SUMMARY
N-methyl-D-aspartate (NMDA) receptors constitute a major subtype of glutamate receptors at extra-synaptic sites that link multiple intracellular catabolic processes responsible for irreversible neuronal death. Here, we report that cerebral ischemia recruits death-associated protein kinase 1 (DAPK1) into the NMDA receptor NR2B protein complex in the cortex of adult mice. DAPK1 directly binds with the NMDA receptor NR2B C-terminal tail consisting of amino acid 1292–1304 (NR2BCT). A constitutively active DAPK1 phosphorylates NR2B subunit at Ser-1303 and in turn enhances the NR1/NR2B receptor channel conductance. Genetic deletion of DAPK1 or administration of NR2BCT that uncouples an activated DAPK1 from an NMDA receptor NR2B subunit in vivo in mice blocks injurious Ca2+ influx through NMDA receptor channels at extrasynaptic sites and protects neurons against cerebral ischemic insults. Thus, DAPK1 physically and functionally interacts with the NMDA receptor NR2B subunit at extra-synaptic sites and this interaction acts as a central mediator for stroke damage.
INTRODUCTION
Glutamate is the major excitatory transmitter in the mammalian central nervous system (CNS) and plays an essential role in neural development, excitatory synaptic transmission, and plasticity (Greengard, 2001). Immediately following ischemia, however, glutamate accumulates at synapses (Drejer et al., 1985; Rossi et al., 2000), resulting in extensive stimulation of its receptors that can eventually be toxic to neurons (Lipton, 2006; Lo et al., 2003). Glutamate activates three classes of ionophore-linked postsynaptic receptors, namely N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate receptors. NMDA receptor toxicity is dependent on extracellular Ca2+, and reflects a large amount of Ca2+ influx directly through the receptor-gated ion channels (Simon et al., 1984; for review, see Lipton, 2006; Lo et al., 2003). As most AMPA receptor channels have poor Ca2+ permeability, injury may result primarily from indirect Ca2+ entry through voltage-gated Ca2+ channels, Ca2+-permeable acid-sensing ion channels (Xiong et al., 2004), and possibly via a cleavage of Na+/Ca2+ exchangers (Bano et al., 2005). Ca2+ overload triggers several downstream lethal reactions including nitrosative stress (Aarts et al., 2003; Uehara et al., 2006), oxidative stress (Kinouchi et al., 1991) and mitochondrial dysfunction (Krajewski et al., 1999; Fiskum et al., 1999). Thus, over-stimulation of glutamate receptors can be considered as a primary intracellular event that induces neuronal death in stroke.
NMDA receptors constitute the major subtype of glutamate receptors, and normally participate in rapid excitatory synaptic transmission. To date, a variety of NMDA receptor subunit proteins (NR1, NR2A–D) have been cloned (Madden, 2002). Native NMDA receptors appear to be heteroligomeric complexes consisting of an essential NR1 subunit and one or more regulatory NR2 subunits (NR2A–D) and possibly the more recently identified NR3 subunit (Chatterton et al., 2002). Since the NR2C-(and possibly NR2D-) containing receptors have very low probability for their channel opening (Sprengel et al., 1998), both NR2A- and NR2B-containing NMDA receptors are considered as the main types of functional NMDA receptor channels in CNS neurons (Madden, 2002). Activation of NMDA receptors requires two coincident events: 1) binding to the coagonists of glutamate and glycine, and 2) simultaneous membrane depolarization, which removes the Mg2+ blockade of the channel pore, leading to the influx of Ca2+. Under physiological conditions, the entrance of Ca2+ produces partial inhibition of NMDA receptors via Ca2+-dependent inactivation, thereby preventing the intracellular Ca2+ overload (Krupp et al., 1999). Under pathological conditions, however, this negative feedback in Ca2+ regulation of NMDA receptors is disabled, resulting in excessive Ca2+ influx through the receptor channels (Wang et al., 2003a). Ca2+ overload triggers multiple intracellular catabolic processes, and thus induces an irreversible death of neuronal cells in the brain (for review, see Lipton, 2006; Lo et al., 2003).
Although over stimulation of NMDA receptors contributes to ischemic neuronal death, blocking them totally could be deleterious to animals and humans because targeting these receptors also blocks the physiological actions of the receptor as well. In the present study, we report that DAPK1, or death-associated protein kinase 1, acts as a specific “cell death signal” that couples NMDA receptor channels at extrasynaptic sites to ischemic neuronal death. We also demonstrate that uncoupling of an activated DAPK1 from the NMDA receptor complex protects against brain damage in stroke without affecting the physiological actions of the NMDA receptors. Thus, targeting DAPK1-NMDA receptor interaction can be considered as a practical strategy for stroke therapy.
RESULTS
Cerebral Ischemia Recruits DAPK1 into NMDA Receptor NR2B Protein Complex
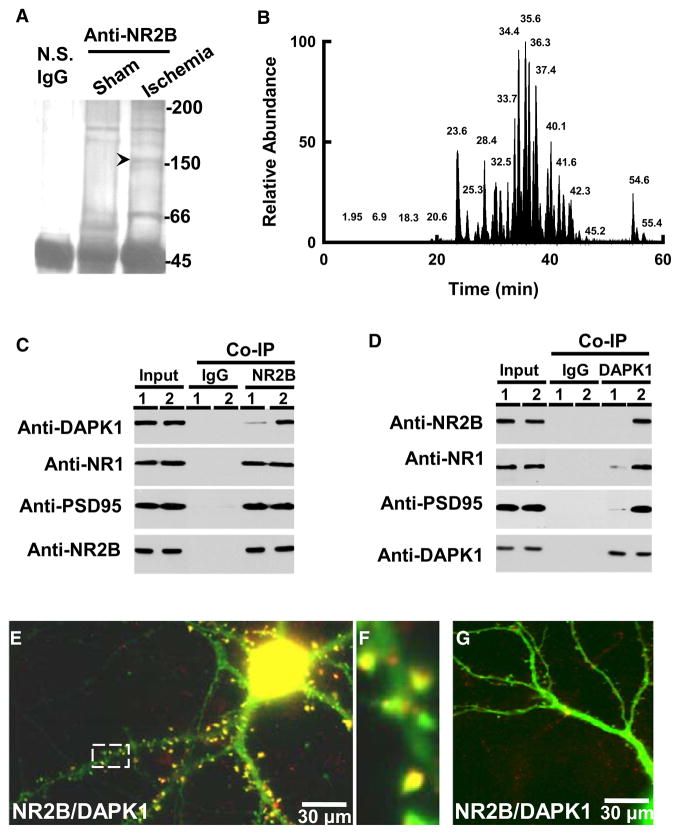
To explore the cell death signals that are linked with NMDA receptors in stroke damage, we used anti-NMDA receptor NR2B antibodies to precipitate the NMDA receptor complex in the membrane extracts from the cortex of mice that had been subjected to focal cerebral ischemia or sham operation (for details, see Experimental Procedures). The precipitates from both groups were separated on the SDS-PAGE and stained with Coomassie blue (Figures 1A and 1B). Compared to the control group, the additional protein bands on the gel with estimated size of 66 kDa and 150 kDa were observed in the ischemic group. We then analyzed the protein components in these bands using a reverse phase Nano-Liquid Chromatography-Tandem Mass Spectrometry (NLC/MS) approach. Five peptide sequences: AEQHEHVAGLLAR, DKSGETALAVAAR, GLFFQQLRPTQNLQPR, HYLSPQQLR, and RGVSREDIER, were found to match death-associated protein kinase 1 (DAPK1, see Tables S1 and S2). We next probed the precipitates with antibody against DAPK1 and confirmed the existence of endogenous DAPK1 in the NMDA receptor complex (Figure 1C, see also Figure S1). We also carried out the reciprocal coimmuno-precipitation, in which anti-DAPK1 was used to precipitate NMDA receptor NR1, NR2B subunits, and their functional interacting partner PSD-95, and CaMK-II (Figure 1D). Single neuronal labeling demonstrated a colocalization of DAPK1 with NMDA receptor NR2B subunits at the post-synaptic spines (Figures 1E–1G). Thus, DAPK1 is physically associated with NMDA receptors.
Figure 1. Ischemia Recruits DAPK1 into NMDA Receptor Complex.
(A) The immune-precipitates with nonspecific IgG (N.S. IgG), or antibody against NMDA receptor NR2B subunit (anti-NR2B) in the membrane extracts (5 mg proteins) from the cortex of mice 2 hr after operation for sham or MCAO (Ischemia) were stained with Coomassie blue.
(B) Base peak chromatogram from trypsin-digested products of a selected protein band (arrow head in [A]).
(C and D) The membrane extracts (1 mg proteins) from the cortex of mice 2 hr after sham (lane 1) or MCAO (lane 2) were precipitated with nonspecific IgG (IgG) or anti-NR2B (C) or anti-DAPK1 (D) and probed with anti-DAPK1, anti-NR1, anti-PSD95 or anti-NR2B. Input: 20 μg of protein was loaded in each lane except the PSD95 lane, in which 10 μg proteins were loaded.
