Abstract
The Dana-Farber Cancer Institute (DFCI) ALL Consortium has been conducting multi-institutional clinical trials in childhood ALL since 1981. The treatment backbone has included 20–30 consecutive weeks of asparaginase during intensification and frequent vincristine/corticosteroid pulses during the continuation phase. Between 1985–2000, 1457 children aged 0–18 years were treated on four consecutive protocols: 85-01 (1985–7), 87-01 (1987–91), 91-01 (1991–5) and 95-01 (1996–2000). The 10-year event-free survival (EFS) ± standard error by protocol was 77.9 ± 2.8% (85-01), 74.2± 2.3 (87-01), 80.8 ± 2.1% (91-01) and 80.5 ± 1.8% (95-01). Approximately 82% of patients treated in the 1980s and 88% treated in the 1990s were long-term survivors. Both EFS and overall survival (OS) rates were significantly higher for patients treated in the 1990s compared with the 1980s (p=0.05 and 0.01, respectively). On the two protocols conducted in the 1990s, EFS was 79–85% for T-ALL patients and 75–78% for adolescents (age 10–18 years). Results of randomized studies revealed that dexrazoxane prevented acute cardiac injury without adversely impacting EFS or OS in high-risk patients and frequently-dosed intrathecal chemotherapy was an effective substitute for cranial radiation in standard-risk patients. Current studies continue to focus on improving efficacy while minimizing acute and late toxicities.
Keywords: Acute lymphoblastic leukemia, long-term follow-up, asparaginase, anthracycline
Introduction
The Dana-Farber Cancer Institute (DFCI) began conducting randomized clinical trials in childhood ALL in 1972. Studies performed during the 1970’s demonstrated improved event-free survival (EFS) for children who received doxorubicin (in addition to vincristine and prednisone) during the remission induction phase,(1) and for those who received weekly E.coli asparaginase during post-remission consolidation.(2)
In 1981, DFCI and several other institutions in the United States and Canada formed the DFCI ALL Consortium. The therapeutic backbone of Consortium trials included an intensive, multiagent induction phase, 20–30 weeks of asparaginase during post-remission consolidation, and frequent vincristine/corticosteroid pulses during the continuation phase. On Protocol 81-01 (1981–5), the first study conducted by the Consortium, patients were stratified for the first time into two risk groups. Therapy was de-intensified for patients considered at lower risk of relapse based on age, leukocyte count and immunophenotype; such patients received lower cumulative doses of both anthracycline and corticosteroid. The overall EFS for patients enrolled on that study (74% at 5 years) was relatively favorable compared with contemporaneous childhood ALL trials, especially for children with T-cell ALL (5-year EFS 77%).(3)
Clinical trials conducted between 1985–2000 focused on improving survival rates while minimizing acute and late toxicities.(4–7) Strategies which were tested to improve survival included: substitution of dexamethasone for prednisone during post-induction therapy (Protocol 91-01),(6) use of high-dose intravenous (IV) instead of standard-dose oral 6-mercaptopurine during the first of year of treatment (Protocol 91-01),(6) addition of high-dose methotrexate during the remission induction phase,(5) and intensification of treatment for patients considered at very high risk of relapse, including patients with presenting leukocyte counts > 100 × 109/L and infants (Protocols 85-01, 87-01 and 91-01).(4, 5, 8)Attempts to reduce toxicity included: testing administration of doxorubicin by continuous infusion (Protocol 91-01) and the addition of a cardioprotectant, dexrazoxane (Protocol 95-01) in high risk patients to minimize anthracycline-associated cardiotoxicity,(6, 7) testing alternative preparations of asparaginase (Protocols 91-01 and 95-01),(6, 7) and substituting intrathecal chemotherapy for cranial radiation in lower risk patients (Protocols 87-01 and 95-01).(5, 7) In this report, we update the results of the four consecutive clinical trials conducted by the DFCI ALL Consortium between 1985–2000.
Patients and Methods
Between 1985–2000, 1457 children aged 0–18 years with newly diagnosed ALL (excluding mature B-cell ALL) were enrolled on four consecutive DFCI ALL Consortium protocols: 85-01 (1981–5, N=220), 87-01 (1987–91, N=369), 91-01 (1991–5, N=377) and 95-01 (1996–2000, N=491). Patients were enrolled from the following DFCI ALL Consortium institutions: DFCI/Children's Hospital Boston (1985–2000), Hospital Sainte Justine, Montreal (1987–2000), Le Centre Hospitalier de L'Universite Laval, Quebec (1991–2000), Maine Medical Center/Maine Children's Cancer Program (1985–2000), McMaster Children's Hospital, Ontario (1985–2000), Mount Sinai Medical Center (1985–2000), Ochsner Clinic, New Orleans (1985–2000), San Jorge Children's Hospital, Puerto Rico (1991–2000), University of Massachusetts Medical Center (1985–1995), University of Puerto Rico, San Juan (1985–1991), and University of Rochester Medical Center, New York (1985–2000). The institutional review board of each participating institution approved all protocols. Informed consent was obtained from parents or guardians prior to instituting therapy.
Therapy
Details of therapy have been previously published.(4–7) Treatment was assigned based on risk group classification determined at the time of diagnosis (Table 1). On all protocols, there were four phases of therapy: Remission Induction, CNS-directed treatment, Intensification and Continuation. Details of each phase of therapy are displayed in Table 2. On Protocols 85-01, 87-01 and 91-01, the remission induction phase was preceded by an investigational window, which consisted of a single agent given 3–5 days before the initiation of multiagent chemotherapy. Results of investigational windows have been previously reported.(9–11)
Table 1.
Risk Group Classification on DFCI ALL Consortium Protocols (1985–2000)
| SR: All of the following |
HR: Any of the following |
VHR: Any of the following |
|
|---|---|---|---|
| Age | 1985–95: 2 to < 9 yrs | 1985–95: ≥ 9 yrs |
1985–2000: < 12 months |
| 1995–2000: 1 to < 10 yrs | 1995–2000: ≥ 10 yrs | ||
| WBC (× 109/L) | 1985–95: < 20 | 1985–91: 20 to < 100 | 1985–91: ≥ 100 |
| 1995–2000: < 50 | 1991–5: ≥ 20 | ||
| 1995–2000: ≥ 50 | |||
| Phenotype | B-precursor | T-cell | |
| CNS disease* | Absent | Present | |
|
Mediastinal Mass |
Absent | Present | |
| t(9;22) | Absent | Present | |
CNS defined as CNS-3 only from 1985–91 and as CNS-2 or CNS-3 from 1991–2000.
Table 2.
Therapy on DFCI ALL Consortium Protocols: 1985–2000
| Induction (4 weeks) | vincristine 1.5 mg/m2 q week (maximum=2 mg) (days 0, 7, 14, 21) |
| prednisone 40 mg/m2/day (days 0–28) | |
| doxorubicin 30 mg/m2/dose (days 0 and 1) | |
| Protocol 95-01: randomized +/− dexrazoxane 300 mg/m2 (HR only) | |
| Methotrexate × 1 dose (day 2): dose per protocol | |
| Protocol 85-01: 40 mg/m2 | |
| Protocol 87-01: 40 mg/m2 or 4 gram/m2 with leucovorin (randomized) | |
| Protocols 91-01 + 95-01: 4 gram/m2 with leucovorin | |
| Asparaginase | |
| Protocol 85-01: E.coli ASP × 1 dose (investigational window; 5 days pre-day 0) | |
| Protocol 87-01: E. coli, Erwinia or PEG ASP × 1 dose (randomized; investigational window; 5days pre-day 0) | |
| Protocol 91-01: None | |
| Protocols 95-01: E.coli or Erwinia ASP 25,000 IU/m2 × 1 dose (randomized; day 4) | |
| IT cytarabine* × 1 dose (day 0), IT chemotherapy day 14 | |
| CNS therapy (3 weeks) | vincristine 2.0 mg/m2 IV day 1 (maximum=2 mg) |
| 6MP 50 mg/m2/day orally days 1–15 | |
| HR only: doxorubicin 30 mg/m2 on day 1 | |
| Protocol 95-01: randomized +/− dexrazoxane 300 mg/m2 | |
| IT chemotherapy twice weekly × 4 doses | |
| Cranial Radiation per protocol (beginning day 1) | |
| Protocol 85-01: SR-18Gy; HR-24 Gy | |
| Protocol 87-01: SR-No XRT; HR-18 Gy | |
| Protocol 91-01: SR girls-No XRT; SR boys and HR-18 Gy. | |
| Protocol 95-01: SR: randomized-No XRT versus 18 Gy; HR-18 Gy | |
|
Intensification (20–30 weeks) |
Every 3 week cycles: |
| SR: vincristine 2.0 mg/m2 IV day 1 (maximum=2 mg) | |
| prednisone 40 mg/m2/day orally days 1–5 | |
| Protocol 91-01: dexamethasone 6 mg/m2/day instead of prednisone | |
| methotrexate 30 mg/m2 IV or IM days 1, 8, 15 | |
| 6MP 50 mg/m2/day orally days 1–15 | |
| Protocol 91-01: Randomized oral 6MP vs IV 6MP 1000 mg/m2 on days 1 and 8 of each cycle for first 12 months of treatment | |
| Asparaginase IM according to protocol: | |
| Protocols 85-01 + 87-01: E.coli ASP 25,000 IU/m2 weekly | |
| Protocol 91-01 (randomized): E.coli ASP 25,000 IU/m2 weekly or PEG ASP 2500 IU/m2 every 2-weeks | |
| Protocol 95-01 (randomized): E.coli ASP 25,000 IU/m2 weekly or Erwinia ASP 25000 IU/m2 weekly | |
| IT chemotherapy per text | |
| HR: same as SR patients, except prednisone dose higher (120 mg/m2/day orally days 1–5), no methotrexate, doxorubicin 30 mg/m2 day 1 of each cycle (cumulative dose of 300 mg/m2), randomized to be given alone or with dexrazoxane 300 mg/m2/dose | |
| Protocol 91-01: dexamethasone 18 mg/m2/day instead of prednisone | |
| Protocol 95-01: doxorubicin +/− dexrazoxane 300 mg/m2 (randomized) | |
|
Continuation (until 24 months CCR) |
Every 3 week cycles: |
| SR: same as intensification, except no asparaginase | |
| HR: same as SR patients | |
| IT chemotherapy per text | |
Abbreviations: IT intrathecal, SR standard risk, HR high risk, 6MP: 6-mercaptopurine, IV: intravenous, IM: intramuscular, CCR= continuous complete remission
IT cytarabine dosed according to age.(6) Patients with CNS leukemia at diagnoses (CNS-2 and CNS-3) received twice weekly doses of IT cytarabine until CSF was clear of blasts cells on three consecutive examinations.
The major differences in therapy among the trials were as follows:
Asparaginase: On Protocols 85-01 and 87-01, patients received 20 weeks of intramuscular E.coli asparaginase during the Intensification phase at a dose of 25,000 IU/m2/week. On Protocol 91-01, patients received 30 weeks of asparaginase during the Intensification phase and were randomized to receive either E.coli asparaginase 25,000 IU/m2/week or PEG asparaginase 2500 IU/m2 every 2 weeks. On Protocol 95-01, patients were randomized to receive either E.coli or Erwinia asparaginase 25000 IU/m2/week for 20 weeks during the Intensification phase.
Doxorubicin: The cumulative dose of doxorubicin for standard risk (SR) patients on all protocols was 60 mg/m2. For high risk (HR) patients, the cumulative dose of doxorubicin was 360 mg/m2 on Protocols 85-01, 87-01, and 91-01, and was 300 mg/m2 on Protocol 95-01. On Protocol 91-01, HR patients were randomized to receive doxorubicin 30 mg/m2/dose either as an IV bolus or a 48-hour continuous infusion. On Protocol 95-01, HR patients were randomized to receive doxorubicin 30 mg/m2/dose IV bolus alone or immediately preceded by dexrazoxane 300 mg/m2/dose.
