity of the PEP mutase RhiH
The rhiH gene encoding putative PEP mutase was expressed in E. coli as a fusion protein with an N-terminal hexahistidine tag. Recombinant RhiH-N-His was purified to near homogeneity using metal affinity chromatography. The reversible reaction catalyzed by PEP mutase favors the formation of PEP (Seidel et al., 1988; Bowman et al., 1988). Subsequent decarboxylation of PnPy to PnAA catalyzed by PnPy decarboxylase provides the necessary driving force in many phosphonate pathways. Therefore, the enzymatic activity of RhiH-N-His was tested using a coupled assay with PnPy decarboxylase from Bacteroides fragilis (Zhang et al., 2003b) prepared as a C-terminally His-tagged protein (Ppd-Bf-His).
Assay conditions were based on published procedures (Zhang et al., 2003a) (Blodgett et al., 2007) and are described in detail in the Supplemental Information. Briefly, the assay mixture containing PEP, catalytic TPP cofactor, and Mg2+ was incubated with RhiH-N-His and Ppd-Bf-His. The extent of the reaction was analyzed using 31P NMR spectroscopy. Upon incubation, PEP (−0.2 ppm) was converted to PnAA as demonstrated by the appearance of a new peak at 9.9 ppm in the 31P NMR spectrum, consistent with the reported value (Blodgett et al., 2007). Upon prolonged storage, PnAA undergoes a non-enzymatic degradation as attested by the appearance of a broad peak at 15.4 ppm in the 31P NMR spectrum consistent with previously reported behavior (Zhang et al., 2003a).
RhiH-N-His, together with Ppd-Bf-His, were used for the enzymatic preparation of PnAA. Due to labile nature of PnAA, the enzyme-free reaction mixture was used as a source of PnAA without further purification.
Investigation of RhiG catalytic activity
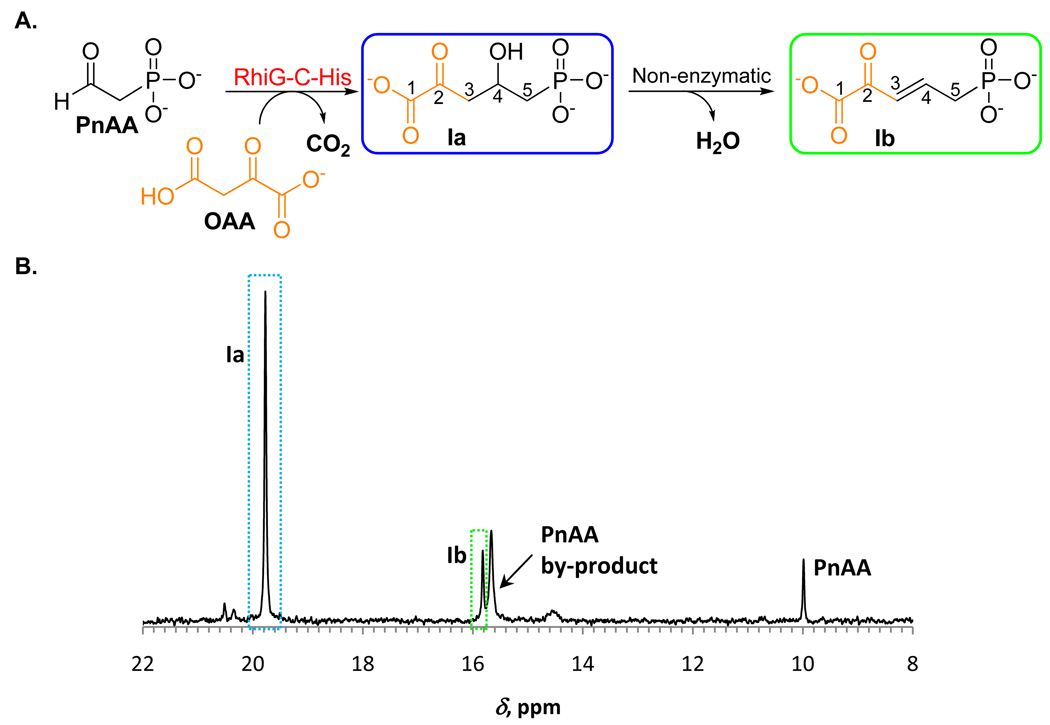
To obtain additional support for the biosynthetic proposal depicted in Figure 4, we set out to experimentally confirm the function of RhiG. Obtaining the product of RhiG via an enzymatic reaction would also provide the substrate for biochemical investigation of subsequent biosynthetic steps. RhiG was purified as a C-terminal fusion with a hexahistidine tag, RhiG-C-His (MW 38.7 kDa), using metal affinity chromatography. The purified protein contained no chromophore as attested by a UV-vis spectrum transparent above 300 nm. Native RhiG-C-His appears to be a homodimer (native MW 75 kDa) as determined by size-exclusion chromatography.
