Abstract
Chronic, non-healing wounds and inadequate tissue repair characterized by excessive fibrosis continue to have a major negative impact on health and quality of life. Understanding the molecular events required for adequate healing, including the transcriptional control of wound repair, will be important for the development of future therapies. We previously showed that loss of Hoxb13 from murine skin results in enhanced cutaneous wound healing, suggesting that Hoxb13 has a negative effect on wound repair. To test this, we generated skin-specific Hoxb13 transgenic mice that overexpress Hoxb13 in the basal layer of the epidermis via the human keratin 14 promoter. Using these mice, we evaluated the effects of Hoxb13 overexpression on cutaneous wound healing. Transgenic wounds were characterized by persistence of the fibrin clot and prolonged inflammation. Notably neutrophils, which had cleared from wild-type wounds, were still pronounced in transgenic wounds. Marked epidermal hyperplasia was observed at transgenic wound edges and dermal vessels were grossly abnormal compared to wild-type. Both VEGF and TNF-α were upregulated in Hoxb13 transgenic skin. Together, our results identify Hoxb13 as a potential important clinical target in wound healing and other pathologies characterized by abnormal or excessive inflammation, angiogenesis, or epidermal proliferation.
INTRODUCTION
Wound healing is an elaborate process that requires precise orchestration and communication between keratinoctyes, fibroblasts, endothelial cells, inflammatory cells, and the extracellular matrix (Arbiser, 1996; Eming et al., 2007; Gailit and Clark, 1994; Raja et al., 2007; Santoro and Gaudino, 2005). Disruption of these interactions can severely inhibit repair. Non-healing wounds (or abnormal healing characterized by excessive scarring) continue to be a major health problem, so understanding the molecular events required for adequate healing is a major research focus. During the past several years, much has been learned about the regulation of wound healing by growth factors and cytokines (Bryan et al., 2005; McKay and Leigh, 1991; Wahl et al., 1989). By contrast, much less is known about transcriptional regulation at the wound site (Schafer and Werner, 2007). One group of regulators that has recently been shown to play important roles in wound repair is the Hox family of transcription factors.
Hox proteins are best known for their key roles as regulators of axial and organ patterning during embryonic development (Grier et al., 2005; Hombria and Lovegrove, 2003; Krumlauf, 1994; Manak and Scott, 1994; Martinez and Amemiya, 2002). To date, thirty-nine Hox genes have been identified in the vertebrate genome. In mouse and humans, they reside in four complexes (A–D in humans; a–d in mice) located on 4 different chromosomes. On the basis of sequence similarity and position, corresponding genes in the four complexes can be aligned with each other in 13 paralogous groups whose functions are often overlapping. In addition to their early developmental roles, it has become increasingly evident over the last several years that Hox gene activity is important in adult tissues (Morgan, 2006). The majority of the known 39 Hox genes have been reported to be expressed in adult skin (Chang et al., 1998; Detmer et al., 1993; Stelnicki et al., 1998b). It is in this organ that the functional requirements for Hox gene activity in adults have been most studied, particularly in the area of cutaneous wound repair. Members of the Hox3 paralogus group have been shown to function as positive regulators of angiogenesis, to promote endothelial as well as epithelial migration, and to enhance collagen deposition in the wound bed (Mace et al., 2005; Myers et al., 2000). In contrast, HoxA5 and HoxD10 have been shown to inhibit angiogenesis (Myers et al., 2002; Rhoads et al., 2005).
We have previously shown that another member of the Hox family, Hoxb13, also influences cutanous wound healing (Mack et al., 2003). Hoxb13 is expressed in unperturbed fetal and adult skin (Stelnicki et al., 1998b), but is significantly downregulated in fetal wounds that heal without a scar compared to adult wounds (Stelnicki et al., 1998a). This result suggested that downregulating Hoxb13 in adult wounds could lead to a more effective repair. To that end, we determined that cutaneous wounds in Hoxb13 knockout mice healed faster and with less scar compared to wounds in wild-type mice (Mack et al., 2003). We further determined that Hoxb13 knockout skin contained significantly higher levels of hyaluronan, a high molecular weight glycosaminoglycan that has been implicated as an important factor in fetal scarless wound healing (McCallion and Ferguson, 1996).
To further examine the effects of Hoxb13 on cutanous wound repair, in this paper we have generated Hoxb13 transgenic mice, utilizing the human keratin 14 promoter (K14-Hoxb13 mice). This promoter is highly active in the basal layer of stratified squamous epithelia and in the outer root sheath of the hair follicles (Vassar et al., 1989). Young adult Hoxb13 transgenic mice were fertile with no apparent skin abnormalities. However, when wounded, they demonstrated significantly delayed healing, exemplified by persistence of the wound eschar, a protracted inflammatory response, enlarged vessels, and grossly abnormal epidermal histology. We further determined that vascular endothelial growth factor (VEGF) and tumor necrosis factor αlpha (TNF-α) expression are upregulated in K14-Hoxb13 transgenic skin, suggesting that these molecules may be responsible in part for the atypical angiogenesis and inflammation, respectively, observed in transgenic wounds. Together, these data indicate that overexpression of Hoxb13 has a negative impact on wound healing and implicate Hoxb13 as a possible transcriptional activator of VEGF and/or TNF-α.
