Abstract
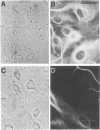
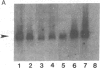
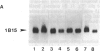
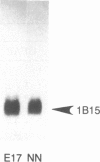
Expression of preproenkephalin mRNA by developing glia and neurons was examined in cultures of embryonic and neonatal rat brain. Cultured glia from specific regions of embryonic day 17 and neonatal day 1 rat brain were identified as astrocytes on the basis of both morphology and expression of immunoreactivity for glial fibrillary acidic protein. The level of preproenkephalin mRNA in cultured neonatal hypothalamic astrocytes was comparable to levels present in cultured embryonic striatal and hypothalamic neurons. Levels of the mRNA were significantly higher in astrocytes derived from neonatal hypothalamus compared to astrocytes derived from other areas of the brain. Thus, there is heterogeneity among astrocytes with respect to preproenkephalin expression. Levels of preproenkephalin mRNA in cultured neonatal striatal astrocytes were only one-third as high as levels in embryonic striatal astrocytes; this observation suggests that glial expression of the gene may be down-regulated during development. Although cultured hypothalamic neurons contained substantial levels of prodynorphin mRNA, levels of this mRNA were not detectable in cultured astrocytes from any brain region or in cultured striatal neurons. Thus, glia do not express all opioid peptide genes during development. These observations suggest that expression of the preproenkephalin gene by astrocytes may play a role in development of the brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bowman C. L., Kimelberg H. K. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984 Oct 18;311(5987):656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A., Crain S. M. Maturation of opioid sensitivity of fetal mouse dorsal root ganglion neuron perikarya in organotypic cultures: regulation by spinal cord. Neuroscience. 1986 Apr;17(4):1181–1198. doi: 10.1016/0306-4522(86)90086-2. [DOI] [PubMed] [Google Scholar]
- Chamak B., Fellous A., Glowinski J., Prochiantz A. MAP2 expression and neuritic outgrowth and branching are coregulated through region-specific neuro-astroglial interactions. J Neurosci. 1987 Oct;7(10):3163–3170. doi: 10.1523/JNEUROSCI.07-10-03163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Douglass J., Goldstein A., Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Brockes J. P., Mirsky R., Wendon L. M. Cell surface markers for distinguishing different types of rat dorsal root ganglion cells in culture. Cell. 1978 May;14(1):43–51. doi: 10.1016/0092-8674(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Effect of catecholamines on the adenosine 3':5'-cyclic monophosphate concentrations of clonal satellite cells of neurons. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2165–2168. doi: 10.1073/pnas.68.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glial-neurone interactions. J Exp Biol. 1981 Dec;95:1–240. doi: 10.1242/jeb.95.1.1. [DOI] [PubMed] [Google Scholar]
- Grumet M., Rutishauser U., Edelman G. M. Neuron-glia adhesion is inhibited by antibodies to neural determinants. Science. 1983 Oct 7;222(4619):60–62. doi: 10.1126/science.6194561. [DOI] [PubMed] [Google Scholar]
- Guenther J., Nick H., Monard D. A glia-derived neurite-promoting factor with protease inhibitory activity. EMBO J. 1985 Aug;4(8):1963–1966. doi: 10.1002/j.1460-2075.1985.tb03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten M. E., Liem R. K. Astroglial cells provide a template for the positioning of developing cerebellar neurons in vitro. J Cell Biol. 1981 Sep;90(3):622–630. doi: 10.1083/jcb.90.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn F. A., Hamberger A. Glial cell function: uptake of transmitter substances. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2686–2690. doi: 10.1073/pnas.68.11.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967 Jun 6;168(1010):1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G., Orkand R. K. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Alpah-adrenergic receptor modulation of beta-adrenergic, adenosine and prostaglandin E1 increased adenosine 3':5'-cyclic monophosphate levels in primary cultures of glia. J Cyclic Nucleotide Res. 1978 Feb;4(1):15–26. [PubMed] [Google Scholar]
- Opler L. A., Makman M. H. Mediation by cyclic AMP of hormone-stimulated glycogenolysis in cultured rat astrocytoma cells. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1140–1145. doi: 10.1016/s0006-291x(72)80093-7. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Repke H., Maderspach K. Muscarinic acetylcholine receptors on culture glia cells. Brain Res. 1982 Jan 28;232(1):206–211. doi: 10.1016/0006-8993(82)90627-8. [DOI] [PubMed] [Google Scholar]
- Ritchie T., Glusman S., Haber B. The filum terminale of the frog spinal cord, a nontransformed glial preparation: II. Uptake of serotonin. Neurochem Res. 1981 Apr;6(4):441–452. doi: 10.1007/BF00963859. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Schachner M. Cell type specificity of a neural cell surface antigen recognized by the monoclonal antibody A2B5. Cell Tissue Res. 1982;224(3):625–636. doi: 10.1007/BF00213757. [DOI] [PubMed] [Google Scholar]
- Semenoff D., Kimelberg H. K. Autoradiography of high affinity uptake of catecholamines by primary astrocyte cultures. Brain Res. 1985 Nov 25;348(1):125–136. doi: 10.1016/0006-8993(85)90368-3. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B. The NG2 antigen, a putative lineage marker: immunofluorescent localization in primary cultures of rat brain. Dev Biol. 1981 Apr 15;83(1):154–165. doi: 10.1016/s0012-1606(81)80018-8. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Bloom F. E., Lerner R. A. Common 82-nucleotide sequence unique to brain RNA. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4942–4946. doi: 10.1073/pnas.79.16.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Shinnick T. M., Bloom F. E. Identifying the protein products of brain-specific genes with antibodies to chemically synthesized peptides. Cell. 1983 Jul;33(3):671–682. doi: 10.1016/0092-8674(83)90010-7. [DOI] [PubMed] [Google Scholar]
- Tardy M., Costa M. F., Fages C., Bardakdjian J., Gonnard P. Uptake and binding of serotonin by primary cultures of mouse astrocytes. Dev Neurosci. 1982;5(1):19–26. doi: 10.1159/000112658. [DOI] [PubMed] [Google Scholar]
- Varon S. S., Somjen G. G. Neuron-glia interactions. Neurosci Res Program Bull. 1979 Feb;17(1):1–239. [PubMed] [Google Scholar]
- Yoshikawa K., Sabol S. L. Expression of the enkephalin precursor gene in C6 rat glioma cells: regulation by beta-adrenergic agonists and glucocorticoids. Brain Res. 1986 Jul;387(1):75–83. doi: 10.1016/0169-328x(86)90022-7. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Rhodes R. E., McLaughlin P. J. Distribution of enkephalin immunoreactivity in germinative cells of developing rat cerebellum. Science. 1985 Mar 1;227(4690):1049–1051. doi: 10.1126/science.3883485. [DOI] [PubMed] [Google Scholar]