Abstract
This study investigated the relationship of skin conductance response (SCR) to a child psychopathy measure. Blunted electrodermal activity is a theoretically important characteristic of psychopathy, but has not been fully explored in preadolescents or females. We tested the hypothesis that reduced SCR magnitude is associated with psychopathic-like traits in boys and girls. Participants were drawn from an ethnically diverse community sample of 9-10 year old twins. Given the fact that members of each twin pair were rated by the same individual (i.e., their caregiver) on the Child Psychopathy Scale, we examined individual differences at the within-family level. Skin conductance data were collected during a passive auditory task consisting of 75-dB tones as well as miscellaneous sounds (e.g., baby cries, bird noises, and speech-like stimuli). Reduced SCR magnitude (hyporeactivity) was only characteristic of boys with higher psychopathy scores. More specifically, electrodermal hyporeactivity was linked to the interpersonal facet of psychopathy, suggesting that it is a biological marker of a manipulative and deceitful orientation in males. No association was found between SCRs and psychopathic traits in girls, indicating the importance of sex specific etiologies of psychopathy in childhood.
Keywords: psychopathy, electrodermal activity, skin conductance, orienting, sex differences
Psychopathy is a constellation of personality and behavioral dispositions that appears to demonstrate validity in childhood (Farrington, 2005; Lynam, 1997). Most instruments assess psychopathy as a combination of several facets, including arrogant/deceitful traits, callous/unemotional traits, and behavioral disinhibition (Hare, 2003; Cooke & Michie, 2001). The psychophysiological literature is equivocal in terms of whether reduced electrodermal responding is associated with certain facets more than others (Fowles, 2000). This is partly due to variation across measuring instruments and samples, as well as the diversity of task stimuli that have been employed (Benning, Patrick, & Iacono, 2005).
Traditionally, lower responding (hyporeactivity) to aversive stimuli is interpreted as reflecting unusual fearlessness or emotional callousness in psychopathic subjects (Fowles, 1993). This perspective views hyporeactivity as an outcome of an underactive Behavioral Inhibition System, rather than due to the sensation-seeking and impulsive traits related to the Behavioral Approach System (Lykken, 1995). However, there may be a second deficit in which impulsive/aggressive individuals are hyporeactive to neutral stimuli (Benning et al., 2005; Fowles, 1993). This could stem from a broader underarousability that predisposes individuals to disinhibitory problems and chronic antisocial behavior (Raine, 2002; Herpertz et al., 2001; Herpertz et al, 2005; but see Zahn & Kruesi, 1993).
Models of electrodermal hyporeactivity have been successfully extended from forensic settings to community samples in which psychopathic characteristics are more benign (Benning et al., 2005; Bare, Hopko & Armento, 2004). That is, normal-functioning individuals with relatively high levels of psychopathic traits show reductions in electrodermal activity (EDA). However, the designs have been limited to signal stimuli (i.e., conditioned or emotionally-charged stimuli), so it remains unclear whether reduced skin conductance reactivity extends to nonsignal orienting stimuli.
Skin Conductance Orienting Responses
In most individuals, skin conductance increases following the presentation of novel or significant stimuli, which is considered a component of the orienting response (Dawson, Schell, & Filion, 2007). However, individuals with prefrontal impairment are hypothesized to suffer attention deficits that interfere with the normal processing of stimuli in their environment, ultimately predisposing them to antisocial behavior (Raine, 2002). Reduced orienting responses have been found in conduct-disordered youth (Herpertz et al., 2001; Delamater & Lahey, 1983), and have been prospectively associated with criminal behavior in a community sample of male adolescents (Raine, Venables, & Williams, 1990).
Although reduced orienting responses are associated with delinquency and aggression, there is less support that this applies to psychopathy per se (see Raine, 1993 for a review). This may be due to the nature of the typical orienting paradigm, in which a series of meaningless tones is presented until habituation (Lykken, 1988). As a result, only the SCR amplitude to the initial (novel) stimulus illuminates group differences. There is some evidence that psychopathy-prone adolescents have lower SCR amplitude to the initial tone (Borkovec, 1970; Siddle, Nicol, & Foggitt, 1973; but see Raine & Venables, 1984, for many failures). Lower reactivity to the first trial of a bell series, although not for a less intense tone series, was found in undersocialized aggressive children (Schmidt, Solant & Bridger, 1985). Despite these positive findings regarding initial hyporeactivity, mean SCR magnitude across trials did not differ between psychopathy-prone juveniles and controls. In the present study, the stimulus paradigm included several trials that were novel and meaningful, such that group differences could emerge beyond the first stimulus.
EDA Correlates in Children
According to a comprehensive meta-analysis by Lorber (2004), children with conduct problems exhibit reduced EDA for tasks involving nonnegative (e.g., orienting) stimuli. For adult psychopaths, a different pattern is found; skin conductance activity is reliably diminished only in tasks involving negative stimuli. Hence, it is unclear which electrodermal profile characterizes psychopathy-prone children, as the construct of child psychopathy is not synonymous with conduct disorder. Additionally, the developmental antecedents of adult psychopathy are poorly understood. In a longitudinal study, Glenn et al. (2007) found that individuals who scored high on self-reported psychopathy at age 28 showed higher orienting responses at age 3. The authors noted that this result was contrary to predictions, but reasoned that increased reactivity may be a protective factor enabling ‘successful psychopaths’ to avoid detection.
Only a few electrodermal studies have focused on psychopathy in children. Fung et al. (2005) found that adolescents scoring high on the Child Psychopathy Scale (Lynam, 1997) showed reduced anticipatory fear and lower electrodermal responsivity to bursts of white noise. However, electrodermal hyporesponsivity was not a product of psychopathy per se, but could be attributed to differences in antisocial behavior between adolescent groups. In other words, individuals who engaged in delinquent behavior were characterized by reduced responsivity, regardless of psychopathy status.
Similarly, boys with psychopathic tendencies were found to exhibit smaller electrodermal responses to distress cues (Blair, 1999). It should be noted that these boys were institutionalized due to severe emotional and behavioral difficulties. Likewise, Fung et al. (2005) exclusively sampled male adolescents at risk for antisocial outcomes. Although these juvenile findings are consistent with the adult psychopathy literature, results need to be replicated using more representative samples. In particular, there is a gap in the literature regarding the correlates of psychopathy in females.
Psychobiological Correlates in Females
The existing literature on the biological correlates of psychopathy/antisocial behavior in females is fragmented and inconsistent. Sutton, Vitale, and Newman (2002) observed a trend in which incarcerated women with high psychopathy scores had lower skin conductance responses to various pictures. There was also evidence that low-anxious psychopaths in this sample lacked the normal augmentation of the startle reflex when viewing unpleasant stimuli, a pattern similar to that observed in male psychopaths (Patrick, 1994). In a community sample, however, Justus and Finn (2007) found that sex moderated the association between psychopathy and affective modulation of the startle blink reflex. Men with higher psychopathy scores failed to show an increase in the startle blink reflex when viewing aversive pictures, whereas there was no effect of psychopathy in women. Additionally, the endocrinal correlates of psychopathy appear to be sex-specific (Loney et al., 2006; O'Leary, Loney, & Eckel, 2007); the expected negative association between psychopathy level and cortisol is confined to males.
