Abstract
Associations between medication use and the development of Alzheimer’s disease have been investigated since the late 1900s. Thyroid hormone supplementation is rarely a studied medication class in this area of research. We examined data from participants enrolled in longitudinal studies at the Washington University Alzheimer’s Disease Research Center for associations between thyroid disease, thyroid hormone supplementation therapy, and subsequent development of dementia of the Alzheimer type (DAT). Data collected between April 1992 and June 2008 from 499 participants, 184 men and 315 women, were analyzed. Mean age was 76.9 years (S.D. = 9.2). At baseline, 61 participants reported thyroid medication use and 87 were identified as having a history of thyroid dysfunction. These participants progressed to a DAT diagnosis more rapidly than individuals not taking thyroid medication (HR: 1.67, 95% CI: 0.99–2.78, p = 0.054). While an interesting trend was seen, baseline thyroid disease was not significantly (p=.093) associated with time to DAT diagnosis. Our findings suggest that utilization of thyroid medication may be associated with the development of DAT.
Keywords: thyroid, dysfunction, hormone replacement, Alzheimer’s, dementia
1. INTRODUCTION
Alzheimer’s disease (AD) has received significant attention by the medical world because it accounts for the majority of dementia cases (Katzman, 2008). In an attempt to unravel the mysteries of AD etiology and progression, some researchers sought to find an association with prior or concurrent medication use. Past research has focused on relationships between dementias and a broad spectrum of drug classes, including estrogens (Slooter et al., 1999), antidepressants (Mayeux and Sano, 1999), antihypertensives (Yasar et al., 2006), and statins (Hayden et al., 2005). However, a void exists regarding thyroid hormone supplementation. Our study attempts to help fill this research void by examining possible associations between use of thyroid medications and AD onset.
2. BACKGROUND
The theoretical existence of a link between thyroid hormone supplementation via prescribed medications and AD development is well-founded. Thyroid agents are simply preparations of natural or synthetic thyroxine (T4) or triiodothyronine (T3) designed to mimic the action of endogenous T4 and T3 in the body (Gold Standard, Inc., 2008). The probable roles of endogenous thyroid hormones in AD pathophysiology have already been established, primarily in the area of beta amyloid plaque accumulation (Jaffe et al., 1994; Latasa et al., 1998; Lima et al., 2001; Mallet et al., 2002; O’Barr et al., 2006; Travis, 1999). Therefore, thyroid medications could affect plaque deposition in the same ways, expressed by three primary physiological mechanisms.
(2.1) Amyloid beta precursor protein (APP)
APP serves as the predecessor of beta amyloid fragments that aggregate to form plaques found in the brains of patients with AD. Thyroid hormones have the ability to regulate the production of different APP isoforms (Latasa et al., 1998). Specifically, the presence of T3 is related to an increase in production of the APP770 isoform while an equivalent reduction in the amount of the APP695 isoform is observed (Latassa et al., 1998). O’Barr, et al. (2006) confirmed these findings were consistent in both in vitro and in vivo conditions. If future research can determine which APP isoform is most directly related to plaque formation and AD onset, the role of thyroid hormone supplementation as a causative or preventative therapy may follow.
(2.2) Secretase synthesis and activity
Secretases are the enzymes responsible for cleaving beta amyloid fragments from APP molecules. Hormonal control is a factor in the synthesis and activity of these secretases because estrogen has been established as a factor in APP metabolism (Jaffe et al., 1994). Since estrogen and thyroid hormone are members of the same nuclear receptor hormone superfamily (Latassa et al., 1998) it is possible that T3 regulates the synthesis and activity of alpha, beta, and gamma secretase as well. Thus, a connection between thyroid hormone level and beta fragment cleavage is plausible.
(2.3) Microglial removal of beta amyloid plaque
Under normal conditions beta amyloid plaque is opsonized by the immune system and removed from the brain by microglia and monocytes (Travis, 1999). Thyroid hormones play a significant role in the production and survival of microglia (Lima et al., 2001; Mallet et al., 2002). Lima et al (2001) reported that artificially induced hypothyroidism among rodents showed markedly less microglial density and processing when compared to euthyroid controls. Since neuronal and astroglial cells are targets of thyroid hormone, lack of this hormone may create an environment unable to support development, differentiation, and survival of microglial cells due to a lack of growth factor secretion from developing neurons and astrocytes. Thus, the effects of insufficient thyroid hormone may be indirect. This thesis is supported by Mallet, et al. (2002) who found microglial processes to be promoted by the presence of T3 in vitro.