(E–G) DAPK1+/+ (E) and DAPK1−/− (G) cultured neurons were stained with anti-DAPK1 (red) and anti-NR2B (green). A high magnified image (F) from a selected area in (E). In panels A–G, similar results were observed in each of the four experiments.
See also Figure S1.
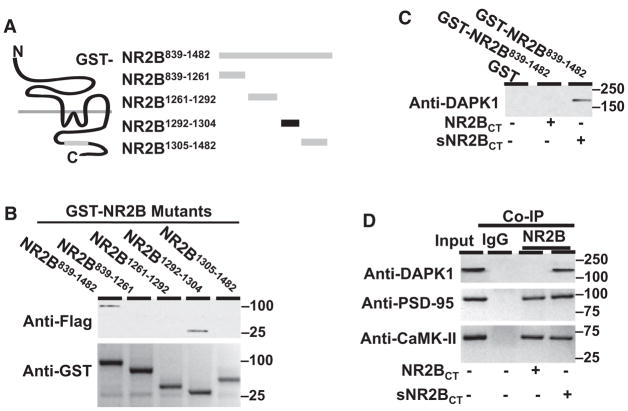
To identify a direct binding region of NMDA receptor NR2B subunit with DAPK1, we generated a series of NMDA receptor NR2B C-terminal deletion mutants fused with glutathione-S-transferase (GST, Figure 2A). We found that only the NR2B1292–1304 fragment was capable of binding with DAPK1 (Figure 2B). The NR2B1292–1304 fragment contains a consensus binding motif (R/K[R/K][R/KXXRXXSX]) of DAPK1 with only one mismatch residue at asparagine-1296 (Schumacher and Schavocky, 2004). We subsequently synthesized a NR2B C-terminal tail peptide of 1292KKNRNKLRRQHSY1304 (NR2BCT). Application of 50 μM NR2BCT uncoupled an activated DAPK1 from NMDA receptor NR2B protein complex (Figure 2C), but it did not affect the association of PSD-95 or CaMK-II with the NMDA receptor NR2B subunit (Figure 2D).
Figure 2. DAPK1 Directly Binds to NMDA Receptor NR2B Subunit.
(A) Representation illustrates a series of GST fusion proteins with the NR2B C-terminal mutants. (B) Affinity binding assay shows that flag-tagged catalytic domain of DAPK1 binds with GST-NR2B839–1482 (~100 kDa) and GST-NR2B1292–1304 (~30 kDa). The blots show anti-Flag (top) and anti-GST (bottom).
(C) NR2BCT suppresses DAPK1 association with NMDA receptor NR2B subunit. Membrane extracts (500 μg proteins) from the forebrain of mice 2 hr after MCAO were incubated with GST or the GST-NR2BCT839–1482 in the presence of 50 μM NR2BCT or sNR2BCT and probed with anti-DAPK1.
(D) NR2BCT blocks NMDA receptor association with DAPK1 but not with PSD-95. Membrane extracts were precipitated with nonspecific IgG (IgG) or anti-NR2B in the presence of 50 μM NR2BCT or sNR2BCT and probed with anti-DAPK1, or anti-PSD-95, or anti-CaMK-IIα. Input: 50 μg of protein was loaded in each lane. In (B)–(D), similar results were observed in each of the four experiments.
DAPK1 Activation Enhances the NR1/NR2B Channel Conductance
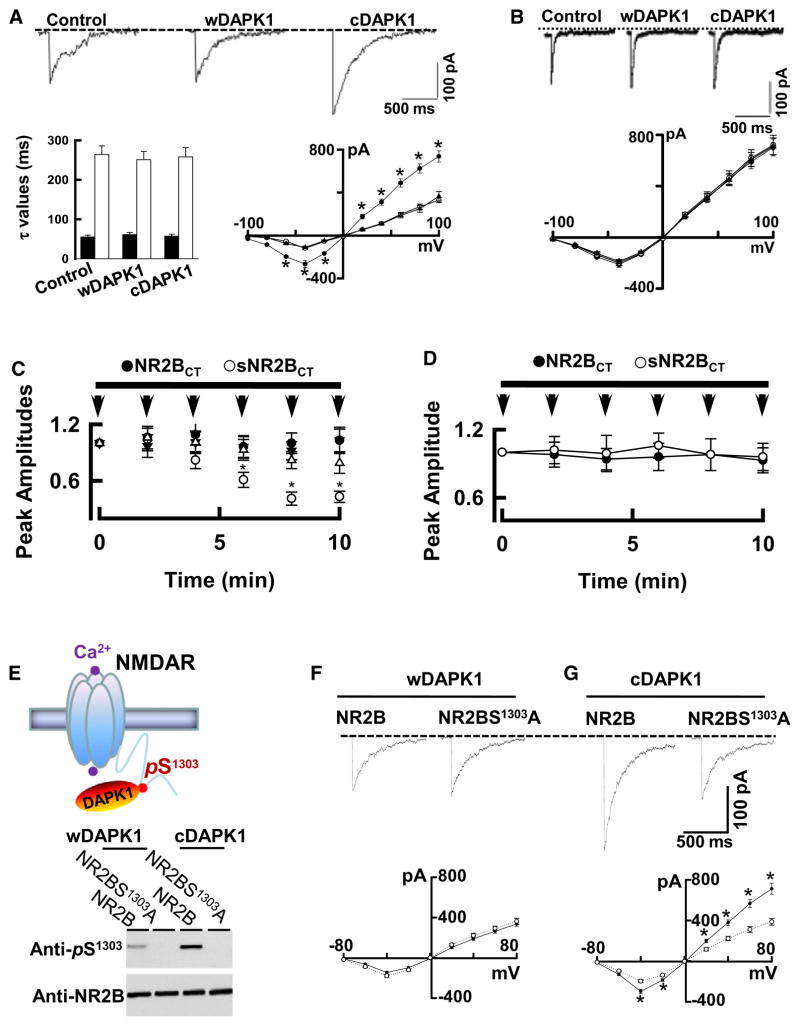
Having determined a binding domain of DAPK1 with the NMDA receptor NR2B subunit, we then examined whether DAPK1 functionally regulates NMDA receptor channel activity. We coexpressed the NR1/NR2B receptors with a constitutively active DAPK1 (cDAPK1), or the wild-type DAPK1 (wDAPK1) in HEK293 cells. We found that cDAPK1 increased the peak amplitude of the recombinant NR1/NR2B receptor currents (Figure 3A), but not the NR1/NR2A receptor currents (Figure 3B), at a range of holding potentials from −100 to +100 mV. Since the decay time constants and the reversal potentials were not changed (Figure 3A), cDAPK1 specifically increases the recombinant NR1/NR2B receptor channel conductance. To determine whether enhancement of NR1/NR2B receptor currents is mediated directly by DAPK1-NR2B interaction, we made use of NR2B subunit C-terminal peptide, NR2BCT, at a concentration of 10, 25, or 50 μM, which was diffused directly into the recording cells via path electrode (Figure 3C). After a 10 min diffusion of 50 μM NR2BCT, the peak amplitude of the whole-cell currents decreased by 0.56 ± 0.086 of the baseline (mean ± standard error of the mean [SEM], Figure 3C). A peptide with the same amino acid composition but in random order scrambled NR2BCT (sNR2BCT) served as control, and did not reduce the recombinant NR1/NR2B receptor currents (0.98 ± 0.11 of the baseline, mean ± SEM). Whole-cell currents in HEK293 cells coexpressing NR1/NR2B with wDAPK1 were not affected by 50 μM NR2BCT (Figure 3D). Thus, enhancement of the recombinant NR1/NR2B receptor channel conductance is mediated by activated DAPK1 binding with NR2B subunit.
Figure 3. DAPK1 Phosphorylates NR2B at Ser-1303 and Increase the Channel Conductance.
(A) Whole-cell currents at holding potential of −30 mV in HEK293 cells coexpressing NR1/NR2B receptors without (control) or with wild-type DAPK1 (wDAPK1) or constitutively active DAPK1 (cDAPK1). Bar graph shows decay time constants of the receptor currents fitted with two exponential components with τfast (75%, filled bars) and τslow (25%, open bars). The peak currents were plotted against holding potentials without (open circles) or with expression of wDAPK1 (triangles, n = 21 cells) or cDAPK1 (filled circles, n = 22 cells).
(B) Expression of cDAPK1 does not alter NR2A receptor currents. Whole-cell currents are at a holding potential of −60 mV in HEK293 cells coexpressing the NR1/NR2A receptors without (control) or with wDAPK1 or cDAPK1. The peak currents were plotted against holding potentials without (open circles, n = 18 cells) or with expression of wDAPK1 (filled circles, n = 18 cells) or cDAPK1 (triangles, n = 18 cells).