Corticosteroid: On Protocols 85-01, 87-01 and 95-01, prednisone was used during the Intensification and Continuation phases. On Protocol 91-01, dexamethasone was used instead of prednisone during these phases.
Methotrexate during Induction: On Protocol 85-01, patients received low-dose methotrexate (40 mg/m2) as a single dose during the remission induction phase. On Protocol 87-01, patients were randomized to receive an induction dose of methotrexate as either low-dose or high-dose (4 gm/m2 over 1 hour, followed by leucovorin rescue). On Protocols 91-01 and 95-01, a single dose of high-dose methotrexate was given during induction.
6-Mercaptopurine(6-MP): On Protocol 91-01, patients were randomized to receive either standard, oral 6-MP (50 mg/m2/day on days 1–14 every 3-weeks) or high-dose, intravenous 6-MP (1,000 mg/m2/dose over 20 hours weekly × 2 every 3 weeks) for one year after completion of the remission induction phase; thereafter, all patients received standard, oral 6-MP. On Protocols 85-01, 87-01, and 95-01, all patients received standard, oral 6-MP during all post-induction phases of treatment.
- CNS-directed therapy:
- Protocol 85-01: SR patients received 18 Gy cranial radiation; HR patients received 24 Gy cranial radiation (22 Gy for patients aged 12–24 months). Cranial radiation was delayed until age 12 months for patients diagnosed during infancy. Total percentage of patients receiving cranial radiation was 100%.
- Protocol 87-01: All SR patients were initially treated without cranial radiation. Because of a higher than expected incidence of CNS relapse in SR boys, the protocol was amended in 1992 to allow one year of additional therapy for any SR boy in first remission, as previously described.(5) Additional therapy included 18 Gy cranial radiation. Forty of 60 eligible SR boys received this additional therapy. HR patients received 18 Gy cranial radiation in either daily or twice-daily fractions (randomized). Total percentage of patients receiving cranial radiation (including SR boys who received additional CNS-directed therapy after protocol amendment) was 66%.
- Protocol 91-01: SR girls were treated without radiation. SR boys and all HR patients received 18 Gy cranial radiation in either daily or twice-daily fractions (randomized). Total percentage of patients receiving cranial radiation was 76%.
- Protocol 95-01: SR patients were randomized to receive either intrathecal chemotherapy alone (every 9 weeks × 6 doses, then every 18 weeks) without radiation or 18 Gy cranial radiation. SR girls who met Protocol 91-01 SR criteria were directly assigned to receive no radiation. HR patients received 18 Gy cranial radiation in either daily or twice-daily fractions (randomized). Total percentage of patients receiving cranial radiation was 60%.
Philadelphia chromosome; t(9;22): Beginning in 1989, patients with t(9;22) were treated with allogeneic transplantation in first remission. This was the only indication for transplantation in first remission on all studies conducted after that date. The percentage of patients who were transplanted in first remission by study was 0% (Protocol 85-01), <1% (Protocol 87-01), 2% (Protocol 91-01) and <1% (Protocol 95-01).
Investigational Window: On Protocol 85-01, patients received a single dose of E.coli asparaginase, randomized to either 25,000 IU/m2 or 2,500 IU/m2, administered 5 days prior to the initiation of the remission induction phase. On Protocol 87-01, patients received a single dose of asparaginase, randomized to E.coli 25,000 IU/m2, Erwinia 25,000 IU/m2, or PEG 2,500 IU/m2, given 5 days prior to the initiation of the remission induction phase. On Protocol 91-01, patients received 3 days of corticosteroids, immediately followed by the remission induction phase. Those patients were randomized to receive prednisone 40 mg/m2/day, or dexamethasone 6, 18, or 150 mg/m2/day for three days. Protocol 95-01 did not have an Investigational Window.
Statistical Analysis
Outcome events included induction failure, induction death, death during remission, relapse, and second malignancy (meningiomas, basal cell carcinomas, and benign tumors were not considered second malignancies). For Protocols 85-01, 87-01 and 91-01, induction failure was defined as the failure to achieve complete remission (CR) at day 52 after diagnosis. For Protocol 95-01, induction failure was defined as persistent leukemia at day 30 after diagnosis. Event-free survival (EFS) was measured from the date of complete remission to the first event or until the date of last contact for event-free survivors. For EFS, induction failure and induction death were considered events at time zero. Overall survival (OS) was measured from the date of starting treatment to death from any cause. EFS and OS were estimated by the method of Kaplan and Meier and compared with the log-rank test.(12) Multivariable regression was performed using the Cox proportional hazard model to assess prognostic factors for EFS and OS for each protocol.(13)
Cumulative incidence functions of any CNS relapse, isolated CNS (no other site involved), and any testicular relapse were constructed using the method of Kalbfleish and Prentice(14) and compared with Gray’s test(15) for patients who achieved complete remission. In the estimation of these functions, all other failures were considered competing events.
Results
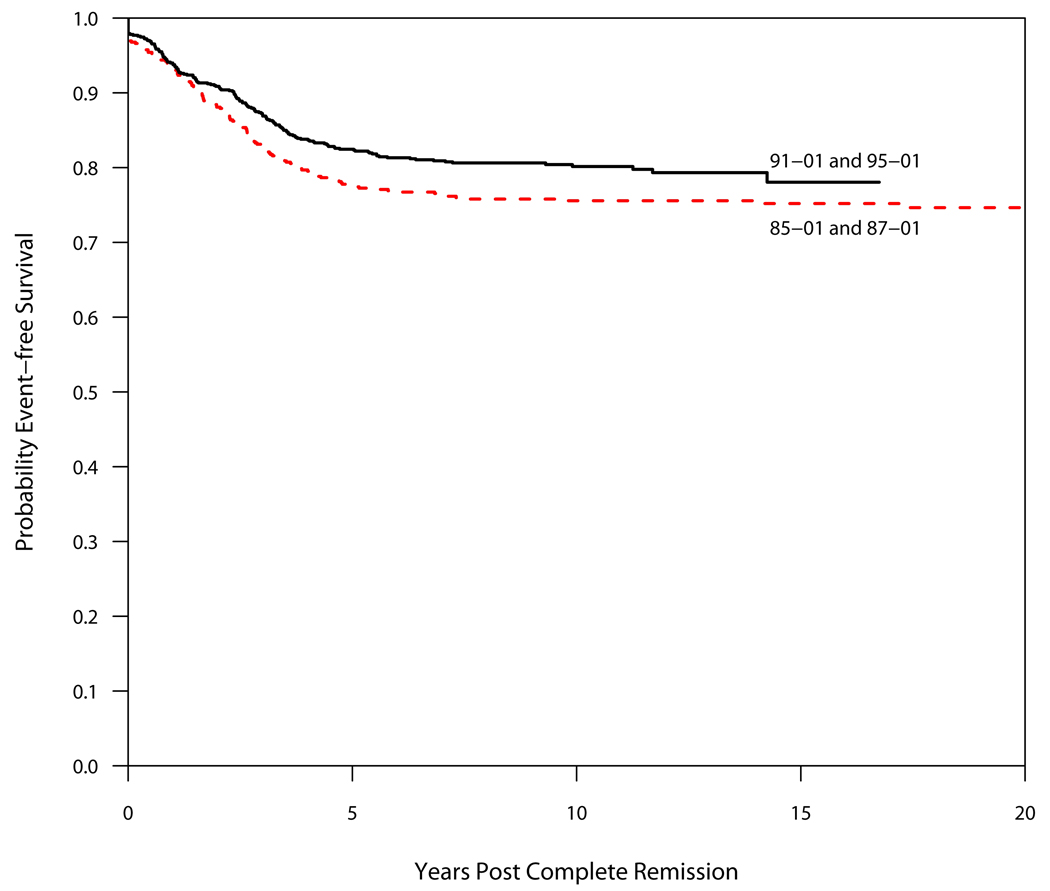
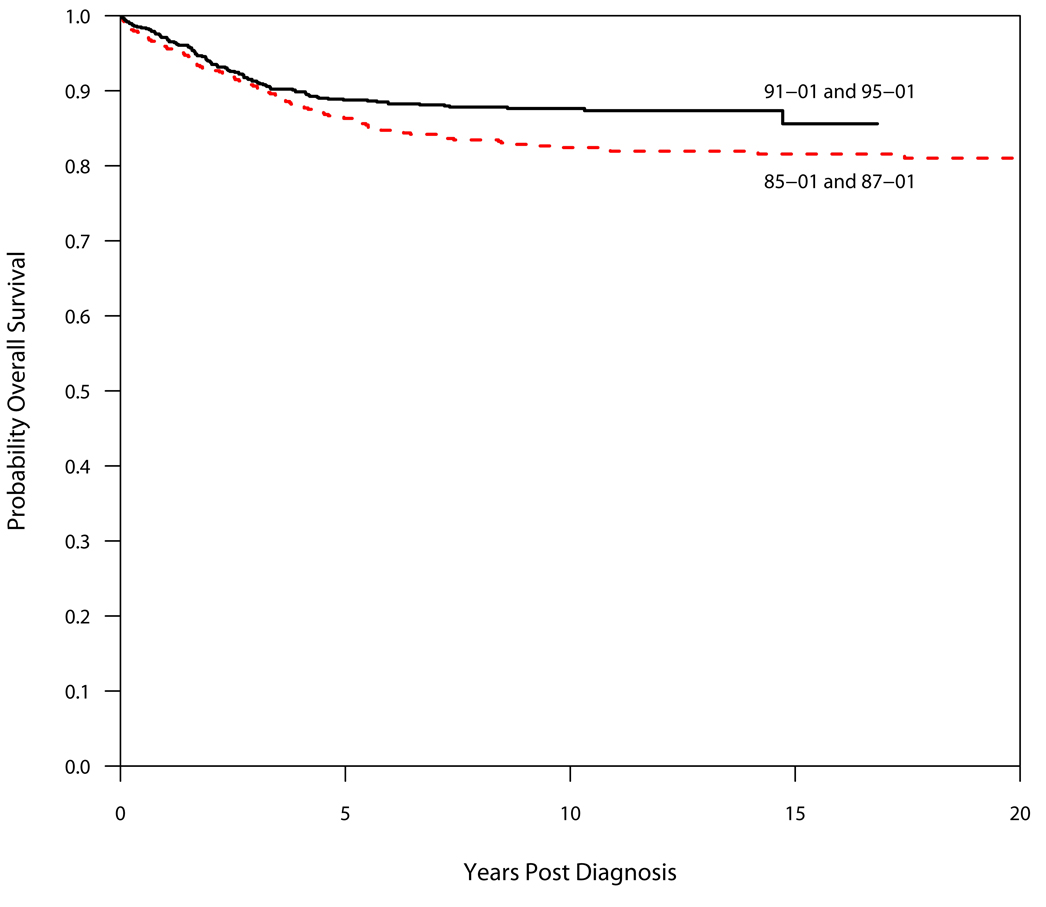
Between 1985–2000, 1457 children were enrolled on DFCI ALL Consortium Protocols. Table 3 and Table 4 summarize outcome by protocol. Of the 1423 patients who achieved complete remission (CR), 241 patients (17%) relapsed. All but three relapses occurred within 7.4 years from diagnosis. Thirty patients (2.1%) died in first remission and 8 patients (0.6%) were diagnosed with a second malignant neoplasm (SMN) as their first event. As of December 2008, 1246 patients (86%) are alive, of whom 1144 have never relapsed or been diagnosed with a SMN. Both the EFS and OS improved significantly from the clinical trials conducted in the 1980s (Protocol 85-01 and 87-01) to those in the 1990s (Protocols 91-01 and 95-01) (p=0.05 and 0.01, respectively; Figure 1 and Figure 2).
