Abstract
Few studies have examined the subjective value attributed to drug rewards specifically as it compares with the value attributed to primary non-drug rewards in addicted individuals. The objective of this study is to assess ‘liking’ and ‘wanting’ of expected ‘drug’ rewards as compared to ‘food’ and ‘sex’ while respondents report about three different situations (‘current’, and hypothetical ‘in general’, and ‘under drug influence’). In all, 20 cocaine-addicted individuals (mean abstinence = 2 days) and 20 healthy control subjects were administered the STRAP-R (Sensitivity To Reinforcement of Addictive and other Primary Rewards) questionnaire after receiving an oral dose of the dopamine agonist methylphenidate (20 mg) or placebo. The reinforcers’ relative value changed within the addicted sample when reporting about the ‘under drug influence’ situation (drug > food; otherwise, drug < food). This change was highest in the addicted individuals with the youngest age of cocaine use onset. Moreover, ‘drug’ ‘wanting’ exceeded ‘drug’ ‘liking’ in the addicted subjects when reporting about this situation during methylphenidate. Thus, cocaine-addicted individuals assign the highest subjective valence to ‘drug’ rewards but only when recalling cue-related situations. When recalling this situation, they also report higher ‘drug’ ‘wanting’ than hedonic ‘liking’, a motivational shift that was only significant during methylphenidate. Together, these valence shifts may underlie compulsive stimulant abuse upon pharmacological or behavioural cue exposure in addicted individuals. Additional studies are required to assess the reliability of the STRAP-R in larger samples and to examine its validity in measuring the subjective value attributed to experienced reinforcers or in predicting behaviour.
Keywords: cue reactivity, methylphenidate, motivation, primary rewards, reinforcement, relative valence, salience
Introduction
Few studies have examined the subjective value attributed to drug rewards specifically as it compares with the value attributed to primary non-drug rewards in addicted individuals. In the current study, we, therefore, asked the following question: how do addicted individuals subjectively value expected drug versus non-drug reward? The literature suggests three possibilities: (A) Animal research suggests that after chronic drug administration the value of a drug reward is increased (Ahmed, et al., 2002; Ahmed and Koob, 1998), whereas that of a non-drug reward is decreased (Grigson and Twining, 2002). Similarly, human cocaine-addicted subjects but not controls showed reduced activation of corticolimbic brain areas when viewing an erotic (non-drug) video than when exposed to a cocaine video (Garavan, et al., 2000). (B) In contrast, other human studies show blunted subjective responses to drug rewards (intravenous methylphenidate) suggesting reductions in the subjective value of drug reward in addicted individuals (Volkow, et al., 1997). (C) Yet, another possibility is that of a generally drug-sensitised brain reward circuit where heightened drug motivation may ‘spillover’ to non-drug rewards (Robinson and Berridge, 2003). Here, evidence from animal studies suggests that drug sensitization can increase the incentive value of other rewards, such as sucrose or other foods, a sexually receptive female (for male rats), and conditioned stimuli for such rewards (Fiorino and Phillips, 1999a; b; Nocjar and Panksepp, 2002; Taylor and Horger, 1999; Wyvell and Berridge, 2001). Similarly, in human addicted individuals, evidence suggests that some cocaine-addicted individuals are hypersexual (Washton and Stone-Washton, 1993) and some substance-dependent individuals may be hyper-responsive to money rewards (Bechara, et al., 2002), rating $10 to be equally valuable to $1000 (Goldstein, et al., 2007).
These discrepancies may in part relate to the dissociation between the subjective value of an expected reward (before it is received) and the perception of the reward at time of consumption (when it is received/experienced). These discrepancies may also relate to how valence/salience is defined. For example, in most self-administration or neuroimaging studies, drug-related valence is assessed as craving or drug ‘wanting’. In contrast, in theoretical accounts of drug addiction, the incentive motivational aspects of drugs are hypothesized to be dissociated from their hedonic effects; ‘wanting’ drugs (e.g., how much an animal will work to acquire a drug) increases to pathological levels without a parallel increase in drug ‘liking’ (Robinson and Berridge, 1993; 2001; 2003). This specific hypersensitivity (i.e., sensitization) to the incentive motivational (i.e., ‘wanting’) effects of drugs (and drug-related stimuli) is hypothesized to ultimately lead to increasingly compulsive patterns of drug-seeking and drug-taking behaviour.