(C and D) NR2BCT antagonizes the cDAPK1-NR2B interaction. The averaged whole-cell currents are plotted against time with the intracellular perfusion of 10 (filled circles), 25 (open triangles) or 50 μM NR2BCT (open circles) or 50 μM sNR2BCT (filled triangles) in HEK293 cells coexpressing NR1/NR2B with cDAPK1 (C). In recordings from HEK293 cells coexpressing NR1/NR2B with wDAPK1 (D), 50 μM NR2BCT (open circles) or sNR2BCT (filled circles) were applied. The peak currents were normalized to baseline (defined as 1.0, n = 22 cell per group, *p < 0.01). Horizontal above the graphs indicate the period of peptide administration. Arrows indicate the time of application of 1 ms pulse of 500 μM glutamate.
(E) Illustration (top) shows that DAPK1 targets to NR2B C-terminal at Ser-1303. Bottom: the NR1/NR2B (lane: NR2B) or the mutant NR1/NR2BS1303A (lane: NR2BS1303A) that were coexpressed with wDAPK1 or cDAPK1 in HEK293 cells were subjected to western blot with anti-pS1303 or anti-NR2B. Similar results were observed in each of four experiments.
(F and G) Whole-cell currents are recorded at holding potential of −30 mV in HEK293 cells coexpressing NR1/NR2B or the mutant NR1/NR2BS1303A receptors with wDAPK1(F) or cDAPK1 (G). The peak currents were plotted against holding potentials in HEK293 cells with expression of NR1/NR2B (filled circles, n = 23 cells) or the mutant NR1/NR2BS1303A (open circles, n = 21 cells).
In panels A–G, data are mean ± SEM, *p < 0.01.
DAPK1 Activation Phosphorylates NR2B Subunit at Ser-1303
We next examined whether an activated DAPK1 phosphorylates NR2B subunit at Ser-1303, (Figure 3E), a residue corresponding to a phosphorylation site (KXXRXXSX) of DAPK1 (Schumacher and Schavocky, 2004). We coexpressed wDAPK1 or cDAPK1 in HEK293 cells with the recombinant NR1/NR2B or the mutant NR1/NR2BS1303A receptors. In the mutant NR2BS1303A, a Ser-1303 residue was replaced with an alanine (A). The levels of phosphorylation at Ser-1303 (pS1303) were measured using antibody against the pS1303 (anti-pS1303). The anti-pS1303 recognized the recombinant wide NR2B subunit, but not with the mutant NR2BS1303A, demonstrating the specificity of anti-pS1303 reaction (Figure 3E). To determine whether phosphorylation of NR2B at Ser-1303 is sufficient to modulate the NR1/NR2B receptor channel activity, whole-cell currents were recorded from HEK293 cells coexpressing the NR1/NR2B or the mutant NR1/NR2BS1303A with wDAPK1 (Figure 3F) or cDAPK1 (Figure 3G). We found that cDAPK1 increased the peak amplitude of the recombinant NR1/NR2B receptor currents, but not the mutant NR1/NR2BS1303A receptor currents, at a range of holding potentials from −80 to +80 mV (Figure 3G). Thus, an activated DAPK1 enhances NR1/NR2B receptor channel conductance by phosphorylating NR2B subunit at Ser-1303.
Generation of Null Mutant Mice Lacking the DAPK1 Gene
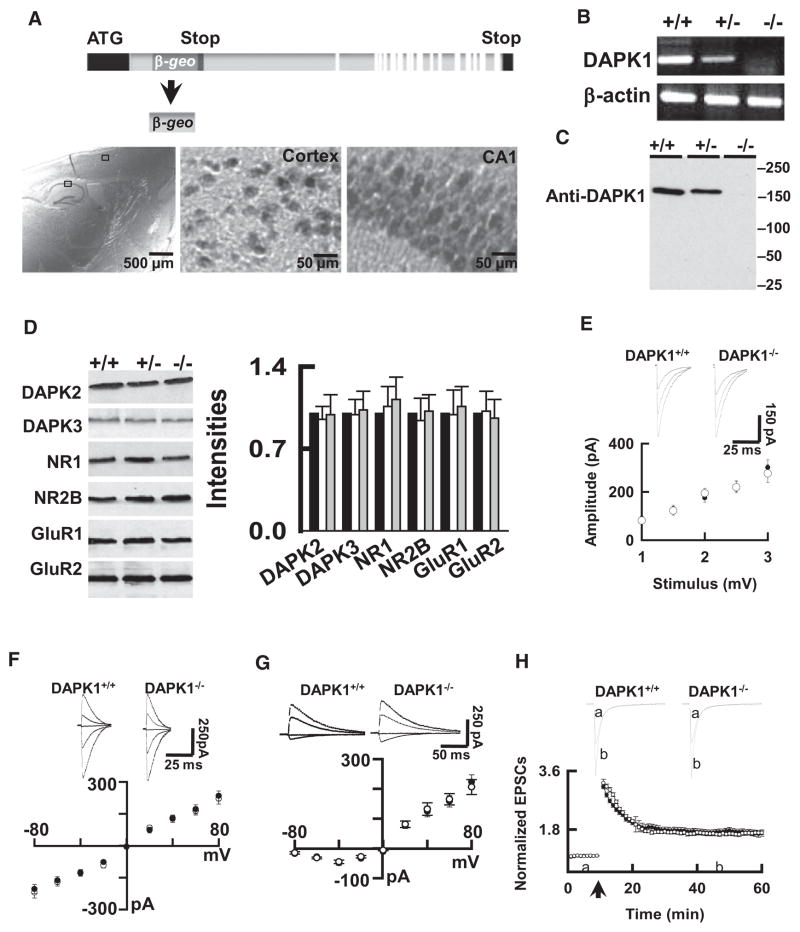
To determine whether an activated DAPK1 physically and functionally interacts with synaptic NMDA receptor NR2B subunit in vivo, we generated null mutant mice with deficiency in expression of DAPK1 (DAPK1−/− mice). β-gal, a marker for DAPK1 deletion, was observed throughout the brain of the DAPK1−/− mice, including the cortex and hippocampus (Figure 4A). RT-PCR and western blot analysis revealed undetectable levels of DAPK1 mRNA (Figure 4B) and proteins (Figure 4C) in the DAPK1−/− mice. No difference of DAPK2 and DAPK3 proteins was observed between DAPK1−/− and DAPK1+/+ mice (Figure 4D).
Figure 4. The Mutant Mice Lacking the DAPK1 Gene Have Normal Synaptic Transmission.
(A) A promoterless gene trapping vector fused into a reporter gene (β-geo) was inserted into the first intron of DAPK1. Staining on a sagittal brain section from an adult DAPK1−/− mouse shows the β-geo protein in the cortex and hippocampus.
(B and C) Representatives show the RT-PCR and western blots of DAPK1 mRNA (B) and proteins (C) in the brain of the homozygous (DAPK1−/−), heterozygous (DAPK1−/+) and their wild-type littermates (DAPK1+/+) mice.
(D) Blots show DAPK2, DAPK3, and synaptic proteins in the brains of DAPK1+/+, DAPK1+/−, and DAPK1−/− mice. The intensities of the blots (bar graph) were normalized to the respective blots in DAPK1+/+ mice (defined as 1.0). Data are mean ± SEM (n = 4 mice/group).
(E) The DAPK1−/− mice have normal input-output responses. The EPSCs (top) were recorded from CA1 pyramidal neurons at holding potential of −70 mV. The mean amplitudes from DAPK1+/+ (open circles) and DAPK1−/− (filled circles) mice, as summarized in graph (bottom), were plotted against the intensities of stimulus. Data are mean ± SEM (n = 8 cells/4 mice per group).
(F and H) Sweeps (top) and the current-voltage relations of the excitatory post-synaptic currents (EPSCs) mediated by AMPA (EPSCsAMPA, F) and the NMDA receptors (EPSCsNMDA, G) at holding potentials of −80, −40, 0 +20, or +40 mV were recorded in CA1 neurons from DAPK1+/+ (open circles) and DAPK1−/− (filled circles) mice. Data are mean ± SEM (n = 12 cells/6 mice per group).
(H) A plot represents the normalized peak amplitude of the EPSCsAMPA in CA1 neurons from DAPK1+/+ (open circles) and DAPK1−/− mice (filled circles). The traces above the graph are averages of three sweeps taken at the time points indicated by the letter above the x axis. Arrow indicates the time of tetanus. Data are mean ± SEM (n = 12 cell/6 mice per group).
We next surveyed several synaptic proteins, including AMPA receptor subunits GluR1 and GluR2, and NMDA receptor subunits NR1, NR2A, and NR2B and PSD-95 (Figure 4D). We confirmed that genetic deletion of DAPK1 does not alter the overall synaptic protein compositions in the brain. We next examined synaptic transmission in the CA1 hippocampus. We analyzed excitatory post-synaptic currents (EPSCs). By plotting the mean amplitude of EPSCs in CA1 pyramidal neurons versus the stimulus intensities, we determined that the input-output curves were identical between genotypes (Figure 4E). The current-voltage (I–V) relations of AMPA receptor- and NMDA receptor-mediated EPSCs (EPSCsAMPA, Figure 4F, and EPSCsNMDA, Figure 4G), and the induction of long-term potentiation (LTP, Figure 4H), also were not affected in the DAPK1−/− mice.