RESULTS
Generation of K14 - Hoxb13 transgenic mice
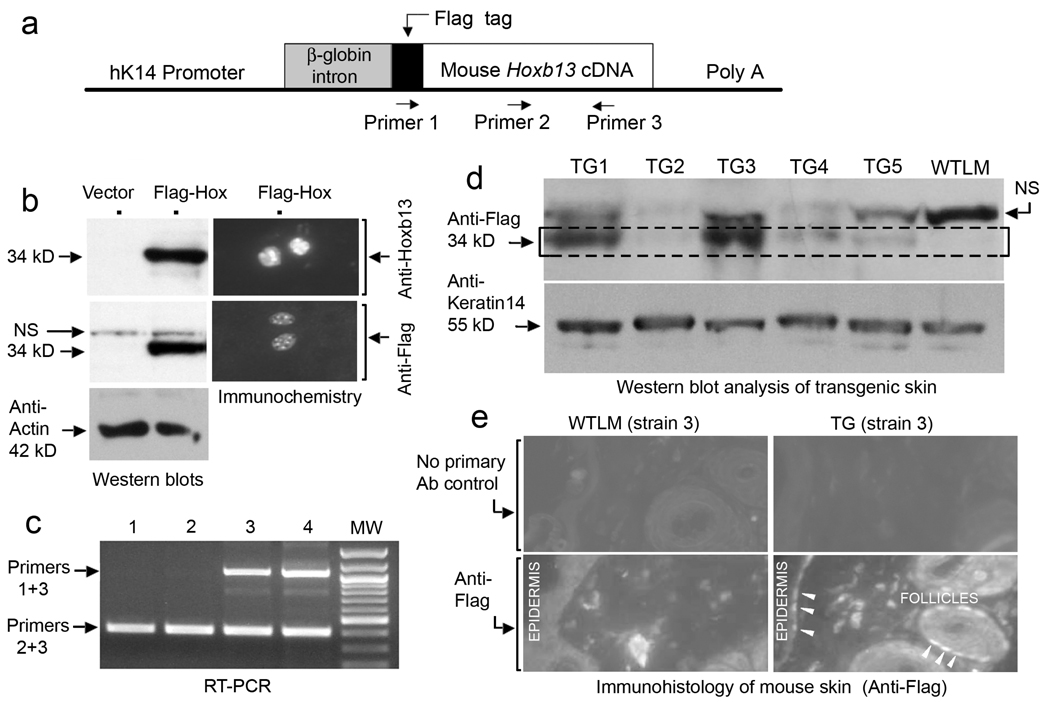
The Hoxb13 cDNA with a 5’ Flag tag epitope was inserted into a targeting vector containing sequence from the human keratin 14 (K14) promoter (Figure 1a). In mice, the human K14 promoter is highly active in the basal layer of the epidermis and the outer root sheath of the hair follicle, matching that of endogenous K14 (Vassar et al., 1989). To test for expression, the K14-Flag-Hoxb13 vector (Figure 1a) or K14 vector alone was transiently transfected into a rat epidermal keratinocyte cell line which exhibits robust K14 expression (data not shown). Western blot analysis showed a strong band of the expected 34 kDa size after staining with anti-Flag or anti-Hoxb13 antibody (Figure 1b, left panels). Immunohistochemical analysis demonstrated nuclear staining with both antibodies (Figure 1b, right panels). Transgenic founders were identified by PCR using forward Flag and reverse Hoxb13 primers (see Figure 1a). Figure 1c shows a sample genotyping; the upper band represents the transgene. Six independent transgenic (TG) lines were established and designated K14-Hoxb13 TG strains #1–6. Western blot analysis was performed on skin protein extracts from TG mice and wild-type littermates (WTLM) using anti-Flag antibody (Figure 1d). The antibody recognized Flag-Hoxb13 (Figure 1d, boxed region) as well as a nonspecific band also observed in protein extracts from transfected cells (Figure 1b). K14-Hoxb13 TG strains 1 and 3 were relatively high expressors compared to TG strains 2, 4, and 5. The absence of a signal in the WTLM samples confirmed the specificity of the Flag-epitope detection. K14-Hoxb13 TG strain 6 was also a high expressor (data not shown). Skin sections from K14-Hoxb13 TG strain 3 stained with anti-Flag antibody showed signal in the nuclei of the epidermal basal layer and outer root sheath of the hair follicle (Figure 1e, arrowheads). No signal was obtained in sections from WTLM. K14-Hoxb13 TG mice appeared healthy. There were no differences in skin morphology between TG mice and WTLM by gross inspection, by histological staining with hematoxylin and eosin (H & E), or by immunostaining for epidermal proteins keratin 10 and 14 (data not shown).
Figure 1. Generation and verification of K14-Hoxb13 transgenic mice.
(a) Schematic of the K14-Hoxb13 transgene construct. Primers 1 and 3 identify the Hoxb13 transgene; Primers 2 and 3 identify both the transgene and the endogenous Hoxb13 gene. (b) Verification of expression of the transgene by Western blot (left) and immunostaining (right) of transiently transfected rat epidermal keratinoctyes with anti-Flag and anti-Hoxb13 antibodies; anti-actin, internal loading control. (c) PCR genotyping of selected founder mice; lanes 3 and 4 are positive for the Hoxb13 transgene (upper bands). The lower band indicates both the transgene and endogenous Hoxb13. (d) Western blot of Hoxb13 transgenic skin extracts with anti-Flag antibody; anti-keratin-14, internal loading control. The lower band (within the dashed box) represents the Flag-Hoxb13 fusion protein; note complete absence from the lane containing protein from wild-type littermate (WTLM) skin. NS, non-specific bands. A similar sized NS band was also seen in the Western blot shown in panel b. Transgenic strains 1 and 3 (TG1, TG3) express relatively high levels of transgene product compared to strains 2, 4, and 5. (e) Immunostaining of Hoxb13 transgenic strain 3 (TG3) with anti-Flag antibody. Transgene product is detected in the nuclei of basal keratinocytes of the epidermis and outer root sheath of hair follicles (white arrowheads).
Wound healing is delayed in response to Hoxb13 overexpression in the epidermis
To evaluate the effects of Hoxb13 overexpression following injury, we created 5-mm diameter, full-thickness excisional wounds on each of the six K14-Hoxb13 TG strains and their WTLM and monitored the wounds for 11 days. Typical results are illustrated in Figure 2a. By day 11 post-wounding, the fibrin clot (crust) had resolved and the wound was completely closed in the WTLM. In contrast, the wound area of the TG mouse was still covered by a large fibrin crust that remained tightly adhered to the skin. TG strains 1 and 6 (the other two high expressors) also retained a large crust at day 11 post-wounding (data not shown). To compare the efficacy of healing in the low Hoxb13 expressors and the high Hoxb13 expressors, we measured the area of the wound/crust over time (Figure 2b). The involved area was larger in all Hoxb13 overexpressors relative to WTLM up to 7 days post-wounding. A large crust still covered the wounds of all high Hoxb13 expressors at day 11 post-wounding. In a follow-up study, the adherent crusts in Hoxb13 high expressors persisted until day 14–15 (data not shown). These findings suggest that wound healing is substantially delayed as a result of Hoxb13 overexpression in the epidermis.
Figure 2. Transgenic mice expressing high levels of epidermal Hoxb13 exhibit abnormal wound healing compared to low expressors or wild-type littermates.