Our primary goal in the current study was to design a brief questionnaire of the perceived subjective value attributed to expected/hypothetical drugs and other primary reinforcers (food and sex) by cocaine-addicted individuals. We also aimed to distinguish subjective appraisal of drug ‘wanting’ from drug ‘liking’ (hedonic ratings of pleasantness). Given that reward value may differ depending on the availability of drug-related cues (Shaham, et al., 2003), we inquired not only about the ‘current’ (laboratory) setting but also about two real-life situations (‘in general’ and ‘under drug influence’; the latter hypothetical situation was presumed to be most cue reactive). We hypothesized that cocaine-addicted individuals would provide (1) overall higher ratings for drug versus food or sex, especially when recalling the ‘under drug influence’ situation; (2) higher drug ‘wanting’ than drug ‘liking’ ratings, especially during the effects of oral methylphenidate. This latter hypothesis rests on previous results from our laboratory showing that methylphenidate enhances saliency of events by increasing dopamine in both drug-addicted (Volkow, et al., 1999a) and drug-naive (Volkow, et al., 1999b) individuals.
Methods
Participants
The sample consisted of 20 cocaine-addicted subjects and 20 healthy comparison subjects. The groups did not differ in distributions of sex and race or in mean education and general intellectual functioning (Table 1). Group differences in age and history of cigarette smoking were accounted for as further described in Results. Cocaine-addicted subjects were those who met DSM-IV criteria for active cocaine dependence and had at least a 6-month history of cocaine abuse (at least 2 g of cocaine per week - smoked or intravenous routes of administration) (see Table 1 for drug use variables). Exclusion criteria were history of a neurological disease of central origin, head trauma causing loss of consciousness > 30 min, psychiatric disease (apart from cocaine dependence for the cocaine-addicted subjects), medical conditions that may have altered cerebral function, glaucoma, cardiovascular disorders, arrhythmia and hypertension as verified by a medical and neurological examination of all subjects. Subjects were also excluded for presence of any psychoactive drugs or their metabolites (other than cocaine for the cocaine-addicted subjects, indicating cocaine use within the past 72 h) as verified by a urine drug screen (a triage urine panel, Biopsych™) performed the morning of each study day. Women who were pregnant (urine pregnancy test: STAT) or breastfeeding were also excluded. Exclusion criteria for the control subjects were the same, except any history of drug abuse or dependence or a positive urine screen for any drugs was prohibitive. Subjects were fully informed of the nature of the research and provided a written consent for their involvement in this study in accordance with the local Institutional Review Board.
Table 1.
Frequencies, means and standard deviations for demographic and drug use variables in the complete study sample
| Cocaine addicted (n = 20) | Healthy control (n = 20) | |
|---|---|---|
| Demographics | ||
| Gender (female/male) | 6/14 | 9/11 |
| Race (African American/Caucasian/Other) | 17/2/1 | 10/5/5 |
| M ± SD | M ± SD | |
|---|---|---|
| Age (Years)* | 42.0 ± 6.0 | 33.1 ± 6.4 |
| Education (Years) | 13.1 ± 1.4 | 14.1 ± 1.4 |
| Reading: Wide Range Achievement Test-Revised IIIa | 92.5 ± 13.7 | 92.9 ± 16.6 |
| Matrix Reasoning: Wechsler Abbreviated Scale of Intelligencea | 10.0 ± 3.9 | 10.2 ± 3.8 |
| Drug use | ||
| Number of cocaine use days in the past month | 17.4 ± 7.6 | — |
| Average grams per cocaine use occasion | 3.3 ± 2.1 | — |
| Length of abstinence from cocaine at time of study (number of days, averaged across all four study days) | 2.2 ± 1.3 | — |
| Age of onset of cocaine use (years) | 23.3 ± 6.1 | — |
| Duration of cocaine use (years) | 16.5 ± 6.4 | — |
| History of cigarette smoking (non-smoker/current smoker/former smoker)* | 2/17/1 | 11/7/2 |
M, mean; SD, standard deviation.
n = 39 (one control subject is missing data).
Independent t-tests (continuous variables) or chi-square tests (categorical variables) were conducted.
p < 0.01.
Procedure
A brief measure (Sensitivity To Reinforcement of Addictive and other Primary Rewards; STRAP-R) was devised (Table 2). Subjects were asked to think about their favourite food, sexual activity and drug or alcohol without reporting the exact stimulus/activity to the interviewer such that privacy was maintained (and demand characteristics reduced) at all times. For ‘liking’, subjects rated ‘How pleasant would it be to eat it (food), do it (sex) or use/drink it (drug)’.For ‘wanting’, subjects rated ‘How much do you want to eat it (food), do it (sex) or use/drink it (drug)’.The same questions were repeated for three different situations: ‘current’, ‘in general’, and hypothetically while ‘under drug influence’ of their favourite drug.a A Likert-type scale was used for all questions, ranging from 1 (‘somewhat’) to 5 (‘extremely’). Question order was fixed across all study subjects (Table 2).
Table 2.