DAPK1 Activation Enhances Extrasynaptic NMDA Receptor Channel Activity
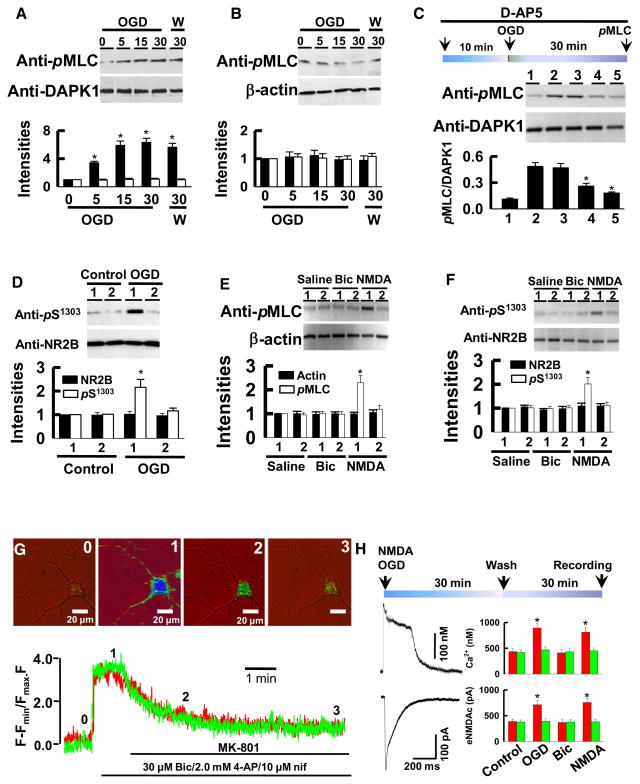
Having demonstrated that genetic deletion of DAPK1 did not alter NMDA receptor physiology, we next examined the functional impacts of DAPK1 deletion in NMDA receptor toxicity. First, we treated the cortical neurons from DAPK1−/− and DAPK1+/+ mice with oxygen/glucose deprivation (OGD). Catalytic activity of DAPK1 was measured using immune-complex kinase assay with myosin light chain (MLC) as an endogenous substrate. We found that enzymatic activity of DAPK1 began to increase 5 min after OGD, and reached a peak 30 min later (Figure 5A). Activation of DAPK1 was maintained for over 30 min after OGD was washed out (Figures 5A and 5B). Activation of DAPK1 was not affected by applying AMPA receptor antagonist (10 μM CNQX), but was completely eliminated either by applying NMDA receptor antagonist (50 μM D-AP5) or by removing extracellular Ca2+ (Ca2+-free, Figure 5C). Catalytic activity of DAPK1 is controlled by its autophosphorylation at serine-308 (pS308); dephosphorylation of pS308 allows DAPK1 to bind with CaM, thereby activating DAPK1 (Shohat et al., 2001). Consistent with this previous report, we found that DAPK1 became dephosphorylated at pS308 in cortical neurons following OGD exposure. Significantly, we showed that dephosphorylation of DAPK1 at pS308 was catalyzed by calcineurin (CaN, see also Figure S2).
Figure 5. Activation of DAPK1 Induces Ca2+ Influx through Extrasynaptic NMDA Receptors.
(A and B) DAPK1+/+ (A) and DAPK1−/− (B) cortical neurons were exposed to OGD for 0, 5, 15, or 30 min. Immediately after OGD (OGD) or 30 min after OGD washout (w), DAPK1 complex was precipitated and blotted with anti-pMLC or anti-DAPK1. The intensities of the anti-pMLC (filled bars) and anti-DAPK1 (open bars) blots were normalized to their respective signals at 0 min OGD (defined as 1.0, n = 16).
(C) DAPK1+/+ neurons were treated with saline (lane 1 and lane 2), 10 μM CNQX (lane 3), 50 μM D-AP5 (lane 4), or Ca2+-free (lane 5) for 10 min, and then challenged with sham control (lane 1) or with OGD (lane 2 through lane 5) for 30 min. DAPK1 complex was then precipitated and blotted with anti-pMLC or anti-DAPK1. The ratio of anti-pMLC versus anti-DAPK1 blots were calculated (n = 12 cultures/4 mice) and summarized in bar graph.
(D) DAPK1+/+ (lane 1) and DAPK1−/− (lane 2) neurons were exposed to shame or OGD for 30 min. NMDA receptor complex was precipitated with anti-NR1 and blotted with anti-pS1303 or anti-NR2B. The intensities of blots were normalized to their respective controls (defined as 1.0, n = 12 cultures/4 mice).
(E and F) NMDA treatment activates DAPK1 (E) and increases the levels of pS1303 in cortical neurons (F). DAPK1+/+ (lane 1) and DAPK1−/− (lane 2) cortical neurons were challenged with saline, 10 μM bicuculline (Bic), or 50 μM NMDA/10 μM glycine for 30 min. Endogenous DAPK1 and NMDA receptor protein complexes were then precipitated and blotted, as indicated. The intensities of the blots were normalized to their respective controls (defined as 1.0), and summarized in bar graphs (n = 12 cultures/4 mice).
(G) Images are taken at the time points indicated by the numbers above the traces from single DAPK1+/+ neuron with pre-OGD (Red) or sham (Green). Ca2+ transients were evoked by 30 μM Bic and 10 μM nifedipine (Nif) and blocked by 10 μM MK-801.
(H) Cortical neurons were exposed to control, OGD, 10 μM Bic, or 50 μM NMDA/10 μM glycine for 30 min. 30 min after washout Ca2+ transients and whole-cell currents (eNMDAc) were evoked by a 5-ms pulse of 100 μM NMDA/10 μM glycine. Data (*p < 0.01) in bar graphs are DAPK1+/+ (red bars) and DAPK1−/− (green bars) neurons (n = 22 cells per group).
In panels A–H, data in bar graphs are mean ± SEM. See also Figure S2.
Next, we investigated the functional impacts of an activated DAPK1 on NMDA receptor channels in vivo. We challenged the cortical neurons with OGD or with 50 μM NMDA/10 μM glycine for 30 min to activate an endogenous DAPK1. Western blots of the precipitates showed that the levels of pS1303, but not the NR2B protein, were elevated in DAPK1+/+ cortical neurons (Figure 5D). An increase of pS1303 in response to OGD (Figure 5D) or to NMDA challenging (Figures 5E and 5F) was not observed in DAPK1−/− neurons (Figure 5E). Thus, an activated DAPK1 is a response to phosphorylation of NR2B subunit at Ser-1303.
To determine whether an increase of the pS1303 level by an activated DAPK1 enhances synaptic NMDA receptor channel activity, we performed fluorescence ratio-metric Ca2+ image combined with whole-cell patch clamp recordings. We found that Ca2+ influx through synaptic NMDA receptor channels were not affected by an activated DAPK1; the peak amplitudes of Ca2+ transients were identical between groups (Figure 5G). Since DAPK1 enhances the channel conductance of the recombinant NR1/NR2B receptors, which are known to be expressed predominantly in the extrasynaptic sites (Tovar and Westbrook, 1999; Brickley et al., 2003), we reasoned that an activated DAPK1 may functionally interact with extrasynaptic NMDA receptors. To test this possibility, we recorded the extrasynaptic NMDA receptor-mediated currents (eNMDAc) and Ca2+ transients by blocking synaptic NMDA receptor channels using MK-801 trapping protocol (for details see Experimental Procedure). We treated the single cortical neurons with OGD or 50 μM NMDA for 30 min to activate endogenous DAPK1. After a 30 min recovery period, the whole-cell currents and Ca2+ transients were recorded (Figure 5H). A greater amplitude of eNMDAc and Ca2+ transients was observed in DAPK1+/+ neurons, compared to controls. Thus, an activated DAPK1 increases Ca2+ influx through extrasynaptic NMDA receptor channels.