Following general anesthesia and shaving of the dorsal hair, a single 5-mm full-thickness excisional wound was made on the upper back of six pairs of mice [a K14-Hoxb13 transgenic (TG) mouse and its respective WTLM] derived from six different founder strains. The wounds were photographed every other day. (a) Wounds from TG strain 3 (a high Hoxb13 expressor) are shown, along with its WTLM. Note that in the WTLM at day 11 post-wounding, the crust had resolved and the wound was completely closed. By contrast, a large fibrin crust persisted at the wound site in the Hoxb13 TG mouse. (b) At the times indicated, the area of the wound and crust was measured using photography and digital analysis. Data from three strains that weakly expressed the Hoxb13 transgene were pooled (Low-expressors, left graph, open circle), as were their corresponding WTLM (left graph, closed circle). The strains that strongly expressed the transgene were grouped in a similar fashion (High-expressors, right graph). Note that for all times up to day 7, all Hoxb13 TG mice showed a larger wound-crust area than the WTLM. This difference persisted beyond day 7 and was statistically significant in the high-expressing Hoxb13 TG mice; *P<0.05, **P<0.01, and ***P<0.0005.
Wound morphology is grossly abnormal in mice expressing high levels of Hoxb13
For histological analysis, we stained 11-day-old wound sections with H & E (Figure 3). WTLM wounds (Figure 3a and b) were characterized by a defined region of granulation tissue (1), a closed and moderately hyperplastic epidermis (2), and a loosely woven stratum corneum (s.c.,3). The wounds of low Hoxb13 expressors (TG strain 4 is shown here, Figure 3c) displayed a healthy granulation bed, but in comparison to the WTLM contained an increased number of inflammatory cells (4, area within dashed white line). In addition, epidermal irregularities were occasionally noted such as increased acanthosis and small compact areas of hyperkeratosis (5). The wound histology of the high Hoxb13 expressors (Figure 3d and e) was severely abnormal compared to the WTLM and the low Hoxb13 expressors. The large and tightly adhered eschar present at the wound site (corresponding to morphology of Figure 2a) contained numerous inflammatory cells (6). The epidermis was highly irregular, with central atrophic areas (7) and severely hyperplastic regions with elongated ridges at the wound edge (8). A well-defined region of granulation tissue was absent in the wounds of the high Hoxb13 expressors (Figure 3d and e) as compared to WTLM (Figure 3a and b). Within the dermal area of the healing wounds, the number of inflammatory cells was grossly increased in high Hoxb13 expressing TG mice relative to WTLM (9, area within white dashed line). Together, these findings indicate that overexpression of Hoxb13 in the epidermis is detrimental to wound healing.
Figure 3. Hoxb13 overexpression in the epidermis leads to a highly abnormal wound morphology.
Hematoxylin-eosin stains of 11-day-old excisional wounds are illustrated as follows: (a, b), WTLM; (c), a low-expressing Hoxb13 TG mouse; (d, e), high-expressing Hoxb13 TG mice. The founder strains are indicated. (a, b) At 11 days post-wounding, a healthy bed of granulation tissue (1) and a completely intact epidermis (2) with a loosely woven stratum corneum (3) was observed in WTLM wounds. (c) Wounds from a low-expressing transgenic mouse contained a granulation bed that was healthy but contained increased inflammatory cells (4; area within the dashed region), along with epidermal abnormalities such as increased acanthosis and compact hyperkeratosis (5). (d, e) Wounds from Hoxb13 high-overexpressors remained covered with a dense eschar that contained numerous polymorphonuclear cells (6). Epidermal morphology at those wound sites showed epidermal atrophy overlying the wound bed (7) and irregular epidermal hyperplasia with elongated ridges at the wound edges (8). An increased inflammatory cell infiltrate was observed in the dermal portion of the wound bed in the TG mice (9). Scale bar, 100 µm.
Overexpression of Hoxb13 enhances and prolongs the wound inflammatory response
To better characterize the increased inflammatory cell infiltrates observed in the wounds of K14-Hoxb13 TG mice, skin sections from 11 day-old excisional wounds were stained for neutrophils, macrophages, and mast cells (Figure 4). A neutrophil-specific antibody revealed occasional neutrophils in the wound beds of WTLM (Figure 4a and c) or low Hoxb13 expressors (Figure 4b). In contrast, wound beds of high Hoxb13 expressors contained large numbers of neutrophils (Figure 4d), comprising a 6-fold elevation over WTLM or low Hoxb13 expressors (Figure 4e). Macrophages, detected by antibody F4/80, were increased by approximately two-fold in the Hoxb13 expressors relative to WTLM or to low Hoxb13 expressors (Figure 4f–j). Mast cells at and adjacent to the wound site were evaluated by toluidine blue staining (Figure 4k and l; open arrowheads). There was no difference in mast cell numbers between low Hoxb13 expressors and matched WTLM (Figure 4m, left). However, in high Hoxb13 expressors, mast cells were ~3-fold more abundant than in their WTLM (Figure 4m, right). Overall, these results indicate that overexpression of Hoxb13 in the epidermis dramatically prolongs the inflammatory response in wounds.
Figure 4. Wounds in Hoxb13 high-expressor mice contain significantly more inflammatory cells than in wild-type mice.
Eleven day-old full-thickness excisional wounds stained with the following antibodies: (a–d) neutrophil-specific, RB6-8C5; (f–i) macrophagespecific, F4/80; (k, l) toluidine blue, for mast cells (arrowheads). Scale bar, 100 µm. Mouse TG strains 4 and 5 (low-expressors) and strains 1 and 3 (high-expressors) are illustrated in the panels, while the graphs (e, j, m) show aggregate data for all strains. P values are indicated for significant differences. NS, not significant. Results represent mean ± SEM from at least 9 optical fields each from the low Hoxb13 expressors, high Hoxb13 expressors, and WTLM.
Transgenic mice expressing high levels of Hoxb13 exhibit atypical vessel biology
H & E stained wounds of high Hoxb13-expressing mice appeared highly vascularized compared to WTLM. To examine vessel subtypes, wound sections were stained with antisera to platelet endothelial cell adhesion molecule-1 for blood vessels ( PECAM-1, Figure 5a and b) or lymphatic vessel endothelial receptor-1 (LYVE-1; Figure 5c and d) for lymphatics (Banerji et al., 1999). Blood and lymphatic vessels were quantified by microscopy and computer-assisted image analysis (Table 1). Uninjured skin showed a slight trend toward higher vessel density in Hoxb13 overexpressors (Table 1, first row). Wounding led to a large increase in numbers of blood vessels by 11 days, and both the size (area) and length of these vessels was increased 2-to 3-fold in Hoxb13 overexpressors vs. WTLM (Figure 5 a and b; Table 1, rows 1, 2). Lymphatic vessels were significantly influenced by expression of the Hoxb13 transgene in both unwounded and wounded skin (Figure 5c and d; Table 1, rows 4–6). The total density of lymph vessels in skin was significantly increased in the K14-Hoxb13 TG mice (3-fold in unwounded skin, 13-fold in wounded skin) through a combination of increased number of vessels and enlargement of their lumens. Thus, overexpression of Hoxb13 has a marked affect on both angiogenesis and lymphangiogenesis.