The Sensitivity To Reinforcement of Addictive and other Primary Rewards (STRAP-R) questionnaire
| Use the following rating scale when answering the questions below: |
|||||||
|---|---|---|---|---|---|---|---|
| 1, somewhat | 2, slightly | 3, moderately | 4, very | 5, extremely | |||
| PL time: _:_ | MP time: _:_ | ||||||
| A. Think about your most favourite food | |||||||
| Currently | 1. How pleasant would it be to eat it right now? | ||||||
| 2. Do you want to eat it right now? | |||||||
| In general | 3. How pleasant is eating it in general? | ||||||
| 4. How much do you want to eat in general? | |||||||
| Under the influence/high | 5. How pleasant was eating it the last time you were high/buzzed? | ||||||
| 6. How much did you want to eat it the last time you were high/buzzed? | |||||||
| B. Think about your most favourite sexual activity | |||||||
| Currently | 1. How pleasant would it be to do it right now? | ||||||
| 2. Do you want to do it right now? | |||||||
| In general | 3. How pleasant is doing it in general? | ||||||
| 4. How much do you want to do it in general? | |||||||
| Under the influence/high | 5. How pleasant was doing it the last time you were high/buzzed? | ||||||
| 6. How much did you want to do it the last time you were high/buzzed? | |||||||
| C. Think about your most favourite drug or alcohol | |||||||
| Currently | 1. How pleasant would it be to use/drink it right now? | ||||||
| 2. Do you want to use/drink it right now? | |||||||
| In general | 3. How pleasant is using/drinking it in general? | ||||||
| 4. How much do you want to use/drink it in general? | |||||||
| Under the influence/high | 5. How pleasant was using/drinking it the last time you were high/buzzed? | ||||||
| 6. How much did you want to use/drink it the last time you were high/buzzed? | |||||||
| Raters comments: | |||||||
| Do not ask for or write down the food, sexual activity or drug/alcohol the subject thinks about. | |||||||
PL, placebo; MP, methylphenidate.
All subjects in the current study participated in one of two positron emission tomography (PET) protocols. The STRAP-R was administered before the PET-related experimental manipulations, which consisted of solving arithmetic problems (controls) or watching neutral or cocaine-related videos (cocaine-addicted subjects), as reported elsewhere (Volkow, et al., 2004; Volkow, et al., 2003; Volkow, et al., 2006).b In both protocols, subjects received a 20 mg oral dose of methylphenidate chloride or placebo (100 mg dose of thiamine) 60 min before the administration of the STRAP-R; this time interval was used to capture the peak stimulant effects of methylphenidate on behavioural responses (Volkow, et al., 1998). The order of placebo versus methylphenidate was counterbalanced between the different study days across subjects (time between scans was at least 1 day with the exception that 1 week had to elapse after the methylphenidate scans). Subjects were fasting for all protocols; ‘current’ ratings, therefore, reflect a food-deprived state.
Statistical analysis
A mixed 3 × 2 × 3 × 2 × 2 analysis of variance (ANOVA) was conducted, with four within-subjects factors: reward (food, sex, drug), medication (methylphenidate, placebo), situation (‘current’, ‘in general’, ‘under drug influence’) and question (‘liking’, ‘wanting’) and one between-subjects factor: group (cocaine, control). In cases where the assumption of sphericity was not met (as tested by Mauchly’s test of sphericity), the Greenhouse-Geisser correction was used. Follow-up paired and independent t-tests were conducted for all significant omni-bus effects. To protect against Type I error, a significance level of 0.01 was set for all analyses.
Results
Mixed five-way ANOVA
Results of the five-way ANOVA showed main effects for (A) reward (food = sex > drug); (B) situation (‘in general’ > ‘under drug influence’ > ‘current’) and (C) question (‘liking’ > ‘wanting’) (Fs > 18.6, ps < 0.0001). Although there was no main effect for group, interactions with group showed that these three main effects differed as a function of drug addiction. Thus, all two-way interactions (with group, Fs > 11.1, ps < 0.001) and a three-way interaction (group × reward × situation, F4,35 = 5.2, p < 0.01) were significant.c A related four-way interaction (group × reward × situation × question) approached the nominal significance level (F4,35 = 3.7, p < 0.05). An additional four-way interaction also approached significance (drug × group × reward × situation, F4,35 = 2.8, p < 0.05). To follow up on these complex results and four-way interactions, we performed four 3 × 3 × 2 (reward × situation × question) ANOVAs separately for each study group and drug. Note that this decision was justified given the differences between the protocols for the two study groups and our a priori hypothesis for methylphenidate effects.
