Abstract
MiR-15a/16-1 and miR-15b/16-2 clusters have been shown to play very important roles in regulating cell proliferation and apoptosis by targeting cell cycle proteins and the antiapoptotic Bcl-2 gene. However, the physiological implications of those two clusters are largely elusive. By aligning the primary miR-15a/16-1 sequence among 44 vertebrates, we found that there was a gap in the homologous region of the rat genome. To verify that there was a similar miR-15a/16-1 cluster in rats, we amplified this region from rat genomic DNA using PCR and found that a 697-bp sequence was missing in the current rat genome database, which covers the miR-15a/16-1 cluster. Subsequently, we also investigated the expression pattern of individual miRNAs spliced from miR-15a/16-1 and miR-15b/16-2 clusters, including miR-15a, miR-15a*, miR-15b, miR-15b*, miR-16-1/2, and miR-16-1/2* from various rat tissues, and found that all of those miRNAs were expressed in the investigated tissues. MiR-16 was most expressed in the heart, followed by the brain, lung, kidney, and small intestine, which indicates tissue specificity for individual miRNA expression from both clusters. Our results demonstrated that both miR-15a/16-1 and miR-15b/16-2 clusters are highly conserved among mammalian species. The investigation of the biological functions of those two clusters using transgenic or knockout/knockdown models will provide new clues to understanding their implications in human diseases and finding a new approach for miRNA-based therapy.
Introduction
MicroRNAs (miRNAs) are noncoding, endogenous, small RNAs that regulate gene expression by either degrading mRNA or inhibiting protein translation at the post-transcriptional level (Suzuki et al. 2009). MiRNAs have been implicated in various human diseases, including cancer and cardiovascular, developmental, viral, and neurodegenerative diseases (Bilen et al. 2006; Cordes et al. 2009; Cullen 2009; Hammoud et al. 2009; Kota et al. 2009; Lawler and Chiocca 2009; Legesse-Miller et al. 2009; Suzuki et al. 2009; Valastyan et al. 2009; Yokota 2009). MiR-15a/16-1 and miR-15b/16-2 clusters have been shown to regulate cell cycle and apoptosis by targeting cyclin D3 (CCND3), cyclin E1 (CCNE1), CDK6 (Linsley et al. 2007; Liu et al. 2008), and the antiapoptotic gene Bcl-2 in various cancer cells (Aqeilan et al. 2009; Bandi et al. 2009; Cimmino et al. 2005; Guo et al. 2009; Xia et al. 2008). The miR-15a/16-1 cluster was found to be frequently deleted or downregulated in more than two thirds of chronic lymphocytic leukemia (CLL) cases (Cimmino et al. 2005; Wang et al. 2008). Using cDNA microarray and proteomics analysis, the miR-15a/16-1 cluster was recently shown to target PDCD4, RAB21, IGSF4, SCAP2 or Bcl-2, and Wt1 (Calin et al. 2008). In addition, this cluster also regulates early embryonic patterning, acting at the crossroads of fundamental signaling cascades in Xenopus by targeting the Nodal type II receptor Acvr2a, which is a core component of Nodal signaling (Martello et al. 2007). The miR-16-2 gene in the miR-15b/16-2 cluster has the same mature sequence as miR-16-1, whereas there are four nucleotide differences between miR-15b and miR-15a. However, it shares the same seed sequence, which indicates a potential similarity in function. The growing experimental evidence demonstrates that both miR-15a/16-1 and miR-15b/16-2 clusters are important gene regulators that fine tune the complicated gene networks in a variety of cell lines. The pathophysiological functions of both clusters have not been investigated yet, however. We aligned the primary miR-15a/16-1 and miR-15b/16-2 clusters' sequences among mammalian species and found a gap in the homologous region of the miR-15a-16-1 cluster in the rat genome database. In view of the importance of this cluster, we postulated that there should be a miR-15a/16-1 cluster in the rat genome. To verify that this is the case, we cloned and sequenced the rat primary miR-15a/16-1 cluster and found that there was a miR-15a/16-1 cluster in the rat that shares homologous sequence with mouse and human. In this study we identified the 796-bp primary miR-15a/16-1 cluster sequence in the rat, which aligned with mouse primary miR-15a/16-1. In addition, we detected the expression of each miRNA spliced from the miR15a/16-1 and miR-15b/16-2 clusters from various tissues of adult rats. We also analyzed the conservation, host genes, chromosome locations, and functional implications for both clusters.