Figure 5. Blood vessels and lymphatic vessels are enlarged in the skin of K14-Hoxb13 transgenic mice expressing high levels of the transgene.
Staining of frozen sections from 11-day-old full-thickness excisional wounds in K14-Hoxb13 TG3 or matched WTLM, using antisera to the following: (a, b) PECAM-1 (blood vessels); (c, d) LYVE-1 (lymphatic vessels). All images are taken from the middle of the wound bed. Dotted lines, epidermal-dermal junction. Arrows, lymphatic vessels. Scale bar, 100 µm.
TABLE I.
Summary of Blood Vessels and Lymphatics in Normal and Wounded Skin of Hoxb13 Mice
| --------- --UNWOUNDED SKIN *------------ | ------ ----WOUNDED SKIN (11 DAYS) *--- ----- | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wildtype | Transgenic | Increase | Wildtype | Transgenic | Increase | ||||||
| ANGIOGENESIS (PECAM-1): | Mean | ± SD (n) | Mean | ± SD (n)P | (-fold) | Mean | ± SD (n) | Mean | ± SD (n)P | (-fold) | |
| Blood vessel area | (% of dermal area) | 1.5 | ± 0.66 (9) | 2.4 | ± 1.3 (9)a | 1.6 | 2.5 | ± 1.3 (6) | 8.7 | ± 5.0 (6)b | 3.5 |
| Blood vessel length | (µm) | ND | ND | ND | 43.2 | ± 6.8 (8) | 92.3 | ± 11.8 (8)d | 2.1 | ||
| Number of vessels | (vessels / mm2) | 13 | ± 3.1 (9) | 16 | ± 9.2 (9) | 1.2 | 150 | ± 49 (8) | 132 | ± 25 (8) | 0.9 |
| LYMPHANGIOGENESIS (LYVE1-1): | |||||||||||
| Lymph vessel area | (% of dermal area)** | 0.23 | ± 0.01 (13) | 0.64 | ± 0.13 (14)d | 2.8 | 0.22 | 0.23 (12) | 2.90 | 1.3 (12)d | 13.2 |
| Lumen Size | (µm2) | 23 | ± 28 (13) | 149 | ± 147 (14)c | 6.5 | 73 | 95 (12) | 402 | 527 (12)c | 5.5 |
| Number of vessels | (vessels / mm2) | 8.8 | ± 3.4 (13) | 13 | ± 4.5 (14)c | 1.5 | 8.7 | ± 7.7 (8) | 35 | ± 14 (8)d | 4.1 |
The data are from unperturbed skin or from skin at 11 days post-wounding. TG mice and WTLM from strains 3 and 6 were used for these experiments.
The upper dermis, above the bulge region of hair follicles, was analyzed.
(n), number of fields (images) that were analyzed.
P-values for comparison of transgenic vs. wildtype are indicated as superscripts:
P < 0.05
P < 0.01
P < 0.005
P < 0.0005
ND, not done
VEGF and TNF-α are upregulated in K14-Hoxb13 transgenic skin
To determine molecular mechanisms for the increased angiogenesis and inflammation in K14-Hoxb13 TG wounded skin, we examined the expression of VEGF and TNF-α, two molecules known to play key roles in angiogenesis and inflammation, respectively. Overexpression of VEGF in murine skin via the K14-promoter (K14-VEGF) has been reported to upregulate both angiogenesis and lymphangiogenesis (Hong et al., 2004; Nagy et al., 2002; Xia et al., 2003). TNF-α has been shown to be a potent chemoattractant for neutrophils (Lukacs et al., 1995; Sayers et al., 1988; Widegren et al., 2008). We evaluated VEGF and TNF-α expression in both unwounded and wounded TG (high expressor) and WTLM skin by Western blot (Figure 6a). In unwounded skin, bands of 43 kDa and 25 kDa, corresponding to VEGF and TNF-α, respectively were detected that were markedly more intense in the TG lanes (black asterisks). Compared to the GAPDH loading controls, there was a 3-fold increase in VEGF levels and a 4.5-fold increase in TNF-α levels in TG unwounded skin compared to WTLM (Figure 6b). In lysates from wounded skin at 7 days, the intensity of the VEGF 43 kDa band was similar between TG and WTLM. However, new bands of ~58 kDa and 80 kDa were present that were stronger in the transgenic lanes (arrows). We believe these larger bands may represent post-translationally modified VEGF (see Discussion). As in unwounded skin, the 25 kDa TNF-α band was significantly more intense in lysates from 7 day TG wounds (black asterisks). The elevated VEGF and TNF-α expression in K14-Hoxb13 TG mice suggests these molecules are responsible in part for the increased vascularity and inflammation, respectively, observed in TG unwounded and/or wounded skin.
Figure 6. VEGF and TNF-α are differentially upregulated in the skin of Hoxb13 transgenic mice.
(a) Western blot of protein extracts from unwounded skin and from 7 day full-thickness excisional wounds stained with antibodies to VEGF, TNF-α, and GAPDH as an internal control. Locations of molecular weight (MW) markers, in kDa, are shown on the right. The VEGF antibody detects a 43 kDa band in unwounded and wounded skin, but the intensity of this band is much greater in the K14-Hoxb13 TG unwounded lanes (asterisks) as compared to the strain-matched WTLM (WT). In wounded skin, high-MW VEGF bands are observed that are significantly more intense in the transgenic lanes (arrows). The TNF-α antibody detects a 25 kDa band that is significantly more intense in the K14-Hoxb13 TG unwounded and wounded lanes. (b) Densiometric analyses of the 43 kDa VEGF, 25 kDa TNF-α, and GAPDH Western blot signals from unwounded skin (n=3 independent K14-Hoxb13 TG mice and 3 matching WTLM).
DISCUSSION
We have previously shown that loss of Hoxb13 from skin results in enhanced cutaneous wound healing (Mack et al., 2003). Here we demonstrate that overexpression of Hoxb13 in the epidermis: 1) has a deleterious effect on wound healing, 2) results in protracted inflammation and atypical vessel remodeling, and 3) is associated with an upregulation of both VEGF and TNF-α in the skin of K14-Hoxb13 TG mice.