It is unclear if there are sex differences in the autonomic profile of children with conduct problems. In a prospective study of Mauritian children, Raine et al. (1997) found that various associations between antisocial behavior and SCR orienting frequency held for girls as well as boys. Nevertheless, more recent literature suggests that the autonomic correlates of conduct problems differ between boys and girls (Beauchaine, Marsh, & Hong, 2008). In the latter study, aggressive females had higher skin conductance arousal (i.e., more nonspecific fluctuations) than female controls, whereas electrodermal arousal failed to differ between male controls and aggressive boys with conduct problems. However, skin conductance orienting responses were not assessed by Beauchaine et al. (2008).
Purpose and Hypotheses
The present study addresses several important issues. First, it explores the electrodermal correlates of psychopathic-like traits in a mixed-gender community sample of preadolescents. It sheds light on whether there are sex differences in the association between SCR orienting and psychopathy. We did not commit to specific hypotheses regarding sex effects, as the available psychophysiological literature on females is sparse and equivocal. Second, this study clarifies whether hyporeactivity is present for nonnegative stimulus conditions. Although hyporeactivity to orienting stimuli is not well established in psychopaths (Raine, 1993), the present study introduces a new orienting paradigm that provided subjects with a wide array of stimuli, ranging from neutral tones to novel and/or meaningful sounds at moderately high intensities. We hypothesized that the association between psychopathy and electrodermal hyporeactivity would be strengthened as a function of stimulus novelty. That is, we expected to find reduced SCR for the more novel and meaningful stimuli.
As a useful personality construct, psychopathy should predict electrodermal hyporeactivity over and above the effects of co-morbid externalizing and internalizing problems. Moreover, the construct of psychopathy is composed of several facets that may be differentially associated with external variables such as autonomic reactivity (Verona et al., 2004) and negative affect (Harpur, Hare, & Hakistan, 1989; Hicks & Patrick, 2006). In the present study, we used subscales from a psychopathy instrument that were previously subjected to an exploratory factor analysis, revealing two oblique factors (Bezdjian, Raine, & Baker, 2009) One factor contained the interpersonal characteristics of psychopathy involving deceit and manipulation of others, while the second factor contained affective as well as impulsive features. Thus, a secondary goal of this study is to investigate the empirical structure of these factors with regard to external variables such as internalizing problems and, more importantly, electrodermal reactivity.
Method
Participants
The subjects were participants in a comprehensive twin study of risk factors for antisocial behavior (see Baker et al., 2006 for a detailed description of the study). The overall sample included 605 sets of twins and triplets (n = 1219 children) age 9-10 years old. The children were recruited from schools located throughout Greater Los Angeles, and the ethnic distribution of the sample is representative of local demographics. Assessment of psychopathy and behavior problems was provided by caregivers, the vast majority of whom (91.4%) were biological mothers (N = 553). Caregivers were administered the questionnaires in either English (N = 492) or Spanish (N = 113), depending on their language proficiency and preference. A total of 791 subjects (comprised from 455 families) with complete data for the orienting task and psychopathy ratings were included, of which 47.9% were male. Of these 791 subjects, 23.8% were White, 39.7% were Hispanic, 13.3% were Black, 4.6% were of East Asian origin, and 18.6% were of mixed/other origin. The mean age at the time of assessment was 9.56 years (SD = 0.58).
Instruments
All questionnaires/interviews were completed at the time that children underwent physiological testing. Caregivers completed a modified version of the Child Psychopathy Scale (CPS; Lynam, 1997) to assess the degree of psychopathy in children. A self-report version of the CPS was also administered, but the present analyses are restricted to the parent ratings. Caregivers also completed the Child Behavior Checklist (CBCL; Achenbach, 1991), which provides information about externalizing and internalizing problems, as well as other behavioral concerns. The externalizing and internalizing scales were transformed to normalized T scores (Mean = 50, S.D. = 10) using the CBCL data conversion program. Although several CPS items were selected from the CBCL, the latter instrument was not designed to tap into the personality features of psychopathy. Nonetheless, there is considerable overlap in item content between the CPS and two subscales of the CBCL: Aggression and Delinquency.
In order to provide an independent measure of antisocial behavior, caregivers were administered the Diagnostic Interview Schedule for Children (DISC-IV; Schaffer et al., 2000) to assess the lifetime number of conduct disorder symptoms. There were a total of 16 possible symptoms. More than half (54.5%) of the boys had at least one symptom, and 33.7% of boys had at least 2 symptoms in their lifetime. By contrast, only 39.2% of girls had one or more conduct disorder symptoms.
Child Psychopathy Scale
The CPS was designed to operationalize in childhood the personality traits of psychopathy, as measured in adults via the Psychopathy Checklist-Revised (PCL-R; Hare, 1991). Several domains from the adult instrument (e.g., promiscuous sexual behavior) were omitted in order to produce a developmentally appropriate measure of psychopathy (Lynam, 1997). Items reflecting blatant antisocial behavior (e.g., criminal versatility) were also excluded in order to produce a more personality-based conceptualization (Lynam et al., 2005). As a result, the CPS only assesses 14 of the 20 PCL-R criteria: Glibness, Grandiosity, Boredom Susceptibility, Untruthfulness, Manipulation, Lack of Guilt, Poverty of Affect, Callousness, Impulsiveness, Parasitic Lifestyle, Behavioral Dyscontrol, Lack of Planning, Unreliability, and Failure to Accept Responsibility,
The original 41-item CPS has undergone several revisions and expansions. We set out using a modified version of the CPS (for details, see Falkenbach, Poythress, & Heide, 2003) which contains good criterion-related validity among youth offenders. The revised CPS is a true-false questionnaire (“no” = 1, “yes” = 2) of 58 items that tap into 14 of the PCL-R constructs. The CPS total score is computed by averaging the mean scores of the 14 subscales. These subscales can be rationally organized into two broad factors (Falkenbach et al., 2003; Lynam et al., 2005) that reflect the traditional factor structure in adults (Hare, 1991).
Upon submitting these subscales to a factor analysis with oblique rotation, however, Bezdjian et al. (2009) found that the two-factor solution was not structured according to Hare's original conceptualization. The first extracted factor contained a composite of affective and impulsive behavioral traits (Callous/Disinhibited; see Table 1). The next factor appeared to tap into a Manipulative/Deceitful orientation: glibness, untruthfulness, manipulation, failure to accept responsibility, and parasitic lifestyle. (Grandiosity and ‘lack of guilt’ failed to load on either factor). Confirmatory factor analyses (see Bezdjian et al., 2009) demonstrated that this structure was superior to the original PCL-R framework and to Cooke and Michie's (2001) three-factor structure. Example items from each subscale are presented in Table 1. The composite factor scores that are used for subsequent analyses are based on the mean of the constituent subscale means.