Table 3.
Outcome by Protocol (1985–2000)
| Protocol | 85-01 | 87-01 | 91-01 | 95-01 |
|---|---|---|---|---|
| N | 220 | 369 | 377 | 491 |
| Median f/u years | 13.8 | 13.3 | 12.5 | 8.6 |
| Remission (%) | 217 (99) | 356 (96) | 370 (98) | 480 (98) |
| Induction Failure (%) | 2 (0.9) | 9 (2.4) | 5 (1.3) | 7 (1.4) |
| Induction Death (%) | 1 (0.5) | 4 (2.2) | 2 (0.5) | 4 (0.8) |
| Remission Death (%) | 8 (3.6) | 7 (1.9) | 12 (3.2) | 3 (0.6) |
| Relapse (%) | 37 (16.8) | 72 (19.5) | 53 (14) | 79 (16) |
| Second Malignancy (%) | 1 (0.5) | 3 (0.8) | 1 (0.3) | 3 (0.6) |
| 10-yr CI Marrow Relapse (isolated or combined) |
13.1% ± 2.3% | 15.9% ± 2.0% | 12.2% ± 1.7% | 15.9% ± 1.8% |
| 10- yr CI Any CNS Relapse (isolated or combined) |
3.7% ± 1.3% | 5.9% ± 1.3% | 4.2% ± 1.1% | 3.8% ± 1.0% |
| 10- yr Isolated CNS Relapse | 2.8% ± 1.1% | 4.2% ± 1.1% | 1.1% ± 0.5% | 0.7% ± 0.4% |
| 10-yr CI Any Testicular Relapse (males only) |
0.9% ± 0.9% | 1.0% ± 0.7% | 1.5% ± 0.9% | 1.9% ± 0.9% |
| 10-yr CI Second Malignancy | 0.5 ± 0.5% | 0.9 ± 0.5% | 0.3 ± 0.3% | 1.1 ± 0.9% |
| 10 yr EFS ± SE (%) † | 77.9 ± 2.8 | 74.2 ± 2.3 | 80.8 ± 2.1 | 79.0 ± 2.1 |
| 10 yr OS ± SE (%) † | 80.9 ± 2.7 | 83.3 ± 2.0 | 86.2 ± 1.8 | 88.9 ± 1.5 |
CI: Cumulative Incidence; SE: Standard Error
Table 4.
Timing of Events for Patients Achieving a CR
| Protocol | 85-01 | 87-01 | 91-01 | 95-01 | |
|---|---|---|---|---|---|
| Patients achieving CR | 217 | 356 | 370 | 480 | |
| Events 0–5 years in CCR | 44 (20%) | 72 (20%) | 55 (15%) | 77 (16%) | |
| Relapse | 36 | 65 | 43 | 73 | |
| SMN | 1 | 2 | 0 | 1 | |
| Remission Death | 7 | 5 | 12 | 3 | |
| Events 5–10 years in CCR | 1 (0.5%) | 9 (2.5%) | 9 (2.4%) | 7 (1.5%) | |
| Relapse | 1 | 7 | 8 | 6 | |
| SMN | 0 | 1 | 1 | 1 | |
| Remission Death | 0 | 1 | 0 | 0 | |
| Events ≥10 years in CCR | 1 (0.5%) | 1 (0.3%) | 2 (0.5%) | 1 (0.2%) | |
| Relapse | 0 | 0 | 2 | 0 | |
| SMN | 0 | 0 | 0 | 1 | |
| Remission Death | 1 | 1 | 0 | 0 | |
| # Free from Adverse Events | 171 (79%) | 274 (77%) | 304 (82%) | 395 (82%) | |
| # Alive | 178 | 306 | 324 | 438 | |
Figure 1.
Event-Free Survival (EFS) by Decade. The EFS of protocols conducted in the 1990s (91-01 and 95-01) was superior to that of protocols conducted in the 1980s (85-01 and 87-01), p=0.05.
Figure 2.
Overall Survival (OS) by Decade. The OS of protocols conducted in the 1990s (91-01 and 95-01) was superior to that of protocols conducted in the 1980s (85-01 and 87-01), p=0.01.
Protocol 85-01(1985–1987)
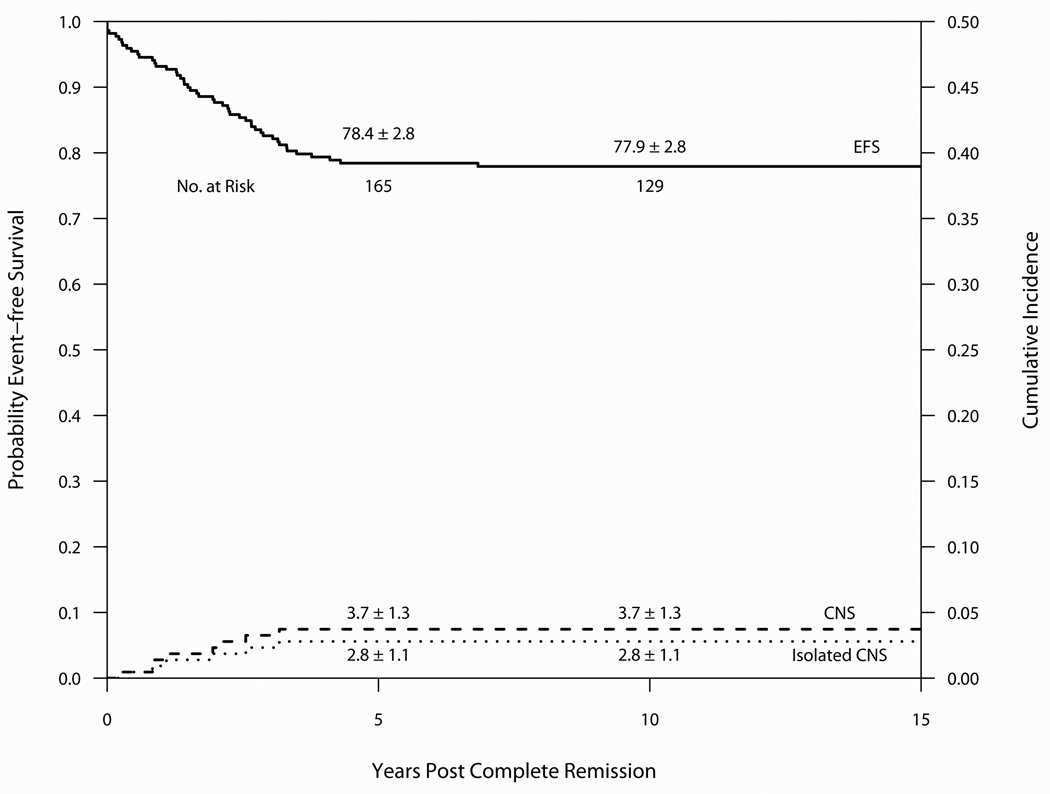
Outcome on Protocol 85-01 is summarized in Table 3 and Figure 3. Median follow-up was 13.8 years. Of the 220 evaluable patients, 217 entered CR (99%), 37 relapsed (16.8%) and 8 patients died in CR (3.6%). 171 (78%) remain alive and free of adverse events. The 10-year cumulative incidence estimates for isolated marrow and any marrow relapses were 12.2 ± 2.2% and 13.1 ± 2.3%, respectively. The 10-year cumulative incidence estimates for isolated CNS and any CNS relapses were 2.8 ± 1.1% and 3.7 ± 1.3%, respectively. Of the 116 evaluable male patients, the 10-year cumulative incidence of any testicular relapse was 0.9 ± 0.9% (1 patient had an isolated testicular relapse). One patient experienced a SMN (AML) as a first event. One other patient developed a basal cell carcinoma in a previous radiation field. Two patients (1.0%) experienced their first event after 5-years of complete continuous remission (CCR) (1 relapse and 1 remission death).(Table 4) The 10-year EFS and OS were 77.9 ± 2.8% and 80.9 ± 2.7%, respectively. For SR patients, the 10-year EFS and OS rates were 88.8 ± 3.5% and 92.4 ± 3.0%, and the EFS and OS rates for HR/VHR patients were 71.6 ± 3.9% and 74.2 ± 3.8%. Univariate predictors of outcome are displayed in Table 5. Multivariable regression analysis including age, sex, presenting leukocyte count, phenotype and CNS status at diagnosis identified only presenting WBC > 100K as an adverse independent predictor of both EFS (Hazard Ratio 5.08, p<0.01) and OS (Hazard Ratio 5.71, p<0.01).
Figure 3.
Event-free survival and Cumulative Incidence of isolated or any CNS relapse for 220 patients treated on Protocol 85-01 (1985–7). Median follow-up was 13.8 years.
Table 5.