Materials and methods
Amplification of rat primary miR-15a/16-1 cluster
PCR was performed to amplify the miR-15a/16-1 cluster from Sprague-Dawley rat genomic DNA using the primers 5′-GATACTCGAGCAGAAGTTTGGCTAATTTAATAA TC-3′ (forward) and 5′-GCGAATTCGCCAAGGATGA CCTTAAGCCTC-3′ (reverse). The PCR conditions were as follows: denatured at 94°C for 3 min, followed by 30 cycles of 94°C for 30 sec, 60°C for 45 sec, and 72°C for 1 min, and final extension for 5 min at 72°C. The PCR product was purified from agarose gel and cloned into the plasmid pCR2.1 TA vector (Invitrogen; Carlsbad, CA). The insert was verified by restriction enzyme and sequencing.
Bioinformatics analysis
The individual mature miRNA sequences spliced from miR-15a/16-1 and miR-15b/16-2 in human, mouse, and rat (except rat miR-15a/16-1) clusters were downloaded from the Wellcome Trust Sanger Institute miRBase, release 13.0 (Griffiths-Jones et al. 2006, 2008). The conservation alignment for the miR-15a/16-1 and miR-15b/16-2 clusters was performed using UCSC Genome Bioinformatics (http://genome.ucsc.edu). The chromosomal locations of miR-15a/16-1 and miR-15b/16-2 in mouse, rat, and human were determined using the Ensembl database (http://www.ensembl.org). The sequence alignment was performed using ClustalW (http://www.ebi.ac.uk/Tools/clustalw2/). Precursor sequences were analyzed for secondary structure using MFOLD (http://mfold.bioinfo.rpi.edu).
Detection of miRNA expression using polyA tailing PCR
Total RNA was extracted from the brain, heart, liver, lung, kidney, spleen, small intestine, and testes of 2-month-old Sprague-Dawley rat with Trizol Reagent (Invitrogen). For polyA tailing reverse transcriptase PCR (RT-PCR), 5 μg of total RNA from each tissue was treated with DNase I (Invitrogen) for 15 min at room temperature and then polyadenylated using polyA polymerase (NEB; Ipswich, MA) at 37°C for 1 h. The final reaction mixtures were extracted with phenol/chloroform, precipitated with isopropanol, and redissolved in 25 μl of diethylpyrocarbonate (DEPC)-treated water. Of the polyA tailed RNA, 6 μl were reverse transcribed into first-strand cDNA using Superscript III transcriptase (Invitrogen) with the oligo-dT adapter primer: 5′-GCGAGCACAGAATTAATACGACT CACTATAGGTTTTTTTTTTTTVN-3′. For PCR, 1 μl of RT product was diluted three times and used as a template in each reaction. The reverse primer for each miRNA was the same tailing sequence: 5′-GCGAGCACAGAATTAATACGACTCAC-3′. The forward primer was specific to individual miRNA mature sequences (Supplementary Table 1), and U6 snRNA sequences were amplified as an internal control using the primers 5′-GCTTGCTTCG GCAGCACATATAC-3′ (forward) and 5′-TGCATGTCATCCTTGCTCAGGG-3′ (reverse). The polyA tailing RT-PCR was performed to detect the expression of miR-15a/16-1 and miR-15b/16-2 clusters. The final PCR product was visualized in a 2% agarose gel.