Table 1.
Examples of items from each subscale within the two CPS factors: Callous/Disinhibited and Manipulative/Deceitful
| Callous/Disinhibited (C/D) Factor | Manipulative/Deceitful (M/D) Factor |
|---|---|
| Unreliability Can people count on him/her? |
Manipulation Does s/he try to take advantage of other people? |
| Poverty of Affect Do his/her feelings sometimes seem fake? |
Failure to accept Responsibility Does s/he try to blame other people for things that s/he has done? |
| Lack of Planning Does s/he plan things ahead? |
Glibness Does s/he tell stories to make him/herself look good? |
| Boredom Susceptibility Does s/he need to have things be exciting? |
Parasitic Lifestyle Does s/he take a lot and not give much in return? |
| Impulsivity Does s/he think before doing or saying something? |
Untruthful Is s/he a good liar? |
| Behavioral Dyscontrol Does s/he get irritated or mad over little things? |
|
| Callousness Does s/he try not to hurt other people's feelings? |
The correlation between the two composite CPS factors was 0.43. The internal consistency of the Callous/Disinhibited (C/D) and Manipulative/Deceitful (M/D) factors were both adequate (α=0.79 and α=0.77, respectively). The internal consistency for the total composite from all 58 items was good (Cronbach's α = 0.83), and the six month test-retest correlation was .87. CPS total scores were positively skewed (skewness = 1.07), ranging from 1.05 to 1.83, with a mean of 1.26 and a standard deviation of 0.12. A rank normalization procedure (Blom, 1958) rendered a normal distribution of CPS scores. For ease of interpretation, only raw values are reported, as the substantive results of our analyses did not differ according to whether we used raw or transformed scores.
Procedure
We collected electrodermal, electrocortical, and cardiovascular measures during a psychophysiological testing session. The psychophysiological protocol lasted approximately two hours and included several tasks. The orienting task occurred at the very beginning of the protocol, during which the child was instructed to sit still and relax. Some electrodermal data were unusable due to technical problems (equipment malfunction, earphones falling out, excessive movement) or refusal to participate in the psychophysiological component of the study. Adjustments were also made to the task following preliminary analyses of the first 100 pairs who participated in the study, and thus these subjects were not included in the present analyses.
Orienting Task Paradigm
Following three minutes of rest, we recorded the subjects' SCRs to a series of 12 sounds delivered through earphones. There were three consecutive trials within each of four stimulus types. The four types of stimuli were presented in the following order: 1) pure 1000-Hz tones, 2) consonant-vowel (“da”) sounds, 3) novel sounds (cuckoo clock, bird chirping, rooster crowing), and 4) baby cries. The interstimulus intervals ranged from 18 to 28 seconds, with a mean of 22.5 seconds. The stimuli consisted of complex waveforms that never exceeded a sound pressure level of 105 decibels (dB). The ranges of intensity for each block of stimuli are as follows: tones (75 dB), consonant-vowels (65 – 95 dB), novel sounds (70 – 105 dB), and baby cries (90 – 95 dB). The duration of the baby cries was exceptionally long (8 seconds) in order to be more ecologically valid, whereas the durations of the other stimuli never exceeded a second.
Psychophysiological Recording
Subjects were seated in a room adjacent to the experimenter. The average temperature of the laboratory was 74.05 °F (SD = 1.98). Skin conductance activity was recorded from bipolar leads on the distal phalanges of the index and middle fingers using silver-silver chloride electrodes, which were placed on the non-dominant hand. The conducting medium was K-Y lubricating jelly, surrounded by an adhesive electrode collar (measuring 1 cm in diameter) that maintained full contact with the skin. The electrodes were further fastened in place using waterproof tape.
Skin conductance level (SCL) was recorded through a bioamplifier manufactured by the James Long Company (Caroga Lake, NY), with a low-pass filter set to 10 Hz. The signal was digitized at a sampling rate of 512 Hz with 12 bits of resolution (corresponding to a step size of < .01 microsiemens). Responses elicited by the stimuli were scored through the James Long Company software (SCOR component of the Orientation Response Analysis System). A rise in SCL was judged a response if it occurred within a window of 1.5 – 4.5 seconds after stimulus onset. Additionally, the slope in SCL was required to exceed the baseline slope (at stimulus onset) by a minimum of 0.05 μS/second. SCR amplitude was defined as the peak change in skin conductance occurring within seven seconds of response initiation. If no response was detected, then the magnitude for that particular trial assumed a value of zero rather than being omitted. We decided against confining our analyses to nonzero values (i.e., amplitudes) in order to exploit the full set of observations.
Statistical Analyses
Linear mixed-effects regression models were employed to account for the paired nature of the twin data. Since Type-1 error can be inflated in correlational research when observations are not independent (i.e., nested within twin pairs), it was necessary to treat individual scores as repeated measures in the pair. The mean of each pair was then treated as a random intercept. This was achieved in accordance with Singer's (1998) application of the SAS PROC MIXED statistical program. The degrees of freedom, which are determined by the Satterthwaite approximation, can be fractional, and will lie somewhere between the number of twin pairs and the total number of participants in the study (Campbell & Kashy, 2002). For all analyses, the degrees of freedom were rounded to the nearest integer.
Rather than regarding the clustered nature of twin data as a statistical nuisance, it can be exploited to deal with a much greater nuisance in correlational research: confounding variables. The use of twin data allows one to directly control for between-family variables such as race, acculturation, and socioeconomic status. As a result, we used a multilevel regression technique (Carlin et al., 2005) to test whether the association between psychopathy score and SCR magnitude is due to within-pair (level-I) and/or between-pair (level-II) sources of variation. If the within-pair effect is significant, then the sibling with the higher psychopathy rating should exhibit a relatively smaller SCR magnitude.
For the sake of brevity, we generally omitted reporting the multi-level ‘breakdown’ of results from the regression model. Unless stated otherwise, we pooled the within-pair (level-I) and between-pair (level-II) effects, while continuing to treat each twin's observation as a repeated measure. This was considered appropriate because the pooled effect was typically in the same direction as the within-pair effect.
For all analyses, we assumed that the variance-covariance structure was equivalent across members of each pair (Twin 1 and Twin 2). That is, we assumed a compound symmetry in the variance components. When examining ‘within-subjects’ effects on orienting responses, we performed a repeated measures regression analysis in which trial # and stimulus type (i.e., dummy codes for tones, baby cries, etc.) were the independent variables. The dependent variables were the twelve measures of skin conductance magnitude obtained from each set of three trials within the four stimulus types. Due to the paired structure of twin data, there were effectively 24 repeated measures in this type of analysis.