Protocol 85-01 Outcome by Patient Characteristics
| Event-free Survival ± SE (%) | Overall Survival ± SE (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protocol | N | 5-yr EFS | 10-yr EFS | 12-yr EFS | p-value | 5-yr OS | 10-yr OS | 12-yr OS | p-value |
| Overall | 220 | 78.4 ± 2.8 | 77.9 ± 2.8 | 77.9 ± 2.8 | 83.9 ± 2.5 | 80.9 ± 2.7 | 80.9 ± 2.7 | ||
| Immunophenotype | |||||||||
| B-precursor | 200 | 79.3 ± 2.9 | 78.7 ± 2.9 | 78.7 ± 2.9 | 0.262 | 85.3 ± 2.5 | 82.0 ± 2.8 | 82.0 ± 2.8 | 0.122 |
| T cell | 20 | 70.0 ± 10.3 | 70.0 ± 10.3 | 70.0 ± 10.3 | 70.0 ± 10.3 | 70.0 ± 10.3 | 70.0 ± 10.3 | ||
| B-precursor† | |||||||||
| NCI Standard | 130 | 84.4 ± 3.2 | 84.4 ± 3.2 | 84.4 ± 3.2 | 0.035 | 89.8 ± 2.7 | 88.1 ± 2.9 | 88.1 ± 2.9 | 0.015 |
| NCI High | 60 | 71.6 ± 5.8 | 69.8 ± 6.0 | 69.8 ± 6.0 | 79.9 ± 5.2 | 72.7 ± 5.9 | 72.7 ± 5.9 | ||
| T-lineage† | |||||||||
| NCI Standard | 6 | 83.3 ± 15.2 | 83.3 ± 15.2 | 83.3 ± 15.2 | 0.356 | 83.3 ± 15.2 | 83.3 ± 15.2 | 83.3 ± 15.2 | 0.354 |
| NCI High | 14 | 64.3 ± 12.8 | 64.3 ± 12.8 | 64.3 ± 12.8 | 64.3 ± 12.8 | 64.3 ± 12.8 | 64.3 ± 12.8 | ||
| Sex | |||||||||
| Male | 116 | 74.7 ± 4.1 | 73.8 ± 4.1 | 73.8 ± 4.1 | 0.198 | 83.4 ± 3.5 | 78.7 ± 3.9 | 78.7 ± 3.9 | 0.552 |
| Female | 104 | 82.6 ± 3.7 | 82.6 ± 3.7 | 82.6 ± 3.7 | 84.5 ± 3.6 | 83.5 ± 3.7 | 83.5 ± 3.7 | ||
| Age at Diagnosis | |||||||||
| <1 year | 10 | 60.0 ± 15.5 | 60.0 ± 15.5 | 60.0 ± 15.5 | 0.122 | 60.0 ± 15.5 | 60.0 ± 15.5 | 60.0 ± 15.5 | 0.043 |
| 1–9 years | 168 | 80.7 ± 3.1 | 80.0 ± 3.1 | 80.0 ± 3.1 | 86.1 ± 2.7 | 84.2 ± 2.8 | 84.2 ± 2.8 | ||
| ≥10 years | 42 | 73.7 ± 6.8 | 71.2 ± 7.0 | 71.2 ± 7.0 | 80.8 ± 6.1 | 72.6 ± 7.1 | 72.6 ± 7.1 | ||
| WBC (× 109/L) | |||||||||
| <10 | 98 | 86.6 ± 3.5 | 85.5 ± 3.6 | 85.5 ± 3.6 | 0.0004 | 90.7 ± 3.0 | 88.4 ± 3.3 | 88.4 ± 3.3 | 0.0002 |
| 10–49 | 77 | 76.2 ± 4.9 | 76.2 ± 4.9 | 76.2 ± 4.9 | 84.0 ± 4.2 | 79.7 ± 4.7 | 79.7 ± 4.7 | ||
| 50–99 | 18 | 83.3 ± 8.8 | 83.3 ± 8.8 | 83.3 ± 8.8 | 83.3 ± 8.8 | 83.3 ± 8.8 | 83.3 ± 8.8 | ||
| ≥100 | 27 | 51.9 ± 9.6 | 51.9 ± 9.6 | 51.9 ± 9.6 | 59.3 ± 9.5 | 55.6 ± 9.6 | 55.6 ± 9.6 | ||
| CNS leukemia | |||||||||
| CNS-3 | 4 | 50.0 ± 25.0 | 50.0 ± 25.0 | 50.0 ± 25.0 | 0.057 | 50.0 ± 25.0 | 50.0 ± 25.0 | 50.0 ± 25.0 | 0.035 |
| Other | 216 | 79.0 ± 2.8 | 78.4 ± 2.8 | 78.4 ± 2.8 | 84.5 ± 2.5 | 81.5 ± 2.7 | 81.5 ± 2.7 | ||
| t(9;22) | |||||||||
| Present | 3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | <0.0001 | 33.3 ± 27.2 | 33.3 ± 27.2 | 33.3 ± 27.2 | 0.011 |
| All others | 217 | 79.5 ± 2.8 | 79.0 ± 2.8 | 79.0 ± 2.8 | 84.6 ± 2.5 | 81.6 ± 2.7 | 81.6 ± 2.7 | ||
| DFCI Risk Group | |||||||||
| Standard | 82 | 88.8 ± 3.5 | 88.8 ± 3.5 | 88.8 ± 3.5 | 0.003 | 93.7 ± 2.7 | 92.4 ± 3.0 | 92.4 ± 3.0 | 0.0008 |
| High/Very High | 138 | 72.4 ± 3.8 | 71.6 ± 3.9 | 71.6 ± 3.9 | 78.1 ± 3.5 | 74.2 ± 3.8 | 74.2 ± 3.8 | ||
Excludes and infants <1 year age.
Protocol 87-01 (1987–1991)
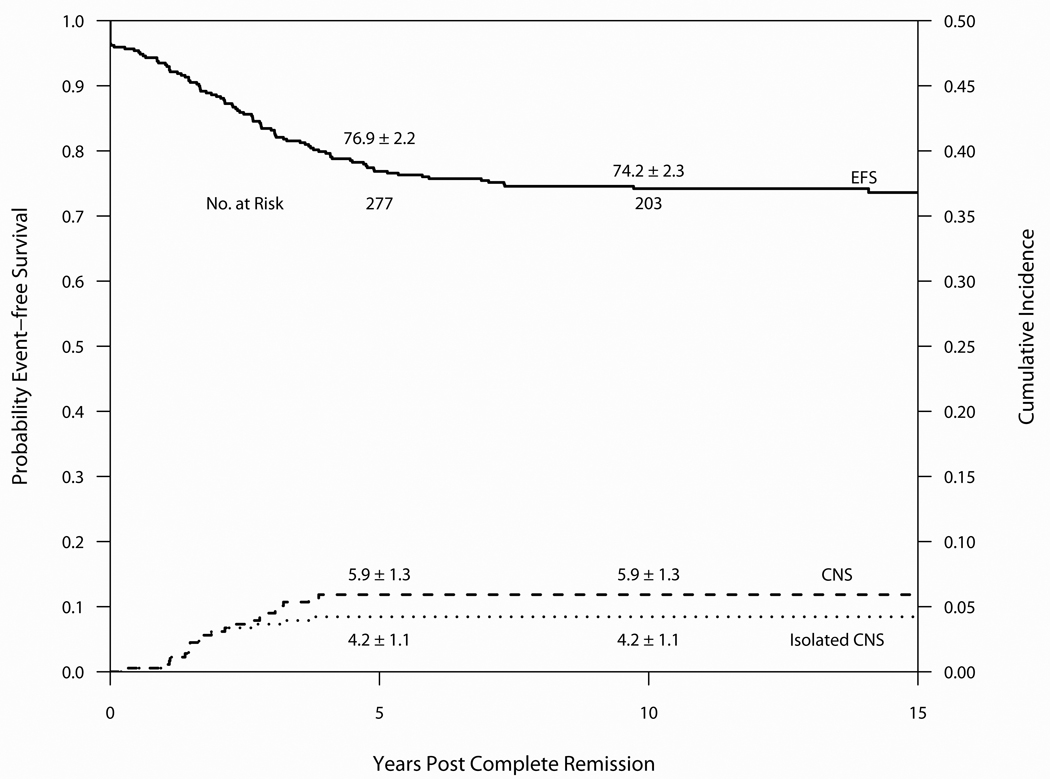
Outcome on Protocol 87-01 is summarized in Table 3 and Figure 4. Median follow-up was 13.3 years. Of the 369 evaluable patients, 356 entered CR (96%), 72 relapsed (19.5%) and 7 patients died in CR (1.9%). 274 (74%) remain alive and free of adverse events. The 10-year cumulative incidence estimates for isolated marrow and any marrow relapses were 13.1 ± 1.8% and 15.9 ± 2.0%, respectively. The 10-year cumulative incidence estimates for isolated CNS and any CNS relapses were 4.2 ± 1.1% and 5.9 ± 1.3%, respectively. Of the 216 evaluable male patients, the 10-year cumulative incidence of any testicular relapse was 1.0 ± 0.7% (no isolated testicular relapses observed). Three patients experienced a SMN as a first event (two cases of AML and one parotid gland carcinoma). The parotid gland had been previously irradiated as part of CNS-directed therapy. Other tumors arising within previous fields of radiation included a meningioma in one patient and a benign fibrous tumor of the left orbit in another patient. Ten patients (2.7%) experienced their first event after 5-years of CCR (7 relapses, 1 SMN, 2 remission deaths).(Table 4) The 10-year EFS and OS were 74.2 ± 2.3% and 83.3 ± 2.0%, respectively. For SR patients, the 10-year EFS and OS rates were 77.4 ± 3.5% and 92.1 ± 2.3%, and the rates for HR/VHR patients were 72.2 ± 3.0% and 77.8 ± 2.8%.
Figure 4.
Event-free survival and Cumulative Incidence of isolated or any CNS relapse for 369 patients treated on Protocol 87-01 (1987–91). Median follow-up was 13.3 years.
Outcome by patient characteristics is presented in Table 6. On univariate analysis, male sex, in addition to age and presenting leukocyte count, was identified as a significant predictor of both EFS and OS, primarily due to a high rate of CNS relapses in SR boys compared with SR girls (10-year CI of 20.3 ± 4.6% for boys compared with 4.8 ± 2.7% for girls, p<0.01). Multivariable regression analysis including age, sex, presenting leukocyte count, phenotype and CNS status at diagnosis identified male sex (Hazard Ratio 1.79, p=0.01), WBC 10–49K (Hazard Ratio 2.33, p<0.01) and WBC ≥ 100K (Hazard Ratio 2.69, p<0.01) as independent adverse predictors of EFS. WBC ≥ 100K (Hazard Ratio 3.04, p<0.01), WBC 10–49K (Hazard Ratio 2.22, p<0.01) and age ≥ 10 years (Hazard Ratio 2.11, p < 0.01), but not male sex, were independent adverse predictors of OS.
Table 6.
Protocol 87-01 Outcome by Patient Characteristics
| Event-free Survival ± SE (%) | Overall Survival ± SE (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protocol | N | 5-yr EFS | 10-yr EFS | 12-yr EFS | p-value | 5-yr OS | 10-yr OS | 12-yr OS | p-value |
| Overall | 369 | 76.9 ± 2.2 | 74.2 ± 2.3 | 74.2 ± 2.3 | 87.7 ± 1.7 | 83.3 ± 2.0 | 82.5 ± 2.0 | ||
| Immunophenotype | |||||||||
| B-precursor | 326 | 77.5 ± 2.3 | 74.5 ± 2.4 | 74.5 ± 2.4 | 0.600 | 89.5 ± 1.7 | 84.5 ± 2.0 | 83.6 ± 2.1 | 0.067 |
| T cell | 38 | 73.7 ± 7.1 | 73.7 ± 7.1 | 73.7 ± 7.1 | 76.3 ± 6.9 | 76.3 ± 6.9 | 76.3 ± 6.9 | ||
| Unknown | 5 | 60.0 ± 21.9 | N/A | N/A | 60.0 ± 21.9 | 60.0 ± 21.9 | N/A | ||
| B-precursor† | |||||||||
| NCI Standard | 225 | 78.1 ± 2.8 | 76.7 ± 2.8 | 76.7 ± 2.8 | 0.340 | 90.2 ± 2.0 | 88.3 ± 2.2 | 87.6 ± 2.3 | 0.011 |
| NCI High | 95 | 76.6 ± 4.4 | 69.6 ± 4.8 | 68.0 ± 4.8 | 88.2 ± 3.3 | 75.4 ± 4.6 | 74.0 ± 4.7 | ||
| T-lineage† | |||||||||
| NCI Standard | 10 | 80.0 ± 12.7 | 80.0 ± 12.7 | 80.0 ± 12.7 | 0.876 | 80.0 ± 12.7 | 80.0 ± 12.7 | 80.0 ± 12.7 | 0.896 |
| NCI High | 26 | 76.9 ± 8.3 | 76.9 ± 8.3 | 71.4 ± 8.3 | 80.8 ± 7.7 | 80.8 ± 7.7 | 80.8 ± 7.7 | ||
| Sex | |||||||||
| Male | 216 | 71.6 ± 3.1 | 69.1 ± 3.2 | 69.1 ± 3.2 | 0.005 | 84.7 ± 2.5 | 80.1 ± 2.8 | 79.4 ± 2.8 | 0.042 |
| Female | 153 | 84.2 ± 3.0 | 81.5 ± 3.2 | 81.5 ± 3.2 | 92.1 ± 2.2 | 87.9 ± 2.7 | 87.0 ± 2.8 | ||
| Age at Diagnosis | |||||||||
| <1 year | 8 | 50.0 ± 17.7 | 50.0 ± 17.7 | 50.0 ± 17.7 | 0.048 | 62.5 ± 17.1 | 62.5 ± 17.1 | 62.5 ± 17.1 | 0.003 |
| 1–9 years | 291 | 78.3 ± 2.4 | 76.8 ± 2.5 | 76.8 ± 2.5 | 89.7 ± 1.8 | 86.8 ± 2.0 | 86.2 ± 2.1 | ||
| ≥10 years | 70 | 74.0 ± 5.3 | 66.0 ± 5.8 | 61.2 ± 5.8 | 82.4 ± 4.6 | 70.7 ± 5.7 | 68.5 ± 5.9 | ||
| WBC (× 109/L) | |||||||||
| <10 | 170 | 85.8 ± 2.7 | 83.3 ± 2.9 | 82.3 ± 2.9 | 0.0004 | 93.5 ± 1.9 | 90.1 ± 2.4 | 89.2 ± 2.5 | 0.004 |
| 10–49 | 121 | 67.7 ± 4.3 | 64.8 ± 4.4 | 64.8 ± 4.4 | 83.4 ± 3.4 | 79.1 ± 3.7 | 77.9 ± 3.9 | ||
| 50–99 | 36 | 83.3 ± 6.2 | 80.0 ± 6.8 | 80.0 ± 6.8 | 91.7 ± 4.6 | 82.8 ± 6.4 | 82.8 ± 6.4 | ||
| ≥100 | 42 | 61.9 ± 7.5 | 61.9 ± 7.5 | 61.9 ± 7.5 | 73.8 ± 6.8 | 68.8 ± 7.2 | 68.8 ± 7.2 | ||
| CNS leukemia | |||||||||
| CNS-3 | 2 | 50.0 ± 35.4 | 50.0 ± 35.4 | 50.0 ± 35.4 | 0.288 | 50.0 ± 35.4 | 50.0 ± 35.4 | 50.0 ± 35.4 | 0.211 |
| Other | 363 | 76.8 ± 2.2 | 74.1 ± 2.3 | 74.1 ± 2.3 | 87.8 ± 1.7 | 83.3 ± 2.0 | 82.5 ± 2.1 | ||
| Unknown | 4 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | ||
| t(9;22) | |||||||||
| Present | 7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | <0.0001 | 14.3 ± 13.2 | 14.3 ± 13.2 | 14.3 ± 13.2 | <0.0001 |
| All others | 362 | 78.4 ± 2.2 | 75.6 ± 2.3 | 75.6 ± 2.3 | 89.2 ± 1.6 | 84.7 ± 1.9 | 83.8 ± 2.0 | ||
| DFCI Risk Group | |||||||||
| Standard | 142 | 78.2 ± 3.5 | 77.4 ± 3.5 | 77.4 ± 3.5 | 0.347 | 93.7 ± 2.0 | 92.1 ± 2.3 | 91.0 ± 2.5 | 0.001 |
| High/Very High | 227 | 76.0 ± 2.9 | 72.2 ± 3.0 | 72.2 ± 3.0 | 84.0 ± 2.4 | 77.8 ± 2.8 | 77.2 ± 2.9 | ||
| Methotrexate-induction dose (randomized) |
|||||||||
| High-dose | 175 | 78.7 ± 3.1 | 76.1 ± 3.3 | 76.1 ± 3.3 | 0.622 | 86.7 ± 2.6 | 83.3 ± 2.9 | 82.5 ± 3.0 | 0.662 |
| Low-dose | 178 | 76.3 ± 3.2 | 73.4 ± 3.3 | 73.4 ± 3.3 | 89.9 ± 2.3 | 84.6 ± 2.7 | 83.8 ± 2.8 | ||
Excludes unknown lineage and infants <1 year age.