Thus, thyroid hormones are likely involved in the physiological mechanisms associated with AD pathology. Research has established the theoretical roadmap between thyroid dysfunction and irreversible brain degradation and cognitive decline, setting the stage for determination of whether thyroid dysfunction, or medication used to treat that dysfunction, is associated with AD development in the real world.
3. RECENT RESEARCH
A review of recent literature reveals inconsistency concerning the impact of thyroid dysfunction on cognitive decline. Studies have found both hypo- and hyperthyroidism to be related to increased dementia of the Alzheimer type (DAT) risk. It is important to consider both forms of thyroid dysfunction because although hypothyroidism is most common epidemiologically (Hollowell et al., 2002), treatment of this disorder with thyroid medications can often result in hyperthyroidism secondary to overcorrection (Choi and Manning, 2009).
(3.1) Hypothyroidism and DAT
For some time, hypothyroidism has been assumed a possible risk factor for AD (Rocca, 1994). Hypothyroidism has been associated with a decrease in memory retrieval and worsening of overall functional memory (Burmeister et al., 2001). A recent case-control study found that participants with hypothyroidism were at more than double the risk for developing DAT as their euthyroid counterparts (Suhanov et al., 2006). Data from the Maastricht Aging Study (MAAS) revealed that hypothyroidism predicted decreased cognitive performance in a random sample of 120 MAAS participants, although the association was weak (van Boxtel et al., 2004). The Framingham Study reported a strong association between hypothyroidism and DAT risk, stating that overt thyroid dysfunction translated to a two-fold greater risk for development of DAT when compared to euthyroid controls (Tan et al., 2008).
(3.1) Hyperthyroidism and DAT
Hyperthyroidism is reported to affect DAT risk in older adults as well, such that increased levels of T4 and free T4 increase one’s risk of developing DAT (de Jong et al., 2007; Kalmijn et al., 2000; Tan et al., 2008). De Jong, et al. (2007) reported a 30% increase in the risk of DAT risk per standard deviation increase of free T4. Of the 665 participants, 143 were followed to autopsy where participants with both hyperthyroidism and AD had more neurofibrillary tangles and neuritic plaques than participants with either condition alone, or neither condition. This is consistent with the findings of a study which examined longitudinal data from 45 participants of the Kungsholmen Project and concluded that hyperthyroidism led to cognitive deficits in late life (Wahlin et al., 2005); and an earlier study reporting a three-fold increase in DAT risk among all participants with hyperthyroidism (Kalmijn et al., 2000).
Other studies have found no significant association between any type of thyroid dysfunction and DAT onset (Lindeman et al., 1999; Luboshitzky et al., 1996; van der Cammen et al., 2003). Given this lack of consensus, the impact of thyroid disease and thyroid hormone replacement therapy on AD risk is unclear. If thyroid disorder, or the use of thyroid medications, is related to developing DAT, it would support the idea of an underlying link between these diseases.
We were unable to find any literature addressing the use of thyroid medications on risk of DAT. However, if thyroid medication use is associated with greater DAT risk, the risks and benefits of thyroid supplementation therapy should be carefully weighed prior to prescription. The purpose of this study was to examine whether such a relationship exists. We also examined associations between a reported history of thyroid disease and development of DAT.
4. MATERIALS AND METHODS
Data were obtained from participants enrolled in longitudinal studies at the Washington University in St. Louis School of Medicine’s Alzheimer’s Disease Research Center (ADRC). These longitudinal studies were initiated in 1979, and include participants with no cognitive impairment as well as dementia. Participants are volunteers, most of whom reside in the St. Louis metro and bi-state (Missouri and Illinois) areas. Given the longstanding nature of ADRC studies, many participants learn of these studies through word-of-mouth from a friend or family member. Other recruitment efforts include media appearances by ADRC staff and educational outreach efforts. Referral by a primary care physician or other health professional accounts for approximately 20% of participants. Exclusion criteria included medical, psychiatric, and neurological illnesses that may interfere with performance on psychometric testing or longitudinal follow-up (e.g., uncontrolled diabetes, renal failure requiring dialysis, treatment for active invasive cancer).