As seen for other class II aldolases, we expected that the activity of RhiG is dependent on a divalent metal cation, most likely Mg2+ or Mn2+. Since the PnAA solution prepared with RhiHN-His and Ppd-Bf-His already contains Mg2+, no additional metals were supplied to the reaction. Incubation of a PnAA solution with pyruvate and RhiG-C-His did not produce new phosphonate compounds when examined by 31P NMR spectroscopy. We then evaluated OAA as substrate for the aldol reaction with PnAA. Indeed, incubation of PnAA with OAA and RhiG-C-His resulted in the formation of a new compound, denoted Ia, as demonstrated by the appearance of a new peak (19.8 ppm) in the 31P NMR spectrum (Figure 5 B). Approximately 80% of the PnAA was converted to Ia, as estimated by integration of the 31P NMR signals. The product Ia was observed only when OAA and RhiG-C-His were both added to the assay. No new phosphonates were detected when 2-ketoglutaric acid was used in place of OAA. Interestingly, upon storage of the enzyme-free assay mixture, a slow conversion occurred of the phosphonate Ia to another phosphonate-containing compound (15.8 ppm), denoted Ib. Degradation of Ia and its highly polar nature complicated its purification by HPLC. Therefore, the structures of compounds Ia and Ib were determined using spectroscopic analyses of a crude assay mixture.
Figure 5.
Reaction catalyzed by RhiG-C-His. A) Scheme of reaction catalyzed by RhiG-C-His. B) 31P NMR spectrum of the RhiG-C-His assay with unlabeled substrates.
A comprehensive NMR analysis of the RhiG-C-His reaction mixtures prepared with unlabeled and 13C-labeled PEP and OAA substrates (Supplemental Information and Figures S3 and Figure S4) allowed for the unequivocal assignment of the structures Ia and Ib (Figure 5 A). The structure of Ia was further supported by LC-MS analysis (Supplemental Information and Figure S5). The trans configuration of the double bond in Ib, and not a cis double bond as seen in APPA, suggests non-enzymatic formation of Ib with anti-elimination of water from Ia resulting in a trans-isomer. Therefore, the involvement of a specific enzyme (other than RhiG) is necessary for the dehydration during rhizocticin biosynthesis.
RhiG-C-His also catalyzes the formation of pyruvate from OAA in the absence of PnAA. This conversion was complete after incubation with RhiG-C-His at room temperature for 15 minutes, whereas only 13% of OAA was converted to pyruvate in the absence of enzyme (Supplemental Information).
Summary of the RhiG reaction
RhiG catalyzes the formation of 2-keto-4-hydroxy-5-phosphonopentanoic acid from PnAA and OAA. Carbon dioxide is presumably a second product of this reaction. The requirement for divalent metal cation was not investigated at this point but is assumed based on the studies of known class II aldolases. Clearly, if required, Mg2+ present in the assay was sufficient for successful conversion to I. OAA serves as a surrogate of pyruvate and the corresponding three-carbon moiety is incorporated into the final product. We propose that OAA coordinates to a divalent metal cation (Mg2+ in our assay) via its 1-carboxylate and 2-ketone moieties and undergoes decarboxylation to produce the enolate form of pyruvate. The enolate is stabilized by the divalent cation acting as an electron sink. Subsequent attack of the enolate on the electrophilic carbonyl moiety of PnAA furnishes the carbon-carbon bond of I (Figure 6 A).
Figure 6.
Proposed mechanism for the RhiG catalyzed transformation. Amino acid residues coordinating divalent metal cation (panel A, RhiG numbering, W denotes water) are based on the alignment with the homolog DmpG shown in panel B (conserved residues are in bold, ligands to M2+ are labeled with arrows).