DAPK1 Deletion Protects against Cerebral Ischemic Neuronal Death
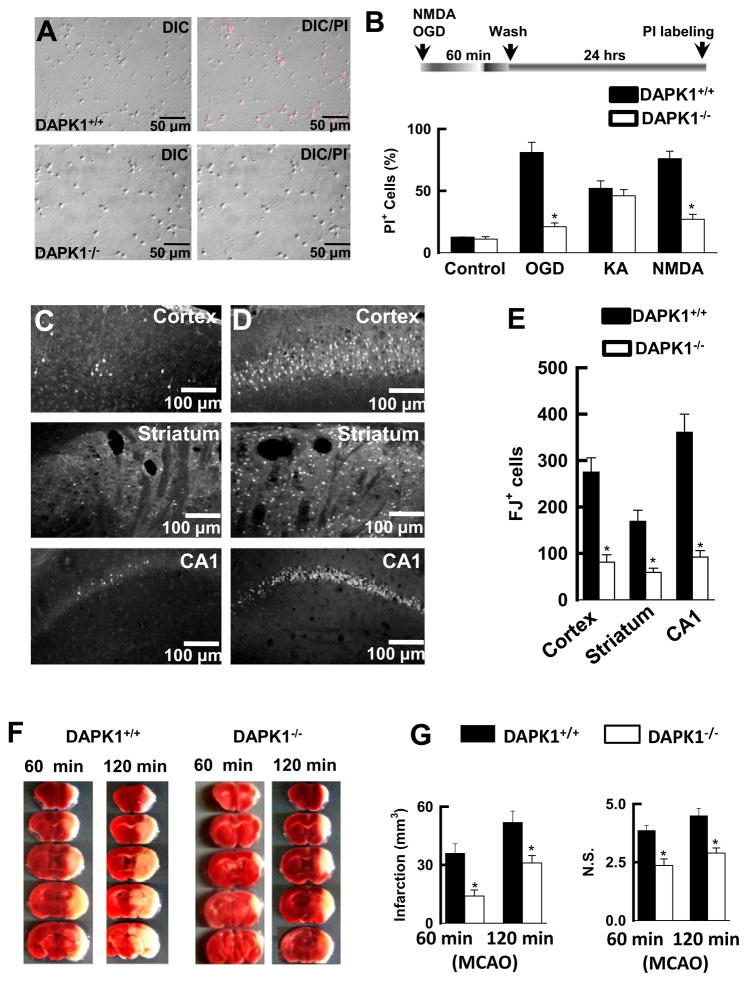
We next examined whether ischemic insult is mediated by DAPK1 induction of injurious Ca2+ influx through extrasynaptic NMDA receptor channels. We challenged cortical cultures with OGD or 50 μM NMDA/10 μM glycine for 60 min. 24 hr after washout cell death was examined. Propidium iodide (PI) staining showed that genetic deletion of the DAPK1 gene sufficiently protected against the insults induced by either OGD or NMDA challenging (Figures 6A and 6B). Next, we subjected the DAPK1−/− and DAPK1+/+ mice with transient global ischemia by occlusion of the common carotid artery for 20 min. Six days after reperfusion, brain sections were stained with Fluoro-Jade (FJ), a marker for degenerating neurons. An unbiased stereological analysis was used to count the FJ-labeled (FJ+) neurons in the CA1 area of the hippocampus, the layer 3 of the cortex, and the striatum. These three groups of neurons were examined because they are known to be vulnerable to transient global ischemic insult (Simon et al., 1984; Pellegrini-Giampietro et al., 1997; Wang et al., 2003a; Liu et al., 2004; Peng et al., 2006). In the DAPK1+/+ mice, the majority of these neurons had degenerated (275 ± 32 FJ+ cells/0.2 mm3 in the cortex, 169 ± 26 FJ+ cells/0.2 mm3 in the striatum, and 361 ± 38 FJ+ cell/0.2 mm3 in the CA1 region, n = 7 mice, Figures 6C–6E). This severe neuronal injury was observed only in the DAPK+/+ mice; markedly less damage was detected in the DAPK−/− mice (81 ± 14 FJ+ cells/0.2 mm3 in the cortex, 59 ± 9 FJ+ cells/0.2 mm3 in the striatum, and 92 ± 7 FJ+ cell/0.2 mm3 in the CA1 region, n = 6 mice, p < 0.01, Figure 6E). We also operated on mice to induce focal cerebral ischemia with middle cerebral artery occlusion (MCAO) for 60 min or 120 min. After 24 hr reperfusion, the brain infarction was analyzed using TTC staining. We found that the DAPK−/− mice had a dramatic reduction of the brain infarction (14.2 ± 2.1 mm3 at 60 min MCAO; 29.6 ± 3.3 mm3 at 120 min MCAO, n = 11 mice/group), compared to DAPK1+/+ mice (34.3 ± 4.6 mm3 at 60 min MCAO; 52.9 ± 6.2 mm3 at 120 min MCAO; n = 11 mice/group, p < 0.001, Figures 6F and 6G). This reduction of the brain infarction was associated with improvement of the neurological scores in DAPK1−/− mice (p < 0.01, n = 11 mice per group, Figure 6G). Thus, DAPK1 can be considered as a primary mediator in brain damage of stroke.
Figure 6. Genetic Deletion of DAPK1 Protects against Ischemic Neuronal Death.
(A) DAPK1 deletion protects cortical neurons against OGD insult. DAPK1+/+ and DAPK1−/− cortical cells were challenged with OGD for 60 min. 24 hr after washout the cultures were stained with propidium iodide (PI, red), and single DIC or double DIC/PI images were collected. (B) Bar graph summarizes the percentage of PI+ cells (n = 12 cultures). Data are mean ± SEM.
(C–E) Images of cortex, striatum and the hippocampus from DAPK1−/− (C) and DAPK1+/+ (D) mice that were stained with Fluoro-Jade 6 days after transient global ischemia. Bar graph (E) shows summarized Fluoro-Jade-labeled cells (n = 7). Data are mean ± SEM.
(F and G) Images of the brain sections (1.0 mm, F) from DAPK1+/+ and DAPK1−/− mice 24 hr after operation for 60 min or 120 min MCAO that were stained with TTC. Bar graphs show the infarct volume and neurological scores (N.S.). Data are mean ± SEM (*p < 0.01).
Treatment of Stroke by Uncoupling DAPK1 from NMDA Receptor NR2B Subunit
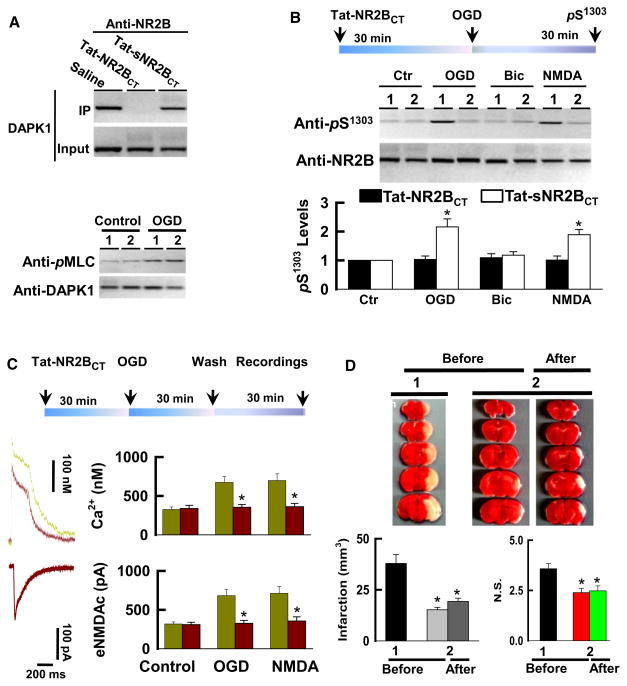
DAPK1 physically and functionally interacts with the NMDA receptor NR2B subunit, and this interaction might act as an essential intracellular event in stroke damage. To investigate this, we examined whether blocking DAPK1-NR2B interaction achieves the therapeutic effects in mouse models with stroke. To do so, we generated cell membrane permeable NR2BCT peptide by fusing the NR2BCT to the cell-membrane transduction domain of the HIV-1 Tat protein (Tat-NR2BCT, for details see Experimental Procedures). Western blots of the precipitates revealed that application of Tat-NR2BCT sufficiently uncoupled an activated DAPK1 from the NMDA receptor NR2B subunit complex (Figure 7A). Although Tat-NR2BCT did not affect the catalytic activity of DAPK1 (Figure 7A), it completely blocked; [1]) an association between an activated DAPK1 and the NMDA receptor NR2B subunit; [2]) NR2B phosphorylation at Ser-1303 (Figure 7B, see also Figure S3); and [3]) Ca2+ influx through NMDA receptor channels at extrasynaptic sites (Figure 7C).
Figure 7. Inhibition of DAPK1-NMDA Receptor Interaction Protects against Stroke Damage.
(A) Tat-NR2BCT treatment disturbs the DAPK1-NMDA receptor association without affecting DAPK1 activity. DAPK1+/+ cortical neurons were treated with 50 μM Tat-NR2BCT or Tat-sNR2BCT or saline for 30 min and exposed to OGD for 30 min. NMDA receptor complex was precipitated with anti-NR2B (top) or anti-DAPK1 (bottom) and blotted with anti-DAPK1 or anti-pMLC, as indicated.
(B) DAPK1+/+ cortical neurons were treated with 50 μM Tat-sNR2BCT (lane 1) or Tat-NR2BCT (lane 2) for 30 min and were then exposed to control, OGD, 10 μM bicuculline (Bic), or 50 μM NMDA/10 μM glycine for 30 min. Anti-NR1 precipitates were blotted with anti-pS1303 or anti-NR2B, as indicated. Data in bar graph are mean ± SEM (n = 16 cultures/4 mice, *p < 0.01).
(C) DAPK1+/+ cortical neurons were treated with 50 μM Tat-sNR2BCT (purple traces) or Tat-NR2BCT (green traces) for 30 min and were then exposed to control, OGD or 50 μM NMDA/10 μM glycine for 30 min. 30 min after washout and Ca2+ transients (top traces) whole-cell currents (bottom traces) were recorded. Data from Tat-NR2BCT (purple bars) and Tat-sNR2BCT (green bars) in bar graphs are mean ± SEM (n = 16 recordings per group, *p < 0.01).