Results
Descriptive Statistics
Only 27 subjects (3.4%) responded electrodermally to every trial during the orienting task, while 8.0% (n = 63) of the sample failed to produce a single SCR to any of the 12 stimuli. The percentage of non-responders for each of the four blocks of stimuli are as follows: 24.9% (tones), 14.0% (consonant-vowels), 16.7% (novel sounds), and 26.2% (baby cries). As is typical of electrodermal data, SCR magnitudes were positively skewed (Dawson et al., 2007). A square root transformation rendered a more normal distribution of magnitudes. We opted to report only the raw magnitudes, however, as the use of transformed values led to results no different than those obtained from raw data analyses.
In order to reduce the SCR data into a manageable number of variables for preliminary analyses (prior to the repeated measures analysis), we ran a principal components analysis on the 12 magnitudes. Two components with eigenvalues above one were extracted. The first component explained 42% of the variance in SCR magnitude, whereas the second component explained a mere 9% of the variance. Subjects' regression scores on the first dimension correlated .91 with the arithmetic mean of the twelve SCR magnitudes, indicating that ‘mean SCR’ is an acceptable representation of electrodermal reactivity.
There were 55 individuals who underwent psychophysiological testing on two occasions, separated by an interval of approximately six months. Information from the 2nd test was used to calculate the stability of electrodermal activity over time, but was omitted from all other analyses. The six-month test-retest stability of mean SCR magnitude was r = .80. We further examined the retest stability of responses to each stimulus type (averaging across the three trials). The test-retest correlations for tones, consonant-vowels, novel sounds, and baby cries were .62, .77, .72, and .49, respectively. Thus, with the possible exception of baby cries, all stimulus types elicited SCRs on a reliable basis.
Preliminary Analyses
A paired-samples t-test on opposite-sex twins indicated that boys received higher total scores on the CPS than did their female siblings: 1.28 (0.12) vs. 1.24 (0.12) [t(146) = 3.36, p < .01]. We examined the effects of sex (1 = male, 0 = female), CPS total score, and their interaction in a regression equation predicting mean SCR magnitude. Interestingly, males were more reactive to the stimuli [b = 0.06, t(749) = 2.08, p = .04]. The main effect of CPS score was not significant (t = 0.20), but there was a sex-by-psychopathy interaction [b = −0.53, t(790) = −2.28, p = .02]. Upon stratifying the sample by sex, we found that the effect of psychopathy was restricted to males [b = −0.50, t(386) = −3.02, p < .01]; there was no effect in females (b = .00).
In order to understand the basis of the link between psychopathy and electrodermal reactivity, we employed multilevel models that partition the effect into within-family and between-family sources. The details of this two-level regression model can be found in Carlin et al. (2005). We examined the significance of two coefficients: 1) a within-pair coefficient (BW) representing the effect of each individual's deviation from the mean CPS score of his/her twin pair, and 2) a between-pair coefficient (BB) representing the effect of the twin pair's mean score on the individual's SCR magnitude. If the former coefficient (BW) is significant, then it indicates that the relative difference in siblings' psychopathy scores (due to individual-specific factors) can account for the hyporeactivity phenomenon.
In females, neither BW nor BB was significant (p > 0.53). In males, the within-pair coefficient (BW = −0.66) was significant [t(211) = −2.86, p < .01]. However, the between-pair coefficient (BB = −0.37) – which is orthogonal to BW – did not reach statistical significance [t(239) = −1.57, p = .12]. These results demonstrate that boys with relatively high psychopathy scores have lower SCR magnitudes. Family-wide influences are not responsible for this association.
Repeated Measures Regression
Hyporeactivity could be due to the fact that boys with higher CPS scores habituated more rapidly to the stimuli, rather than necessarily having lower initial reactivity. Therefore, we examined whether hyporeactivity in males was specific to certain stimuli and/or trials, as well as whether the habituation trends differed as a function of psychopathy score. We ran a series of mixed-model regressions in which the dependent variable contained all twelve SCR measures. These magnitudes were organized into two within-subjects factors: stimulus block and trial (4 × 3). Three consecutive trials were presented for each stimulus type in the following order: tones, consonant-vowel (“da”) sounds, novel sounds (cuckoo clock, bird chirp, rooster crow), and baby cries. Given that we already found a sex-specific association, we ignored the main effect of psychopathy score, and instead modeled separate interaction terms for males and females.
We tested three within-subjects hypotheses: 1) SCR magnitude should decline across trials due to habituation, 2) the stimulus block with novel stimuli (Block 3) should show the least evidence of habituation, and 3) Block 3 should elicit the largest responses. In support of the first hypothesis, SCR magnitude generally declined across the three trials (averaging across blocks); there was a reduction of −0.14 microsiemens across adjacent trials, t(9091) = −22.28. In fact, response magnitude declined within all four blocks of stimuli, but, as hypothesized, the rate of decline was greater for tones (p < .01), speechlike stimuli (p < .01), and baby cries (p < .01) than for the stimuli in Block 3. However, there was little evidence that the rate of habituation differed as a function of psychopathy score. One exception to this was that, in females, psychopathy score interacted with trial number in predicting responses to consonant-vowel sounds (Block 2). A unit increase in CPS score was associated with a smaller decrease (less habituation) in SCR magnitude across adjacent trials of consonant-vowel stimuli in girls; t(9078) = 2.21, p = .03.
As for the third hypothesis, we expected that Block 3 would elicit the largest responses, as all three stimuli were novel and rather intense. This hypothesis was only partially supported, as can be seen in Table 2. The mean SCR magnitude was smaller for tones and baby cries, but larger for consonant-vowel stimuli, relative to Block 3. In males, the association between psychopathy score and SCR was consistently negative, generalizing across all stimulus types. A follow-up test revealed that the negative association in males was significantly stronger in Block 3 than in Block 4 (p < .01). However, hyporeactivity in Block 3 was no greater than in relation to Block 1 (p = .06 ) or Block 2 (p = .10), indicating that the hyporeactivity phenomenon is not specifically tied to novel and/or intense stimuli. When all 12 SCRs were examined separately, hyporeactivity was found in all but two instances - the 2nd and 3rd trials of baby cries. In girls, psychopathy score did not relate to SCR magnitude in any instance.
Table 2.
