Abstract
Impaired ability to remember what has gone before is one of the most distressing aspects of the aging process. Odor recognition memory is particularly vulnerable to the effects of aging, yet the underlying neural substrate is unknown. This study investigated the neural substrate of cross-modal olfactory recognition memory in young and older adults using fMRI. Participants were presented with 16 familiar odors immediately before entering the scanner, and were then tested for retrieval with words, either names of odors previously presented (targets) or names of new odors (foils), while being scanned at 3T. Activation was reduced in the old subjects, both in regions involved in episodic memory retrieval and in regions involved in olfactory processing. Greater activation in the cerebellum of older adults was observed, suggesting increased response to attentional demands or compensatory mechanisms. Unlike in a number of studies in other sensory modalities, no increase in activation in frontal areas in older adults was observed during retrieval.
Keywords: aging, cognition, cognitive neuroimaging, odor, odor memory
1. Introduction
The ability to remember that a stimulus or an event has previously occurred is a critical human competency and its loss one of the most devastating effects of aging. Elucidating the underlying substrates of human memory, and specifically of age-related memory dysfunction, is a major challenge for cognitive neuroscience. Striking impairment of odor memory and odor naming in Alzheimer's disease has sparked interest in the role of mesial temporal lobe structures in odor memory. Given the critical importance of age-related memory impairment and the need to understand its underlying substrate, the present study investigated age-related changes in the neural substrate for recognition memory in young and older adults by using a cross-modal olfactory memory paradigm to specifically challenge mesiotemporal lobe structures.
Older adults show significant impairment in odor memory, in recall (Murphy et al. 1997), recognition (Murphy, et al. 1991, 1997), source memory (Gilbert et al., 2006) and associative memory (Gilbert et al., 2007). Olfactory event-related potential (OERP) measurements have shown that older adults exhibit longer latencies and smaller amplitudes for the cognitive component P3 (Morgan et al, 1997, 1999; Murphy et al. 2000), suggesting slower updating of memory.
Functional magnetic resonance imaging of brain activity during tasks where odor is presented in the scanner have shown reduced activation in orbital frontal cortex in older adults (Cerf-Ducastel and Murphy, 2003; Suzuki, et al. 2001; Wang et al. 2005; Yousem et al. 1999). Further, Cerf-Ducastel and Murphy (2003) found that when presented with odors in the scanner, young and older adults showed fMRI activation in similar regions, but activation was attenuated in older adults in a number of regions that process olfactory information (amygdala, piriform cortex, entorhinal cortex), and this result has been replicated by Wang and colleagues (2005). Although the localization is necessarily less clear, results with PET are consonant (Kareken et al. 2003).
Early projections of the olfactory system involve brain regions participating in emotion and memory processes, such as the hippocampus, entorhinal cortex, and amygdala (Carmichael and Price, 1996). Those regions are significantly affected by aging (Jernigan et al, 1991), potentially mediating deficits observed in olfactory recognition memory. FMRI studies of aging and memory have investigated activation during performance of visual or verbal tasks; however, the neurophysiological substrate of olfactory memory in aging is not well established.
The primary aim of the present study was to investigate the neural substrate of impairment in recognition memory for odor in older adults. We have investigated the cortical substrate of cross-modal odor recognition memory in young adults (Cerf-Ducastel and Murphy, 2006). In order to investigate the neural substrate of impairment in older adults, the present study used the same paradigm, under the same scanning conditions, in healthy older adults and compared the results to those of the young adults in the published study (Cerf-Ducastel and Murphy, 2006).
2. Results
Results have shown that brain activation patterns in this task significantly differ across runs for young subjects (Cerf-Ducastel and Murphy, 2006) suggesting a shift in the task performed. Therefore, the results reported here are based solely on run1.
Results from psychophysical measures
To facilitate odor encoding during the memory task, individuals with anosmia and serious hyposmia were excluded from participation. Mean olfactory threshold was better in young adults (7.3±1.6) than in older adults ( 4.9±2.3), t (16) = 2.7, p = 0.016. Odor identification scores collected on eight young and six old subjects showed that young participants correctly identified an average of 7.5 ±0.8 odors whereas older adults averaged 5.0 ±2.4, t (12) = 2.2, p = 0.05. On the AST alcohol detection test collected on eight young and nine old subjects, young participants showed a tendency toward detecting the alcohol at a greater distance, mean = 22.5±6.8 cm, than the older adults, mean = 15.7±7 cm, t (15) = 2.1, p = 0.051; but this difference did not reach statistical significance.
Behavioral performance
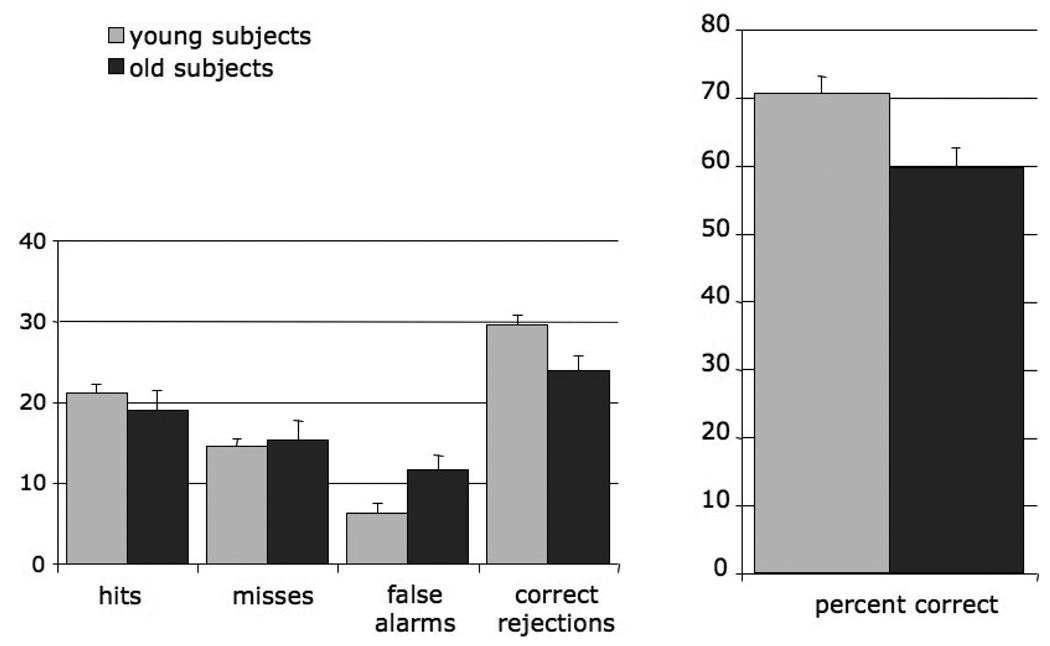
Percent correct (PC) was calculated as the ratio of the sum of hits (H) and correct rejections (CR) over the total number of words multiplied by 100 (PC = (H + CR) x 100 / (total number of words)). A one way ANOVA conducted on the percent correct values showed a reduction in percent correct in older adults, F(1,18)=7.53, p=0.013. Similarly, ANOVA on d' showed an effect of age, F(1,17=5.52, p=0.031, (M (Young) =1.22±0.53, M (Old) = 0.65±0.53). A group (young and older adults) by type of response (hit, miss, correct rejection and false alarm) multivariate analysis of variance (MANOVA) showed a main effect of age (F(4,15)=3.92, p=0.023), a main effect of memory response (F (1,16) = 642.9, p<0.001), and an interaction between age and measure (F (1,16) = 8.9, p = 0.009). Age differences were found for correct rejections (M (Young) =3 0.4±2.45, M (Old) = 23.5±6.02, p=0.016) and false alarms (M (Young) = 5.44±2.60, M (Old) = 12.0±6.04, p = 0.029), suggesting a greater tendency for older adults to respond "yes," but not for hits (M (Young) = 21.1±3.54, M (Old) = 19.0±7.57, p = 0.437) or misses (M(Young)=140.4±3.27, M(Old)=15.2±7.77, p=0.768) (Figure 1).