The proposed RhiG mechanism draws from the homology of RhiG to 4-hydroxy-2-oxovalerate aldolase DmpG. Particularly, the residues comprising Mn2+ ligands in DmpG are also conserved in RhiG (Figure 6B) (Manjasetty et al., 2003). A crystal structure of DmpG contains either pyruvate (a product) or oxalate (a structural analog of pyruvate enolate) as an equatorial bidentate ligand to Mn2+ (Manjasetty et al., 2003). An analogous position could be occupied by OAA in RhiG as depicted in Figure 6 A.
The first step in this mechanistic proposal, decarboxylation of OAA, is analogous to that catalyzed by macrophomate synthase from the fungus Macrophoma commelinae (Watanabe et al., 2000), (Ose et al., 2003). The pyruvate enolate generated by macrophomate synthase is stabilized by coordination to Mg2+ and carries out either a Diels-Alder or Michael-type aldol reaction, the specifics still being a matter of debate. Macrophomate synthase was recently reported to also possess promiscuous aldolase activity catalyzing an aldol reaction of OAA with aldehydes, analogous to the RhiG transformation (Serafimov et al., 2008). Interestingly, the amino acid sequence of RhiG has no homology to that of macrophomate synthase.
Significance
Despite the demonstrated utility of phosphonates as therapeutics and agricultural chemicals, our knowledge of phosphonate biosynthetic pathways remains limited to a handful of examples (Metcalf and van der Donk, 2009). We report here the identification of the gene cluster responsible for the biosynthesis of rhizocticins in B. subtilis ATCC6633 and biochemical characterization of early transformations in the biosynthetic pathway. In addition to gaining valuable insight into the details of phosphonate biosynthesis, access to the pathway intermediate allows further investigation of the subsequent steps in the pathway and ultimately may provide a source of biologically active APPA or APPA-containing peptides.
The majority of currently known phosphonate biosynthetic pathways share PnAA as a common intermediate (Metcalf and van der Donk, 2009). The metabolic fate of PnAA is either transamination or reduction. Transamination produces 2-aminoethylphosphonate (AEP) which is incorporated into a variety of structural macromolecules, such as lipids and polysaccharides. Reduction, on the other hand, generates 2-hydroxyethylphosphonate (HEP) and leads to antibiotics such as fosfomycin, PTT, and dehydrophos. In this work, we discovered a third route by which phosphonate natural products are made from PnAA – through an aldol reaction with oxaloacetate. This type of transformation may prove to be a gateway to greater diversity of natural phosphonate compounds.
Experimental Procedures
Materials
Chemical reagents used in this study were the products of Sigma-Aldrich (St. Louis, MO) or Thermo Fisher Scientific (Pittsburgh, PA) and were used without further purification. 13C-labeled phosphoenolpyruvic acid potassium salt and aspartate were from Isotec of Sigma-Aldrich group. Media components were purchased from Thermo Fisher Scientific or VWR (West Chester, PA). Bacterial strains and PCR primers are listed in Tables S1 and Table S2, respectively (Supplemental Information).
Preparation of rhizocticin heterologous producer B. subtilis MMG272
The spectinomycin cassette was incorporated into 2–11E fosmid (Supplemental Information) using λ Red mediated recombination (Datsenko and Wanner, 2000) with modifications as described below. A spectinomycin resistance cassette was amplified by PCR using primers Spec-red-fwd2 and Spec-red-rev2 using pAIN750 as a template. The primers were designed to contain 51 bp regions of homology to the sequences flanking the site of Spec insertion in fosmid 2–11E. The PCR product (Spec fragment, 1247 nt) was digested with DpnI and purified from an agarose gel. Electrocompetent E. coli MMG194 was transformed with pKD46, plated on LB agar containing 12 µg/mL chloramphenicol (Cm) and 100 µg/mL ampicillin (Amp), and grown at 30 °C overnight. One of the transformants was picked and grown overnight at 30 °C in LB-Cm, Amp. The culture was then diluted 100-fold into SOB medium containing Cm, Amp, and 2 mM arabinose (to induce λ recombinase) and grown to OD600 ~ 0.6 at 30 °C. The cells were made electrocompetent by extensive washing with ice-cold 10% glycerol and concentrated 100-fold. These cells (50 µL aliquot) were transformed with the PCR fragment (35 ng) via electroporation, recovered in SOC medium at 37 °C for 2 h, and plated on LB agar containing 7 µg/mL Cm and 100 µg/mL spectinomycin (Spec). Several colonies were inoculated into LB-Cm, Spec and grown overnight at 37 °C. The fosmid DNA was isolated using QIAprep kit and analyzed by PCR amplification of the rhiC, rhiM genes and Spec fragment. The amplification of the DNA fragments of the desired size confirmed the incorporation of Spec into 2–11E and formation of fosmid 2–11E+Spec.