Hox genes are best known for their highly conserved roles in embryonic patterning (Krumlauf, 1994; Manak and Scott, 1994). We are just beginning to elucidate their functions in adult tissues. Despite their abundance in both fetal and adult tissues, relatively few direct targets of Hox transcriptional activity have been identified. Of those that have been characterized, several have been assigned biological roles that are important in wound healing, including angiogenesis (Bruhl et al., 2004; Wu et al., 2003), cell migration (Daftary et al., 2002), and inflammation (Bandyopadhyay et al., 2007; Mori et al., 2008; Shi et al., 2001). In the context of cutaneous wound healing, HoxD3 has been shown to induce the expression of type 1 collagen in wounds (Hansen et al., 2003) and HoxA3 and D3 have been reported to promote angiogenesis in murine skin following wounding (Mace et al., 2005; Myers et al., 2000).
VEGF is a major mediator of angiogenesis (Ferrara and Davis-Smyth, 1997). Here we show that VEGF is a potential target of Hoxb13. In normal mice, VEGF is expressed at low levels in the epidermis, but is upregulated in wounded epidermis for 2–3 days post-injury (Brown et al., 1992). Epidermal VEGF is thought to promote dermal angiogenesis via a paracrine response. As cited earlier, angiogeneisis and lymphangiogenesis are upregulated in K14-VEGF skin/wounds (Hong et al., 2004; Nagy et al., 2002; Xia et al., 2003). The murine K14 promoter is still very active at day 11 post-wounding (data not shown), indicating that K14-Hoxb13 expression is almost certainly high at this time point. Therefore, it is likely that Hoxb13-induced VEGF is in part responsible for the increased angiogenesis and lymphangiogenesis observed in the wound bed of transgenic mice. However, we cannot rule out the involvement of other factors such as VEGF-C or VEGF-D which have been identified as critical cytokines responsible for generating the lymphatic vascular system (Jussila and Alitalo, 2002; Oliver and Detmar, 2002).
Our western blots showed a significant increase in the intensity of the VEGF 43 kDa band in unwounded K14-Hoxb13 TG skin (Figure 6). This was not observed in lysates from wounded skin. However, we detected bands of a higher molecular weight than that predicted by amino acid sequence alone that were more intense in lysates from TG wounds. We postulate that these represent alternate forms of VEGF. VEGF can be modified post-translationally though such events as ADP-ribosylation, glycosylation, and heparin binding (Brandner et al., 2006; Ghani et al., 2003), all of which can have a marked influence on its biological activity. We are not yet certain what influence Hoxb13 induced expression of VEGF is having on wound healing per se. It has been reported that epidermal VEGF is required for the development of hyperplasia in response to a sustained barrier disruption injury (Elias et al., 2008). This suggests that the marked epidermal hyperplasia observed at the wound edges of K14-Hoxb13 TG wounds (see Figure 3) may be due in part to abnormally high levels of epidermal VEGF. Interestingly, Wilgus et al. recently reported that addition of VEGF to fetal wounds that normally heal in a scarless fashion results in increased vascularity and a scarring phenotype, while neutralization of VEGF in adult wounds reduced vascularity and scar formation (Wilgus et al., 2008). We have preliminary data indicating increased scarring and fibrosis in K14-Hoxb13 TG wounds compared to the wounds of WTLM (data not shown). Therefore, it is possible that increased and prolonged expression of VEGF in TG wounds may be contributing to excessive scarring.
Another important feature of K14-Hoxb13 TG mice is the upregulation of the pro-inflammatory cytokine TNF-α. In normal healthy skin, TNF-α is expressed at low levels in epidermal keratinocytes (Strickland et al., 1997). In injured or diseased tissue, TNF-α levels are greatly augmented by activated macrophages (Chen and Goeddel, 2002) and mast cells (Gordon and Galli, 1990). TNF-a levels are significantly higher in both TG unwounded and wounded skin. We have found no evidence of increased inflammation in unwounded TG skin (data not shown), suggesting that the source of the increased TNF-α is epidermal in nature. In wounded TG skin, higher TNF-α levels are most likely due to the increased and protracted presence of macrophages and mast cells at the wound site. However, we cannot rule out the possibility that Hoxb13 is abnormally upregulating epidermal TNF-α in response to wounding. TNF-α is a potent inducer of neutrophil adhesion (Bevilacqua et al., 1987; Gotsch et al., 1994) and infiltration into damaged tissues (Lukacs et al., 1995; Widegren et al., 2008). Several studies have demonstrated that addition of exogenous TNF-α to wounds is detrimental to wound healing (Buck et al., 1996; Rapala et al., 1991; Salomon et al., 1991). In addition, mice deficient for the tumor necrosis factor receptor p55 (TNF-Rp55), which is normally upregulated following cutaneous wounding, show accelerated wound healing in association with markedly reduced recruitment of neutrophils and macrophages (Mori et al., 2002). These data suggest that increased TNF-α in K14-Hoxb13 TG wounds, interacting with TNF-Rp55, is significantly contributing to the exacerbated inflammatory response and delayed healing.
A notable phenotype of high expressing K14-Hoxb13 TG excisional wounds is the persistence of the fibrin clot, which normally resolved by approximately seven days post-wounding in WTLM, but remained large and tightly adherent in Hoxb13-high expressors for as long as 15 days. Both VEGF and TNF-α are strong inducers of vascular permeability, allowing vessels to leak plasma proteins and promote fibrin deposition (Brett et al., 1989; Keck et al., 1989; Nawroth et al., 1988; Senger et al., 1990). The combined activity of high levels of VEGF and TNF-α in TG wounds may be responsible for the persistence of the fibrin clot. Alternatively, it is also possible that the clot may be lacking substances required for its lysis. Fibrin clots harbor growth factors and cytokines (Clark, 2003). Based on this observation, it is conceivable that K14-Hoxb13 TG wounds are being subjected to prolonged exposures of growth factors and/or cytokines which may be deleterious to the wound environment.
The mechanisms by which Hoxb13 upregulates VEGF and TNF-α are not currently clear. As a transcription factor, Hoxb13 could be promoting expression by direct binding and activation of their promoters. Both the VEGF and TNF-α promoter contain potential Hoxb13 consensus binding sites (data not shown). Alternatively, Hoxb13 could be indirectly influencing their expression through the regulation of upstream genes. In the case of VEGF, one potential candidate is hypoxic inducible factor-1 (HIF-1), a master regulator of VEGF expression (Dery et al., 2005). Interestingly, TNF-α can also induce VEGF expression in a HIF-1 dependent manner.