Figure 1. Performance on the olfactory recognition memory task.
Mean and standard errors for hits (recognizing a target), misses (rejecting a target), false alarms, (FA, incorrectly choosing a foil), correct rejections, (CR, rejecting a foil), no response (No Re), pressing the wrong key (W Key), and percent correct, (PC = (H + CR) x 100 / (total number of words)). Older adults produced significantly more false alarms than young adults and their percent correct was lower than that of the young adults.
The responses of subjects in the debriefing indicated that none of the subjects had perceived a regular pattern in the presentation of the foils and targets.
Imaging results - Group analysis
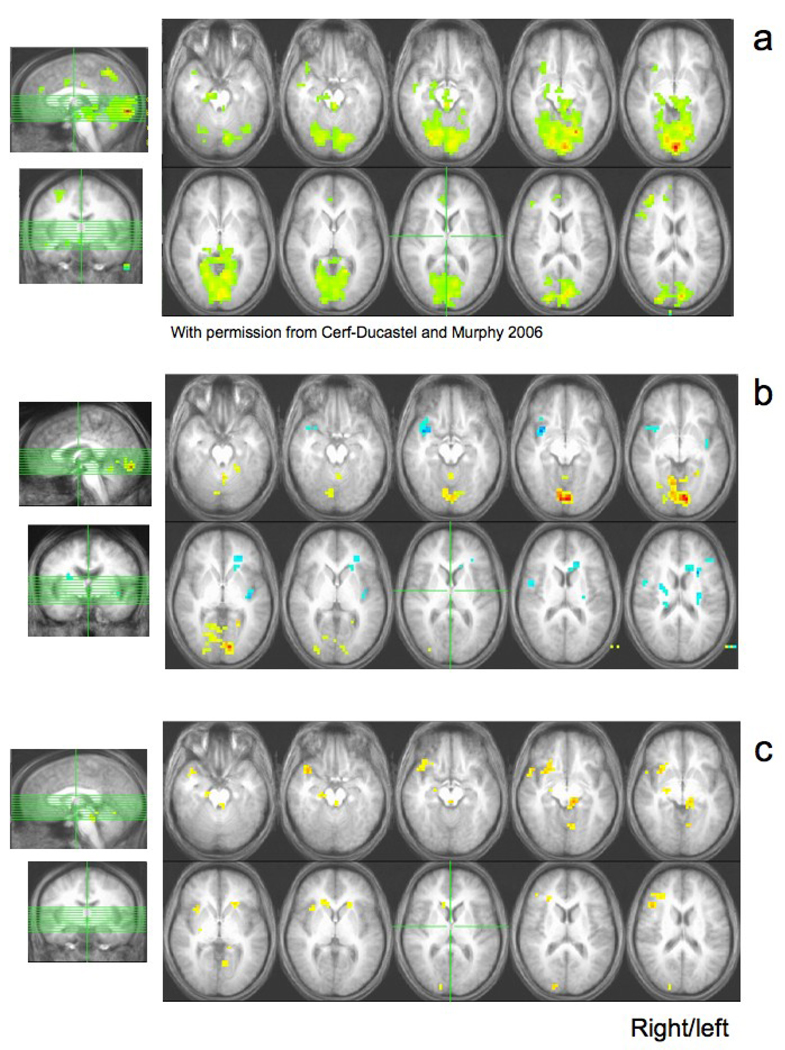
In young adults performing this task, activated regions had included right hippocampus, piriform/amygdalar area, superior temporal gyrus, anterior cingulate gyrus, inferior frontal/orbito frontal gyrus, superior/medial frontal gyrus, and bilateral parahippocampal gyrus, inferior parietal lobule, supramarginal gyrus, cerebellum, lingual/fusiform area, and middle/posterior cingulate gyrus (Figure 2a, from Cerf-Ducastel and Murphy, 2006, with permission).
Figure 2. Activations during olfactory recognition.
The right side of the brain appears on the left side of the image according to the radiological convention. Group images were obtained by calculating a one-sample t test on the Fisher transformed correlation coefficients at each voxel. Correction for multiple comparisons: voxels with a p<0.015 and belonging to clusters of at least six voxels were considered as activated.
Figure 2a: Group activation in the young subjects.
This figure is reproduced with permission from Cerf-Ducastel and Murphy, 2006, NeuroImage for comparison with the activation in older adults. Activation was observed in the cerebellum (slices 1, 2, 3), in the right parahippocampal gyrus (slices 4 and 5, the right amygdala (slice 6), at the limit of the right insula and the right orbitofrontal cortex BA 47 (slice 7), the left and right lingual gyrus and parahippocampal gyri (slices 6 through 10).
Figure 2b: Group activation in the old subjects.
Positive activation can be seen in the cerebellum (slices 2 through 5), in the lingual gyrus and in the parahippocampal gyrus (slices 6 through 9). Negative activation can be seen in the right ventral insula (slices 5 through 8) and the left dorsal insula (slices 8, 9, 10).
Figure 2c: Difference Tmap Young > Old.
With this contrast, positive activation is found in areas where activation was significantly higher for young subjects than for older subjects, or in other terms, in areas where there was a significant decrease in activation in older subjects, compared to the young subjects. Images show significant differences between young and old subjects in the right superior temporal gyrus (slices 3, 4, 5), the orbitofrontal cortex BA 47 (slices 6, 7), the right insula (slices 9, 10), the left parahippocampal gyrus (slices 7, 8), the head of caudate nucleus (slice 10), the right middle frontal gyrus (slices 13, 14, 15).