Results
Cloning and identification of rat miR-15a/16-1 cluster
We aligned the miR-15a/16-1 cluster among vertebrates and found that there was a gap in the homologous region of the rat genome (Fig. 1a). To verify that there is a similar miR-15a/16-1 cluster in the rat genome, we performed PCR to amplify the primary miR-15a/16-1 sequence from rat genome DNA. The PCR product was cloned into the pCR2.1 vector and sequenced. We found that a 796-bp sequence that includes the miR-15a/16-1 cluster is missing in the current rat genome database. The primary miR-15a/16-1 sequence was aligned with the mouse genome in the same region (Supplementary Fig. 1). The verified sequence has been submitted to GenBank (accession No. GQ903793).
Fig. 1.
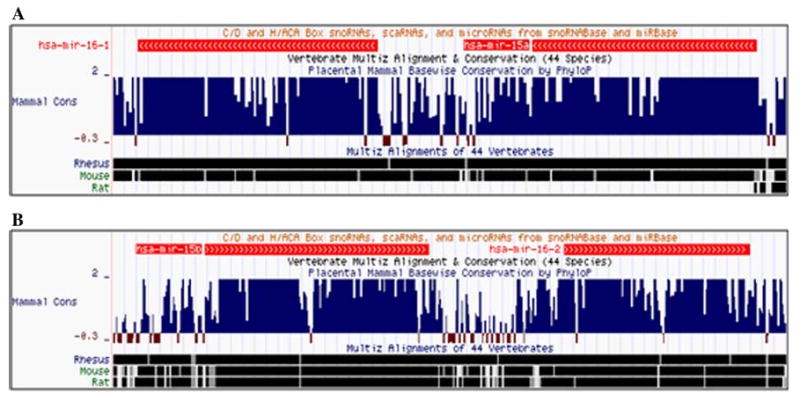
Conservation of miR-15a/16-1 and miR-15b/16-2 clusters. a The miR-15a/16-1 cluster is highly conserved among mammalian species except that it is missing in the rat genome database. b The sequence of the miR-15b/16-2 cluster was aligned among 44 vertebrates and showed conservation
miR-15a/16-1 and miR-15b/16-2 are highly conserved clusters
The miR-15a/16-1 cluster is located in chromosome 13 in humans, 14 in mice, 15 in rats, and 17 in rhesus monkeys, whereas the miR-15b/16-2 cluster is in chromosome 3 in humans and mice and 2 in rats and rhesus monkeys. The miR-15a/16-1 cluster produces miR-15a, miR-15a*, miR-16-1, and miR-16*-1, whereas the miR-15b/16-2 cluster generates miR-15b, miR-15b*, miR-16-2, and miR-16*-2. The individual mature miRNA for both clusters contains 22 nucleotides. These two clusters are highly conserved through the alignment of genomes among 44 vertebrates (Fig. 1a, b). We aligned the mature miR-15a/16-1 and miR-15b/16-2 clusters among humans, mice, rats, and rhesus monkeys. MiR-15a, miR-15b, and miR-16-1/2 have the same sequences among these species except that (1) miR-15a* in the mouse has one mismatch with rats, humans, and rhesus monkeys and (2) miR-16* in the mouse and rat has one mismatch with both humans and rhesus monkeys, although this miRNA has the same sequence between mice and rats and between humans and rhesus monkeys (Fig. 2).
Fig. 2.

Alignment of mature miRNAs spliced from miR-15a/16-1 and miR-16-2 clusters. Mature miRNA sequences from miR-15a, 15a*, 15b, 15b*, 16, and 16* were aligned among humans, mice, and rats. The different nucleotides are indicated in underlined italics
Host genes for miR-15a/16-1 and miR-15b/16-2 clusters
The miR-15a/16-1 cluster is hosted by the tumor suppressor gene DLEU2, which is frequently deleted in CLL, myeloma, and mantle cell lymphoma (Lerner et al. 2009). There are eight transcripts of DLEU2, none of which encode the protein in humans. In mice, this cluster is hosted in the intergenic region of two unidentified genes, AK080165.1 and AK134888.1. These two genes, however, share more than 77% homology in DNA sequence with the human DLEU2 gene. In rats, the gene hosting this cluster has not yet been identified. However, the sequence alignment showed that the 679-bp sequence that we identified from rat genomic DNA has more than 90% homology between rats and mice (Supplementary Fig. 1), which indicates a DLEU2 homologous gene in this region. The miR-15a/16-1 cluster is in the reverse strand and has the same transcriptional orientation as the host gene DLEU2 (Fig. 3a).