In conclusion, we have shown that overexpression of Hoxb13 in the murine epidermis via the K14 promoter results in abnormal wound healing, a prolonged inflammatory response, vascular enlargement, and upregulation of VEGF and TNF-α. The latter two molecules play a central role not only in wound healing but also in tumor growth, and have been associated with skin pathologies such as psoriasis (Kristensen et al., 1993; Xia et al., 2003). We previously reported that overexpression of Hoxb13 in an organotypic epidermal model resulted in many tissue characteristics reminiscent of psoriasis (Mack et al., 2005). Interestingly, it should also be noted that Hoxb13 is upregulated in cancer (Cantile et al., 2003; Lopez et al., 2006; Svingen and Tonissen, 2003; Yamashita et al., 2006) and promotes ovarian cancer progression (Miao et al., 2007). As part of our ongoing study, we will also investigate the expression of other key molecules important in cutaneous healing and disease such as basic fibroblast growth factor and transforming growth factor beta (Arbiser et al., 1998; Li et al., 2004). Together, our current data suggest that Hoxb13 may be an important clinical target in wound healing and in other pathological skin conditions that involve excessive inflammation, angiogenesis and epidermal hyperproliferation.
MATERIALS and METHODS
Generation and identification of K14-Hoxb13 transgenic mice
The full-length mouse Hoxb13 cDNA was obtained from Open Biosystems (Huntsville, AL). An N-terminal Flag-tagged Hoxb13 with flanking Xba sites and a Kozak sequence directly upstream of the Flag tag was generated by PCR using the following primers: Forward 5’-CTCTAGAGCCACCATGGATTACAAGGATGACGACGATAAGGAGCCCGGCAATTATGCC-3’; Reverse – 5’- AGTGCTCAACAGAGCTCTAGATAGAA-3’ and subcloned into the pCR2.1 vector using the TOPO cloning Kit (Invitrogen, Carlsbad, CA). The Flag-Hoxb13 sequence was then subcloned into the Xba site of the human K14 promoter vector (kindly provided by Dr. Xiao-Jing Wang, University of Colorado Denver). Transgenic mice were generated at the Case Transgenic and Targeting Facility (Case Western Reserve Univ., Cleveland, OH) and identified by PCR analysis of tail DNA. The Flag-Hoxb13 transgene was identified using a Flag-specific forward primer, 5’- GGATTACAAGGATGACGACGATAAGG-3’ and a Hoxb13-specific reverse primer, 5’- AGGTTCTTCAGAACCGTAATGGA -3’. The PCR product positive for the Flag-Hoxb13 transgene was approximately 1083 bp. The mice were maintained as hemizygotes.
Preparation of nuclear extracts
Rat epidermal keratinocytes were transiently transfected with 5 ug of the K14 promoter vector alone or with 5 ug of the K14-Flag-Hoxb13 vector using Gene Porter (Genlantis, San Diego, CA) and incubated at 37°C in 5% CO2 for 48 hours. Cells were then washed in cold PBS, scraped and resuspended in 1 ml of PBS, and centrifuged. The pellet was resuspended in cold hypotonic buffer (10 mM Hepes, pH 7.9, 10 mM KCL, 0.1 mM EDTA, 0.1 mM EGTA, and 1 mM DTT), incubated on ice for 15 min followed by the addition of 25 µl of 1% NP-40, and vortexed for 10 sec. Lysed cells were spun for 1 minute at full speed and the pellet resuspended in 50 µl of a cold hypertonic buffer (20 mM Hepes, pH 7.9, 0.4 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 20% glycerol, 1mM DTT, and 1mM PMSF), followed by shaking for 15 minutes at 4°C. The lysate was then spun at full speed for 5 minutes, and the supernatant containing nuclear proteins transferred to a clean tube. Protein concentration was determined by Bradford Assay (Bio-Rad, Hercules, CA) and the samples stored at −80°C.
Preparation of skin protein lysates
Full-thickness skin specimens were placed in Brij lysis buffer (1 ml of 1 M Tris, 0.4 ml of 0.5 M EDTA, 3 ml of 5 M NaCl, 8.75 ml of 10% Brij 97, 1.25 ml of 10% NP40, diluted to 100 ml with H2O) or Cell Lysis Buffer from Ray Bio™ (Norcross, GA) each containing a protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO), homogenized, and briefly sonicated. Samples were then centrifuged at 13,000 rpm for 10 minutes at 4°C and the supernatant transferred to new tubes on ice. Protein concentrations were determined by Bradford Assay (Bio-Rad) and the samples stored at −80°C.
Western blot analysis
Proteins were electrophoresed on NuPAGE™ 4–12% Bis-Tris gels (Invitrogen) and blotted according to standard protocol. Blots were blocked for 1 hour at RT (blocking buffer: 5% milk, 50 mM Tris pH 7.5, 150 mM NaCl), and incubated overnight with the following antibodies: mouse monoclonal Hoxb13 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal Flag (1:500, Sigma Aldrich), rabbit anti-mouse beta-actin (1:500; Santa Cruz Biotechnology), rabbit anti-mouse keratin-14 (1:2000; Covance, Denver, PA), rabbit anti-mouse VEGF (1 ug/ml; AbCam, Cambridge, MA), rat monoclonal TNF-α (BioXCell, West Lebanon, NH) or rabbit anti-mouse GAPDH (1:500; Santa Cruz Biotechnology) followed by a 1–2 hour incubation with the appropriate HRP-conjugated secondary (1:10,000; Sigma Aldrich). Signal was developed using ECL or ECL Plus Western Blotting Detection System (GE Healthcare, Waukesha, WI).
Wounding protocols
The Cleveland Clinic Institutional Animal Care and Use Committee (IACUC) approved all animal procedures. At the time of this study, K14-Hoxb13 transgenics were backcrossed to C57BL/6 four times. Sex matched K14-Hoxb13 transgenics and wild-type littermates at 8–12 weeks were anesthetized with an IP injection of Pentobarbitol (40–85 mg/kg; Ovation Pharmaceutical, Inc., Deerfield, IL), shaved on the dorsal back, and the area wiped down with 70% alcohol. Each mouse received a 5-mm diameter full-thickness excisonal wound on the upper dorsal back using iris scissors. Wounds were left open to the air. All wounded animals were housed in individual cages for the duration of the study or until the wound was harvested.