By contrast, older adults showed less activation (Figure 2b). Yellow areas correspond to areas more activated during Target periods and blue areas correspond to regions more activated during Foil periods. The difference group image (Figure 2c) shows regions that were significantly more activated in the young subjects than in the older adults (in yellow) and regions that were significantly more activated in the older than in the young subjects (in blue). The corresponding table of activated clusters (Table I) reports the size and coordinates of major clusters differentially activated between young and older subjects. This table shows that young subjects produced more activation than the older adults in the right inferior and superior frontal gyri, inferior parietal lobule/supramarginal gyrus, insula, superior temporal gyrus, posterior cingulate gyrus / precuneus, amygdala, bilaterally in middle frontal gyrus, parahippocampal gyrus, lingual gyrus and caudate, and in the left culmen. Older adults showed only one area more activated than the young in the left cerebellar tonsil / culmen /dentate gyrus.
Table I.
Activated clusters in the difference activation map Young minus Older adults. L: Left, R: Right, BA: Brodman Area, no: number, max int: maximum intensity, X,Y, Z, coordinates of the center of mass of the activated cluster, in Talairach space, according to AFNI’s algorithm (Cox 1996), Y: Young adults, O: Older adults. Bold Italics indicate negative activations, or activations that were stronger in older than in young adults.
| Localization of cluster | proximity of BA | no. voxels | Talairach center of mass | max int | mean | sem | Difference Young -Old | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| R middle frontal gyrus | 9 | 63 | 39 | 18.6 | 27.5 | 4.44 | 3.44 | 0.05 | Y>O |
| R inferior parietal lobule/supramarginal gyrus | 40 | 51 | 47.6 | −38.9 | 43 | 6.74 | 3.81 | 0.11 | Y>O |
| R insula/BA 13/BA 47 | 13/47 | 27 | 27.6 | 16.6 | −4.7 | 4.22 | 3.51 | 0.07 | Y>O |
| R inferior frontal gyrus/BA 47/ R sup temp gyrus | 47/38 | 26 | 40.9 | 15.4 | −13.2 | 4.62 | 3.53 | 0.08 | Y>O |
| L parahippocampal gyrus | 30/27 | 21 | −9.7 | −30.7 | −5.8 | 4.92 | 3.68 | 0.10 | Y>O |
| R superior frontal gyrus /BA 8 /R middle frontal gyrus | 8/9 | 19 | 18.7 | 36.8 | 37.9 | 4.80 | 3.65 | 0.12 | Y>O |
| R posterior cingulate gyrus / precuneus | 31 | 18 | 10 | −43 | 32.5 | 4.06 | 3.38 | 0.07 | Y>O |
| R superior frontal gyrus/middle frontal gyrus | 6/32 | 18 | 18.4 | 12 | 47.9 | 4.89 | 3.59 | 0.13 | Y>O |
| R inferior frontal gyrus/middle frontal gyrus | (46/10) | 12 | 31.2 | 32.6 | 14.6 | 3.88 | 3.34 | 0.07 | Y>O |
| L lingual gyrus | 19 | 11 | −7.5 | −61 | −4.4 | 3.87 | 3.42 | 0.08 | Y>O |
| R superior temporal gyrus | 38/21 | 8 | 39 | 4.5 | −23.5 | 3.75 | 3.39 | 0.09 | Y>O |
| L putamen/caudate | 8 | −21 | 21.6 | 2 | 3.79 | 3.35 | 0.08 | Y>O | |
| R caudate (head) | 8 | 13 | 23.1 | 5 | 3.71 | 3.22 | 0.08 | Y>O | |
| L middle frontal gyrus | 46 | 8 | −34.9 | 27.4 | 22.1 | 4.42 | 3.53 | 0.16 | Y>O |
| L cerebellar tonsil / culmen /dentate | 7 | −16.3 | −38.6 | −29.2 | −3.67 | 3.28 | 0.08 | O>Y | |
| R parahippocampal gyrus | 28 | 7 | 17.5 | −19.3 | −17.7 | 3.88 | 3.29 | 0.11 | Y>O |
| L culmen | 7 | −2.5 | −31.7 | −16.4 | 3.96 | 3.41 | 0.12 | Y>O | |
| R lentiform nucleus/Lateral globus pallidus/amygdala | 7 | 22.5 | −13.6 | −5.2 | 3.75 | 3.31 | 0.09 | Y>O | |
| R cuneus/lingual gyrus | 18 | 7 | 16.3 | −90.7 | 12 | 3.89 | 3.40 | 0.14 | Y>O |
| R precuneus | 7 | 6 | 12.7 | −49.7 | 47.4 | 3.97 | 3.46 | 0.13 | Y>O |
Imaging results – Region of interest analysis
The region of interest analysis was performed on regions selected based on the analysis of ROI activation in young subjects performing this task (Cerf-Ducastel and Murphy, 2006). The ten selected regions of interest can be separated into two groups based on the functions of the areas, as derived from previous studies. Three repeated measures ANCOVAs were performed on (i) on group 1, the six regions more directly involved in memory processes, i.e. anterior and posterior hippocampus, parahippocampal gyrus, lingual gyrus, fusiform gyrus and middle frontal gyrus; (ii) group 2, the four regions more directly involved in olfactory processing, i.e. piriform cortex, amygdala, orbitofrontal cortex BA 47 and BA 11; and (iii) on the composite. Olfactory threshold was used as the covariate in each of the three ANCOVAs.
Each of these ANCOVAs revealed robust effects of age, and the effect was more pronounced in group 2 regions, i.e. “olfactory” regions (F(1,15)=5.85, p=0.029, Eta2 = .28) than in group 1 regions, i.e. “memory” regions (F(1,15)=4.68, p=0.047, Eta2 = .24) or in the composite (F(1,15)=5.45, p=0.034, Eta2 = .27). As expected, the effect of olfactory threshold was not significant for any of the three ANCOVAs (p=0.86, p=0.81 and p=0.83 respectively for the group 1, group 2 and the composite). A significant interaction of age and hemisphere for the composite set of regions (F(1,15)=5.30, p=0.036, Eta2 = .26) showed that young subjects exhibited more activation in right hemisphere (M=0.180) than the left (M=0.092), but that old subjects did not (M=0.008 and M=0.003 respectively in the right and left hemispheres).
3. Discussion
The primary aim of this study was to identify the neural correlates of cross-modal olfactory recognition memory in older adults. In order to specifically challenge mesial temporal lobe circuits, the investigation used a cross-modal recognition task that was based on the presentation of odors for encoding, and the visual presentation of odor names for retrieval.
The present study identified regions that were significantly more activated when participants viewed mostly targets, i.e. names of odors presented to them during the pre-scanning encoding session (T periods), than when participants viewed mostly foils, i.e. name of odors that had not been presented to them during the pre-scanning session (F periods). Note that activation patterns were not completely specific for targets and foils since foils were also included in T periods and targets in F periods, following the procedure of Stark and Squire (2000).