(D) The adult male DAPK1+/+ mice were received a single injection (10 mg/kg, i.v.) of Tat-NR2BCT (1) or Tat-sNR2BCT (2) 60 min before or 60 min after MCAO (After). The infarction volume and neurological scores (N.S.) were summarized in the bar graphs (G). Data are mean ± SEM (*p < 0.001).
See also Figure S3.
We next operated on mice with MCAO for 60 min, and analyzed enzymatic activity of DAPK1. Our data revealed that DAPK1 in the forebrain of mice became activated 60 min, and reached a peak 120 min after reperfusion. Activation of DAPK1 was closely associated with phosphorylation of NR2B subunit at Ser-1303 (see also Figure S3). Blots of the anti-NR1 precipitates in the forebrain extracts showed Tat-NR2BCT at a dose of 10 mg/kg that did not alter the catalytic activity of DAPK1, but that sufficiently uncoupled an activated DAPK1 from NMDA receptor NR2B complex (see also Figure S3). By measuring the cerebral infarction 24 hr after MCAO operation using TTC staining, we found that the total infarction volume in the Tat-NR2BCT -treated mice was reduced to 15.2 ± 1.2 mm3, compared to 37.9 ± 4.3 mm3 in control, in which mice were injected with Tat-sNR2BCT (mean ± SEM, n = 11 mice per group, ANOVA, F = 12.98, p < 0.001, Figure 7D). Furthermore, a significant reduction of the cerebral infarction was also observed even when Tat-NR2BCT (10 mg/kg, i.v.) was administered 60 min after MCAO operation (19.3 ± 1.9 mm3 in the Tat-NR2BCT post-treatment, and 36.1 ± 3.7 mm3 in Tat-sNR2BCT-post-treatment, n = 8 mice/group, Figure 7D). A reduction of brain infarction was closely associated with improved neurological behaviors (Figure 7D). Together, our data demonstrate that DAPK1 physically and functionally interacts with the NMDA receptor NR2B subunit and that this interaction mediates stroke damage.
DISCUSSION
NMDA receptor NR2B subunits have recently become the major focus of studies in the treatment of stroke because of their unique features (Hardingham et al., 2002; Simpkins et al., 2003; Liu et al., 2007). First, in the adult CNS neurons NMDA receptor NR2B subunits are localized predominantly at extra-synaptic sites (Tovar and Westbrook, 1999; Brickley et al., 2003). This feature enables the receptor to detect extrasynaptic glutamate spillover in the brain during ischemic insults (Drejer et al., 1985; Rossi et al., 2000). Second, extrasynaptic NMDA receptors are reported to link the specific signals that induce neuronal death in ischemia (Finkbeiner, 2000; Hardingham et al., 2002, Takasu et al., 2002; Liu et al., 2007). However, it is now believed that NMDA receptor NR2B subunits may be also combined with NR1/NR2A subunits to form NR1/NR2A/NR2B complex receptors at synapses (Thomas et al., 2006). Thus, using NMDA receptor NR2B subunit antagonists for selectively inhibiting extra-synaptic NMDA receptors still could be problematic.
Alternatively, recent studies have focused on NMDA receptor NR2B subunit downstream cell death signaling via interaction with PSD-95 (Aarts et al., 2002). Disturbing NR2B-PSD-95 interaction by expression of a peptide fragment of the NR2B C-terminal tail (KLSSIESDV) uncouples neuronal nitric synthase (nNOS) activation, protecting cerebral ischemic infarction in adult rats (Aarts et al., 2002). This finding suggests that blocking NR2B-PSD-95 interaction would be able to eliminate NMDA receptor NR2B subunit toxicity. However, this strategy has been challenged by its parallel inhibition of synaptic plasticity (El-Husseini et al., 2002). Also, some other studies indicated that inhibition of PSD-95 increased brain damage in stroke (Gardoni et al., 2002).
Multiple intracellular signals are able to regulate NMDA receptor channel activity (Ghosh, 2002) and induce NMDA receptor-dependent cell death (Simon et al., 1984; Wang et al., 1999). Some including calcineurin (CaN) (Wang et al., 1999), Ca2+/CaM-activated protein kinase II (CaMK-II) (Mabuchi et al., 2001), protein kinase C (Sun et al., 2008), cycline-dependetn kinase 5 (Cdk5) (Wang et al., 2003a), tyrosine kinases (Khanna et al., 2007), and nNOS (Eliasson et al., 1999), however, also control the physiological processes of gene expression, as well as synaptic plasticity (Ghosh, 2002; Salter and Kalia, 2004), and thus are not promising targets for stroke therapy.
DAPK1 belongs to a Ca2+/CaM-dependent protein kinase family, and comprises a kinase domain, a CaM-binding motif, eight ankyrin repeats, and a death domain (for reviews, see Bialik and Kimchi, 2006; Van Eldik, 2002; Velentza et al., 2001). The kinase domain resides at the amino terminus and precedes the CaM-binding motif, which consists of a CaM binding site and an autoinhibitory region. CaM binding to the CaM binding site induces a conformation change. Ths conformation change is known to relieve the steric block of the active site by the autoinhibitory region (Van Eldik, 2002), and possibly leads to an activated DAPK1 binding with the NMDA receptor NR2B subunit. Consistent with this idea, our data revealed that activation of DAPK1 following ischemic insult phosphorylates the NMDA receptor NR2B subunit at Ser-1303. Interestingly, the NR2B at Ser-1303 is the same residue as that catalyzed by CaMK-II (Omkumar et al., 1996). Thus, DAPK1 may have a functional analogous to that of CaMK-II. CaMK-II is one of the most abundant Ca2+/CaM-dependent enzymes in the central neurons and participates in synaptic plasticity (Lisman et al., 2002) as well as in the cellular events responsible for ischemic neuronal death (Waxham et al., 1996). However, CaMK-II was found to become inactivated in the brain in response to ischemic insults (Hanson et al., 1994; Waxham et al., 1996; Petersen et al., 2003). Inhibition of CaMK-II enhances the vulnerability of brain cells to stroke damage (Waxham et al., 1996). In this context, CaMK-II can be viewed as a neuronal survival mediator. Thus, it is unlikely that CaMK-II plays a role in the DAPK1-NR2B interaction for ischemic neuronal death.
In the present study, we used Tat-NR2BCT to disrupt the interaction between an activated DAPK1 and NMDA receptor NR2B. Tat-NR2BCT, but not its scrambled control, protected cortical neurons from NMDA receptor-mediated insults without affecting catalytic activity of DAPK1 or the NMDA receptor physiology. Thus, systematic administration of Tat-NR2BCT could serve as an advantage over NMDA receptor antagonists in stroke therapy. Indeed, our data demonstrated that Tat-NR2BCT, even when it was administered one hour after stroke onset, dramatically reduced brain infarction and improved neurological functions in mice. Thus, targeting the interaction between an activated DAPK1 and NMDA receptor NR2B could be considered a promising strategy for the treatment of stroke. It must be noted, however, that even though we found that Tat-NR2BCT disrupted DAPK1-NR2B interaction without interfering with the association of NR2B with PSD-95 or CaMK-II, we could not exclude the possibility that inhibition of other binding proteins, rather than DAPK1 interaction with NMDA receptor NR2B by Tat-NR2BCT, could be responsible for its neuronal protective effects.
A main question arising from this study is how DAPK1 becomes activated in response to cerebral ischemic insults. Catalytic activity of DAPK1 is thought to be controlled by its autophosphorylation at serine-308 (pS308); dephosphorylation of pS308 allows DAPK1 to bind with CaM, thereby activating DAPK1 (Shohat et al., 2001). In the present study, we showed that DAPK1 becomes dephosphorylated at pS308 in cortical neurons following ischemic insults (Figure S2). We also demonstrated that dephosphorylation of pS308 in response to ischemic insults can be eliminated by genetic mutation of calcineurin (CaN, see also Figure S2). Thus, CaN can be considered as an upstream activator of DAPK1. It has been known that enzymatic activity of CaN depends on Ca2+ influx through NMDA receptor channels (Marshall et al., 2003). Consistent with this previous report, our data revealed that challenging cortical neurons with NMDA induced dephosphorylation of pS308 (Figures 5C–5E). Conversely, application of NMDA receptor antagonist or removal of extracellular Ca2+ inhibits DAPK1 dephosphorylation at pS308 and inactivates DAPK1 (Figure 5C, see also Figure S2). Thus, the most parsimonious explanation for our findings is that under physiological conditions, an initial Ca2+ influx through NMDA receptor channels produces partial inhibition of NMDA receptors via Ca2+-dependent inactivation, thereby preventing the intracellular Ca2+ overloading (Krupp et al., 1999). Under pathological conditions such as ischemic insults, however, Ca2+ influx through NMDA receptor channels activates CaN and in turn dephosphorylates DAPK1 at pS308, leading to activation of DAPK1. An activated DAPK1 is recruited into the NMDA receptor NR2B subunit at extrasynaptic sites and phosphorylates NR2B subunit at Ser-1303 and subsequently induces injurious Ca2+ influx through NMDA receptor channels, resulting in an irreversible neuronal death. Thus, DAPK1 can be considered as a signaling amplifier of NMDA receptors at extrasynaptic sites for mediating brain damage in stroke.