Fig. 3.
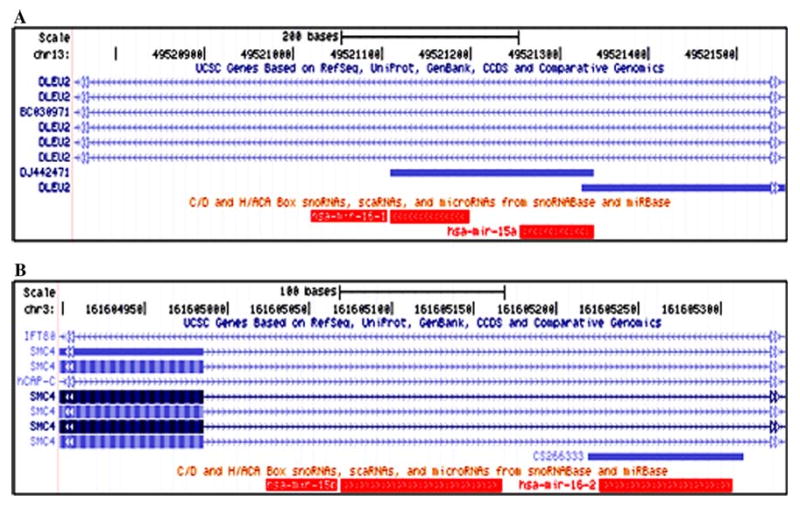
miR-15a/16-1 and miR-15b/16-2 host genes. a The miR-15a/16-1 cluster is in the reverse strand and has the same transcriptional orientation as the host gene DLEU2. There are eight different transcripts of DLEU2, none of which encodes proteins. b The miR-15b/16-2 cluster is in the forward strand and has the same transcriptional orientation as the host gene SMC4. There are four different transcripts of SMC4, all of which encode protein
The miR-15b/16-2 cluster is hosted in the fourth or fifth intron of structural maintenance of the chromosome protein 4 (SMC4) genes. This cluster is in the forward strand and has the same transcriptional orientation as SMC4 (Fig. 3b). There are four transcripts, SMC4-201, 202, 203, and 204, in humans, all of which encode proteins. There is one transcript, SMC4l1, in rats and there are two, SMC4-001 and SMC4-004, in mice. The SMC4 genes have been implicated in maintaining chromosome stability and dynamics. The genomic region of the miR15b/16-2 cluster is in a locus that is implicated in blood pressure, diabetes, and prostate cancer (http://www.ensembl.org).
Expression patterns of miR-15a/16-1 and miR-15b/16-2 clusters
Expressions of the miR-15a/16-1 and miR-15b/16-2 clusters were detected using polyA tailing RT-PCR from adult rats. We found that individual miR-15a, miR-15a*, miR-15b, miR-15b*, miR-16, and miR-16* were all expressed in brain, heart, liver, spleen, lung, kidney, small intestine and testis under physiological conditions. MiR-16 was found to be highly expressed in the heart, brain, small intestine, lung, and kidney, whereas miR-16* was not as highly expressed as miR-16 in the same tissues, although both were spliced from the same pre-miRNA. MiR-16 was transcribed from the first stem of the hairpin loop, whereas miR-16* was transcribed from the second stem after the hairpin loop (Figs. 4 and 5).
Fig. 4.