Three-way ANOVAs
I. Placebo, healthy control subjects
The three main effects (reward: food > sex > drug; situation: ‘current’ < ‘under drug influence’ = ‘in general’; question: ‘liking’ > ‘wanting’; Fs > 7.2, ps < 0.01) and a reward × situation interaction (F4,16 = 6.5, p < 0.01) were significant (Figure 1). This interaction was driven by a significant difference between ratings of ‘food’ and ‘sex’ as a function of situation such that ‘food’ > ‘sex’ in the ‘current’ situation only (a result attributed to the ‘current’ fasting requirements and experimental PET environment; all else, ‘food’ = ‘sex’). This interaction was also driven by highest ratings for ‘drug’ in the ‘under drug influence’ situation (although healthy control subjects were not included if they had any history of drug addiction or abuse, drug use per sea was not basis for exclusion). Age was negatively correlated with ‘under drug influence’ ‘sex’ ‘liking’ ratings (r = -0.57, p < 0.01). History of cigarette smoking was associated with ‘current’ ‘sex’ ‘liking’ (subjects with no cigarette smoking history provided higher ratings than subjects with current or past history of cigarette smoking, t18 = 2.9, p < 0.01); there were no significant correlations with mean number of cigarettes smoked per day for the current nicotine smokers.
Figure 1.
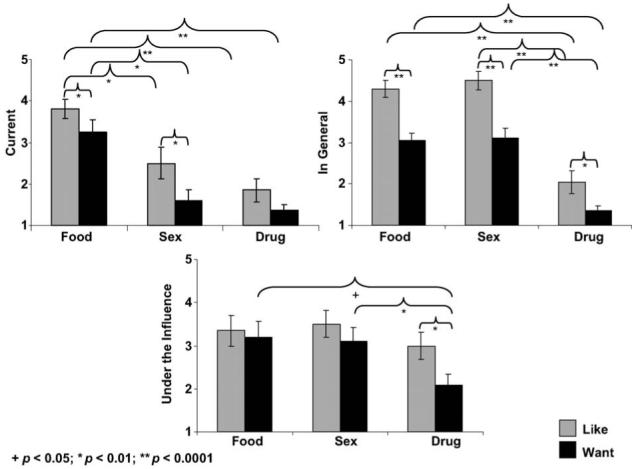
The value of food, sex and drugs in healthy control subjects during placebo. Mean STRAP-R ratings (± standard error of the mean) for three reported situations: (A) current; (B) in general and (C) hypothetical ‘under drug influence’ in 20 healthy control subjects as a function of three reinforcers (food, sex, drug) and two questions (‘liking’, ‘wanting’) during placebo.
II. Methylphenidate, healthy control subjects
All three main effects and interaction remained significant (Fs > 15.3, ps < 0.0001) (Figure 2). Here, associations between the STRAP-R ratings with age or cigarette smoking were not significant; therefore, the similar ANOVA results during methylphenidate (as compared to placebo) indicate that age and cigarette smoking did not significantly impact the STRAP-R results in the control subjects.
Figure 2.
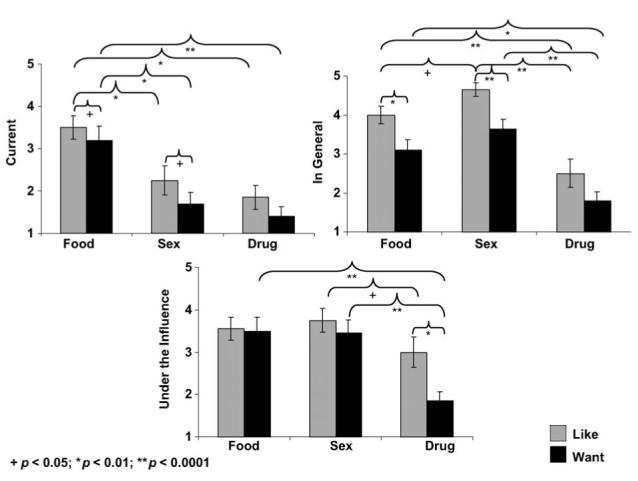
The value of food, sex and drugs in healthy control subjects during methylphenidate. Mean STRAP-R ratings (± standard error of the mean) for three reported situations: (A) current; (B) in general and (C) hypothetical ‘under drug influence’ in 20 healthy control subjects as a function of three reinforcers (food, sex, drug) and two questions (‘liking’, ‘wanting’) during 20 mg oral methylphenidate.
III. Placebo, cocaine-addicted subjects
The main effects for reward (food = sex = drug; F2,18 = 0.8, p > 0.4) and question (F1,19 = 6.6, p > 0.01) were not significant (Figure 3). The situation main effect (‘current’ = ‘under drug influence’ < ‘in general’) and reward × situation interaction were significant (Fs > 22.0, ps < 0.0001). This latter interaction was driven by a different pattern of ratings for all three rewards depending on the situation: similarly to the control subjects, ratings for ‘sex’ and ‘drug’ were lowest in the ‘current’ situation (again, an expected response given the experimental environment). However, in contrast to the control subjects, this interaction in the cocaine group was also driven by (1) ‘food’ ratings in the ‘under drug influence’ situation, now lowest as compared to the other rewards and (2) ‘sex’ ratings that were significantly decreased in the ‘under drug influence’ as compared to the ‘in general’ situation (‘drug’ was similarly rated in both these situations). There were no associations between the STRAP-R ratings during placebo with age or history of cigarette smoking in the cocaine-addicted individuals.