Olfactory and Memory Performance in the Older Adult
Aging can affect olfactory sensory abilities (Murphy, 2002, Schiffman, 1986), although there is considerable variability in the older population (Nordin et al, 2004). Thus, to facilitate odor encoding during the memory task, potential participants for the current study were screened with psychophysical testing and individuals with anosmia and serious hyposmia had been excluded. Nevertheless, it was of interest to compare young and older adult participants on these measures. Although younger participants had statistically better olfactory thresholds, mean detection thresholds for this group of older adults suggested that they were only mildly hyposmic, and they did not differ significantly on the distance detection measure. Olfactory threshold was used as a covariate in the analyses of the imaging results and as expected, the covariate was not significant, p's > .80. Performance on odor recognition memory for old and younger participants showed higher percent correct for younger adults. The main effect of age on type of response was largely due to fewer correct rejections and more false alarms in the older adults. There were no differences in hits or misses. Such a result would be consonant with the view that presented items are encoded by the older adults, but with less precision and appear to match a broader group of stimuli at retrieval. In addition, older adults would be expected to have difficulty with novel items at retrieval if they are matched to a less distinct template.
Pattern of Cortical Activations
Main activated regions in the young subjects had included right hippocampus, piriform/amygdalar area, superior temporal gyrus, anterior cingulate gyrus, inferior frontal/orbito frontal gyrus, superior/medial frontal gyrus, and bilateral parahippocampal gyrus, inferior parietal lobule, supramarginal gyrus, cerebellum, lingual/fusiform area, and middle/posterior cingulate gyrus (Cerf-Ducastel and Murphy, 2006).
These areas were assigned to two subgroups, the first included mesiotemporal lobe (MTL) areas, the prefrontal cortex, the fusiform/parahippocampal gyrus, and the lateral and medial parietal areas. Previous lesion and clinical studies have established the involvement in episodic memory of mesiotemporal areas [e.g., Scoville and Milner, (1957); Cohen and Squire, (1980), for reviews see Gabrieli, (2001); Squire, (1992)] and of prefrontal areas [for a review see Wheeler et al. (1995)]. In neuroimaging studies, prefrontal regions have been found activated in a more consistent manner than the mesiotemporal areas, although MTL activation has been reported during encoding and retrieval (Roland and Gulyas, 1995; Kapur et al. 1995; Daselaar, et al. 2006), and specifically during successful retrieval (Henson et al. 1999). Prefrontal activation has been reported more consistently, during encoding (Dolan and Fletcher, 1997) and retrieval (e.g. Kapur, et al. 1995; Markowitsch, 1995), and more recently during working memory (Mattay et al. 2006; Mitchell et al. 2006). Finally, the fusiform/parahippocampal gyrus is activated during successful recognition memory (Daselaar, et al. 2001). Thus, the first subset of areas included many areas activated in previous fMRI studies of memory. The second subset of regions included the piriform and entorhinal cortex, the amygdala, and the orbitofrontal cortex. The piriform, amygdala and lateral entorhinal cortex receive direct projections from the olfactory bulb, and the hippocampus and orbitofrontal cortex receive projections respectively from the entorhinal and the piriform cortex (Carmichael and Price, 1994). These regions also have been reported in neuroimaging studies of olfactory function with Positron Emission Tomography or fMRI (Zatorre et al. 1992; Savic et al. 2000; Anderson al., 2003; Zald and Pardo, 1997; Royet et al. 1999; Sobel et al. 1998; Yousem et al. 1999; O'Doherty et al. 2000; Wiser et al. 2000; Cerf-Ducastel and Murphy, 2001; Poellinger et al. 2001; Suzuki et al. 2001; Gottfried et al. 2002; Cerf-Ducastel and Murphy, 2003; Murphy et al. 2005; Wang et al. 2005).
The activation of these regions in the young subjects during a recognition memory task is consistent with the notion that (i) some of the regions responsible for the effective encoding of information are the regions that process the actual event (Rugg et al. 2002) and (ii) that some of the regions activated during retrieval success include regions activated during encoding (Nyberg et al. 2000; Rugg et al. 2002; Gottfried et al. 2004).
Reduced Activation in Older Adults
The group analysis revealed that older adults had reduced activation in comparison to the young adults and showed areas of negative activation in a number of regions, including the left and right claustrum, putamen, caudate tail, cingulate cortex, insula cortex, left middle frontal gyrus and right precentral gyrus. To interpret these negative activations, it is useful to be mindful that with the present paradigm, negative activations correspond to F periods, greater activation when subjects were viewing mostly foils; by comparison to T periods, when subjects were viewing mostly targets. Older adults had more false alarms and fewer correct rejections, thus, they were less successful in recognizing novel foils during the F periods, possibly because they are attempting to match novel stimuli (i.e., foils) to a less distinct template than that of young adults. These data suggest that older adults may engage in more effortful processing than the young in order to determine whether foils had been previously presented and thus produce more activation to foils relative to young subjects, resulting in negative activations. Negative activation during a working memory task also has been interpreted as a suppression of activity in an area not required for task performance in order to focus resources on the task at hand (Tomasi et al. 2006). The current results also would be consistent with this hypothesis suggesting that older adults suppress activity in areas activated during retrieval of targets that are less active or less suppressed during processing of foils (i.e., novel stimuli).
The difference image comparing the activation in older and young adults revealed that older adults produced significantly less pronounced activation in several regions including the right prefrontal (middle frontal gyrus), and parietal areas (inferior parietal lobule / supramarginal gyrus). Other areas less strongly activated in the older adults included left parahippocampal gyrus, lingual gyrus, right insula and posterolateral orbitofrontal cortex, right amygdala and globus pallidus, right superior temporal gyrus, and bilateral caudate nucleus.
Imaging results – Region of Interest analysis
In addition to the pattern of activation revealed by the voxel by voxel group analysis, the ROI analysis showed a differential effect discriminating the group 1 (more “memory”) and the group 2 (more “olfactory”) subsets or activated areas. The effect size for the influence of age was greater in the group 2 areas, suggesting that the impairment due to aging was more pronounced in those regions. This is in agreement with the fact that structural changes related to age have been identified in the mesio-temporal lobe including areas critical to olfactory processing such as the amygdala and entorhinal cortex (Jernigan et al. 1991, Insausti et al. 1998, De Toledo-Morrell et al. 2000, Tisserand et al. 2000, Jernigan et al. 2001, Pruessner et al. 2001; Raz et al, 2005).
Hemispheric differences in activation
The voxel by voxel group analysis and the ROI analyses on the composite region showed greater cortical activation in the right hemisphere than in the left in the young subjects, in agreement with other studies of olfactory processing (Savic and Gulyas, 2000), including unimodal odor recognition memory (Dade et al., 2002; Savic Gulyas, Larsson and Roland, 2000).
The results are also in concert with previous studies describing decreased fMRI activation in occipital and mesial temporal regions in older adults (Cabeza et al. 2004); however, there was no significant increase of activation in the prefrontal cortex, often observed in older adults (Bachman et al. 1999; Cabeza, 2001; Cabeza et al. 1997, 2000; Grady et al. 2002); and activation in right prefrontal cortex was significantly greater in young than older adults.