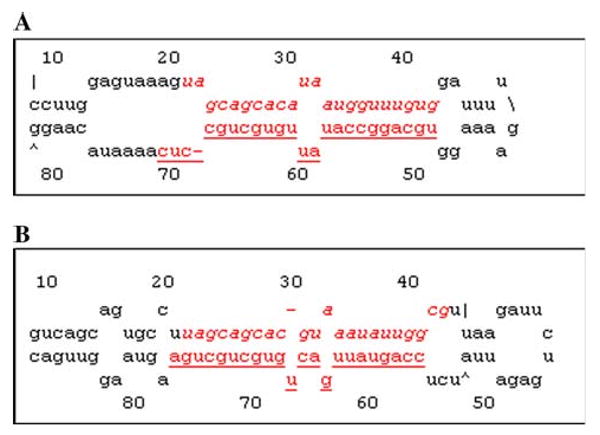
Secondary structure of rat miR-15a/16-1. a The secondary structure of rat pre-miR-15a was predicted using the RNA MFOLD program. The mature miR-15a sequence is displayed in italics; the miR-15* sequence is underlined. b The secondary structure of rat pre-miR-16-1 was predicted using the RNA MFOLD program. MiR-16 is shown in italics; miR-16* is underlined
Fig. 5.
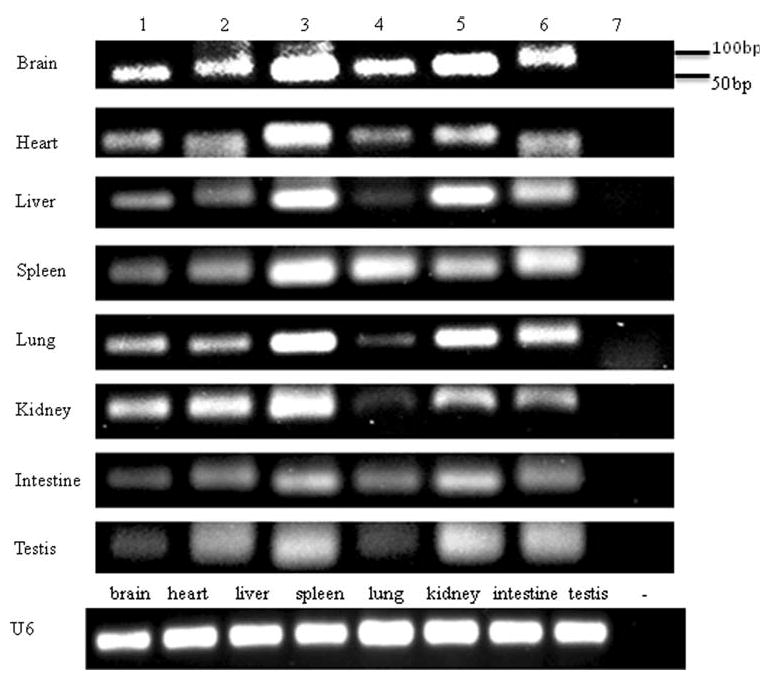
The expression of individual miRNAs spliced from the miR-15a/16-1 and miR-15b/16-2 clusters was detected using polyA tailing RT-PCR from eight different tissues of 2-month-old Sprague-Dawley rats: 1, miR-15a; 2, miR-15a*; 3, miR-15b; 4, miR-15b*; 5, miR-16; 6, miR-16*; 7, RT negative control. U6 noncoding RNA was also amplified as internal controls from different tissues. For RT negative control, the reverse transcription was run simultaneously with other samples without the reverse transcriptase. PCR was performed by detecting only miR-16 from each tissue; no PCR product was visualized from agarose gel. The template for negative control of U6 was selected from heart
Discussion
In this study we cloned and identified the primary miR-15a/16-1 cluster from rat genomic DNA, which is missing in the current rat genome database (http://www.ensembl.org or http://genome.ucsc.edu). Through sequence alignment we showed that both the miR-15a/16-1 and the miR-15b/16-2 cluster are highly conserved (Figs. 1 and 2), which indicates that both clusters might have similar biological functions in different mammalian species. Therefore, the investigation of both clusters using a mouse or rat model may help us understand their roles in a variety of human diseases such as cancer and cardiovascular, developmental, and other diseases.