The fosmid DNA 2–11E+Spec was used to transform E. coli WM4489 to yield E. coli MMG273 strain. This strain was grown in the presence of 10 mM rhamnose to induce a high copy number for the fosmid 2–11E+Spec and the fosmid DNA was re-isolated. The 2–11E+Spec DNA was digested by restriction endonuclease NotI, purified by ethanol precipitation, and used to transform B. subtilis 168 following a published protocol (Henner, 1990). Recombinants were selected on LB agar plates containing 100 µg/mL Spec. The recombination was confirmed by culture PCR of selected recombinant strains as described above for verification of the fosmid 2–11E+Spec. One of the strains, B. subtilis MMG272, was chosen for rhizocticin production analysis as described below.
Rhizocticin B production in B. subtilis MMG272, and analysis by 31P NMR spectroscopy and LC-MS
The heterologous producer B. subtilis MMG272 was grown for metabolite production as described for B. subtilis ATCC6633 (Supplemental Information) with several exceptions. Spectinomycin was added to all of the media at 100 µg/mL. Additionally, PL medium was supplemented with tryptophan at 50 µg/mL and the fermentation culture volume was 2 L. The cell-free supernatant was taken through the same purification steps through Biogel P2 fractionation as described for rhizocoticin purification (Supplemental Information). The P2 fractions corresponding to the rhizocticin B elution volume were analyzed by 31P NMR spectroscopy and compared to an authentic standard. Several phosphonates with chemical shifts in the range 17–27 ppm were detected; fractions eluted from the column with 90–100 mL of water (B7-8) contained a major phosphonate with a chemical shift of 20.7 ppm. The NMR sample of B7-8 was supplemented with 8 mM rhizocticin B and re-analyzed by 31P NMR spectroscopy. Sample B7-8 was analyzed by LC-MS as described for rhizocticin B analysis (Supplemental Information) and its retention time and fragmentation pattern were consistent with the presence of rhizocticin B (Figure 3 B).
RhiG activity assays
A stock of 100 mM oxaloacetic acid was freshly prepared in 100 mM sodium cacodylate buffer pH 7.5. It was added to the PnAA sample (Supplemental Information) to a final OAA concentration of 12 mM. The reaction was initiated by the addition of RhiG-C-His (45 µM, see the Supplemental Information for the details of the protein purification) and the assay mixture was incubated at 30 °C for 1 h. A precipitate that formed during incubation was removed by centrifugation, and soluble proteins were removed by filtration through a Microcon YM-30 unit. We found that adding OAA and RhiG without prior removal of RhiH-N-His and Ppd-Bf-His, or even simultaneously with PnAA formation, reduced the amount of the PnAA degradation product formed. Therefore, the samples intended for extensive NMR characterization were prepared in this manner to reduce the processing time. The Microcon units were sequentially rinsed with 0.1 M sodium hydroxide, water, and finally reaction buffer prior to use to eliminate trace amounts of glycerol because it produced 1H NMR signals in the region of interest. The enzymatic preparation of 13C-labeled compounds and the spectroscopic characterization of compounds Ia, Ib, Ia′,Ia″and Ib′are described in the Supplemental Information section.
Supplementary Material
Acknowledgements
The authors thank Dr. Feng Lin (Keck NMR laboratory, UIUC) for assistance with advanced NMR experiments, Dr. Lucas Li (Biotechnology Center, UIUC) for performing LC-MS analysis, and Nurul Zulkepli for help in preparation of pRhiG-C-His. This work was supported by the National Institutes of Health (GM PO1 GM077596). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Number
The sequence of the insert in fosmid 2–11E containing rhizocticin biosynthetic gene cluster has been deposited in GenBank under accession number FJ935779.
Supplemental Information
Supplemental Information includes five figures, two tables, and Experimental Procedures. It can be found with this article online at (web address to be supplied upon publication).