Mesial temporal and frontal activation
Reductions in activation in mesial temporal lobe areas in older adults have been reported in other memory studies (Gutchess et al. 2005). Since olfactory detection and processing is mediated by structures in the mesial temporal lobe, the reduced activation in these areas in older adults is of interest in the present study. This reduced activation in mesial temporal lobe may impact activation in frontal areas through frontal temporal circuits for memory information processing, and specifically olfactory memory information processing (Ramus et al. 2007; Gottfried et al. 2004). Decreased functional connectivity between frontal and mesial temporal lobe areas has been reported in older adults (Grady et al. 1995). Older adults processing olfactory information showed reduced functional connectivity between orbital frontal cortex and mesial temporal lobe areas including entorhinal cortex and hippocampus (Murphy et al. 2005). Thus, reduced connectivity in older adults performing olfactory processing may oppose increased activation that might result from greater effortful processing or recruitment in frontal areas including the prefrontal and specifically the orbital frontal cortex.
Differences in frontal activity between young and old may reflect age-related differences in the functional neural networks involved in odor recognition memory or the fact that older adults may be engaging in different strategies for retrieval that involve different cortical areas.
Cerebellum activation
Older adults in the present study did show greater activation in the cerebellum than young subjects did. This greater activity on the part of older adults may reflect greater attentional demands, different strategies, more effortful processing, or compensatory recruitment. In a previous fMRI study by Ferdon and Murphy (2003), older adults performing an olfactory task showed a decrease in activation in Crus I and Crus II, areas of the cerebellum that are responsive to increases in stimulus concentration and to trigeminal stimulation and that thus reflect a more sensory based activation. In contrast to activation in the more sensory areas, activation in Lobule VI was not reduced in older adults. Allen, et al. (1997) have proposed a model that postulates an important role for the cerebellum in attentional modulation and Allen et al. (1997) demonstrated that Lobule VI is involved in modulating attentional shifts. Older adults show more difficulty in sustained attentional tasks and are likely to require more effortful activity. Thus, it is possible that the older adults engaged in significant effort in response to the attentional demands in the odor task in Ferdon and Murphy (2003) and in the present study. It thus seems possible that the greater activation in the cerebellum in the present study similarly reflects the older adults' increased effort in response to attentional demands. Backman et al. (1999) compared older adults and Alzheimer's patients performing a memory task and thus have proposed that activation in the left cerebellum in older adults who show memory impairment reflects redistribution of neural activity as a compensatory mechanism to address the difficulty in performing the memory task. It is possible that the increased activation in the cerebellum in older adults in the present study also reflects compensatory use of different neural systems. Direct manipulation of attention and task difficulty in odor memory tasks in older adults would be of interest.
Conclusion
The present study, the first to consider the effects of age on recognition memory for odor using fMRI, investigated the neural substrate of the decline in olfactory memory in elderly adults during a cross-modal recognition task. Activation was reduced in the old subjects, both in regions involved in episodic memory retrieval and in regions involved in olfactory processing. Older adults showed greater activation only in the cerebellum, suggesting greater attentional demands, redistribution of memory processing, or compensatory use of different neural systems. Unlike in a number of studies in other sensory modalities, no increase in activation in frontal areas in older adults was observed during retrieval. We suggest that reduced reactivation of olfactory processing areas in older adults during retrieval and reduced activation of frontal temporal circuits for memory information processing underlie the particular vulnerability of olfactory recognition memory in older adults.
4. Experimental Procedure
Subjects and stimuli
Ten healthy older adults (5 men and 5 women, aged 66 to 86 yrs, mean = 73) participated in this study after giving informed consent. They were compared with ten healthy young subjects (5 men and 5 women, aged 20 to 25 yrs, mean = 22) who had previously been studied in the same paradigm (Cerf-Ducastel and Murphy, 2006). Participants were screened for dementia using either the Dementia Rating Scale (Mattis, 1976) or the Mini Mental State Exam (Folstein, Folstein & McHugh, 1975). Participants were routinely screened for conditions that produce smell loss independent of age: upper respiratory infections, nasal sinus disease, allergic rhinitis (Harris, Davidson, Murphy et al., 2006). The study was approved by Institutional Review Boards at San Diego State University and the University of California San Diego. Subjects gave written consent.
Psychophysical measures
Psychophysical measures included olfactory thresholds (Cain et al, 1983 modified by Murphy et al, 1990), an alcohol detection task (AST, Davidson and Murphy, 1997) and odor identification (Murphy et al. 2002).
Odor Stimuli
Odor stimuli were 16 familiar odorants corresponding to the items of the List A of the California Odor Learning Test (Murphy, Nordin, and Acosta, 1997).
Experimental Procedure
The experimental procedure consisted of the psychophysical assessment, a pre-scanning presentation of odors, one fMRI session conducted on a Varian 3 Tesla whole body MR scanner, and a post-scanning session with de-briefing.
Immediately before entering the scanner, the odors were presented in random order to the subject, who was asked to close the eyes, concentrate on the odor and try to memorize it.
Each imaging session began with the acquisition of functional runs followed by the structural run, to limit the time between the presentation of the stimuli to be remembered and the recognition task. During functional runs, subjects were presented with words on a screen every four seconds, which were either names of odors previously presented (targets) or names of odors that were not presented previously (foils). Subjects responded via button box whether an odor had been presented in the pre-scanning session or not.
Immediately after exiting the fMRI scanner, subjects were asked a series of questions, including degree of confidence in their responses and whether they perceived a pattern in the presentation of targets and foils. None did.
FMRI scanning parameters and FMRI paradigm
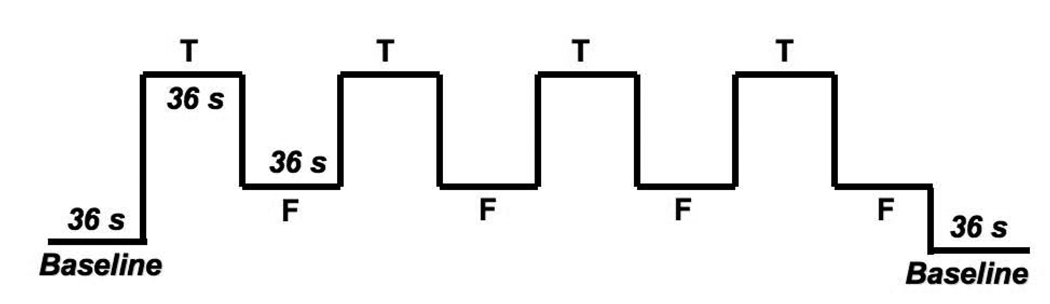
The experimental procedures and the neuroimaging protocol have been described in more detail in Cerf-Ducastel and Murphy (2006). Three functional runs of six minutes were collected using an echo planar sequence (30 axial slices, resolution: 4×4×4 mm3, TR: 4 s). Each run was composed of an initial 36 second baseline period with a fixation cross at the center of the screen, followed by eight 36 second periods alternating Target (T) and Foils (F), and a final 36 second baseline with the fixation cross (Figure 3). Each Target or Foil was presented for 3500 msec with a fixation cross located at the center of the screen. The fixation cross alone was presented for 500 msec in between words. T periods included the presentation of seven targets (names of odors presented before the scan) and two foils (names of odors that were not presented before the scan). F periods included seven foils and two targets. Instructions, fixation cross and names of odors were presented on a screen visible to the subject through a one way mirror. This paradigm was derived from Stark and Squire (2000). As in Stark and Squire (2000), the inclusion of foils in T periods and of targets in F periods was designed to limit the perception by the subjects of a pattern in the presentation of stimuli. Subjects responded via button box.