Histological and immunohistochemical analysis
Fixed skin samples were processed, embedded, and cut into 5 μm sections. Slides were baked overnight at 42°C and stained with hematoxylin and eosin using a standard protocol. Primary antibodies utilized for immunohistochemistry were rat anti-mouse RB6-8C5 for neutrophils (1:100; a gift from Robert Fairchild, Cleveland Clinic, OH), rat anti-mouse F4/80 for macrophages (1:50; Serotec, Raleigh, NC), rabbit anti-OctA (1:100; Santa Cruz; reacts with the Flag epitope), rabbit-anti-mouse K14 (1:1000; Covance), rabbit-anti mouse K10 (1:500; Covance), biotinlyated rabbit polyclonal to LYVE-1 (1 ug/ml; AbCam), and biotinylated rat anti-mouse PECAM-1/CD31 (1:100; BD Bioscience, San Jose, CA). For staining, detection, and mounting, the ABC staining system for rat or rabbit primary antibodies (Santa Cruz Biotechnology) was used with detection by DAB/HRP/H2O2 with mounting in Permount (Vector Laboratories, Burlingame, CA) or streptavidin-Cy3 (Jackson Immunoresearch Laboratories) followed by mounting in Vectashield (Vector Laboratories).
Statistics
All statistical analyses were performed using the Student’s t-test and a P value less than 0.05 was considered statistically significant. Values presented as means ± S.D. or S.E.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants K01-AR051076 (J.A.M.) and R01-AR049249 (E.V.M). We thank Dr. Xiao-Jing Wang for the hK14 promoter vector, Dr. Robert Fairchild for the neutrophil antibody used in this study, and Dimitry Burdjalov for assistance with PCR and immunohistochemistry.
Abbreviations
- TG
transgenic
- WTLM
wild-type littermates
Footnotes
CONFLICT OF INTEREST:
The authors declare no conflict of interest.
REFERENCES
- Arbiser JL. Angiogenesis and the skin: a primer. J Am Acad Dermatol. 1996;34:486–497. doi: 10.1016/s0190-9622(96)90444-2. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Fine JD, Murrell D, Paller A, Connors S, Keough K, et al. Basic fibroblast growth factor: a missing link between collagen VII, increased collagenase, and squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Mol Med. 1998;4:191–195. [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S, Ashraf MZ, Daher P, Howe PH, DiCorleto PE. HOXA9 participates in the transcriptional activation of E-selectin in endothelial cells. Mol Cell Biol. 2007;27:4207–4216. doi: 10.1128/MCB.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner B, Kurkela R, Vihko P, Kungl AJ. Investigating the effect of VEGF glycosylation on glycosaminoglycan binding and protein unfolding. Biochem Biophys Res Commun. 2006;340:836–839. doi: 10.1016/j.bbrc.2005.12.079. [DOI] [PubMed] [Google Scholar]
- Brett J, Gerlach H, Nawroth P, Steinberg S, Godman G, Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989;169:1977–1991. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhl T, Urbich C, Aicher D, Acker-Palmer A, Zeiher AM, Dimmeler S. Homeobox A9 transcriptionally regulates the EphB4 receptor to modulate endothelial cell migration and tube formation. Circ Res. 2004;94:743–751. doi: 10.1161/01.RES.0000120861.27064.09. [DOI] [PubMed] [Google Scholar]
- Bryan D, Walker KB, Ferguson M, Thorpe R. Cytokine gene expression in a murine wound healing model. Cytokine. 2005;31:429–438. doi: 10.1016/j.cyto.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Buck M, Houglum K, Chojkier M. Tumor necrosis factor-alpha inhibits collagen alpha1(I) gene expression and wound healing in a murine model of cachexia. Am J Pathol. 1996;149:195–204. [PMC free article] [PubMed] [Google Scholar]
- Cantile M, Pettinato G, Procino A, Feliciello I, Cindolo L, Cillo C. In vivo expression of the whole HOX gene network in human breast cancer. Eur J Cancer. 2003;39:257–264. doi: 10.1016/s0959-8049(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Chang PY, Kozono T, Chida K, Kuroki T, Huh N. Differential expression of Hox genes in multistage carcinogenesis of mouse skin. Biochem Biophys Res Commun. 1998;248:749–752. doi: 10.1006/bbrc.1998.9076. [DOI] [PubMed] [Google Scholar]
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Clark RA. Fibrin is a many splendored thing. J Invest Dermatol. 2003;121:xxi–xxii. doi: 10.1046/j.1523-1747.2003.12575.x. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16:571–579. doi: 10.1210/mend.16.3.0792. [DOI] [PubMed] [Google Scholar]
- Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Detmer K, Lawrence HJ, Largman C. Expression of class I homeobox genes in fetal and adult murine skin. J Invest Dermatol. 1993;101:517–522. doi: 10.1111/1523-1747.ep12365890. [DOI] [PubMed] [Google Scholar]
- Elias PM, Arbiser J, Brown BE, Rossiter H, Man MQ, Cerimele F, et al. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasis. Am J Pathol. 2008;173:689–699. doi: 10.2353/ajpath.2008.080088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Gailit J, Clark RA. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994;6:717–725. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Ghani QP, Wagner S, Hussain MZ. Role of ADP-ribosylation in wound repair. The contributions of Thomas K. Hunt, MD. Wound Repair Regen. 2003;11:439–444. doi: 10.1046/j.1524-475x.2003.11608.x. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Gotsch U, Jager U, Dominis M, Vestweber D. Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-alpha in vivo. Cell Adhes Commun. 1994;2:7–14. doi: 10.3109/15419069409014198. [DOI] [PubMed] [Google Scholar]
- Grier DG, Thompson A, Kwasniewska A, McGonigle GJ, Halliday HL, Lappin TR. The pathophysiology of HOX genes and their role in cancer. J Pathol. 2005;205:154–171. doi: 10.1002/path.1710. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Myers CA, Charboneau A, Young DM, Boudreau N. HoxD3 accelerates wound healing in diabetic mice. Am J Pathol. 2003;163:2421–2431. doi: 10.1016/S0002-9440(10)63597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombria JC, Lovegrove B. Beyond homeosis--HOX function in morphogenesis and organogenesis. Differentiation. 2003;71:461–476. doi: 10.1046/j.1432-0436.2003.7108004.x. [DOI] [PubMed] [Google Scholar]
- Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. Faseb J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Chu CQ, Eedy DJ, Feldmann M, Brennan FM, Breathnach SM. Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin Exp Immunol. 1993;94:354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;7:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Li AG, Wang D, Feng XH, Wang XJ. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. Embo J. 2004;23:1770–1781. doi: 10.1038/sj.emboj.7600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R, Garrido E, Vazquez G, Pina P, Perez C, Alvarado I, et al. A subgroup of HOX Abd-B gene is differentially expressed in cervical cancer. Int J Gynecol Cancer. 2006;16:1289–1296. doi: 10.1111/j.1525-1438.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Chensue SW, Widmer M, Kunkel SL. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol. 1995;154:5411–5417. [PubMed] [Google Scholar]