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Artymiuk PJ, Poirrette AR, Rice DW, Willett P. Biotin carboxylase comes into the fold. Nat. Struct. Biol. 1996;3:128–132. doi: 10.1038/nsb0296-128. [DOI] [PubMed] [Google Scholar]
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett JA, Thomas PM, Li G, Velasquez JE, van der Donk WA, Kelleher NL, Metcalf WW. Unusual transformations in the biosynthesis of the antibiotic phosphinothricin tripeptide. Nat. Chem. Biol. 2007;3:480–485. doi: 10.1038/nchembio.2007.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett JA, Zhang JK, Metcalf WW. Molecular cloning, sequence analysis, and heterologous expression of the phosphinothricin tripeptide biosynthetic gene cluster from Streptomyces viridochromogenes DSM 40736. Antimicrob. Agents Chemother. 2005;49:230–240. doi: 10.1128/AAC.49.1.230-240.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E, McQueney M, Barry RJ, Dunaway-Mariano D. Catalysis and thermodynamics of the phosphoenolpyruvate/phosphonopyruvate rearrangement. Entry into the phosphonate class of naturally occurring organophosphorus compounds. J. Am. Chem. Soc. 1988;110:5575–5576. [Google Scholar]
- Cicchillo RM, Zhang H, Blodgett JA, Whitteck JT, Li G, Nair SK, van der Donk WA, Metcalf WW. An unusual carbon-carbon bond cleavage reaction during phosphinothricin biosynthesis. Nature. 2009;459:871–874. doi: 10.1038/nature07972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diddens H, Dorgerloh M, Zahner H. Metabolic products of microorganisms. 176. On the transport of small peptide antibiotics in bacteria. J. Antibiot. 1979;32:87–90. doi: 10.7164/antibiotics.32.87. [DOI] [PubMed] [Google Scholar]
- Eliot AC, Griffin BM, Thomas PM, Johannes TW, Kelleher NL, Zhao H, Metcalf WW. Cloning, expression, and biochemical characterization of Streptomyces rubellomurinus genes required for biosynthesis of antimalarial compound FR900098. Chem. Biol. 2008;15:765–770. doi: 10.1016/j.chembiol.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot AC, Kirsch JF. Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Annu. Rev. Biochem. 2004;73:383–415. doi: 10.1146/annurev.biochem.73.011303.074021. [DOI] [PubMed] [Google Scholar]
- Fan C, Moews PC, Shi Y, Walsh CT, Knox JR. A common fold for peptide synthetases cleaving ATP to ADP: glutathione synthetase and d-alanine:d-alanine ligase of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1995;92:1172–1176. doi: 10.1073/pnas.92.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Moews PC, Walsh CT, Knox JR. Vancomycin resistance: structure of d-alanine:d-alanine ligase at 2.3 A resolution. Science. 1994;266:439–443. doi: 10.1126/science.7939684. [DOI] [PubMed] [Google Scholar]
- Galperin MY, Koonin EV. A diverse superfamily of enzymes with ATP-dependent carboxylate-amine/thiol ligase activity. Protein Sci. 1997;6:2639–2643. doi: 10.1002/pro.5560061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pastor JE, Hobbs EC, Losick R. Cannibalism by sporulating bacteria. Science. 2003;301:510–513. doi: 10.1126/science.1086462. [DOI] [PubMed] [Google Scholar]
- Graupner M, Xu H, White RH. Identification of the gene encoding sulfopyruvate decarboxylase, an enzyme involved in biosynthesis of coenzyme M. J. Bacteriol. 2000;182:4862–4867. doi: 10.1128/jb.182.17.4862-4867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgadottir S, Rosas-Sandoval G, Soll D, Graham DE. Biosynthesis of phosphoserine in the Methanococcales. J. Bacteriol. 2007;189:575–582. doi: 10.1128/JB.01269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner DJ. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- Hidaka T, Goda M, Kuzuyama T, Takei N, Hidaka M, Seto H. Cloning and nucleotide sequence of fosfomycin biosynthetic genes of Streptomyces wedmorensis. Mol. Gen. Genet. 1995;249:274–280. doi: 10.1007/BF00290527. [DOI] [PubMed] [Google Scholar]