Figure 3. FMRI olfactory recognition memory paradigm.
Before entering the scanner, subjects were presented with 16 familiar odors. In the scanner, subjects viewed words displayed on a screen, which were either names of odors previously presented to them (targets) or names of odors that were not previously presented to them (foils). During a run 4 T and 4 F periods of 36 s composed of 9 words each were alternated. T periods were composed of 7 targets and 2 foils in order to limit the perception of blocks. F periods were composed of 7 foils and 2 targets. Responses were collected through a button box and analyzed with a matlab program that calculated hits, misses, correct rejections, false alarms and percent correct for each run.
A program written in Matlab was used to present words, to collect responses, and to calculate the numbers of hits, misses, false alarms, and correct rejections. The scanning session ended with the acquisition of high-resolution anatomical images to allow accurate localization of activations (MPRAGE, 180 axial slices, resolution: 1×1×1 mm3).
FMRI Data processing and statistical analyses
Functional data were processed with AFNI [Analysis of Functional Neurolmages] software (Cox, 1996). Each functional run (echo planar image) was composed of 84 temporal volumes (number of repetitions) of 30 axial slices each, with the two first excluded from analysis. Each run's temporal series were temporally smoothed and motion corrected. Resulting motion correction equations indicated that movement did not exceed two mm in translation or rotation for any subject. Low signal intensity voxels outside the brain were discarded by a clipping function.
Analysis of individual runs and group analysis
FMRI activity was correlated with a template based on the alternating T and F periods and extracting voxels or ROIs responding significantly more during T than F periods (Stark and Squire, 2000). This method of data processing aimed at identifying areas involved in recognition, which would be more activated during the recognition of already presented items than during periods of new items. A ramp corresponding to 2 TR (8 seconds) was added to the square wave design to account for the hemodynamic rise time at the transitions between the T and F periods (Figure 1). Within the AFNI Program (Cox, 1996), the activity in each voxel or ROI is cross correlated with a series of six versions of an idealized reference function, representing the alternating T and F periods, that differ in that they are time-shifted in three 1 sec shifts backward and three 1 sec shifts forward in time. The best fitting reference function is determined for each voxel or ROI for a given subject. This method allows the program to use the information about the time course of the hemodynamic response to optimize the quantification of activation. The same template is used for a given voxel or ROI for all of the image analysis, although analysis of the activation in a given subject may employ different templates for different voxels or ROIs, accounting for potential differences in the lag between the hemodynamic response and neural activity in different brain areas in different subjects. This takes into consideration (i) the fact that functional slices were acquired in interleaved fashion, creating a delay of up to four seconds between the first and last functional slice collected, and (ii) differences in the hemodynamic rise time in different brain regions.
For group analyses, each participant’s datasets were transformed to Talairach space. A one-sample t test was calculated on the Fisher transformed correlation coefficients at each voxel. Voxels presenting a p<0.015 and belonging to clusters of at least six voxels were considered as activated. These parameters were based on 10,000 Monte Carlo simulations processed with the AlphaSim program (Ward, 1997). The program estimates the probability of occurrence of clusters composed of voxels with a specific p value (i.e. 0.001), separated by no more than one voxel width (i.e., 4 mm, i.e. activated voxels in the same cluster had one complete side in common), for images spatially blurred with a 4 mm kernel (FWHM) Gaussian filtering. The analysis indicated that with the study parameters, less than 5 % of clusters would be activated by chance in the explored brain volume. Images were then normalized to fit the Talairach coordinate reference system (Talairach and Tournoux, 1993) using the AFNI algorithm. Activated areas were identified using Talairach coordinates and human brain atlases (Talairach and Tournoux, 1993; Mai et al, 1997).
Region Of Interest (ROI) analysis
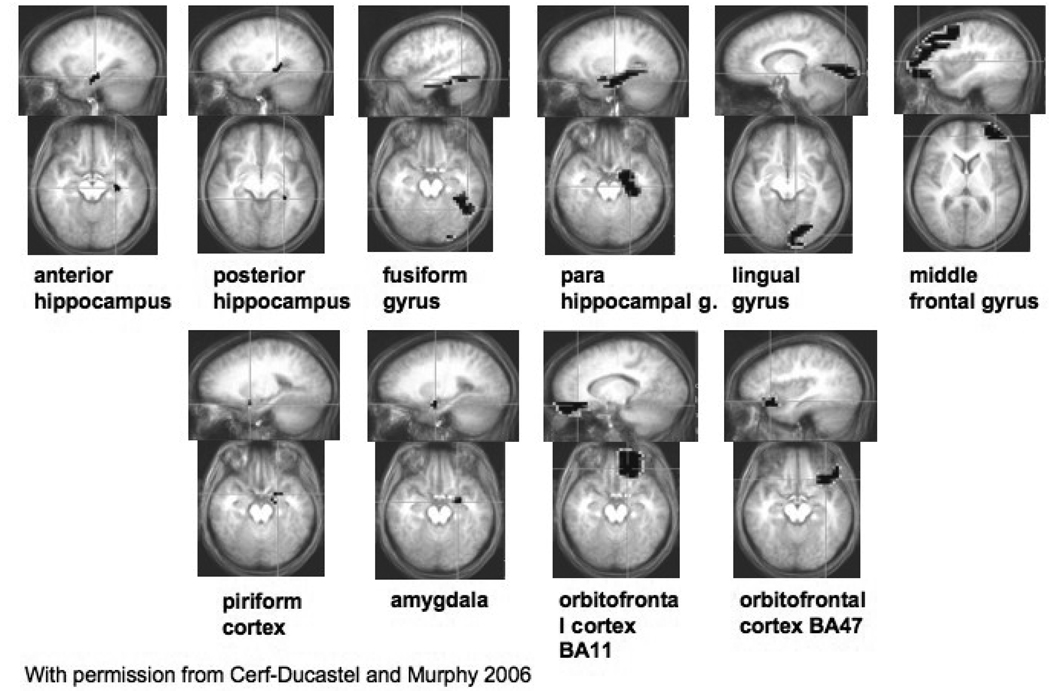
Ten regions of interest were selected (Figure 4), based on the results of the study of young adults (Cerf-Ducastel and Murphy, 2006) and on their potential interest suggested by previous neurophysiological and fMRI studies. In particular, we selected for the first group areas known to be involved in recognition memory i.e. mesiotemporal (MTL) lobe areas, prefrontal lobe areas, the fusiform/parahippocampal area, and lateral and medial parietal areas; and for the second group, areas more specifically involved in olfactory processing, i.e. piriform cortex, amygdala and orbitofrontal cortex (Cerf-Ducastel and Murphy, 2001, 2003, 2006; Eichenbaum, 1998, Ergorul and Eichenbaum, 2004, Gottfried et al. 2002, O'Doherty et al. 2000, Poellinger et al. 2001, Royet et al. 1999, Savic et al. 2000, Zald and Pardo, 1997; Zatorre et al. 1992). Regions of interest included anterior and posterior hippocampus, lingual gyrus, fusiform gyrus, parahippocampal gyrus, middle frontal gyrus, amygdala, piriform cortex, orbitofrontal cortex BA 11, orbitofrontal cortex BA 47. These regions were selected from ROIs available in AFNI and modified to fit the present experiment: the hippocampal region was divided in half into anterior and posterior; contours of the orbitofrontal areas were manually filled. The ROIs were considered independently in right and left hemispheres. The signal from each subject and from each run was averaged spatially over the volume of the ROIs, and detrended. The Fisher transforms of correlation coefficients calculated between an average ROI signal and the template were used to run statistical analyses, i.e., t-tests and repeated measures ANOVAs.