Repeated measures regression analysis predicting skin conductance response (SCR) magnitude across four different types (blocks) of stimuli.
| Predictors | SCR Magnitude b (se) |
|---|---|
| Male | .07 (.02) ** |
| Block 1a | −.13 (.01) ** |
| Block 2a | .13 (.01) ** |
| Block 4a | −.18 (.01) ** |
| Male × CPS × Block 1 | −.56 (.14) ** |
| Male × CPS × Block 2 | −.70 (.14) ** |
| Male × CPS × Block 3 | −.98 (.14) ** |
| Male × CPS × Block 4 | −.37 (.14) * |
| Female × CPS × Block 1 | −.10 (.16) |
| Female × CPS × Block 2 | .32 (.16) |
| Female × CPS × Block 3 | .04 (.13) |
| Female × CPS × Block 4 | −.04 (.13) |
Parameter estimate (b) is the difference from the mean SCR magnitude in Block 3 Notes: Block 1 = tones; Block 2 = consonant-vowels; Block 3 = novel sounds; Block 4 = baby cries; SCR magnitude is measured in microsiemens (μS)
CPS = Child Psychopathy Scale total score
p < .05;
p < .01
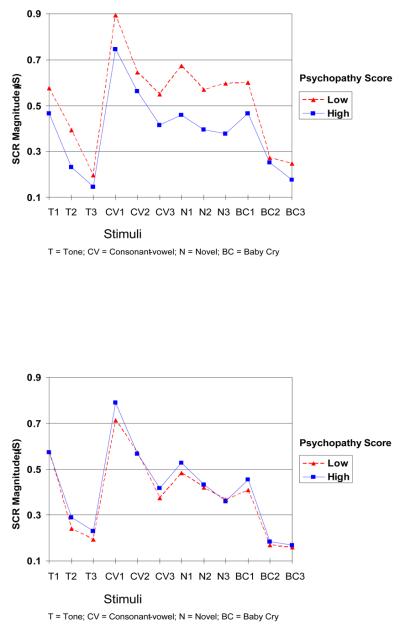
For illustrative purposes, we have graphed the nature of our results in Figure 1, for boys and girls, respectively. Despite this categorical representation of psychopathic traits, in which members of each sex are grouped into high and low-scoring groups on the basis of a median split on the CPS, the picture is consistent with the pattern of results obtained from the mixed model regression analyses (in which psychopathic traits are treated as a continuum). That is, habituation within each stimulus type did not generally differ as a function of psychopathy level. Rather, boys with lower psychopathy scores had uniformly higher reactivity to the stimuli. There was no effect of psychopathy on SCR in girls, as indicated by the superimposed nature of the two lines for high and low psychopathy scores.
Figure 1.
Skin conductance response (SCR) magnitudes to four types of stimuli in males (top panel) and females (lower panel). Each stimulus type was presented across three trials. High and low psychopathy groups were formed by a median split on the total CPS score.
Two-Factor Structure
Thus far, we have shown that the total psychopathy score is related to electrodermal hyporeactivity in boys. However, previously reported confirmatory factor analyses of the CPS subscales indicated that a two-factor solution was superior to a one-factor solution (see Bezdjian et al., 2009). The idea that there are multiple pathways to antisocial behavior is deeply embedded in the psychopathy literature, dating from the first descriptions of primary vs. secondary psychopathy (Lykken, 1995; Karpman, 1941). There is widespread evidence that the external correlates of the interpersonal facet (i.e., Factor 1) and the impulsiveness facet (i.e., Factor 2) are distinct. For example, the former is negatively associated with internalizing problems, whereas the latter is positively related to Neuroticism (Blonigen et al., 2005, Harpur et al., 1989; Hicks & Patrick, 2006). It is thus conceivable that the hyporeactivity-psychopathy relationship may differ for different factors of psychopathy.
In the present sample, a Manipulative/Deceitful (M/D) factor and a Callous/Disinhibited (C/D) factor had been extracted by Bezdjian et al. (2009) using oblique rotation. The mean sex difference in the M/D factor was minimal, as demonstrated by a paired samples t-test on the scores of opposite-sex twins: 1.30 (0.19) in males vs. 1.28 (0.20) in females [t(146) = 1.30, p > .05]. However, males scored significantly higher than their female siblings on the C/D factor: 1.26 (0.18) vs. 1.18 (0.15) [t(146) = 4.97, p < .01].
One of our goals was to examine whether electrodermal hyporeactivity was specific to psychopathic traits, or whether it could be explained by externalizing and internalizing problems. However, it was first necessary to determine which of the two factors was related to electrodermal hyporeactivity. Since the two factors were moderately correlated (r = .43), we regressed mean SCR magnitude on both M/D and C/D, as well as their interactions with sex (1 = male, 0 = female). Only the interaction term of M/D and sex was significant [t(768) = −2.81, p = .01]. Upon stratifying the sample by sex, there was a significant negative association between M/D and mean SCR magnitude in males (b = −0.40, p < .01), but no relationship in females (see Table 3).
Table 3.
Unstandardized partial regression coefficients (b) and their standard errors (se) for each psychopathy factor while predicting orienting responses (mean SCR magnitude), number of conduct disorder (CD) symptoms, and internalizing behavior problems (T-scores on the CBCL).
| Criterion | Boys | Girls | ||
|---|---|---|---|---|
| Manipulative/ Deceitful b (se) |
Callous/ Disinhibited b (se) |
Manipulative/ Deceitful b (se) |
Callous/ Disinhibited b (se) |
|
| SCR Magnitude (μS) | −0.40 (0.13)** | 0.01 (0.13) | 0.03 (0.11) | −0.02 (0.14) |
| CD symptoms | 4.05 (0.42)** | 2.33 (0.44)** | 2.78 (0.33)** | 2.24 (0.39)** |
| CBCL Internalizing | −4.72 (2.33)* | 18.64 (2.42)** | −0.32 (2.23) | 17.75 (2.65)** |
| -Withdrawn | −4.81 (1.42)** | 10.15 (1.47)** | −0.17 (1.33) | 9.78 (1.59)** |
| -Anxious/Depressed | 0.37 (1.43) | 7.34 (1.48)** | 0.78 (1.30) | 11.46 (1.54)** |
| -Somatic Complaints | −0.36 (1.59) | 7.34 (1.65)** | −0.83 (1.54) | 9.36 (1.83)** |
p < .05;
p < .01
Given that there was an effect on SCR for the M/D but not the C/D factor, we explored how these two psychopathy factors related to additional criterion variables such as conduct disorder symptoms and internalizing problems. In support of the convergent validity of primary and secondary psychopathy, both factors were positively related to the DISC-IV symptom count for conduct disorder when controlling one for the other (see Table 3). A divergent pattern, however, emerged with respect to the Internalizing scale of the Child Behavior Checklist (CBCL). The C/D factor was positively and significantly (p < 0.01) related to the overall Internalizing problems scale and all three subscales (Withdrawn, Anxious-depressed, and Somatic complaints), both in boys and girls. The M/D factor, for the most part, was unrelated to internalizing problems, although there was a negative association in boys with respect to the overall Internalizing scale (p = .04) and the Withdrawn subscale (p < .01). This latter finding is not surprising, as there is direct overlap between the M/D factor and Withdrawn on at least one item (“Is he/she shy?”). On the other hand, there is no overlapping content between C/D and any of the internalizing subscales.