- Higgins LJ, Yan F, Liu P, Liu HW, Drennan CL. Structural insight into antibiotic fosfomycin biosynthesis by a mononuclear iron enzyme. Nature. 2005;437:838–844. doi: 10.1038/nature03924. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Kino K, Kotanaka Y, Arai T, Yagasaki M. A novel l-amino acid ligase from Bacillus subtilis NBRC3134, a microorganism producing peptide-antibiotic rhizocticin. Biosci. Biotechnol. Biochem. 2009;73:901–907. doi: 10.1271/bbb.80842. [DOI] [PubMed] [Google Scholar]
- Kino K, Nakazawa Y, Yagasaki M. Dipeptide synthesis by l-amino acid ligase from Ralstonia solanacearum. Biochem. Biophys. Res. Commun. 2008a;371:536–540. doi: 10.1016/j.bbrc.2008.04.105. [DOI] [PubMed] [Google Scholar]
- Kino K, Noguchi A, Nakazawa Y, Yagasaki M. A novel l-amino acid ligase from Bacillus licheniformis. J. Biosci. Bioeng. 2008b;106:313–315. doi: 10.1263/jbb.106.313. [DOI] [PubMed] [Google Scholar]
- Koonin EV. Conserved sequence pattern in a wide variety of phosphoesterases. Protein Sci. 1994;3:356–358. doi: 10.1002/pro.5560030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler M, Loeffler W, Rapp C, Kern A, Jung G. Rhizocticin A, an antifungal phosphono-oligopeptide of Bacillus subtilis ATCC6633: biological properties. Arch. Microbiol. 1990;153:276–281. doi: 10.1007/BF00249082. [DOI] [PubMed] [Google Scholar]
- Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- Laber B, Lindell SD, Pohlenz HD. Inactivation of Escherichia coli threonine synthase by dl-Z-2-amino-5-phosphono-3-pentenoic acid. Arch. Microbiol. 1994;161:400–403. doi: 10.1007/BF00288949. [DOI] [PubMed] [Google Scholar]
- Lauer B, Russwurm R, Schwarz W, Kalmanczhelyi A, Bruntner C, Rosemeier A, Bormann C. Molecular characterization of co-transcribed genes from Streptomycestendae Tu901 involved in the biosynthesis of the peptidyl moiety and assembly of the peptidyl nucleoside antibiotic nikkomycin. Mol. Gen. Genet. 2001;264:662–673. doi: 10.1007/s004380000352. [DOI] [PubMed] [Google Scholar]
- Li Y, Zeng H, Tan H. Cloning, function, and expression of sanS: a gene essential for nikkomycin biosynthesis of Streptomyces ansochromogenes. Curr. Microbiol. 2004;49:128–132. doi: 10.1007/s00284-004-4260-4. [DOI] [PubMed] [Google Scholar]
- Maddocks SE, Oyston PC. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology. 2008;154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- Manjasetty BA, Powlowski J, Vrielink A. Crystal structure of a bifunctional aldolase-dehydrogenase: sequestering a reactive and volatile intermediate. Proc. Natl. Acad. Sci. USA. 2003;100:6992–6997. doi: 10.1073/pnas.1236794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Verstraete I, Deutscher J, Galinier A. Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signalling pathway for the Bacillus subtilis levanase operon. J. Bacteriol. 1999;181:2966–2969. doi: 10.1128/jb.181.9.2966-2969.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PK, Hale TI, Christen P. Aminotransferases: demonstration of homology and division into evolutionary subgroups. Eur. J. Biochem. 1993;214:549–561. doi: 10.1111/j.1432-1033.1993.tb17953.x. [DOI] [PubMed] [Google Scholar]
- Metcalf WW, van der Donk WA. Biosynthesis of phosphonic and phosphinic acid natural products. Annu. Rev. Biochem. 2009;78:65–94. doi: 10.1146/annurev.biochem.78.091707.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener HD, Snell N. Two antifungal substances from Bacillus subtilis cultures. Arch. Biochem. 1949;22:208–214. [PubMed] [Google Scholar]
- Morett E, Saab-Rincon G, Olvera L, Olvera M, Flores H, Grande R. Sensitive genome-wide screen for low secondary enzymatic activities: the YjbQ family shows thiamin phosphate synthase activity. J. Mol. Biol. 2008;376:839–853. doi: 10.1016/j.jmb.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Ose T, Watanabe K, Mie T, Honma M, Watanabe H, Yao M, Oikawa H, Tanaka I. Insight into a natural Diels-Alder reaction from the structure of macrophomate synthase. Nature. 2003;422:185–189. doi: 10.1038/nature01454. [DOI] [PubMed] [Google Scholar]