Protocol 91-01 (1991–1996)
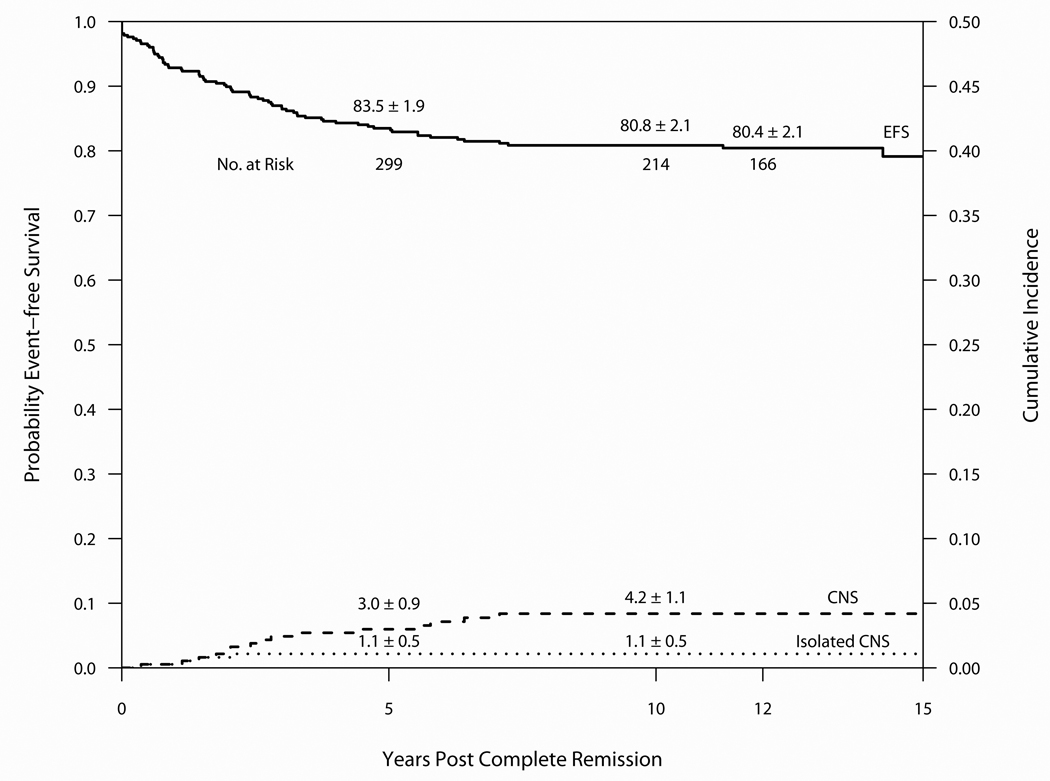
Outcome on Protocol 91-01 is summarized in Table 3 and Figure 5. Median follow-up was 12.5 years. Of the 377 evaluable patients, 370 entered CR (98%), 53 relapsed (14.1%) and 12 patients died in CR (3.2%). 304 (81%) remain alive and free of adverse events. The 10-year CI estimates for isolated marrow and any marrow relapses were 8.8 ± 1.5% and 12.2 ± 1.7%, respectively. The 10-year CI estimates for isolated CNS and any CNS relapses were 1.1 ± 0.5% and 4.2 ± 1.1%, respectively. Of the 199 evaluable male patients, the 10-year cumulative incidence for isolated or any testicular relapse was 1.0 ± 0.7% and 1.5 ± 0.9%. One patient experienced a SMN as a first event (malignant brain tumor in a previously irradiated patient) and two others were diagnosed with meningiomas (both previously irradiated). Eleven patients (3.0%) experienced their first event after 5-years of CCR (10 relapses, 1 SMN).(Table 4) The 10-year EFS and OS were 80.8 ± 2.1% and 86.2 ± 1.8%, respectively. For SR patients, the 10-year EFS and OS rates were 84.3 ± 3.2% and 91.0 ± 2.5, and the rates for HR/VHR patients were 78.9 ± 2.7% and 83.5 ± 2.4%.
Figure 5.
Event-free survival and Cumulative Incidence of isolated or any CNS relapse for 377 patients treated on Protocol 91-01 (1991–5). Median follow-up was 12.5 years.
Outcome by patient characteristic is presented in Table 7. Multivariable regression analysis including age, sex, presenting leukocyte count, phenotype and CNS status at diagnosis identified only CNS-3 status as an independent adverse predictor of EFS (Hazard Ratio 4.36, p=0.02). Although no independent predictors were identified when these same variables were included in a multivariable regression analysis for OS, univariate analysis indicated that HR/VHR patients had a significantly lower OS than SR patients (p=0.04).
Table 7.
Protocol 91-01 Outcome by Patient Characteristics
| Event-free Survival ± SE (%) | Overall Survival ± SE (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protocol | N | 5-yr EFS | 10-yr EFS | 12-yr EFS | p-value | 5-yr OS | 10-yr OS | 12-yr OS | p-value |
| Overall | 377 | 83.5 ± 1.9 | 80.8 ± 2.1 | 80.0 ± 2.1 | 87.8 ± 1.7 | 86.2 ± 1.8 | 85.8 ± 1.8 | ||
| Immunophenotype | |||||||||
| B-precursor | 343 | 83.9 ± 2.0 | 81.0 ± 2.1 | 80.1 ± 2.2 | 0.866 | 88.3 ± 1.7 | 86.6 ± 1.9 | 86.2 ± 1.9 | 0.725 |
| T cell | 28 | 78.6 ± 7.8 | 78.6 ± 7.8 | 78.6 ± 7.8 | 82.1 ± 7.2 | 82.1 ± 7.2 | 82.1 ± 7.2 | ||
| Unknown | 6 | 83.3 ± 15.2 | 83.3 ± 15.2 | 83.3 ± 15.2 | 83.3 ± 15.2 | 83.3 ± 15.2 | 83.3 ± 15.2 | ||
| B-precursor† | |||||||||
| NCI Standard | 239 | 85.7 ± 2.3 | 82.5 ± 2.5 | 81.9 ± 2.5 | 0.370 | 90.3 ± 1.9 | 89.3 ± 2.0 | 88.7 ± 2.1 | 0.061 |
| NCI High | 97 | 80.4 ± 4.0 | 78.0 ± 4.3 | 78.0 ± 4.3 | 84.5 ± 3.7 | 80.9 ± 4.1 | 80.9 ± 4.1 | ||
| T-lineage† | |||||||||
| NCI Standard | 5 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 0.218 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 0.274 |
| NCI High | 23 | 73.9 ± 9.2 | 73.9 ± 9.2 | 73.9 ± 9.2 | 78.3 ± 8.6 | 78.3 ± 8.6 | 78.3 ± 8.6 | ||
| Sex | |||||||||
| Male | 199 | 83.9 ± 2.6 | 81.6 ± 2.8 | 80.7 ± 2.9 | 0.880 | 87.4 ± 2.4 | 85.6 ± 2.5 | 84.8 ± 2.6 | 0.669 |
| Female | 178 | 83.1 ± 2.8 | 80.0 ± 3.0 | 80.0 ± 3.0 | 88.2 ± 2.4 | 86.9 ± 2.6 | 86.9 ± 2.6 | ||
| Age at Diagnosis | |||||||||
| <1 year | 7 | 71.4 ± 17.1 | 71.4 ± 17.1 | 71.4 ± 17.1 | 0.179 | 71.4 ± 17.1 | 71.4 ± 17.1 | 71.4 ± 17.1 | 0.075 |
| 1–9 years | 301 | 84.6 ± 2.1 | 81.7 ± 2.3 | 81.2 ± 2.3 | 89.7 ± 1.8 | 88.1 ± 1.9 | 87.6 ± 2.0 | ||
| ≥10 years | 69 | 79.7 ± 4.9 | 78.1 ± 5.0 | 78.1 ± 5.0 | 81.1 ± 4.7 | 79.5 ± 4.9 | 79.5 ± 4.9 | ||
| WBC (× 109/L) | |||||||||
| <10 | 191 | 82.6 ± 2.8 | 78.1 ± 3.0 | 78.1 ± 3.0 | 0.032 | 86.8 ± 2.5 | 85.6 ± 2.6 | 84.9 ± 2.7 | 0.234 |
| 10–49 | 117 | 87.1 ± 3.1 | 87.1 ± 3.1 | 85.7 ± 3.4 | 90.5 ± 2.7 | 89.6 ± 2.9 | 89.6 ± 2.9 | ||
| 50–99 | 28 | 89.3 ± 5.9 | 89.3 ± 5.9 | 89.3 ± 5.9 | 92.9 ± 4.9 | 89.1 ± 5.9 | 89.1 ± 5.9 | ||
| ≥100 | 41 | 73.1 ± 6.9 | 70.2 ± 7.3 | 70.2 ± 7.3 | 80.5 ± 6.2 | 77.5 ± 6.6 | 77.5 ± 6.6 | ||
| CNS leukemia | |||||||||
| CNS-3 | 5 | 40.0 ± 21.9 | 40.0 ± 21.9 | 40.0 ± 21.9 | 0.008 | 80.0 ± 17.9 | 80.0 ± 17.9 | 80.0 ± 17.9 | 0.384 |