Figure 3.
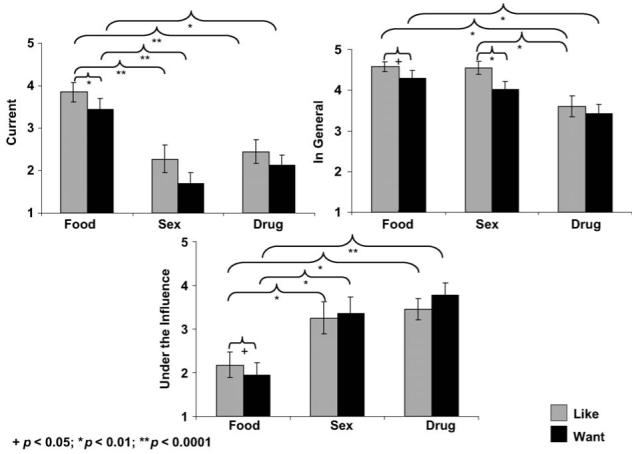
The value of food, sex and drugs in cocaine-addicted individuals during placebo. Mean STRAP-R ratings (± standard error of the mean) for three reported situations: (A) current; (B) in general and (C) hypothetical ‘under drug influence’ in 20 cocaine-addicted subjects as a function of three reinforcers (food, sex, drug) and two questions (‘liking’, ‘wanting’) during placebo.
IV. Methylphenidate, cocaine-addicted subjects
The same pattern of results was observed under methylphenidate, although now a question (‘liking’ > ‘wanting’) and a situation × question interaction were also significant (Fs > 14.9, ps < 0.01) (Figure 4). This latter interaction was explained by higher ‘liking’ than ‘wanting’ ratings across all, but the ‘under drug influence’ situation, where the opposite pattern was observed: here, ‘wanting’ ratings exceeded ‘liking’ ratings (t19 = -3.2, p < 0.01). Follow-up paired t-tests showed that this effect was unique for the ‘drug’ ratings (t19 = -2.3, p < 0.05) (in contrast, recall the main effect of question in the healthy control subjects, where ‘liking’ always exceeded ‘wanting’, even while rating ‘drug’ ‘under drug influence’ during methylphenidate, t19 = 4.1, p < 0.01, Figure 2). There were no associations between the STRAP-R ratings during methylphenidate with age or history of cigarette smoking in the cocaine-addicted individuals.
Figure 4.
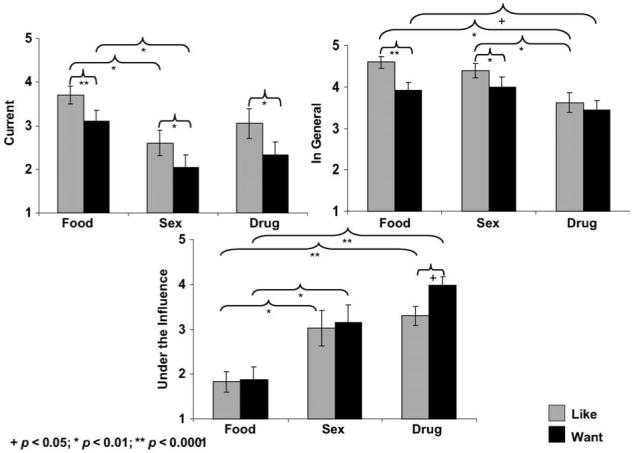
The value of food, sex and drugs in cocaine-addicted individuals during methylphenidate. Mean STRAP-R ratings (± standard error of the mean) for three reported situations: (A) current; (B) in general and (C) hypothetical ‘under drug influence’ in 20 cocaine-addicted subjects as a function of three reinforcers (food, sex, drug) and two questions (‘liking’, ‘wanting’) during 20 mg oral methylphenidate.