- Pan JL, Bardwell JC. The origami of thioredoxin-like folds. Protein Sci. 2006;15:2217–2227. doi: 10.1110/ps.062268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BK, Hirota A, Sakai H. Studies no new antimetabolite produced by microorganism. 3. Structure of plumbemycin-A and plumbemycin-B, antagonists of l-threonine from Streptomyces plumbeus. Agric. Biol. Chem. 1977a;41:573–579. [Google Scholar]
- Park BK, Hirota A, Sakai H. Studies on new antimetabolite N-1409. Agric. Biol. Chem. 1977b;41:161–167. [Google Scholar]
- Peapus DH, Chiu HJ, Campobasso N, Reddick JJ, Begley TP, Ealick SE. Structural characterization of the enzyme-substrate, enzyme-intermediate, and enzyme-product complexes of thiamin phosphate synthase. Biochemistry. 2001;40:10103–10114. doi: 10.1021/bi0104726. [DOI] [PubMed] [Google Scholar]
- Platt A, Shingler V, Taylor SC, Williams PA. The 4-hydroxy-2-oxovalerate aldolase and acetaldehyde dehydrogenase (acylating) encoded by the nahM and nahO genes of the naphthalene catabolic plasmid pWW60-22 provide further evidence of conservation of meta-cleavage pathway gene sequences. Microbiology. 1995;141(Pt 9):2223–2233. doi: 10.1099/13500872-141-9-2223. [DOI] [PubMed] [Google Scholar]
- Rapp C, Jung G, Kugler M, Loeffler W. Rhizocticins - new phosphono-oligopeptides with antifungal activity. Liebigs Ann. Chem. 1988:655–661. [Google Scholar]
- Schell MA. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- Seidel HM, Freeman S, Seto H, Knowles JR. Phosphonate biosynthesis: isolation of the enzyme responsible for the formation of a carbon-phosphorus bond. Nature. 1988;335:457–458. doi: 10.1038/335457a0. [DOI] [PubMed] [Google Scholar]
- Serafimov JM, Gillingham D, Kuster S, Hilvert D. The putative Diels-Alderase macrophomate synthase is an efficient aldolase. J. Am. Chem. Soc. 2008;130:7798–7799. doi: 10.1021/ja8017994. [DOI] [PubMed] [Google Scholar]
- Tabata K, Ikeda H, Hashimoto S. ywfE in Bacillus subtilis codes for a novel enzyme, l-amino acid ligase. J. Bacteriol. 2005;187:5195–5202. doi: 10.1128/JB.187.15.5195-5202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop GL, Rayment I, Holden HM. Three-dimensional structure of the biotin carboxylase subunit of acetyl-CoA carboxylase. Biochemistry. 1994;33:10249–10256. doi: 10.1021/bi00200a004. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Mie T, Ichihara A, Oikawa H, Honma M. Detailed reaction mechanism of macrophomate synthase. Extraordinary enzyme catalyzing five-step transformation from 2-pyrones to benzoates. J. Biol. Chem. 2000;275:38393–38401. doi: 10.1074/jbc.M003119200. [DOI] [PubMed] [Google Scholar]
- Woodyer RD, Shao Z, Thomas PM, Kelleher NL, Blodgett JA, Metcalf WW, van der Donk WA, Zhao H. Heterologous production of fosfomycin and identification of the minimal biosynthetic gene cluster. Chem. Biol. 2006;13:1171–1182. doi: 10.1016/j.chembiol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Kato H, Hata Y, Nishioka T, Kimura A, Oda J, Katsube Y. Three-dimensional structure of the glutathione synthetase from Escherichia coli B at 2.0 A resolution. J. Mol. Biol. 1993;229:1083–1100. doi: 10.1006/jmbi.1993.1106. [DOI] [PubMed] [Google Scholar]
- Zawadzke LE, Bugg TD, Walsh CT. Existence of two d-alanine:d-alanine ligases in Escherichia coli: cloning and sequencing of the ddlA gene and purification and characterization of the DdlA and DdlB enzymes. Biochemistry. 1991;30:1673–1682. doi: 10.1021/bi00220a033. [DOI] [PubMed] [Google Scholar]
- Zhang G, Allen KN, Dunaway-Mariano D. Enzymatic synthesis of radiolabeled phosphonoacetaldehyde. Anal. Biochem. 2003a;322:233–237. doi: 10.1016/j.ab.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Zhang G, Dai J, Lu Z, Dunaway-Mariano D. The phosphonopyruvate decarboxylase from Bacteroides fragilis. J. Biol. Chem. 2003b;278:41302–41308. doi: 10.1074/jbc.M305976200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.