| Other | 365 | 84.3 ± 1.9 | 81.6 ± 2.1 | 81.2 ± 2.1 | 88.2 ± 1.7 | 86.6 ± 1.8 | 86.2 ± 1.9 | ||
| Unknown | 7 | 71.4 ± 17.1 | 71.4 ± 17.1 | 71.4 ± 17.1 | 71.4 ± 17.1 | 71.4 ± 17.1 | 71.4 ± 17.1 | ||
| t(9;22) | |||||||||
| Present | 6 | 50.0 ± 20.4 | 50.0 ± 20.4 | 50.0 ± 20.4 | 0.020 | 50.0 ± 20.4 | 50.0 ± 20.4 | 50.0 ± 20.4 | 0.002 |
| All others | 371 | 84.0 ± 1.9 | 81.3 ± 2.1 | 80.9 ± 2.1 | 88.4 ± 1.7 | 86.8 ± 1.8 | 86.4 ± 1.8 | ||
| DFCI Risk Group | |||||||||
| Standard | 137 | 88.2 ± 2.8 | 84.3 ± 3.2 | 83.2 ± 3.3 | 0.186 | 91.9 ± 2.3 | 91.0 ± 2.5 | 91.0 ± 2.5 | 0.036 |
| High/Very High | 240 | 80.8 ± 2.6 | 78.9 ± 2.7 | 78.9 ± 2.7 | 85.4 ± 2.3 | 83.5 ± 2.4 | 82.9 ± 2.5 | ||
| Randomizations | |||||||||
| 6-MP | |||||||||
| High-dose | 159 | 84.8 ± 2.9 | 82.7 ± 3.0 | 81.7 ± 3.2 | 0.998 | 87.3 ± 2.7 | 85.8 ± 2.8 | 85.8 ± 2.8 | 0.657 |
| Low-dose | 163 | 84.5 ± 2.8 | 81.1 ± 3.1 | 81.1 ± 3.1 | 89.5 ± 2.4 | 87.4 ± 2.7 | 86.5 ± 2.8 | ||
| Asparaginase | |||||||||
| E. Coli | 92 | 83.5 ± 3.9 | 82.4 ± 4.0 | 82.4 ± 4.0 | 0.287 | 87.9 ± 3.4 | 85.2 ± 3.8 | 85.2 ± 3.8 | 0.285 |
| PEG | 106 | 79.1 ± 4.0 | 76.9 ± 4.1 | 76.9 ± 4.1 | 81.9 ± 3.8 | 80.8 ± 3.9 | 80.8 ± 3.9 | ||
| Doxorubicin (HR only) | |||||||||
| Bolus | 102 | 79.4 ± 4.0 | 78.2 ± 4.1 | 78.2 ± 4.1 | 0.239 | 83.3 ± 3.7 | 82.1 ± 3.8 | 82.1 ± 3.8 | 0.308 |
| Continuous Infusion | 102 | 86.1 ± 3.4 | 82.9 ± 3.8 | 82.9 ± 3.8 | 90.1 ± 3.0 | 86.7 ± 3.4 | 85.3 ± 3.7 | ||
Excludes unknown lineage and infants <1 year age.
Protocol 95-01 (1996–2000)
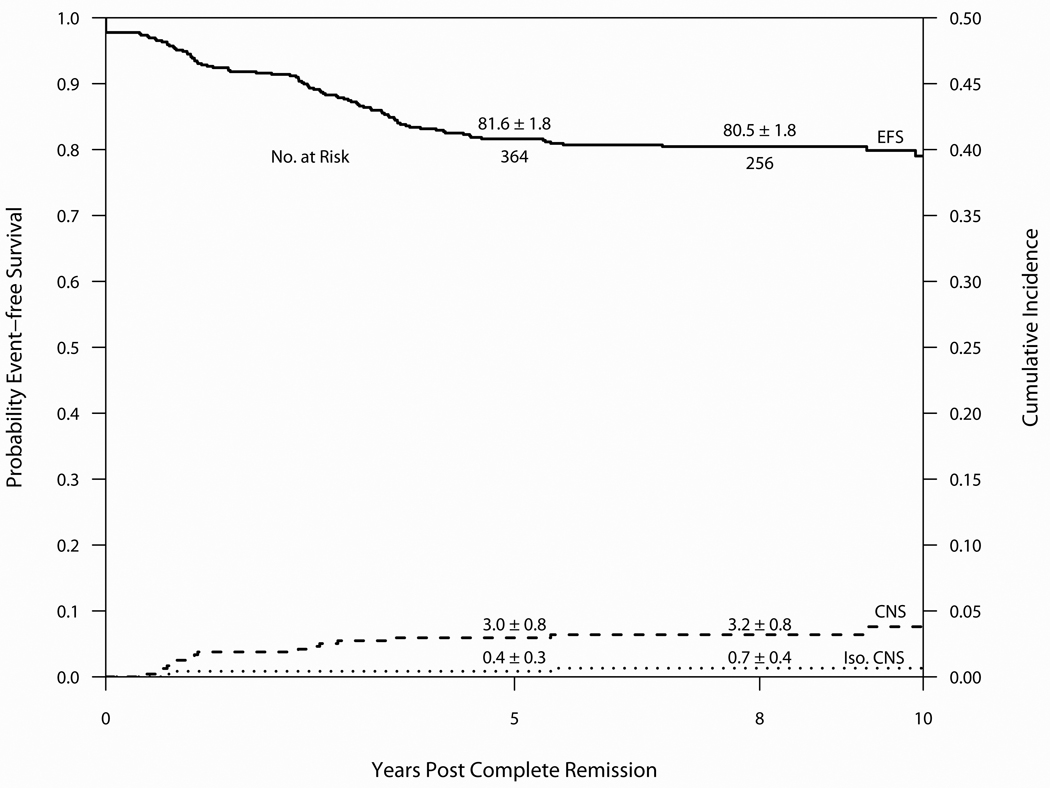
Outcome on Protocol 95-01 is summarized in Table 3 and Figure 6. Median follow-up was 8.6 years. Of the 491 evaluable patients, 480 entered CR (98%), 79 relapsed (16%) and 3 patients died in CR (0.6%). 395 (80%) remain alive and free of adverse events. The 10-year CI estimates for isolated marrow and any marrow relapses were 12.1 ± 1.5% and 15.9 ± 1.8%, respectively. The 10-year CI estimates for isolated CNS and any CNS relapses were 0.7 ± 0.4% and 3.8 ± 1.0%, respectively. Of the 274 evaluable male patients, the 10-year cumulative incidence of any and isolated testicular relapse was 1.9 ± 0.9% and 0.8 ± 0.5%, respectively. Three patients experienced a SMN as a first event (one malignant brain tumor in a previously irradiated patient and two cases of malignant melanoma). Neither case of malignant melanoma occurred in a previous radiation field. Eight patients (1.7%) experienced their first event after 5-years of CCR (6 relapses, 2 SMN).(Table 4) All six of these very late relapses occurred in SR patients. The 10-year EFS and OS were 79.0 ± 2.1% and 88.9 ± 1.5%, respectively. For SR patients, the 10-year EFS and OS rates were 83.1 ± 2.5% and 93.1 ± 2.1%, and the rates for HR/VHR patients were 74.1 ± 3.3% and 83.7 ± 2.5%.
Figure 6.
Event-free survival and Cumulative Incidence of isolated or any CNS relapse for 491 patients treated on Protocol 95-01 (1996–2000). Median follow-up was 8.6 years.
Univariate predictors of outcome are displayed in Table 8. End-induction (Day 30) minimal residual disease (MRD) level was measured by PCR in 284 of 430 (66%) B-precursor patients who achieved morphologic CR(16) and was a significant predictor of both EFS and OS. The 10-year EFS for B-precursor patients with low MRD (<0.001) was 83.9 ± 3.0% versus 24.4 ± 7.1% for those with high MRD (≥0.001), p<0.01. The presence or absence of the TEL/AML1 fusion was also prospectively tested by PCR in 299 of 438 (68%) patients with B-precursor phenotype.(17) The TEL/AML1 fusion was detected in 26% of these patients, and was associated with significantly better OS (p=0.05), but not EFS (p=0.10) Multivariable regression analysis including age, sex, presenting leukocyte count, phenotype and CNS status at diagnosis identified T-cell phenotype as an independent favorable predictor of EFS (Hazard Ratio 0.39, p=0.02) and OS (Hazard Ratio 0.36, p=0.04). Independent adverse predictors of EFS included WBC ≥ 100K (Hazard Ratio 3.40, p<0.01) and age ≥ 10 years (Hazard Ratio 1.66, p=0.04). These two features were also independent adverse predictors of OS (WBC ≥ 100K: Hazard Ratio 5.10, p<0.01; age ≥ 10 years: Hazard Ratio 2.90, p<0.01).
Table 8.