Next, we tested whether internalizing behaviors could account for the link between the M/D factor and reduced SCR in boys. M/D and the three internalizing subscales – Withdrawn, Anxious-depressed, and Somatic – were entered into a regression model predicting SCR magnitude. There was no effect for Anxious-depressed (p = .97) or Somatic complaints (p = .76), but males with higher scores on the Withdrawn subscale were more reactive to the stimuli [t(379) = 2.27, p = .02]. Even while controlling for these internalizing problems, the effect for M/D remained significant [b = −0.39, t(379) = −3.25, p < .01].
Finally, we examined whether antisocial behavior was responsible for the link between M/D and reduced SCR. This was achieved by entering M/D and the DISC-IV symptom count for conduct disorder into a regression model predicting SCR magnitude. M/D was still related to reductions in SCR [b = −0.35, t(381) = −2.69, p = .01], while the effect of conduct problems on SCR was not significant (p = .59). In addition to conduct disorder symptoms, the Externalizing scale of the CBCL contains information about aggressive and delinquent behaviors. The M/D factor continued to predict mean SCR magnitude after controlling for externalizing problems (b = −0.39, t(381) = −2.90, p < .01).
Discussion
We found that boys with higher scores on the Child Psychopathy Scale showed smaller skin conductance responses (SCRs) to unsignaled and nonaversive auditory stimuli. To our knowledge, this is the first study to show that psychopathic traits are associated with lower reactivity (SCR hyporeactivity) to orienting stimuli in a community sample of children. Reduced electrodermal orienting has been reported in children with conduct disorder (Herpertz et al., 2001; Delamater & Lahey, 1983), but not specifically in relation to psychopathic personality traits. Interestingly, the association was mediated by the interpersonal (manipulative/deceitful) factor of psychopathy; neither callous/disinhibited traits nor conduct problems were linked to hyporeactivity in boys. Furthermore, psychopathy did not predict hyporeactivity in girls.
Reduced electrodermal reactivity in higher-scoring boys was found across a wide range of stimuli, including tones, speechlike stimuli, and miscellaneous bird sounds. With the exception of baby cries, there was no evidence that hyporeactivity was confined to ‘novel’ sounds or only to the first trial of a stimulus. This finding is inconsistent with some of the literature on psychopathy-proneness and reactivity to nonsignal stimuli. Most orienting tasks have utilized a habituation series, in which a neutral tone is presented repeatedly until the electrodermal system ceases to respond. Several investigators have found that psychopathy-prone juveniles can be characterized by lower SCR amplitude only to the first stimulus (Borkovec, 1970; Schmidt et al., 1985). In the present study, by contrast, the hyporeactivity effect persisted across multiple presentations of tones.
Reduced electrodermal activity to nonsignal stimuli is thought to reflect deficits in attentional processes (Raine & Venables, 1984; Fowles, 2000), which may in turn be related to inferior functioning of the prefrontal cortex. Antisocial individuals with schizotypal tendencies (who demonstrably suffer cognitive and affective impairments) are exceptionally hyporesponsive to orienting stimuli (Raine, 1993). In the present study, the hypothesis of prefrontal dysfunction was not supported. If prefrontal impairment is responsible for hyporeactivity, then we would have expected to obtain significant relations with callous/disinhibited traits (impulsiveness, behavioral dyscontrol, lack of planning, etc.). This was not the case, as only the Manipulative/Deceitful factor was associated with reduced SCR magnitude. It should be noted, however, that prefrontal regions develop over the course of adolescence, leading to the possibility that prefrontal-related orienting deficits have yet to manifest in our young sample.
The callous/disinhibited features of psychopathy, unlike the interpersonal facets, were associated with greater internalizing problems. This supports the view that a disabling ‘secondary’ syndrome, characterized by poor impulse control and higher anxiety, can coexist with the more socially dominant, ‘primary’ aspects of psychopathy (Lykken, 1995). An important caveat is that ‘secondary’ psychopathy is not traditionally thought to include callous-unemotional traits, whereas factor analyses in the present sample indicated that ‘callousness’ and ‘poverty of affect’ share much in common with impulsive/disinhibited traits. Conversely, ‘failure to accept responsibility’ loaded on the Manipulative/Deceitful factor, which might reflect an element of verbal persuasiveness that facilitates the shifting of blame. Furthermore, the ‘grandiosity’ and ‘lack of guilt’ subscales failed to load on either of the two main factors. These unexpected patterns may be due to psychometric limitations in the Child Psychopathy Scale, or may be a product of the specific developmental (i.e., preadolescent) status of our subjects.
Recent empirical investigations have argued for the separation between callous-unemotional and deceitful interpersonal traits (Hare, 2003; Cooke & Michie, 2001). This has important implications in the framing and interpretation of psychophysiological results. Although the particular stimulus contexts and psychopathy facets that underlie electrodermal hyporeactivity are not straightforward (Benning et al., 2005; Patrick, Cuthbert, & Lang, 1994), studies that have employed aversive stimuli have typically interpreted hyporeactivity as evidence of an emotional disturbance (Blair, 1999). However, it may be necessary to separate the interpersonal (i.e., deceitful and charming) features from the affective (i.e., emotionally detached) features when making inferences about the underlying personality processes. For example, Verona et al. (2004) found that prison inmates who scored high on Factor 1 of the PCL-R (Hare, 1991) had deficient electrodermal responses to emotionally-charged acoustic stimuli. Upon further examination, it appeared that the interpersonal facet, rather than emotional detachment, was related to the criminals' failure in differentiating affective from neutral sounds. This result, coupled with the present findings, suggest that glibness and manipulation should be more carefully examined in relation to electrodermal deficits. It also fuels the speculation that the verbal components of psychopathy (i.e., glibness and manipulation) are related to atypical processing of verbal/acoustic stimuli (Raine & Venables, 1988).
Physiological investigations of psychopathy have relied primarily on male or institutionalized samples. The present study is unique in that it examined a mixed-gender sample of preadolescent twins. We found an interaction of sex and psychopathy level, such that only boys with higher psychopathy scores were electrodermally hyporeactive. This challenges an intuitive explanation regarding the nature of gender differences. According to some theories (e.g. Mednick et al., 1977), biological correlates of antisocial personality should be stronger in females because the ‘social push’ is less prominent. That is, a greater dosage of biological risk factors is necessary to steer a female in a psychopathic direction.
Although few studies have investigated the electrodermal correlates of psychopathic traits in females, a sex-specific biological association is not unique in the psychopathy literature. At the endocrinal level, for example, there is evidence that cortisol level is lower in adolescent males who have a high degree of psychopathic traits (Loney et al., 2006); this association is absent in high-scoring females. Furthermore, the cortisol stress response appears to be uniquely elevated in males with low psychopathy scores (O'Leary, Loney, & Eckel, 2007). Females, regardless of their psychopathy score, show a pattern of cortisol reactivity that is similar to the high-scoring males.