We found that the individual miRNAs spliced from both clusters were all expressed in the tissues we investigated. However, the expression level of the individual miRNAs was significantly different. For example, miR-16 was most expressed in the brain and heart, whereas expression of miR-16* was much lower than that of miR-16 in both tissues. The function of miR-16* is largely elusive and has not been studied yet. The targets of miR-16* may be different from those of miR-16 because the mature sequence of miR-16 is different than that of miR-16*. Therefore, miR-16* might play a different role in pathophysiological conditions by regulating different gene expressions. In the future, miR-16* functions should also be addressed while we study both clusters. The case was similar for miR-15a and miR-15b; we found that both miR-15a* and miR-15b* were expressed in the tissues we investigated. Moreover, the expression of miR-15b was greater than that of miR-15b* in the lung and small intestine, although we do not know the functional implications for the difference yet.
We compared the miR-15a/16-1 and miR-15b/16-2 clusters in terms of their chromosome location, host genes, and targets. The clusters are located in different chromosomes and have different host genes, although they have high sequence homology, which indicates a similar function in targeting the same genes. For example, both clusters were demonstrated to target the anti-apoptotic gene Bcl-2 in several cancers (Bonci et al. 2008; Cimmino et al. 2005; Xia et al. 2008). The miR-15a/16-1 cluster is hosted in a noncoding DLEU2 gene, which is frequently deleted in CLL cancers (Elnenaei et al. 2003; Lerner et al. 2009; Mertens et al. 2009). DLEU2 does not encode protein but encodes an antisense RNA, which may regulate the LEU5 gene that encodes several transcripts with an open reading frame (Corcoran et al. 2004). The miR-15b/16-2 cluster is located in the host gene SMC4, which basically regulates cell chromosome stability, assembly, and segregation by forming a complex with SMC2 (Fujioka et al. 2002; Hirano 2002; Hirano and Hirano 2002; Kulawiec et al. 2008; Losada and Hirano 2005). However, this cluster is found in a locus that may have implications in blood pressure, diabetes, prostate cancer, and other diseases. Interestingly, the miR-15a/16-1 cluster was found to be down-regulated in prostate cancer (Bonci et al. 2008), although we do not know the function of the miR-15b/16-2 cluster in prostate cancer yet. However, considering the same seed sequence and targets of this cluster, it is highly possible that it plays a similar role to that of miR-15a/16-1 in prostate cancer. The same mature miRNA, miR-16, is produced from miR-16-1 and miR-16-2, which are in two different clusters. MiR-16 has been shown to target cell-cycle-regulating proteins such as cyclins D and E and CDK6, which also indicates that both clusters might coordinate or augment the functions of individual miRNAs spliced from both clusters. The major difference between the two clusters is in miR-15a and miR-15b, which have four nucleotides that differ in mature sequence. However, the functional differences between miR-15a and miR-15b have not been revealed yet and need further investigation.
Supplementary Material
Acknowledgments
This project was supported by award R03HD061420 to J. Yue from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the author and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00335-009-9240-3) contains supplementary material, which is available to authorized users.