Protocol 95-01 Outcome by Patient Characteristics
| Event-free Survival ± SE (%) | Overall Survival ± SE (%) | ||||||
|---|---|---|---|---|---|---|---|
| Protocol | N | 5-yr EFS | 10-yr EFS | p-value | 5-yr OS | 10-yr OS | p-value |
| Overall | 491 | 81.6 ± 1.8 | 79.0 ± 2.1 | 89.6 ± 1.4 | 88.9 ± 1.5 | ||
| Immunophenotype | |||||||
| B lineage | 438 | 81.3 ± 1.9 | 78.4 ± 2.2 | 0.755 | 89.5 ± 1.5 | 88.7 ± 1.5 | 0.934 |
| T lineage | 52 | 84.6 ± 5.0 | 84.6 ± 5.0 | 90.1 ± 4.2 | 90.1 ± 4.2 | ||
| Unknown | 1 | N/A | N/A | N/A | N/A | ||
| B-lineage† | |||||||
| NCI Standard | 303 | 85.7 ± 2.1 | 82.8 ± 2.4 | 0.015 | 94.6 ± 1.3 | 93.5 ± 1.5 | 0.0004 |
| NCI High | 121 | 74.9 ± 4.0 | 71.6 ± 5.0 | 82.3 ± 3.5 | 82.3 ± 3.5 | ||
| T-lineage† | |||||||
| NCI Standard | 12 | 83.3 ± 10.8 | 83.3 ± 10.8 | 0.838 | 100.0 ± 0.0 | 100.0 ± 0.0 | 0.201 |
| NCI High | 40 | 84.9 ± 5.7 | 84.9 ± 5.7 | 87.1 ± 5.4 | 87.1 ± 5.4 | ||
| Sex | |||||||
| Male | 274 | 79.9 ± 2.5 | 77.5 ± 2.5 | 0.383 | 88.4 ± 2.0 | 87.6 ± 2.0 | 0.343 |
| Female | 217 | 83.8 ± 2.6 | 80.9 ± 2.9 | 91.0 ± 2.0 | 90.5 ± 2.0 | ||
| Age at Diagnosis | |||||||
| <1 year | 14 | 41.7 ± 13.5 | 41.7 ± 13.5 | <0.0001 | 41.7 ± 13.5 | 41.7 ± 13.5 | <0.0001 |
| 1–9 years | 385 | 84.8 ± 1.9 | 81.5 ± 1.9 | 94.2 ± 1.2 | 93.3 ± 1.3 | ||
| ≥10 years | 92 | 74.5 ± 4.6 | 74.5 ± 4.6 | 77.5 ± 4.4 | 77.5 ± 4.4 | ||
| WBC | |||||||
| <10 | 239 | 86.2 ± 2.3 | 83.2 ± 2.6 | 0.011 | 93.2 ± 1.7 | 92.6 ± 1.7 | 0.0003 |
| 10–49 | 155 | 80.8 ± 3.2 | 80.0 ± 3.3 | 90.0 ± 2.5 | 88.5 ± 2.6 | ||
| 50–99 | 43 | 78.9 ± 6.3 | 70.1 ± 10.0 | 88.4 ± 4.9 | 88.4 ± 4.9 | ||
| ≥100 | 54 | 66.0 ± 6.5 | 66.0 ± 6.5 | 73.1 ± 6.2 | 73.1 ± 6.2 | ||
| CNS leukemia | |||||||
| CNS-1 | 403 | 83.5 ± 1.9 | 81.4 ± 2.1 | 0.147 | 90.6 ± 1.5 | 89.7 ± 1.5 | 0.168 |
| CNS-2 | 49 | 73.1 ± 6.4 | 65.7 ± 9.0 | 89.7 ± 4.4 | 89.7 ± 4.4 | ||
| CNS-3 | 12 | 75.0 ± 12.5 | NA | 75.0 ± 12.5 | 75.0 ± 12.5 | ||
| Traumatic | 19 | 68.4 ± 10.7 | 68.4 ± 10.7 | 79.0 ± 9.4 | 79.0 ± 9.4 | ||
| Unknown | 8 | 87.5 ± 11.7 | 87.5 ± 11.7 | 85.7 ± 13.2 | 85.7 ± 13.2 | ||
| Down Syndrome | |||||||
| Yes | 14 | 71.4 ± 12.1 | 47.6 ± 21.0 | 0.111 | 71.4 ± 12.1 | 71.4 ± 12.1 | 0.024 |
| No | 477 | 81.9 ± 1.8 | 79.9 ± 2.0 | 90.1 ± 1.4 | 89.4 ± 1.4 | ||
| DNA Index | |||||||
| 1.16–1.60 | 59 | 89.1 ± 4.2 | 89.1 ± 4.2 | 0.008 | 96.4 ± 2.5 | 96.4 ± 2.5 | 0.011 |
| Other | 269 | 76.9 ± 2.6 | 74.2 ± 2.7 | 86.0 ± 2.1 | 85.2 ± 2.2 | ||
| Unknown | 163 | 86.8 ± 2.7 | 83.8 ± 3.2 | 93.1 ± 2.0 | 92.4 ± 2.1 | ||
| TEL/AML1* | |||||||
| Positive | 77 | 88.2 ± 3.7 | 88.2 ± 3.7 | 0.098 | 97.4 ± 1.8 | 96.0 ± 2.3 | 0.048 |
| Negative | 222 | 80.1 ± 2.7 | 77.0 ± 3.4 | 88.9 ± 2.1 | 88.4 ± 2.2 | ||
| t(9;22) | |||||||
| Present | 1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.019 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0003 |
| All others | 490 | 81.8 ± 1.8 | 79.2 ± 2.1 | 89.8 ± 1.4 | 89.1 ± 1.4 | ||
| MRD Day 30** | |||||||
| <0.001 | 246 | 87.7 ± 2.1 | 83.9 ± 3.0 | <0.0001 | 96.3 ± 1.2 | 95.3 ± 1.4 | <0.0001 |
| ≥0.001 | 38 | 27.4 ± 7.4 | 24.4 ± 7.1 | 50.5 ± 8.3 | 50.5 ± 8.3 | ||
| DFCI Risk Group | |||||||
| Standard | 272 | 86.3 ± 2.1 | 83.1 ± 2.5 | 0.007 | 94.3 ± 1.4 | 93.1 ± 1.6 | 0.0008 |
| High/Very High | 219 | 75.9 ± 2.9 | 74.1 ± 3.3 | 83.7 ± 2.5 | 83.7 ± 2.5 | ||
| Randomizations | |||||||
| Asparaginase | |||||||
| E. Coli | 147 | 88.1 ± 2.7 | 84.6 ± 3.4 | 0.020 | 93.8 ± 2.0 | 93.1 ± 2.1 | 0.038 |
| Erwinia | 139 | 78.1 ± 3.5 | 75.2 ± 3.8 | 85.3 ± 3.1 | 85.3 ± 3.1 | ||
| Doxurubicin (HR only) | |||||||
| Alone | 100 | 76.8 ± 4.2 | 73.5 ± 5.2 | 0.813 | 85.8 ± 3.5 | 85.8 ± 3.5 | 0.662 |
| With Dexrazoxane | 105 | 76.4 ± 4.2 | 76.4 ± 4.2 | 83.1 ± 3.8 | 83.1 ± 3.8 | ||
| CNS-directed therapy (SR only) |
|||||||
| IT only | 79 | 84.5 ± 4.1 | 80.2 ± 4.6 | 0.206 | 91.0 ± 3.2 | 89.5 ± 3.5 | 0.392 |
| 1800 cGy XRT | 77 | 88.1 ± 3.7 | 88.1 ± 3.7 | 94.7 ± 2.6 | 93.3 ± 2.9 | ||
TEL/AML1 data from B-precursor patients only.
MRD data from B-precursor patients who achieved morphologic CR.
Excludes unknown lineage and infants <1 year age.
Outcome of Randomized Comparisons
Asparaginase
On Protocol 91-01, 198 patients (SR and HR/VHR) were randomized to receive either native E.coli asparaginase (25,000 IU/m2 IM weekly) or PEG asparaginase (2,500 IU/m2 IM every 2-weeks) for a total of 30 weeks during post-induction consolidation. There was no significant difference in EFS (p=0.29) or OS (p=0.29) based on asparaginase type.(Table 7) On Protocol 95-01, 286 patients (SR and HR/VHR) were randomized to receive either native E.coli or Erwinia asparaginase (both dosed at 25,000 IU/m2 IM weekly) for 20 weeks during post-induction consolidation. Patients randomized to receive Erwinia asparaginase had a significantly inferior 10-year EFS (75.2 ± 3.8% versus 84.6 ± 3.4%, p=0.02) and OS (85.3 ± 3.1% versus 93.1 ± 2.1%, p=0.04). (Table 8) More patients randomized to Erwinia experienced a relapse involving the CNS (7% versus 1%, p<0.01)
Doxorubicin
On Protocol 91-01, 204 HR/VHR patients were randomized to receive doxorubicin (30 mg/m2) as either a bolus dose or a 48-hour continuous infusion every 3-weeks to a total cumulative dose of 360 mg/m2 during the post-induction consolidation phase. There was no difference in EFS (p=0.24) or OS (p=0.31) based on infusion duration.(Table 7) On Protocol 95-01, 205 HR/VHR patients were randomized to receive bolus doxorubicin (30 mg/m2) every 3-weeks with or without dexrazoxane (300 mg/m2), a potential cardioprotectant agent. Total cumulative dose of doxorubicin was 300 mg/m2. There was no difference in EFS (p=0.81) or OS (p=0.66) when comparing patients treated with or without dexrazoxane.(Table 8) No SMN’s have been observed in patients randomized to receive dexrazoxane.
CNS-directed Therapy
SR
On Protocol 95-01, 164 SR patients were randomized to receive either frequently-dosed triple IT chemotherapy (methotrexate/cytarabine/hydrocortisone) without radiation or 18 Gy cranial radiation with less frequent IT therapy. There was no difference in EFS (p=0.21), OS (p=0.39) or CI of isolated CNS relapse (p=0.15) between the two randomized groups.(Table 8)
HR
On Protocols 87-01, 91-01 and 95-01, HR/VHR patients were randomized to receive either daily (180 cGy) or twice-daily (90 cGy) fractions of cranial radiation to a total dose of 18 Gy. A total of 591 participated in these randomizations. There was no difference in 10-yr EFS (p=0.47), OS (p=0.59), CI of isolated CNS relapse (p=0.18) or CI of any CNS relapse (p=0.13).
Other Randomizations
On Protocol 87-01, 353 patients (SR and HR/VHR) were randomized to receive either high-dose (4 gm/m2) or low-dose (40 mg/m2) methotrexate during remission induction. There was no difference in EFS (p=0.62) or OS (p=0.66) based on methotrexate dose. (Table 6). On Protocol 91-01, 322 patients (SR and HR/VHR) were randomized to receive high-dose, IV 6-MP or standard, low-dose oral 6-MP during the first year of post-induction therapy. There was no difference in EFS (p=0.99) or OS (p=0.66) based on 6MP dosing. (Table 7).
Discussion
On the four consecutive DFCI ALL Consortium protocols conducted between 1985–2000, we observed long-term EFS rates ranging from 74–81% and OS rates of 81–89%. Both EFS and OS rates significantly improved during the 1990s, with EFS rates exceeding 80% and OS approaching 90% for patients treated during that decade. Although the incidence of marrow-involved relapses was relatively unchanged over the 15-year period, the incidence of isolated CNS relapse decreased, likely contributing to the improvement in EFS in the 1990s. There was also a decrease in the remission death rate, likely secondary to improvements in supportive care. The improvements in OS may have also been due, in part, to improved salvage after relapse during the 1990s, as evidenced by a larger difference between EFS and OS rates for patients treated in the 1990s compared to the 1980s.
The improvement in outcome during the 1990s occurred at the same time that therapy was de-intensified on DFCI ALL Consortium protocols: during that decade, cumulative dosage of doxorubicin was decreased in higher risk patients and fewer patients received cranial radiation. Also, risk group definitions were changed during the 1990s which resulted in more patients being classified as SR and so receiving less intensive therapy than they would have during the 1980s (when they would have been considered HR).
The favorable outcomes on our trials are especially notable for subsets of HR/VHR patients who historically have had worse prognoses, including those with T-cell immunophenotype and adolescents (age 10–18 years at diagnosis). We have previously reported that patients with T-cell ALL treated on our protocols have similar outcomes to those with B-precursor phenotype.(18) In fact, on Protocol 95-01, T-cell phenotype was an independent predictor of favorable EFS and OS on multivariable analysis. We have also previously demonstrated that there was no significant difference in outcome between younger (10–14 years old) and older (15–18 year old) adolescents treated on our trials in the 1990s, with long-term EFS exceeding 75% for both subgroups.(19) Based on the favorable outcomes achieved by older adolescents on our protocols, we are currently piloting our HR regimen in adults with ALL, with promising preliminary results.(20)
While age and phenotype no longer identify patients at highest risk of relapse, very high presenting leukocyte count ≥ 100K remained an independent predictor of adverse outcome throughout this time period. Overall, the prognosis for such patients improved in the 1990s (EFS 66–70% versus 52–62% in the 1980s), perhaps due to some changes in the regimen backbone during this decade. For instance, outcomes for patients with a very high presenting leukocyte count were best on Protocol 91-01 (1991–5), which included a more prolonged asparaginase consolidation phase (30 weeks instead of 20 weeks) and the use of dexamethasone instead of prednisone during all post-induction phases. In fact, Protocol 91-01 was the only trial reported here on which leukocyte count was not a significant prognostic factor.
On Protocol 95-01 (1996–2000), we identified end-induction MRD level as a significant independent predictor of outcome. Patients with high end-induction MRD (≥0.001 as measured by quantitative PCR) had a 10.5-fold greater risk of relapse than those with low MRD.(16) Based upon these results, we have re-defined the VHR group in our current clinical trial to include patients with high end-induction MRD, as well as those with the following adverse chromosomal abnormalities regardless of MRD level: MLL translocation, hypodiploidy (<45 chromosomes), and t(9;22). Approximately 15% of patients are now considered VHR and receive intensified treatment.