This result echoes the present finding that the electrodermal responses of higher-scoring males were not deviantly low, but were simply diminished relative to their lower-scoring male counterparts. In other words, there was indication that boys with lower psychopathy scores were actually responding to the auditory stimuli in a hyperreactive manner - much higher than that observed in girls. This implies that electrodermal hyperreactivity may be a sex-specific protective factor that shields males from the development of a manipulative-deceitful orientation.
A major strength of this study is methodological, in that twin data provides an optimal method of handling potentially noisy or, at worst, confounding variables. Multilevel models indicated that the hyporeactivity phenomenon was largely due to effects occurring within twin pairs, rather than mediated by between-family variables. The latter is plagued by much noise, given that the SCR signal is affected by anatomical properties of the skin as well as atmospheric conditions (Boucsein, 1992; Venables & Mitchell, 1996). Measurement error is reduced at the within-pair level because gross anatomical features (e.g., skin pigmentation) and environmental factors (e.g., temperature/season) are necessarily held constant across twins
The absence of a between-pair source of the hyporeactivity phenomenon may also be due to measurement error in the assessment of psychopathic traits. Different caregivers may have disparate standards, as well as differential susceptibility to social desirability biases, when rating their children. This underscores the fact that a subject's absolute score is less reliable than his relative score (vis-à-vis his sibling). The within-pair deviation in CPS scores effectively boils down to an observational measure (i.e., observations based on a standard rating system), perhaps explaining why hyporeactivity was found only at the within-family level.
Limitations and future directions
It should be noted that the orienting paradigm employed in this study is quite different from that used by previous investigators. In addition to the use of highly novel stimuli, several sounds approached 105 decibels. This is an unusually high intensity for stimuli designed to elicit orienting responses. The implication, therefore, is that reduced SCR magnitude may represent poor defensive reactions as opposed to deficient orienting responses. In adult psychopaths, for example, hyporeactivity was found for 120-dB tones but not for tones at lower intensities (Hare, 1978). Similarly, in children with conduct disorder, hyporeactivity to an initial 90-dB bell sound was found, but not for a 75-dB tone (Schmidt et al., 1985). Nevertheless, it should be noted that the effects found here with the more intense novel sounds presented in Block 3 were also found with less intense tones.
Our findings await replication using an expanded range of stimulus conditions and valences. It will be interesting to see if electrodermal hyporeactivity is attendant to blatantly aversive stimuli in addition to orienting stimuli. In the meantime, this study suggests that the interpersonal facet may be a focal point for uncovering the biological bases of psychopathy in males. It contributes to the emerging perspective (O'Leary et al., 2007; Justus & Finn, 2007) that the biological correlates of psychopathy may be sex-specific.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health to the first author (MH58354), the second author (MH01114-08), and to the fifth author (MH068953). We wish to thank the numerous public and private school personnel for their assistance in recruiting twins, as well as the many research staff members involved in the data collection. We would also like to acknowledge Biing-Jiun Shen for providing valuable feedback on a previous version of this manuscript. Most of all, we deeply appreciate the enormous contributions of the twins and their families who have participated in this project.
References
- Achenbach TM. Manual for the Child Behavior Checklist. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Baker LA, Barton M, Lozano DI, Raine A, Fowler JH. The Southern California twin register at the University of Southern California: II. Twin Research and Human Genetics. 2006;9:933–40. doi: 10.1375/183242706779462912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bare RA, Hopko DR, Armento MEA. The relation of psychopathic characteristics and anxiety in noncriminals: physiological and cognitive responses to guided imagery. Journal of Psychopathology and Behavioral Assessment. 2004;26:225–32. [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning S, Patrick C, Iacono W. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42:753–62. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the psychopathic personality inventory: validity and implications for clinical assessment. Psychological Assessment. 2003;15:340–350. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Bezdjian S, Raine A, Baker LA. Psychopathic personality in children: genetic and environmental contributions. 2009 doi: 10.1017/S0033291710000966. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Responsiveness to distress cues in the child with psychopathic tendencies. Personality and Individual Differences. 1999;27:135–45. [Google Scholar]
- Blom G. Statistical estimates and transformed beta-variables. John Wiley and Sons; New York: 1958. [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Psychopathic personality traits: heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine. 2005;35:637–48. doi: 10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD. Autonomic reactivity to sensory stimulation in psychopathic, neurotic, and normal juvenile delinquents. Journal of Consulting and Clinical Psychology. 1970;35:217–22. doi: 10.1037/h0030119. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal activity. Plenum Press; New York: 1992. [Google Scholar]
- Campbell L, Kashy D. Estimating actor, partner, and interaction effects for dyadic data using PROC MIXED and HLM: a user-friendly guide. Personal Relationships. 2002;9:327–42. [Google Scholar]
- Carlin JB, Gurrin LC, Sterne JAC, Morley R, Dwyer T. Regression models for twin studies: a critical review. International Journal of Epidemiology. 2005;34:1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- Cleckley H. The mask of sanity. Mosby; St. Louis, MO: 19411976. [Google Scholar]
- Cooke DJ, Michie C. Refining the construct of psychopathy: towards a hierarchical model. Psychological Assessment. 2001;13:171–88. [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3rd edition Cambridge University Press; New York: 2007. pp. 159–181. [Google Scholar]
- Delamater AM, Lahey BB. Physiological correlates of conduct problems and anxiety in hyperactive and learning-disabled children. Journal of Abnormal Child Psychology. 1983;11:85–100. doi: 10.1007/BF00912180. [DOI] [PubMed] [Google Scholar]
- Falkenbach DM, Poythress NG, Heide KM. Psychopathic features in a juvenile diversion population: reliability and predictive validity of two self-report measures. Behavioral Sciences and the Law. 2003;21:787–805. doi: 10.1002/bsl.562. [DOI] [PubMed] [Google Scholar]
- Farrington DP. The importance of child and adolescent psychopathy. Journal of Abnormal Child Psychology. 2005;33:489–497. doi: 10.1007/s10802-005-5729-8. [DOI] [PubMed] [Google Scholar]
- Fowles D. Electrodermal activity and antisocial behavior: empirical findings and theoretical issues. In: Roy J-C, Boucsein W, Fowles D, Gruzelier J, editors. Progress in Electrodermal Research. Plenum; London: 1993. pp. 223–38. [Google Scholar]