Correlations
To further understand this pattern of results in the cocaine-addicted individuals, where ‘drug’ ratings were higher than ‘food’ or ‘sex’ ratings ‘under drug influence’ and where ‘drug’ ‘wanting’ exceeded drug ‘liking’ ‘under drug influence’ during methylphenidate, we performed correlations between several selected dependent variables with drug use variables (listed in Table 1). Specifically, we chose to calculate the difference between ‘under drug influence’ ratings for ‘drug’ and the other reinforcers (averaged across placebo and methylphenidate, and across ‘liking’ and ‘wanting’) and also the difference between ‘under drug influence’ ‘wanting’ and ‘liking’ for ‘drug’ vis-à-vis the other reinforcers during methylphenidate only. A correlation between the differential ‘under drug influence’ ‘drug’ versus ‘food’ ratings with age of cocaine use onset was significant (r = -0.70, p < 0.01) (Figure 5); there was a similar trend for duration of use (r = 0.53, p < 0.05). The former correlation survived corrections (with partial correlations) for age, history of cigarette smoking and mean number of cigarettes smoked per day (rs > -0.70, ps < 0.001). The other correlation remained at a trend level across all these analyses (rs > 0.53, p < 0.05).
Figure 5.

A correlation between the STRAP-R and cocaine use onset in cocaine-addicted individuals. Differential STRAP-R ratings for ‘drug’ minus ‘food’ ‘under drug influence’ (averaged across ‘liking’ and ‘wanting, placebo and methylphenidate) plotted against age of onset of cocaine use in 20 cocaine-addicted individuals.
Of interest were also the correlations between ‘liking’ and ‘wanting’, especially ‘under drug influence’ during methylphenidate. In the control subjects, these correlations were significant for all three rewards (rs > 0.63, p < 0.01). In contrast, in the cocaine-addicted individuals, ‘liking’ and ‘wanting’ were significantly intercorrelated for ‘food’ and ‘sex’ only (rs > 0.83, p < 0.0001) but not for ‘drug’ (r = -0.04, p > 0.9) ratings. These correlations provide support for the ANOVA results reported above (IV) further indicative of a dissociation between ‘drug’ ‘wanting’ and ‘liking’ ‘under drug influence’ during methylphenidate in the cocaine-addicted individuals.
Discussion
Using the newly developed STRAP-R questionnaire, we describe two main findings. First, the relative value of the three expected reinforcers (food, sex, drug) was uniquely modulated by the reported situation in the cocaine-addicted individuals. Specifically, ratings of ‘food’ exceeded ratings of ‘drug’ during the ‘current’ situation; similarly, ratings of ‘food’ and ‘sex’ exceeded ratings of ‘drug’ when reporting about an ‘in general’ situation. In contrast, when reporting about the ‘under drug influence’ situation, this pattern was reversed. In this situation ratings of ‘drug’ exceeded ratings of the other expected reinforcers (statistically significant for ‘food’) only in the drug-addicted group. The specificity of this unique reinforcer value shift to the ‘under drug influence’ situation may reflect conditioned responses to cue-induced increases in dopamine; in line with the current results, we previously suggested these conditioned responses to trigger an intense desire for cocaine, possibly exceeding desire for all other non-drug reinforcers (Volkow, et al., 2006). In general, these STRAP-R results add to an impressive body of work on the subjective effects of drugs in addicted individuals (Fox, et al., 2005; Gawin, 1991; Lasagna, et al., 1955; Leyton, et al., 2005; Von Felsinger, et al., 1955). Our current results provide further evidence in support of the possibility that in addiction, drug reward value is increased (Ahmed, et al., 2002; Ahmed and Koob, 1998), whereas non-drug reward value is decreased (Grigson and Twining, 2002). Evidence for the other two possibilities (blunted versus sensitised value) remains to be tested with direct group comparisons and with consumatory (versus expected) rewards.
Of note is the fact that the low ratings of ‘food’ ‘under drug influence’ in the cocaine-addicted subjects may be indicative of cocaine’s acute anorexigenic effects [and followed by episodes of rebound hunger (Williamson, et al., 1997)]. In contrast, in the healthy control subjects, food value may not have decreased when recalling the ‘under drug influence’ situation, as these individuals may have been imagining how they felt under the effects of marijuana. Nevertheless, a significant negative correlation with age of cocaine use onset, whereby the largest drug > food shift characterised the cocaine-addicted individuals with the youngest age of cocaine use onset, suggests this value shift may represent a cumulative (and not acute) effect of drug use. One could also entertain the possibility that this drug > food relative value differential may be a factor that predisposes individuals to more intense early drug experimentation and subsequent development of drug addiction.