Figure 4. Regions of interest.
The ten regions of interest were selected from the regions available in the Talairach database in AFNI and were modified to fit the present experiment: the hippocampal region was divided into an anterior and posterior area; the contours of the orbitofrontal areas were manually filled. Regions were considered independently in the right and in the left hemisphere and are presented here only in the left hemisphere (and appear on the right side of the screen due to the radiological convention). Figure reproduced from Cerf-Ducastel and Murphy, (2006), with permission.
Acknowledgments
Funding
This research was supported by NIH grant # AG04085 from the National Institute on Aging to Claire Murphy.
We thank Lori Haase, Rose Calhoun Haney, Ph.D., Nobuko Kemmotsu, Margaret Chen, M.D., and Mi Ran Wang for expert technical assistance; Paul Gilblrt, Ph.D. and thoughtful reviewers for comments on the manuscript; Giedrius Buraccas, Ph.D. for computer programming and cogent discussion; and Richard Buxton, Ph.D. for radiological expertise and advice.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Backman L, Andersson JL, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer's disease. Neurology. 1999;52:1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc Natl Acad Sci U S A. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand J Psychol. 2001;42:277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. J Cogn Neurosci. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, Mclntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, Mcintosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain WS, Gent J, Catalanotto FA, Goodspeed RB. Clinical evaluation of olfaction. Am J Otolaryngol. 1983;4:252–256. doi: 10.1016/s0196-0709(83)80068-4. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. FMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses. 2001;26:625–637. doi: 10.1093/chemse/26.6.625. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 2003;986:39–53. doi: 10.1016/s0006-8993(03)03168-8. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. Neural substrates of cross-modal olfactory recognition memory: an fMRI study. Neuroimage. 2006;31:386–396. doi: 10.1016/j.neuroimage.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dade LA, Zatorre RJ, Jones-Gotman M. Olfactory learning: convergent findings from lesion and brain imaging studies in humans. Brain. 2002;125:86–101. doi: 10.1093/brain/awf003. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Lazeron RH, Jonker C. Parahippocampal activation during successful recognition of words: a self-paced event-related fMRI study. Neuroimage. 2001;13:1113–1120. doi: 10.1006/nimg.2001.0758. [DOI] [PubMed] [Google Scholar]
- Davidson TM, Murphy C. Rapid clinical evaluation of anosmia. The alcohol sniff test. Arch Otolaryngol Head Neck Surg. 1997;123:591–594. doi: 10.1001/archotol.1997.01900060033005. [DOI] [PubMed] [Google Scholar]
- De Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Using olfaction to study memory. Ann N Y Acad Sci. 1998;855:657–669. doi: 10.1111/j.1749-6632.1998.tb10642.x. [DOI] [PubMed] [Google Scholar]
- Ekman G, Berglund B, Berglund U, Lindvall T. Perceived intensity of odor as a function of time of adaptation. Scand J Psychol. 1967;8:177–186. doi: 10.1111/j.1467-9450.1967.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The Hippocampus and Memory for "What," "Where," and "When". Learn Mem. 2004 doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ, Cui L, Starr A. Olfactory event-related potentials in normal human subjects: effects of age and gender. Electroencephalogr Clin Neurophysiol. 1995;95:293–301. doi: 10.1016/0013-4694(95)00055-4. [DOI] [PubMed] [Google Scholar]
- Ferdon S, Murphy C. The cerebellum and olfaction in the aging brain: a functional magnetic resonance imaging study. Neuroimage. 2003;20:12–21. doi: 10.1016/s1053-8119(03)00276-3. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Functional Neuroimaging of Episodic Memory. In: Kingstone RCaA., editor. Handbook of Functional Neuroimaging of Cognition. Cambridge, MA, London, England: MIT Press; 2001. pp. 253–291. [Google Scholar]
- Gick ML, Cratk FI, Morris RG. Task complexity and age differences in working memory. Mem Cognit. 1988;16:353–361. doi: 10.3758/bf03197046. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Pirogovsky E, Ferdon S, Murphy C. The effects of normal aging on source memory for odors. J Gerontol B Psychol Sci Soc Sci. 2006;61:P58–P60. doi: 10.1093/geronb/61.1.p58. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Pirogovsky E, Ferdon S, Brushfield A, Murphy C. Differential effects of normal aging on memory for odor-place and object-place associations. Experimental Aging Research. 2007;34:437–452. doi: 10.1080/03610730802271914. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Wilson APR, Rugg MD, Dolan RJ. Remembrance of odors past human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42:687–695. doi: 10.1016/s0896-6273(04)00270-3. [DOI] [PubMed] [Google Scholar]
- Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the function nal neuroanatomy of face memory. Psychol Aging. 2002;17:7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Grady CL, Mcintosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Harris R, Davidson TM, Murphy C, et al. Clinical evaluation and symptoms of chemosensory impairment: 1000 consecutive cases from the Nasal Dysfunction Clinic in SanDiego. Am. J Rhinology. 2006;20:101–108. [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Insausti AM, Sobreviela MT, Salinas A, Martinez-Penuela JM. Human medial temporal lobe in aging: anatomical basis of memory preservation. Microsc Res Tech. 1998;43:8–15. doi: 10.1002/(SICI)1097-0029(19981001)43:1<8::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychol Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biol Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD. Functional anatomy of human odor sensation, discrimination, and identification in health and aging. Neuropsychology. 2003;17:482–495. doi: 10.1037/0894-4105.17.3.482. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. New York: Academic Press; 1997. [Google Scholar]
- Markowitsch HJ. Which brain regions are critically involved in the retrieval of old episodic memory? Brain Res Brain Res Rev. 1995;21:117–127. doi: 10.1016/0165-0173(95)00007-0. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Karasu TB, editors. Geriatric Psychiatry. New York: Grune and Stratton; 1976. pp. 77–101. [Google Scholar]
- Mitchell KJ, Raye CL, Johnson MK, Greene EJ. An fMRI investigation of short-term source memory in young and older adults. Neuroimage. 2006;30:627–633. doi: 10.1016/j.neuroimage.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Covington JW, Geisler MW, Polich J, Murphy C. Olfactory event-related potentials: older males demonstrate the greatest deficits. Electroencephalogr Clin Neurophysiol. 1997;104:351–358. doi: 10.1016/s0168-5597(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Geisler MW, Covington JW, Polich J, Murphy C. Olfactory P3 in young and older adults. Psychophysiology. 1999;36:281–287. doi: 10.1017/s0048577299980265. [DOI] [PubMed] [Google Scholar]