EXPERIMENTAL PROCEDURES
Generation of DAPK1 Null Mutant Mice
The null mutant mice with complete deletion of DAPK1 gene were generated using a gene-trapping technique. A mouse (strain C57/Bl6) was cloned from an embryonic stem cell line (IST12766D5; Texas Institute for Genomic Medicine, TIGM), carrying DAPK1 allele disrupted by the insertion of a gene trap vector with a lacZ (β-galactosidase)-neomycin resistance fusion cassette (β-gal, Omnibank Vector 76) in the first intron. The insertion site, as denoted with an asterisk*, was confirmed by genomic sequence, which is available upon requested. The cloned ES cell was used to generate DAPK1 mutant mice with a C57/Bl6 background, which were verified by PCR amplification of the mutant gene in tail DNA from progeny. The homozygous (DAPK1−/−) mice were derived by mating heterozygous DAPK1+/− mice. The PCR primers for wild DAPK1 were as follows: sense 5′TTGCACAACAGCTACACAGC-3″ antisense 5′ ATAGTCCCACTACTCAGGTC-3′.
Focal Cerebral Ischemia and Transient Global Ischemia
DAPK1 mutant mice were fasted overnight, and anesthetized with 4% halothane and maintained with mechanical ventilation (3.5 ml/cycle, 28 cycle/min, 0.5% halothane in a mixture of 30% O2 and 70% N2O). The blood gas conditions were kept at constant levels throughout the surgery (PO2, 120 ± 10 mm Hg; PCO2, 35 ± 3 mm Hg), and the rectal and temporal muscle temperatures were also maintained at 37.5 ± 0.2°C and 37.0 ± 0.1°C, respectively. Body temperature was maintained at 37 ± 0.5°C with heating pads until the animals had recovered from surgery. Focal cerebral ischemia was induced by intraluminal middle cerebral artery occlusion (MCAO), as described previously. Local blood flow was monitored using BLF21C Laser Doppler Flow-meter with LL-M1043 fiber flow-probe (Transonic System Inc, New York), as described before (Wang et al., 2003a; Liu et al., 2004; Peng et al., 2006, see also Extended Experimental Procedures).
Administration of Tat-NR2BCT
Mice were intravenously (i.v.) administered with 10 mg/kg Tat-NR2BCT (Tat-KKNRNKLRRQHSY) or Tat-sNR2BCT (Tat-NRRRNSKLQHKKY) 60 min before or after MCAO operation. To test whether the Tat-peptide could be delivered into the brain, the Tat-NR2NCT-fluorescein isothiocyanate (FITC, 10 mg/kg, i.v.) was injected. 30 min after the injection, the brain sections (30 μm) were imaged under the Axio Observer 200 Apotome microscope (data not shown). For the electrophysiological recordings, 50 μM NR2BCT or sNR2BCT was applied directly into the recording cells via patch electrode. A dose of 10 mg/kg tat peptide or 50 μM NR2BCT peptide was chosen because we found they did not alter DAPK1 activity but uncoupled an activated DAPK1 from NMDA receptor complex. The peptides with 99% purity were synthesized by AnaSpec (San Jose, CA) and Peptide 2.0, Inc (Chantilly, VA), respectively. The peptides were numbered and the experimenters were unaware of which one was applied in all experiments.
Whole-Cell Patch Clamp Recordings
Hippocampal slices (300 μm) were prepared from mice as previously described (Wang et al., 2003a; 2003b; Liu et al., 2004; Peng et al., 2006, see also Extended Experimental Procedures). Slices in the recording chamber were continuously superfused with artificial cerebrospinal fluid (ACSF, 2 ml/min) saturated with 95% O2/5% CO2 at 30 ± 1°C. The composition of ACSF (in mM) was 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1.2 MgCl2, 2 CaCl2, 26 NaHCO3, and 10 dextrose.
Co-IP, Affinity Binding Assay, and Western Blots
Cerebral cortex was homogenized in ice-cold lysis buffer containing (mM) 50 Tris-HCl, 150 NaCl, 1% NP-40, 2 EDTA, 1 Na-orthovanadate, (pH 7.4), and proteinase inhibitor mixture (Sigma, 5 μl/100 mg tissue). After clearing debris by centrifuging at 14,000 × g at 4°C, protein concentration in the extracts was determined by Bradford assay (Bio-Rad, Hercules, CA). The extracts (~500 μg protein) were incubated with nonspecific IgG (2 μg) or polyclonal rabbit anti-NR2B (2 μg; Millipore) or anti-DAPK1 (2 μg, Sigma) overnight at 4°C, followed by the addition of 40 μl of Protein G-Sepharose (Sigma) for 3 hr at 4°C. The precipitates were washed four times with lysis buffer and denatured with SDS sample buffer and separated by 12% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes using a Bio-Rad mini-protein-III wet transfer unit overnight at 4°C. Transfer membranes were then incubated with blocking solution [5% nonfat dried milk dissolved in TBST buffer containing (mM); 10 Tris-HCl, 150 NaCl, and 0.1% Tween-20 for 1 hr at room temperature, washed three times, and incubated with anti-goat primary antibody against NR1 (1:1000, Santa Cruz), anti-rabbit antibody against NR2A (anti-NR2A, 1:1000; Millipore), NR2B (anti-NR2B, 1:1000; Millipore), PSD-95 (anti-PSD-95, 1:1000; Santa Cruz), or anti-DAPK1 (1: 1000, Sigma) for 1 hr at room temperature. Membranes were washed three times with TBST buffer and incubated with the appropriate secondary antibodies (1:1000 dilution) for 1 hr followed by washing four times. Signal detection was performed with an enhanced chemiluminescence kit (Amersham Biosciences, Arlington, IL). The lanes marked “input” were loaded with 10% of the starting material used for immunoprecipitation.
To determine direct binding of DAPK1 with NMDA receptor NR2B C-terminal domain, the GST-NR2B deletion mutants (GST-NR2B839–1482, GST-NR2B839–1261, GST-NR2B1261–1292, GST-NR2B1292–1304, and GST-NR2B1305–1482) were generated and purified. Purified GST fusion proteins were separated on SDS-PAGE and transferred onto a nitrocellulose membrane, which was washed with distilled water and blocked with TBST for 1 hr at room temperature. The membrane was then incubated with affinity binding buffer containing 50 mM Tris-HCl, (pH 7.5), 200 mM NaCl, 12 mM β-mercaptoethanol, 1.0% polyethylene glycol, 10 μg/ml protease inhibitors and 500 μg/ml purified flag-tagged DAPK1 (Invitrogen) for 1 hr at room temperature and washed four times for 5 min with affinity binding buffer. Bound DAPK1 was detected with anti-Flag (1: 2000, Invitrogen).
Protein Identification
Protein bands were excised manually from the Coomassie blue-stained gels and then digested automatically using a Proteineer DP protein digestion station (Bruker-Daltonics, Bremen, Germany). Tryptic products were analyzed using Nano-Liquid Chromatography-Tandem Mass Spectrometry (NLC/MS). Each spectrum was internally calibrated with mass signals of trypsin autolysis ions to reach a typical mass measurement accuracy of ± 10 ppm. Raw data were searched in the SWISS-PROT database.
Cell Cultures and Oxygen-Glucose Deprivation
Hippocampi were isolated from the E20 DAPK1 mice. Cells were dissociated and purified using papain dissociation kit (Worthington Biochemical Corporation) platted with the densities of 100–150 cells/mm for Ca2+ image or 800–1000 cells/mm for OGD on 19 mm coverslips coated with 30 μg/ml poly-D-lysine and 2 μg/ml laminin. Cells were placed in fresh serum-free Neurobasal Medium (21103, GIBCO) plus 2% B27 and fed every 4 days with fresh media and used after 18 days (DIV18). Oxygen/glucose deprivation (OGD) was induced by perfusion (2 ml/min) of a glucose-free bicarbonate-buffer containing (in mM) 120 NaCl, 5 KCl, 2.0 CaCl2, 0.8 MgCl2, 25 NaHCO3, (pH 7.4), at 32°C, saturated with 5% CO2/10% H2/85%N2 for 0, 5, 15, or 30 min. Zero or 30 min after OGD washout, the cultures were used for enzymatic assays. In all NMDA treatment, 10 μM glycine was included. The phosphorylated myosine light chain (MLC) at Thr-18 and Ser-18 (pMLC) that was catalyzed by an activated DAPK1 in the lysates was blotted with anti-pMLC (1:1000, Millipore) or anti-pDAPK1 (1:500, Millipore) that specifically recognizes the phosphorylated DAPK1 at Ser-308. For measurement of phosphorylation of the NMDA receptor NR2B subunit at Ser-1303 (pS1303), the NMDA receptor complex was immunoprecipitated using anti-NR1. The precipitates were then probed using anit-pS1303 (1:1000, anti-phospho-NR2B at Ser1303 with antigene: RRQHpSYDTF, Millipore). The bands were quantified using “the normalized method” of the densitometer Quantity One following the Quantity One user instructions (Bio-Rad). The intensities of the bands marked “0” in each gel were normalized as 1. Each of the other bands in the same gel was then normalized to “0” bands.