References
- Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.69. in press. [DOI] [PubMed] [Google Scholar]
- Bandi N, Zbinden S, Gugger M, Arnold M, Kocher V, et al. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 2009;69:5553–5559. doi: 10.1158/0008-5472.CAN-08-4277. [DOI] [PubMed] [Google Scholar]
- Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell. 2006;24:157–163. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, et al. The miR-15a-miR-16–1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, et al. MiR-15a and miR-16–1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio V, Ferracin M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran MM, Hammarsund M, Zhu C, Lerner M, Kapanadze B, et al. DLEU2 encodes an antisense RNA for the putative bicistronic RFP2/LEU5 gene in humans and mouse. Genes Chromosomes Cancer. 2004;40:285–297. doi: 10.1002/gcc.20046. [DOI] [PubMed] [Google Scholar]
- Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. Viral and cellular messenger RNA targets of viral microRNAs. Nature. 2009;457:421–425. doi: 10.1038/nature07757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnenaei MO, Hamoudi RA, Swansbury J, Gruszka-Westwood AM, Brito-Babapulle V, et al. Delineation of the minimal region of loss at 13q14 in multiple myeloma. Genes Chromosom Cancer. 2003;36:99–106. doi: 10.1002/gcc.10140. [DOI] [PubMed] [Google Scholar]
- Fujioka Y, Kimata Y, Nomaguchi K, Watanabe K, Kohno K. Identification of a novel non-structural maintenance of chromosomes (SMC) component of the SMC5-SMC6 complex involved in DNA repair. J Biol Chem. 2002;277:21585–21591. doi: 10.1074/jbc.M201523200. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J Hepatol. 2009;50:766–778. doi: 10.1016/j.jhep.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 2002;16:399–414. doi: 10.1101/gad.955102. [DOI] [PubMed] [Google Scholar]
- Hirano M, Hirano T. Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J. 2002;21:5733–5744. doi: 10.1093/emboj/cdf575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulawiec M, Safina A, Desouki MM, Still I, Matsui S, et al. Tumorigenic transformation of human breast epithelial cells induced by mitochondrial DNA depletion. Cancer Biol Ther. 2008;7:1732–1743. doi: 10.4161/cbt.7.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S, Chiocca EA. Emerging functions of microRNAs in glioblastoma. J Neurooncol. 2009;92:297–306. doi: 10.1007/s11060-009-9843-2. [DOI] [PubMed] [Google Scholar]
- Legesse-Miller A, Elemento O, Pfau SJ, Forman JJ, Tavazole S, et al. let-7 overexpression leads to an increased fraction of cells in G2/M, direct down-regulation of Cdc34, and stabilization of Wee1 kinase in primary fibroblasts. J Biol Chem. 2009;284:6605–6609. doi: 10.1074/jbc.C900002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M, Harada M, Lovén J, Castro J, Davis Z, et al. DLEU2, frequently deleted in malignancy, functions as a critical host gene of the cell cycle inhibitory microRNAs miR-15a and miR-16–1. Exp Cell Res. 2009;315:2941–2952. doi: 10.1016/j.yexcr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Linsley PS, Schelter J, Burchard J, Kibukawa M, Martin MM, et al. Transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol Cell Biol. 2007;27:2240–2252. doi: 10.1128/MCB.02005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Fu H, Sun F, Zhang H, Tie Y, et al. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008;36:5391–5404. doi: 10.1093/nar/gkn522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 2005;19:1269–1287. doi: 10.1101/gad.1320505. [DOI] [PubMed] [Google Scholar]
- Martello G, Zacchigna L, Inui M, Montagner M, Adorno M, et al. MicroRNA control of Nodal signalling. Nature. 2007;449:183–188. doi: 10.1038/nature06100. [DOI] [PubMed] [Google Scholar]
- Mertens D, Philippen A, Ruppel M, Allegra D, Bhattacharya N, et al. Chronic lymphocytic leukemia and 13q14: miRs and more. Leuk Lymphoma. 2009;50:502–505. doi: 10.1080/10428190902763509. [DOI] [PubMed] [Google Scholar]
- Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, et al. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szász AM, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang M, Tan LP, Dijkstra MK, van Lom K, Robertus JL, et al. miRNA analysis in B-cell chronic lymphocytic leukaemia: proliferation centres characterized by low miR-150 and high BIC/miR-155 expression. J Pathol. 2008;215:13–20. doi: 10.1002/path.2333. [DOI] [PubMed] [Google Scholar]
- Xia L, Zhang D, Du R, Pan Y, Zhao L, et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- Yokota T. MicroRNA and central nervous system. Brain Nerve. 2009;61:167–176. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