- Murphy C. Age-related effects on the threshold, psychophysical function, and pleasantness of menthol. J. Gerontol. 1983;38:217–222. doi: 10.1093/geronj/38.2.217. [DOI] [PubMed] [Google Scholar]
- Murphy C, Anderson JA, Markison S. Psychophysical assessement of chemosensory disorders in clinical populations. In: Kurihara K, Suzuki N, Ogawa H, editors. Olfaction and Taste XI. Tokyo: Springer-Verlag; 1994. pp. 609–613. [Google Scholar]
- Murphy C, Cain WS, Gilmore MM, Skinner RB. Sensory and semantic factors in recognition memory for odors and graphic stimuli: elderly versus young persons. Am J Psychol. 1991;104:161–192. [PubMed] [Google Scholar]
- Murphy C, Cerf-Ducastel B, Calhoun-Haney R, Gilbert PE, Ferdon S. ERP, fMRI and Functional Connectivity Studies of Brain Response to Odor in Normal Aging and Alzheimer's Disease. Chem Senses. 2005;30 Suppl 1:il70–il71. doi: 10.1093/chemse/bjh168. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiol Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Murphy C, Morgan CD, Geisler MW, Wetter S, Covington JW, Madowitz MD, Nordin S, Polich JM. Olfactory event-related potentials and aging: normative data. Int J Psychophysiol. 2000;36:133–145. doi: 10.1016/s0167-8760(99)00107-5. [DOI] [PubMed] [Google Scholar]
- Murphy C, Nordin S, Acosta L. Odor learning, recall, and recognition memory in young and elderly adults. Neuropsychology. 1997;11:126–137. doi: 10.1037//0894-4105.11.1.126. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. J Am Med Assoc. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Nordin S, Brämerson A, Bende M. Prevalence of self-reported poor odor detection sensitivity: the Skövde population-based study. Acta Otolaryngol. 2004;124:1171–1173. doi: 10.1080/00016480410017468. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, Mcintosh AR, Tulving E. Reactivation of encoding-related brain activity during memory retrieval. Proc Natl Acad Sci USA. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11:399–403. doi: 10.1097/00001756-200002070-00035. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Long term memory and aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging. Oxford, New York: Oxford university press; 2005. pp. 218–245. [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles JL, Frieske D, Zwahr M, Gaines CL. Mediators of long-term memory performance across the life span. Psychol Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen BR, Kwong KK. Activation and Habituation in Olfaction-An fMRI Study. Neuroimage. 2001;13:547–560. doi: 10.1006/nimg.2000.0713. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and Gender Predict Volume Decline in the Anterior and Posterior Hippocampus in Early Adulthood. J Neurosci. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Ramos SJ, Davis JB, Donahue RJ, Discenza CB, White AA. Interactions between the orbitofrontal cortex and the hippocampal memory system during the storage of long-term memory. Ann NY Acad Sci. 2007;1121:216–231. doi: 10.1196/annals.1401.038. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Roland PE, Gulyas B. Visual memory, visual imagery, and visual recognition of large field patterns by the human brain: functional anatomy by positron emission tomography. Cereb Cortex. 1995;5:79–93. doi: 10.1093/cercor/5.1.79. [DOI] [PubMed] [Google Scholar]
- Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G, Holley A, Mauguiere F, Comar D, Froment JC. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL, Shaw RJ. Effects of adult age on structural and operational capacities in working memory. Psychol Aging. 1991;6:118–127. doi: 10.1037//0882-7974.6.1.118. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B. Olfactory processing in healthy subjects measured with positron emission tomography. International Symposium for Olfaction and Taste. Brighton. 2000:98. [Google Scholar]
- Savic I, Gulyas B. PET shows that odors are processed both ipsilaterally and contrlaterally to the stimulated nostril. NeuroReport. 2000;11:2861–2866. doi: 10.1097/00001756-200009110-00007. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Age-related changes in taste and smell and their possible causes. In: Meiselman HL, Rivlin RS, editors. Clinical Measurement of Taste and Smell. New York: Macmillan; 1986. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurochem. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998;392:282–286. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. J Neurosci. 2000;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. fMRI activity in the medial temporal lobe during recognition memory as a function of study-test interval. Hippocampus. 2000;10:3329–3337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Critchley HD, Suckling J, Fukuda R, Williams SC, Andrew C, Howard R, Ouldred E, Bryant C, Swift CG, Jackson SH. Functional Magnetic Resonance Imaging of Odor Identification: The Effect of Aging. J Gerontol A Biol Sci Med Sci. 2001;56:M756–M760. doi: 10.1093/gerona/56.12.m756. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Referentially Oriented Cerebral MRI Anatomy, Atlas of Stereotaxic Anatomical Correlations for Gray and White Matter. New York: Thieme Medical Publishers, Inc; 1993. [Google Scholar]
- Tisserand DJ, Visser PJ, van Boxtel MP, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging. 2000;21:569–576. doi: 10.1016/s0197-4580(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60:510–514. doi: 10.1093/gerona/60.4.510. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Biophysics Research Institute, Medical College of Wisconsin. 1997 [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. J Int Neuropsychol Soc. 1995;1:525–536. doi: 10.1017/s1355617700000655. [DOI] [PubMed] [Google Scholar]
- Wilson JL, Jenkinson M, Jezzard P. Optimization of static field homogeneity in human brain using diamagnetic passive shims. Magn Reson Med. 2002;48:906–914. doi: 10.1002/mrm.10298. [DOI] [PubMed] [Google Scholar]
- Wiser A, Cerf-Ducastel B, Murphy C. International Symposium on Olfaction and Taste XIII. Brighton, UK: 2000. Effect of aging on the olfactory network: What fMRI combined with psychophysics can tell us; p. 54. [Google Scholar]
- Yousem DM, Maldjian JA, Hummel T, Alsop DC, Geckle RJ, Kraut MA, Doty RL. The effect of age on odor-stimulated functional MR imaging. AJNR Am J Neuroradiol. 1999;20:600–608. [PMC free article] [PubMed] [Google Scholar]
- Zacks R, Hasher L. Cognitive gerontology and attentional inhibition: a reply to Burke and McDowd. J Gerontol B Psychol Soc Sci. 1997;52:P274–P283. doi: 10.1093/geronb/52b.6.p274. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]