Ca2+ Imaging
For Ca2+ imaging, the neuronal cultures were loaded with fura-2-AM (1 μM, Invitrogen) for 30 min followed by 5 washes for 30 min. The cultures were then subjected to OGD for 30 min (OGD, pre-OGD-treated) or 0 min (control). The 30 min OGD was chosen because it activates DAPK1 without causing morphological changes, thereby ensuring accurate measurement of Ca2+ signals in single neurons. Immediately after OGD washout, the cultures were perfused at 4 ml/min with a glucose-containing bicarbonate buffer 135 NaCl, 5 KCl, 2.0 CaCl2, 25 NaHCO3, and 10 glucose, (pH 7.4), at 32°C, saturated with 5% CO2/10% H2/85%O2. Synaptic NMDA receptors were activated by applying 30 μM bicuculline (Bic)/2 mM 4-AP in the presence of 10 μM nifedipine. One min after Bic/4-AP, MK-801 (20 μM, Sigma) was applied for blocking synaptic NMDA receptor channels. Since MK-801 is an irreversible open channel blocker, activated synaptic NMDA receptor channels remained blocked after MK-801 washout. To ensure these synaptic NMDA receptor channels were not reactivated, we applied AMPA receptor antagonist CNQX (10 μM) to block neuronal spontaneous firing. The extrasynaptic NMDA receptors were activated by a 5 ms pulse of 100 μM NMDA/10 μM glycine (Sigma) in the presence of 10 μM CNQX (Tocris) and 10 μM nifendipine (Sigma). One min later 10 μM ifenprodil was applied using SmartSquirt 4-Micro-Perfusion System with ValveLink8.2 controller (AutoMate Scientific, Berkeley, CA). Fura-2 340 and 380 nm fluorescence imaging pairs were collected through an AxioCam MRM REV3 camera, which was equipped with an Axio Observer 200 microscope with a 63 × Fluor water immersion objective. The images were captured with 30 cycles/second using Axiovision 4.7 software in Carl Zeiss Imaging Station 35A with XP Pro. Calcium concentrations were expressed as a function of the [Ca2+]/Kd = [(F−Fmin)/(Fmax−F)]. The average values that were normalized to basal line (defined as 1.0) were determined for each 20 s period of measurement (60 images).
Supplementary Material
Acknowledgments
This work was supported by the American Heart Association (05553413YL; 0535201YC; and 0625532 XZ), National Institute of Health (NIH/NINDS, R01NS5051383YL and NIH/NIA R01AG033282YL). We thank Ryan Labadens at the Neuroscience Center of Excellence, Louisiana State University School of Medicine Health Sciences Center, and for assistance in manuscript preparation and discussion.
Footnotes
Supplemental Information includes Extended Experimental Procedures, three figures, and two tables and can be found with this article online at doi:10.1016/j.cell.2009.12.055.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, Mac-Donald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Bialik S, Kimchi A. The Death-Associated Protein Kinases: Structure, Function, and Beyond. Annu Rev Biochem. 2006;75:189–200. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci. 2003;23:4958–4966. doi: 10.1523/JNEUROSCI.23-12-04958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Drejer J, Benveniste H, Diemer NH, Schousboe A. Cellular origin of ischemia-induced glutamate release from brain tissue in vivo and in vitro. J Neurochem. 1985;45:145–151. doi: 10.1111/j.1471-4159.1985.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini Ael-D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Bellone C, Viviani B, Marinovich M, Meli E, Pellegrini-Giampietro DE, Cattabeni F, Di Luca M. Lack of PSD-95 drives hippocampal neuronal cell death through activation of an alpha CaMKII transduction pathway. Eur J Neurosci. 2002;16:777–786. doi: 10.1046/j.1460-9568.2002.02141.x. [DOI] [PubMed] [Google Scholar]
- Ghosh A. Neurobiology. Learning more about NMDA receptor regulation. Science. 2002;295:449–451. doi: 10.1126/science.1069391. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Hanson SK, Grotta JC, Waxham MN, Aronowski J, Ostrow P. Calcium/calmodulin-dependent protein kinase II activity in ischemia with reperfusion in rats. Stroke. 1994;25:466–473. doi: 10.1161/01.str.25.2.466. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Khanna S, Roy S, Park HA, Sen CK. Regulation of c-Src activity in glutamate-induced neurodegeneration. J Biol Chem. 2007;282:23482–23490. doi: 10.1074/jbc.M611269200. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and α-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci. 1999;19:1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Ellerby LM, Welsh K, Xie Z, Deveraux QL, Salvesen GS, Bredesen DE, Rosenthal RE, Fiskum G, Reed JC. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci USA. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Liu L, Lau L, Zhu DY, Wei JS, Zou S, Sun HS, Fu YP, Liu F, Lu Y. Expression of Ca2+-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Neuron. 2004;43:43–55. doi: 10.1016/j.neuron.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Kuwabara K, Takasawa K, Ohtsuki T, Xia Z, Storm D, Yanagihara T, Hori M, Matsumoto M. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. J Neurosci. 2001;21:9204–9213. doi: 10.1523/JNEUROSCI.21-23-09204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DR. The structure and function of glutamate receptor ion channels. Nat Rev Neurosci. 2002;3:91–101. doi: 10.1038/nrn725. [DOI] [PubMed] [Google Scholar]
- Marshall J, Dolan BM, Garcia EP, Sathe S, Tang X, Mao Z, Blair LA. Calcium channel and NMDA receptor activities differentially regulate nuclear C/EBPbeta levels to control neuronal survival. Neuron. 2003;39:625–639. doi: 10.1016/s0896-6273(03)00496-3. [DOI] [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulindependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Gorter JA, Bennett MV, Zukin RS. The GluR2 (GluRB) hypothesis: Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- Peng PL, Zhong XF, Tu WH, Soundarapandian MM, Molner P, Zhu DY, Liu SH, Lu Y. ADAR2-Dependent RNA Editing of AMPA Receptor Subunit GluR2 Determines Vulnerability of Neurons in Forebrain Ischemia. Neuron. 2006;49:719–733. doi: 10.1016/j.neuron.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Schumacher AM, Schavocky JP, Velentza AV, Mirzoeva S, Waterson DM. A calmodulin-regulated protein kinase linked to neuron survival is a substrate for the calmodulin-regulated death-associated protein kinase. Biochemistry. 2004;43:8116–8124. doi: 10.1021/bi049589v. [DOI] [PubMed] [Google Scholar]
- Simon RP, Swan JH, Meldrum BS. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984;226:850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Simpkins KL, Guttmann RP, Dong Y, Chen Z, Sokol S, Neumar RW, Lynch DR. Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci. 2003;23:11322–11331. doi: 10.1523/JNEUROSCI.23-36-11322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat G, Spivak-Kroizman T, Cohen O, Bialik S, Shani G, Berrisi H, Eisenstein M, Kimchi A. The pro-apoptotic function of death-associated protein kinase is controlled by a unique inhibitory autophosphorylation-based mechanism. J Biol Chem. 2001;276:47460–47467. doi: 10.1074/jbc.M105133200. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, et al. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- Sun MK, Hongpaisan J, Nelson TJ, Alkon DL. Poststroke neuronal rescue and synaptogenesis mediated in vivo by protein kinase C in adult brains. Proc Natl Acad Sci USA. 2008;105:13620–13625. doi: 10.1073/pnas.0805952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- Van Eldik LJ. Structure and enzymology of a death-associated protein kinase. Trends Pharmacol Sci. 2002;23:302–304. doi: 10.1016/s0165-6147(02)02049-7. [DOI] [PubMed] [Google Scholar]
- Velentza AV, Schumacher AM, Weiss C, Egli M, Watterson DM. A protein kinase associated with apoptosis and tumor suppression: structure, activity, and discovery of peptide substrates. J Biol Chem. 2001;276:38956–38965. doi: 10.1074/jbc.M104273200. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu SH, Fu YP, Wang JH, Lu Y. Cdk5 activation induces CA1 pyramidal cell death by direct phosphorylation of NMDA receptors. Nat Neurosci. 2003a;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Haditsch U, Tu W, Cochrane K, Ahmadian G, Tran L, Paw J, Wang Y, Mansuy I, et al. Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long-term depression at CA1 inhibitory synapses. J Neurosci. 2003b;23:826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Waxham MN, Grotta JC, Silva AJ, Strong R, Aronowski J. Ischemia-induced neuronal damage: a role for calcium/calmodulin-dependent protein kinase II. J Cereb Blood Flow Metab. 1996;16:1–6. doi: 10.1097/00004647-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.