- Fowles D. Electrodermal hyporeactivity and antisocial behavior: does anxiety mediate the relationship. Journal of Affective Disorders. 2000;61:177–89. doi: 10.1016/s0165-0327(00)00336-0. [DOI] [PubMed] [Google Scholar]
- Fowles D, Furuseth A. Electrodermal hyporeactivity and antisocial behavior. In: Routh D, editor. Disruptive behavior disorders in childhood. Plenum Press; New York: 1994. pp. 181–205. [Google Scholar]
- Fung M, Raine A, Lynam D, Venables P, Loeber R, et al. Reduced electrodermal activity in psychopathy-prone adolescents. Journal of Abnormal Psychology. 2005;114:187–96. doi: 10.1037/0021-843X.114.2.187. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Mednick SA, Venables P. Early temperamental and psychophysiological precursors of adult psychopathic personality. Journal of Abnormal Psychology. 2007;116:508–18. doi: 10.1037/0021-843X.116.3.508. [DOI] [PubMed] [Google Scholar]
- Hare R. Psychopathy and electrodermal responses to nonsignal stimulation. Biological Psychology. 1978;6:237–246. doi: 10.1016/0301-0511(78)90026-1. [DOI] [PubMed] [Google Scholar]
- Hare R. The Hare Psychopathy Checklist - Revised. Multi-Health Systems; Toronto, Ontario, Canada: 1991. [Google Scholar]
- Hare R. The Hare Psychopathy Checklist - Revised. 2nd edition Multi-Health Systems; Toronto, Ontario, Canada: 2003. [Google Scholar]
- Harpur T, Hare R, Hakistan A. Two-factor conceptualization of psychopathy: construct validity and assessment implications. Psychological Assessment. 1989;1:6–17. [Google Scholar]
- Herpertz SC, Wenning B, Mueller B, Qunaibi M, Sass H, Herpertz-Dahlmann B. Psychophysiological responses in ADHD boys with and without conduct disorder: implications for adult antisocial behavior. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:1222–30. doi: 10.1097/00004583-200110000-00017. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlmann B. Response to emotional stimuli in boys with conduct disorder. American Journal of Psychiatry. 2005;6:1100–1107. doi: 10.1176/appi.ajp.162.6.1100. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Patrick CJ. Psychopathy and negative emotionality: analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. Journal of Abnormal Psychology. 2006;115:276–287. doi: 10.1037/0021-843X.115.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus AN, Finn PR. Startle modulation in non-incarcerated men and women with psychopathic traits. Personality and Individual Differences. 2007;43:2057–2071. doi: 10.1016/j.paid.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpman B. On the need for separating psychopathy into two distinct clinical types: symptomatic and idiopathic. Journal of Criminology and Psychopathology. 1941;3:112–137. [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–52. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Loney BR, Butler MA, Lima EN, Counts CA, Eckel LA. The relation between salivary cortisol, callous-unemotional traits, and conduct problems in an adolescent non-referred sample. Journal of Child Psychology and Psychiatry. 2006;47:30–36. doi: 10.1111/j.1469-7610.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- Lykken DT. The Antisocial Personalities. Lawrence Erlbaum Associates; Hillsdale, NJ: 1995. [Google Scholar]
- Lykken DT, Iacono WG, Haroian K, McGue M, Bouchard TJ. Habituation of the skin conductance response to strong stimuli: A twin study. Psychophysiology. 1988;24:4–15. doi: 10.1111/j.1469-8986.1988.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Lynam DR. Pursuing the psychopath: capturing the fledgling psychopath in a nomological net. Journal of Abnormal Psychology. 1997;106:425–38. doi: 10.1037//0021-843x.106.3.425. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Caspi A, Moffitt TE, Raine A, Loeber R, Stouthamer-Loeber M. Adolescent psychopathy and the big five: results from two samples. Journal of Abnormal Child Psychology. 2005;33:431–43. doi: 10.1007/s10648-005-5724-0. [DOI] [PubMed] [Google Scholar]
- Mednick S, Kirkegaard-Sorensen L, Hutchings B, Knop J, Rosenberg R, Schulsinger F. An example of bio-social research: the interplay of socioenvironmental and individual factors in the etiology of criminal behavior. In: Mednick SA, Christiansen KO, editors. Biosocial bases of criminal behavior. Gardner; New York: 1977. pp. 9–24. [Google Scholar]
- O'Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32:183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Patrick C. Emotion and psychopathy: startling new insights. Psychophysiology. 1994;31:415–428. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Cuthbert BN, Lang PJ. Emotion in the criminal psychopath: fear image processing. Journal of Abnormal Psychology. 1994;103:523–534. doi: 10.1037//0021-843x.103.3.523. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH. Skin conductance responsivity in psychopaths to orienting, defensive, and consonant-vowel stimuli. Journal of Psychophysiology. 1988;2:221–225. [Google Scholar]
- Raine A, Venables PH, Williams M. Autonomic orienting responses in 15-year-old male subjects and criminal behavior at age 24. The American Journal of Psychiatry. 1990;147:933–37. doi: 10.1176/ajp.147.7.933. [DOI] [PubMed] [Google Scholar]
- Raine A. The psychopathology of crime: Criminal behavior as a clinical disorder. Academic Press; San Diego: 1993. [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology. 2002;30:311–26. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH. Electrodermal nonresponding, antisocial behavior, and schizoid tendencies in adolescents. Psychophysiology. 1984;21:424–33. doi: 10.1111/j.1469-8986.1984.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Raine A, Reynolds C, Venables PH, Mednick S. Biosocial bases of aggressive behavior in childhood. In: Raine A, Brennan PA, Farrington DP, Mednick SA, editors. Biosocial bases of violence. Plenum; New York: 1997. pp. 107–126. [Google Scholar]
- Schaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous version, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Solant MV, Bridger WH. Electrodermal activity of undersocialized aggressive children: a pilot study. Journal of Child Psychology and Psychiatry. 1985;26:653–60. doi: 10.1111/j.1469-7610.1985.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Siddle DAT, Nicol AR, Foggitt RH. Habituation and overextinction of the GSR component of the orienting response in antisocial adolescents. British Journal of Social and Clinical Psychology. 1973;12:303–308. doi: 10.1111/j.2044-8260.1973.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Singer J. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24:323–355. [Google Scholar]
- Sutton SK, Vitale JE, Newman JP. Emotion among women with psychopathy during picture perception. Journal of Abnormal Psychology. 2002;111:610–619. doi: 10.1037//0021-843x.111.4.610. [DOI] [PubMed] [Google Scholar]
- Venables PH, Mitchell DA. The effects of age, sex and time of testing on skin conductance activity. Biological Psychology. 1996;43:87–101. doi: 10.1016/0301-0511(96)05183-6. [DOI] [PubMed] [Google Scholar]
- Verona E, Patrick CJ, Curtin JJ, Bradley MM, Lang PJ. Psychopathy and physiological response to emotionally evocative sounds. Journal of Abnormal Psychology. 2004;113:99–108. doi: 10.1037/0021-843X.113.1.99. [DOI] [PubMed] [Google Scholar]
- Zahn TP, Kruesi MJ. Autonomic activity in boys with disruptive behavior disorders. Psychophysiology. 1993;30:605–614. doi: 10.1111/j.1469-8986.1993.tb02086.x. [DOI] [PubMed] [Google Scholar]