Our second finding is partially consistent with the drug-related sensitization concept of the incentive motivation model (Robinson and Berridge, 1993; 2001; 2003). Consistent with this model, cocaine-addicted individuals reported ‘wanting’ drugs more than ‘liking’ drugs. However, this result was significant only when subjects recalled drug-related situations during methylphenidate (a similar trend was observed during placebo). The specificity of this ‘drug’ ‘wanting’ > ‘liking’ motivational shift to the ‘under drug influence’ situation (recall of the last time the individual was high/buzzed) and its enhancement by methylphenidate, a dopamine agonist and stimulant, suggest the impact on results of the following factors: (A) heightened arousal/autonomic reactions (Carter and Tiffany, 1999; Ehrman, et al., 1992; Glautier and Drummond, 1994; Margolin, et al., 1994; Sinha, et al., 2000); (B) ‘fresher’ memory traces of drug effects (Lee, et al., 2006); (C) increased craving/desire/drug-urges (Garavan, et al., 2000; Madden, et al., 1997; Robbins, et al., 1992; Volkow, et al., 2006); (D) dopaminergic amplification of stimuli salience (Volkow, et al., 2002; Volkow, et al., 2004); or (E) an interaction between these factors (Brody, et al., 2002). Overall, this shift (or dissociation between ‘drug’ ‘wanting’ and ‘liking’) may contribute to compulsive drug use even when the substance is no longer pleasurable (Fischman, et al., 1985).
With few exceptions (Willner, et al., 2005), most human studies in drug users appear to similarly support the incentive motivation model. For example, an alcohol prime (but not a juice prime) increased alcohol ‘wanting’ in heavy and light social-drinkers as measured by increased alcohol consumption; however, priming did not increase alcohol ‘liking’ as measured by taste ratings (Hobbs, et al., 2005). Correspondingly, Lambert, et al. (2006) reported a dissociation of ‘wanting’ from ‘liking’ in adult cocaine users who were studied prospectively from childhood into adulthood. Exposure to both stimulant treatment (for symptoms of attention deficit and hyperactivity disorder) and regular cigarette smoking predicted the highest ‘wanting’ for cocaine (self-report of ‘always wanted more’) and the lowest ‘liking’ (self-reported global positive effects from cocaine) (Lambert, et al., 2006). A recently developed computer-based experimental procedure similarly showed a unique pattern of dissociations between ‘wanting’ (forced-choice photographic procedure) and ‘liking’ (pleasantness ratings) of food stimuli in 60 healthy individuals depending on their state (hungry versus after an ad-libitum meal) (Finlayson, et al., 2007). Our current parallel results indicate that the STRAP-R could provide a rapid alternative to these more time consuming experimental procedures, especially when administered in combination with a salience-enhancing agent (such as the dopamine agonist methylphenidate).
Study limitations: (A) Given the experimental differences between the study groups, we analysed results separately for the cocaine addicted versus control subjects; direct comparisons with a healthy control group undergoing the same experimental protocol remain to be performed; (B) the psychometric properties of this instrument need to be tested in larger samples; it would be of particular interest to study whether the STRAP-R ratings predict behaviour [e.g., selection of drug over monetary rewards (Madden, et al., 1997)] and (C) results need to be tested in other drug using groups, such as those with longer withdrawal periods (Grimm, et al., 2003), in recreational cocaine users and in users of other drugs such as marijuana, alcohol, opiates or methamphetamine (Newton, et al., 2005).
For future uses of the STRAP-R, the following changes could be implemented: (A) ask about specific reinforcers to reduce potential inter-subject variability; (B) administer the questions in a randomised order or consider reversing the order of the questions, asking first about ‘wanting’ then about ‘liking’; (C) allow subjects to rate experiences as negative, which will allow studying reward avoidance or the effect of negative reinforcement; and most importantly (D) obtain the STRAP-R ratings during actual reinforcement experience. For example, the STRAP-R could be used to test reinforcer deprivation (e.g., food-deprived healthy control subjects compared with drug-withdrawn addicted individuals) or reinforcer consumption (eating versus drug intoxication).
In summary, results of this brief questionnaire, the STRAP-R, developed based on translation of principles from basic animal research, suggest a shift in the valuation of drugs as compared to other primary rewards in cocaine addiction. This shift is most clearly expressed when subjects are in a cue-related context (behaviourally: when reporting an ‘under drug influence’ situation; and more so, pharmacologically: during methylphenidate). In this cue-related context, drug valence exceeds that of food or sex, a potent social reinforcer; here, drugs are also wanted more than they are liked. This relative paling of other rewards in the environment, and the increase in the drug’s incentive motivation over its hedonic properties, may predispose the drug-addicted individual to compulsive drug use, uninterrupted by the promise of attaining other no-longer salient rewards. These results, thus, support our working hypothesis that drug-addicted individuals disproportionately attribute salience, or value, to their drug of choice with a concomitant decrease in the value of other primary rewards (Goldstein and Volkow, 2002), an impairment that is expressed when recalling or during a drug cue-induced situation.
Acknowledgements
This study was supported by grants from the National Institute on Drug Abuse (to RZG: 1R01DA023579 and R21DA02062); Laboratory Directed Research and Development from U.S. Department of Energy (OBER); National Institute on Alcohol Abuse and Alcoholism (2RO1AA09481); and General Clinical Research Center (5-MO1-RR-10710). Notice: This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
Footnotes
The meaning of ‘under drug influence’ probably differs as a function of drug use history (control subjects may have thought about marijuana, alcohol, cigarettes or coffee or experimentation with other drugs).