A major component of our therapeutic backbone is the administration of asparaginase for 20–30 consecutive weeks beginning 3 weeks after the completion of the remission induction phase. Toxicities associated with this treatment have included hypersensitivity reactions in 20–30% of patients, pancreatitis in 5–8%, and thrombotic events in 2–5%.(6, 7) After allergy to native E.coli asparaginase, patients have been treated with alternative asparaginase preparations (either weekly PEG or twice-weekly Erwinia, depending on protocol and agent availability during this era); approximately one-third of patients develop hypersensitivity to the second asparaginase preparation. The incidence of pancreatitis and thromboembolic complications, but not asparaginase allergy, is higher in patients 10–18 years of age compared with those younger than 10 years.(19) In an attempt to optimize asparaginase dosing, we have extensively studied toxicities associated with the different asparaginase preparations. On Protocol 95-01, weekly Erwinia asparaginase was associated with a lower incidence of asparaginase-associated toxicity (10% versus 24%), but also with inferior 5-year EFS compared with weekly E.coli asparaginase.(7) This result was likely due to the dosing schedule; because Erwinia asparaginase has a far shorter half-life than E.coli asparaginase, it is probable that fewer Erwinia-treated patients experienced continuous asparagine depletion during the intensification phase. On Protocol 91-01, we demonstrated that IM PEG asparaginase (administered every 2 weeks) was associated with a reduced risk of hypersensitivity compared to weekly E.coli asparaginase without impacting EFS.(6) However, that study was not sufficiently powered to detect small differences in EFS. In our current trial, we are comparing IV PEG asparaginase with native E.coli asparaginase in a larger cohort of patients to determine the tolerability of intravenous administration of PEG asparaginase, as well as the relative toxicity and efficacy of the two preparations.
A major focus of our clinical trials has been to reduce late effects of therapy. To that end, our therapeutic backbone does not include exposure to alkylating agents or epipodophyllotoxins. To minimize the risk of late cardiotoxicity, SR patients receive only 60 mg/m2 cumulative dose of doxorubicin. For HR patients, the cumulative dosage of doxorubicin was reduced from 360 mg/m2 to 300 mg/m2 in 1996. In successive randomized trials, we found that continuous infusion doxorubicin was not cardioprotective,(21) but demonstrated that dexrazoxane prevented acute cardiac injury (as measured by troponin-T elevation) in HR patients without increasing the risk of relapse or second malignant neoplasm.(22, 23) We are currently analyzing long-term echocardiograms (obtained 5 or more years after completion of anthracycline) on patients who participated in that randomization, and continue to use dexrazoxane prior to each dose of doxorubicin in HR/VHR patients.
Between 1985–2000, we also focused on reducing late effects associated with CNS-directed therapy. On Protocol 87-01, all SR patients were treated without cranial radiation; however, no change was made in either intrathecal or systemic chemotherapy to substitute for the absence of radiation. This non-randomized change resulted in an unacceptably high rate of CNS relapses in SR boys, although most of these patients could be salvaged post-relapse.(5) On Protocol 95-01, we were able to successfully eliminate cranial radiation in all SR patients (including boys) by more frequent administration of intrathecal chemotherapy (every 9 weeks) during the first year of treatment.(7) Neurocognitive testing of survivors from that study (median follow-up 6 years from diagnosis) demonstrated that cognitive function for both irradiated and non-irradiated patients was solidly in the normal range, although irradiated patients as a group exhibited a slower rate of information processing.(24) Longer follow-up is necessary to more fully assess the relative long-term neurocognitive and neuroendocrine consequences of these two CNS-directed treatments (with and without radiation). In addition to neurocognitive sequelae, cranial radiation has also been associated with a higher risk of SMNs.(25) On our current trial, we have restricted the use of radiation to those considered to be at the highest risk of CNS relapse (~25–30% of patients), including those with CNS-3 status at diagnosis, T-cell phenotype and/or high end-induction MRD. In an attempt to reduce the risk of radiation-associated late effects for these patients, we are also using a lower dose of cranial radiation (12 Gy instead of 18–24 Gy in earlier studies).
The EFS and OS of the two protocols we conducted in the 1990s were nearly identical. The plateau in survival rates over that decade suggests that we may have reached the limits of currently applied risk factors and conventional chemotherapeutic agents. To improve outcomes, our current studies focus on identifying biologic factors which may supplement or replace the epidemiologic factors currently used to determine risk-based therapy. For instance, microarray gene expression studies from our investigators have identified biologically distinct and prognostically relevant subtypes of ALL based upon gene expression profiles.(26, 27) In addition, research focused on pharmacogenomics has begun to identify patient-related factors that impact outcome and help lead to more individualized therapy.(28) We have also focused on identifying novel, targeted therapies, including inhibitors of the FLT3 tyrosine kinase,(29) the antiapoptotic protein BCL-2, (30) and the mTOR pathway (which has been implicated in glucocorticoid resistance).(31) By identifying new, biologically distinctive patient subsets and devising novel targeted treatments for them, our goal is to improve survival and reduce toxicities for all patients with ALL.
Acknowledgements
These trials were supported in part by a grant from the National Institute of Health (NCI grant 5P01CA068484). We thank the patients, families, physicians, nurses, clinical research coordinators and all others who participated in these trials. We acknowledge the important contributions of Jennifer Cronin RN, Annette Dalton, Virginia Dalton, MS, APRN, Meghan Eaton, and Richard D. Gelber, PhD.
Footnotes
Conflicts of Interest: Dr. Silverman received compensation as an advisory board member for EUSA Pharma, and also received compensation as a consultant for Enzon, Inc. Dr. Sallan received honoraria and research funding from Enzon Inc. and compensation as an advisory board member for EUSA Pharma. Jane O’Brien, Kristen Stevenson, Eileen Whyte O’Holleran, and Drs. Asselin, Barr, Clavell, Cohen, Cole, Kelly, Laverdiere, Michon, Neuberg, Schorin, and Schwartz declare no conflict of interest.
References
- 1.Sallan SE, Cammita BM, Cassady JR, Nathan DG, Frei E., 3rd Intermittent combination chemotherapy with adriamycin for childhood acute lymphoblastic leukemia: clinical results. Blood. 1978;51(3):425–433. [PubMed] [Google Scholar]
- 2.Sallan SE, Hitchcock-Bryan S, Gelber R, Cassady JR, Frei E, 3rd, Nathan DG. Influence of intensive asparaginase in the treatment of childhood non-T- cell acute lymphoblastic leukemia. Cancer Res. 1983;43(11):5601–5607. [PubMed] [Google Scholar]
- 3.Clavell LA, Gelber RD, Cohen HJ, Hitchcock-Bryan S, Cassady JR, Tarbell NJ, et al. Four-agent induction and intensive asparaginase therapy for treatment of childhood acute lymphoblastic leukemia. N Engl J Med. 1986;315(11):657–663. doi: 10.1056/NEJM198609113151101. [DOI] [PubMed] [Google Scholar]
- 4.Schorin MA, Blattner S, Gelber RD, Tarbell NJ, Donnelly M, Dalton V, et al. Treatment of childhood acute lymphoblastic leukemia: results of Dana- Farber Cancer Institute/Children's Hospital Acute Lymphoblastic Leukemia Consortium Protocol 85-01. J Clin Oncol. 1994;12(4):740–747. doi: 10.1200/JCO.1994.12.4.740. [DOI] [PubMed] [Google Scholar]
- 5.LeClerc JM, Billett AL, Gelber RD, Dalton V, Tarbell N, Lipton JM, et al. Treatment of childhood acute lymphoblastic leukemia: results of Dana- Farber ALL Consortium Protocol 87-01. J Clin Oncol. 2002;20(1):237–246. doi: 10.1200/JCO.2002.20.1.237. [DOI] [PubMed] [Google Scholar]
- 6.Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 7.Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007 Feb 1;109(3):896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman LB, McLean TW, Gelber RD, Donnelly MJ, Gilliland DG, Tarbell NJ, et al. Intensified therapy for infants with acute lymphoblastic leukemia: results from the Dana-Farber Cancer Institute Consortium. Cancer. 1997;80(12):2285–2295. [PubMed] [Google Scholar]
- 9.Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol. 1993 Sep;11(9):1780–1786. doi: 10.1200/JCO.1993.11.9.1780. [DOI] [PubMed] [Google Scholar]
- 10.Asselin BL, Kreissman S, Coppola DJ, Bernal SD, Leavitt PR, Gelber RD, et al. Prognostic significance of early response to a single dose of asparaginase in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1999 Jan-Feb;21(1):6–12. doi: 10.1097/00043426-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz CL, Thompson EB, Gelber RD, Young ML, Chilton D, Cohen HJ, et al. Improved response with higher corticosteroid dose in children with acute lymphoblastic leukemia. J Clin Oncol. 2001 Feb 15;19(4):1040–1046. doi: 10.1200/JCO.2001.19.4.1040. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Cox DR, Oates D. Regression models and life tables. J Royal Stat Soc. 1972;B34:187–220. [Google Scholar]
- 14.Klabfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: Wiley; 1980. [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 16.Zhou J, Goldwasser MA, Li A, Dahlberg SE, Neuberg D, Wang H, et al. Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95-01. Blood. 2007 Sep 1;110(5):1607–1611. doi: 10.1182/blood-2006-09-045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loh ML, Goldwasser MA, Silverman LB, Poon WM, Vattikuti S, Cardoso A, et al. Prospective analysis of TEL/AML1 positive patients treated on Dana-Farber Cancer Institute Consortium Protocol 95-01. Blood. 2006;107(11):4508–4513. doi: 10.1182/blood-2005-08-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003 Oct 1;21(19):3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 19.Barry E, DeAngelo DJ, Neuberg D, Stevenson K, Loh ML, Asselin BL, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007 Mar 1;25(7):813–819. doi: 10.1200/JCO.2006.08.6397. [DOI] [PubMed] [Google Scholar]
- 20.DeAngelo DJ, Dahlberg S, Silverman LB, Couban S, Amrein PC, Deftel MD, et al. A multicenter Phase II study using a dose intensified pediatric regimen in adults with untreated acute lymphoblastic leukemia. Blood. 2007;110:181a. [Google Scholar]
- 21.Lipshultz SE, Giantris AL, Lipsitz SR, Kimball Dalton V, Asselin BL, Barr RD, et al. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J Clin Oncol. 2002;20(6):1677–1682. doi: 10.1200/JCO.2002.20.6.1677. [DOI] [PubMed] [Google Scholar]
- 22.Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. Dexrazoxane reduces myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia: Results from a randomized trial. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 23.Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008 Mar 1;26(7):1106–1111. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- 24.Waber DP, Turek J, Catania L, Stevenson K, Robaey P, Romero I, et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01. J Clin Oncol. 2007 Nov 1;25(31):4914–4921. doi: 10.1200/JCO.2007.10.8464. [DOI] [PubMed] [Google Scholar]
- 25.Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007 Mar 21;297(11):1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002 Jan;30(1):41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 27.Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1(1):75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 28.Dulucq S, St-Onge G, Gagne V, Ansari M, Sinnett D, Labuda D, et al. DNA variants in the dihydrofolate reductase gene and outcome in childhood ALL. Blood. 2008 Apr 1;111(117):3692–3700. doi: 10.1182/blood-2007-09-110593. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong SA, Kung AL, Mabon ME, Silverman LB, Stam RW, Den Boer ML, et al. Inhibition of FLT3 in MLL. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003 Feb;3(2):173–183. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 30.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008 Feb 15;111(4):2300–2309. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006 Oct;10(4):331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]