- Mace KA, Hansen SL, Myers C, Young DM, Boudreau N. HOXA3 induces cell migration in endothelial and epithelial cells promoting angiogenesis and wound repair. J Cell Sci. 2005;118:2567–2577. doi: 10.1242/jcs.02399. [DOI] [PubMed] [Google Scholar]
- Mack JA, Abramson SR, Ben Y, Coffin JC, Rothrock JK, Maytin EV, et al. Hoxb13 knockout adult skin exhibits high levels of hyaluronan and enhanced wound healing. FASEB Journal. 2003;17:1352–1354. doi: 10.1096/fj.02-0959fje. [DOI] [PubMed] [Google Scholar]
- Mack JA, Li L, Sato N, Hascall VC, Maytin EV. Hoxb13 up-regulates transglutaminase activity and drives terminal differentiation in an epidermal organotypic model. J Biol Chem. 2005;280:29904–29911. doi: 10.1074/jbc.M505262200. [DOI] [PubMed] [Google Scholar]
- Manak JR, Scott MP. A class act: conservation of homeodomain protein functions. Development Supplement. 1994:61–71. [PubMed] [Google Scholar]
- Martinez P, Amemiya CT. Genomics of the HOX gene cluster. Comparative Biochemistry & Physiology. Part B, Biochemistry & Molecular Biology. 2002;133:571–580. doi: 10.1016/s1096-4959(02)00121-5. [DOI] [PubMed] [Google Scholar]
- McCallion RL, Ferguson MWJ. Fetal Wound Healing and the Development of Antiscarring Therapies for Adult Wounds. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. pp. 561–600. [Google Scholar]
- McKay IA, Leigh IM. Epidermal cytokines and their roles in cutaneous wound healing. Br J Dermatol. 1991;124:513–518. doi: 10.1111/j.1365-2133.1991.tb04942.x. [DOI] [PubMed] [Google Scholar]
- Miao J, Wang Z, Provencher H, Muir B, Dahiya S, Carney E, et al. HOXB13 promotes ovarian cancer progression. Proc Natl Acad Sci U S A. 2007;104:17093–17098. doi: 10.1073/pnas.0707938104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. Hox genes: a continuation of embryonic patterning? Trends Genet. 2006;22:67–69. doi: 10.1016/j.tig.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mori R, Kondo T, Ohshima T, Ishida Y, Mukaida N. Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. Faseb J. 2002;16:963–974. doi: 10.1096/fj.01-0776com. [DOI] [PubMed] [Google Scholar]
- Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C, Charboneau A, Boudreau N. Homeobox B3 promotes capillary morphogenesis and angiogenesis. J Cell Biol. 2000;148:343–351. doi: 10.1083/jcb.148.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C, Charboneau A, Cheung I, Hanks D, Boudreau N. Sustained expression of homeobox D10 inhibits angiogenesis. Am J Pathol. 2002;161:2099–2109. doi: 10.1016/S0002-9440(10)64488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P, Handley D, Matsueda G, De Waal R, Gerlach H, Blohm D, et al. Tumor necrosis factor/cachectin-induced intravascular fibrin formation in meth A fibrosarcomas. J Exp Med. 1988;168:637–647. doi: 10.1084/jem.168.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- Raja, Sivamani K, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–2868. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- Rapala K, Laato M, Niinikoski J, Kujari H, Soder O, Mauviel A, et al. Tumor necrosis factor alpha inhibits wound healing in the rat. Eur Surg Res. 1991;23:261–268. doi: 10.1159/000129163. [DOI] [PubMed] [Google Scholar]
- Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N. A role for Hox A5 in regulating angiogenesis and vascular patterning. Lymphat Res Biol. 2005;3:240–252. doi: 10.1089/lrb.2005.3.240. [DOI] [PubMed] [Google Scholar]
- Salomon GD, Kasid A, Cromack DT, Director E, Talbot TL, Sank A, et al. The local effects of cachectin/tumor necrosis factor on wound healing. Ann Surg. 1991;214:175–180. doi: 10.1097/00000658-199108000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Sayers TJ, Wiltrout TA, Bull CA, Denn AC, 3rd, Pilaro AM, Lokesh B. Effect of cytokines on polymorphonuclear neutrophil infiltration in the mouse. Prostaglandin- and leukotriene-independent induction of infiltration by IL-1 and tumor necrosis factor. J Immunol. 1988;141:1670–1677. [PubMed] [Google Scholar]
- Schafer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol. 2007;23:69–92. doi: 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- Shi X, Bai S, Li L, Cao X. Hoxa-9 represses transforming growth factor-beta-induced osteopontin gene transcription. J Biol Chem. 2001;276:850–855. doi: 10.1074/jbc.M005955200. [DOI] [PubMed] [Google Scholar]
- Stelnicki EJ, Arbeit J, Cass DL, Saner C, Harrison M, Largman C. Modulation of the human homeobox genes PRX-2 and HOXB13 in scarless fetal wounds. J Invest Dermatol. 1998a;111:57–63. doi: 10.1046/j.1523-1747.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- Stelnicki EJ, Komuves LG, Kwong AO, Holmes D, Klein P, Rozenfeld S, et al. HOX homeobox genes exhibit spatial and temporal changes in expression during human skin development. J Invest Dermatol. 1998b;110:110–115. doi: 10.1046/j.1523-1747.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108:763–768. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- Svingen T, Tonissen KF. Altered HOX gene expression in human skin and breast cancer cells. Cancer Biol Ther. 2003;2:518–523. doi: 10.4161/cbt.2.5.441. [DOI] [PubMed] [Google Scholar]
- Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989;86:1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SM, Wong H, McCartney-Francis N. Role of growth factors in inflammation and repair. J Cell Biochem. 1989;40:193–199. doi: 10.1002/jcb.240400208. [DOI] [PubMed] [Google Scholar]
- Widegren H, Erjefalt J, Korsgren M, Andersson M, Greiff L. Effects of intranasal TNFalpha on granulocyte recruitment and activity in healthy subjects and patients with allergic rhinitis. Respir Res. 2008;9:15. doi: 10.1186/1465-9921-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88:579–590. doi: 10.1038/labinvest.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Moser M, Bautch VL, Patterson C. HoxB5 is an upstream transcriptional switch for differentiation of the vascular endothelium from precursor cells. Mol Cell Biol. 2003;23:5680–5691. doi: 10.1128/MCB.23.16.5680-5691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tazawa S, Yawei Z, Katayama H, Kato Y, Nishiwaki K, et al. Suppression of invasive characteristics by antisense introduction of overexpressed HOX genes in ovarian cancer cells. Int J Oncol. 2006;28:931–938. [PubMed] [Google Scholar]