Thus, for the controls, the protocol consisted of a 2-day study (arithmetic, and placebo or methylphenidate), whereas for the cocaine-addicted group it consisted of a 4-day study (video type counterbalanced with medication). Because paired t-tests indicated, as expected, no significant differences between the STRAP-R scores as a function of the subsequent video type (ts < 2.1, ps > 0.05), we averaged the scores on the STRAP-R across both video days in the cocaine-addicted individuals.
The other three-way interaction (group × situation × question) approached nominal significance level (F2,37 = 3.8, p < 0.05). With the exception of reward × question, the other two-way interactions (reward × situation and question × situation) were also significant (Fs > 16.7, ps < 0.0001).
Contributor Information
RZ Goldstein, Department of Medical Research, Center for Translational Neuroimaging, Brookhaven National Laboratory, Upton, New York, USA.
PA Woicik, Department of Medical Research, Center for Translational Neuroimaging, Brookhaven National Laboratory, Upton, New York, USA.
SJ Moeller, University of Michigan, Ann Arbor, Michigan, USA.
F Telang, Department of Medical Research, Center for Translational Neuroimaging, Brookhaven National Laboratory, Upton, New York, USA; National Institute on Alcohol Abuse and Alcoholism, Rockville, Maryland, USA.
M Jayne, Department of Medical Research, Center for Translational Neuroimaging, Brookhaven National Laboratory, Upton, New York, USA.
C Wong, Department of Medical Research, Center for Translational Neuroimaging, Brookhaven National Laboratory, Upton, New York, USA.
GJ Wang, Department of Medical Research, Center for Translational Neuroimaging, Brookhaven National Laboratory, Upton, New York, USA; Mount Sinai School of Medicine, New York, New York, USA.
JS Fowler, Department of Medical Research, Center for Translational Neuroimaging, Brookhaven National Laboratory, Upton, New York, USA; Mount Sinai School of Medicine, New York, New York, USA.
ND Volkow, National Institute on Alcohol Abuse and Alcoholism, Rockville, Maryland, USA; National Institute on Drug Abuse, Bethesda, Maryland, USA.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward. Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Ehrman R, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Finlayson G, King N, Blundell JE. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiol Behav. 2007;90:36–42. doi: 10.1016/j.physbeh.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999a;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior in male rats following d-amphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1999b;142:200–208. doi: 10.1007/s002130050880. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Javaid J, Hatano Y, Davis J. Acute tolerance development to the cardiovascular and subjective effects of cocaine. J Pharmacol Exp Ther. 1985;235:677–682. [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Glautier S, Drummond DC. Alcohol dependence and cue reactivity. J Stud Alcohol. 1994;55:224–229. doi: 10.15288/jsa.1994.55.224. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, et al. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend. 2007;87:233–240. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs M, Remington B, Glautier S. Dissociation of wanting and liking for alcohol in humans: a test of the incentive-sensitisation theory. Psychopharmacology (Berl) 2005;178:493–499. doi: 10.1007/s00213-004-2026-0. [DOI] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Lasagna L, Von Felsinger JM, Beecher HK. Drug-induced mood changes in man. I. Observations on healthy subjects, chronically ill patients, and postaddicts. J Am Med Assoc. 1955;157:1006–1020. doi: 10.1001/jama.1955.02950290026009. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci. 2005;119:1619–1627. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Kosten TR. Cue-elicited cocaine craving and autogenic relaxation: association with treatment outcome. J Subst Abuse Treat. 1994;11:549–552. doi: 10.1016/0740-5472(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav. 2005;82:90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Using cue reactivity to screen medications for cocaine abuse: a test of amantadine hydrochloride. Addict Behav. 1992;17:491–499. doi: 10.1016/0306-4603(92)90009-k. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The rein-statement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology (Berl) 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G-J, Fowler JS, Gatley SJ, Logan J, Ding Y-S, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999a;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999b;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, et al. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry. 2004;161:1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Felsinger JM, Lasagna L, Beecher HK. Drug-induced mood changes in man. II. Personality and reactions to drugs. J Am Med Assoc. 1955;157:1113–1119. doi: 10.1001/jama.1955.02950300041009. [DOI] [PubMed] [Google Scholar]
- Washton AM, Stone-Washton N. Outpatient treatment of cocaine and crack addiction: a clinical perspective. NIDA Res Monogr. 1993;135:15–30. [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Willner P, James D, Morgan M. Excessive alcohol consumption and dependence on amphetamine are associated with parallel increases in subjective ratings of both ‘wanting’ and ‘liking’. Addiction. 2005;100:1487–1495. doi: 10.1111/j.1360-0443.2005.01222.x. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