ADRC participants annually undergo an extensive battery of tests, including interview-based clinical assessments with additional information about the participants’ cognitive and physical status provided by a collateral source, usually a close relative or friend. The assessment protocol includes a basic physical and neurological examination, as well as psychometric testing conducted independently a few weeks after the clinical assessment. The diagnosis of dementia is made after a clinician interview using the Clinical Dementia Rating (CDR) (Berg, 1988; Morris, 1993). Participants are given a functional score in each of six specifically assessed areas, or “domains” of functioning. An algorithm is then used to calculate a global CDR based on the domain scores, with global scores ranging from 0 to 3, representing (0) no dementia, (0.5) very mild, (1) mild, (2) moderate or (3) severe dementia. The presumed etiology of dementia is diagnosed based on standardized criteria (Morris et al., 2006). Additional details about recruitment and assessment are available (Berg et al., 1998; Morris et al., 2001; Villareal et al, 2003).
In April 1992, questions on thyroid disease were added to the portion of the structured interview that assessed health history. Thus, participants who were enrolled between April 1992 (when data on thyroid disease were available) and June 2008 were selected for inclusion. Other inclusion criteria were a diagnosis of no dementia and age of 50 years or greater at the baseline assessment, and participation in at least one subsequent clinical assessment.
Participants who reported either ongoing or previous thyroid dysfunction at the baseline assessment were identified. This was the sole criteria for defining and identifying thyroid disease as information concerning serum thyroid hormone levels was not available. Use of thyroid hormone replacement therapy at the baseline assessment was ascertained, and was defined as any form of exogenous T3 or T4 and included all forms of levothyroxine and liothyronine of both synthetic and natural origin.
(4.1) Statistical Analyses
Kaplan-Meier survival curves were used to examine unadjusted associations between taking a thyroid medication at baseline (Yes vs. No), regardless of whether the participant reported a history of thyroid disease, and rate of receiving a diagnosis of dementia of the Alzheimer type (DAT) with time. Differences between the survival curves were tested using the log-rank test. Data from participants who did not receive a DAT diagnosis over the follow-up period were censored at the date of last clinical assessment. Many potential confounders exist when studying associations related to AD development (Mortimer and Borenstein, 2006). Therefore, Cox proportional hazards models were used to examine the association between taking thyroid medications at baseline and time to a diagnosis of DAT after adjustment for baseline age, sex, educational attainment in years, minority race, and the presence of an apolipoprotein E e4 allele. Age and educational attainment were dichotomized using median splits before inclusion in the Cox proportional hazards models. Data from participants who did not develop DAT over the follow-up period were censored at the date of their last clinical assessment. Similar analyses were conducted using time to a dementia diagnosis generally (CDR>0), regardless of its presumed etiology, as the event of interest.
The analyses were repeated to examine the association between development of DAT and dementia and (1) a variable indicating whether the participant had a reported history of thyroid disease at baseline, and (2) a variable reflecting both thyroid disease and thyroid medication status at baseline.
5. RESULTS
The sample comprised 499 participants, 315 women and 184 men, who were followed between 0.7 and 15.9 years. Demographic information for these participants is presented in Table 1. The mean age at initial assessment was 76.9 years. Of the 499 total participants, 87 (17.4%) reported ongoing or previous thyroid dysfunction at first visit, and 61 (12.2%) were taking a thyroid medication. Of participants with thyroid disease, 59 were concurrently taking at least one thyroid medication, and 28 were not. Only 2 participants without reported thyroid disease were taking thyroid medication. Therefore, for use in the survival analyses, a variable was constructed that reflected combined thyroid disease/thyroid medication status at baseline for 3 groups: no diagnosis/no medication (N=410), diagnosis/no medication (N=28), and diagnosis/medication (N=59).
Table 1.
Demographics (N=499).
| Characteristic | All (N=499) | Thyroid med (N=61) | No thyroid med (N=468) |
|---|---|---|---|
| Age, mean (SD), y | 76.9 (9.2) | 77.7 (9.5) | 76.7 (9.2) |
| Women, No. (%) | 315 (63.1) | 45 (73.8) | 270 (61.6) |
| Minority race, No. (%) | 49 (9.8) | 8 (13.1) | 41 (9.4) |
| MMSE score (SD) | |||
| (Folstein et al, 1975) † | 28.8 (1.4) | 28.7 (1.2) | 28.8 (1.4) |
| ≥1 Apolipoprotein E ε4 allele, No. (%)* | 133 (28.7) | 16 (28.1) | 117 (28.8) |
| Education, mean (SD), y | 14.8 (3.2) | 15.5 (3.2) | 14.7 (3.2) |
| Length of follow-up, mean (SD), y | 5.9 (3.9) | 5.8 (3.7) | 6.0 (3.9) |
| Thyroid disease, No. (%) | 87 (17.4) | 59 (96.7) | 28 (6.4) |
| Thyroid medication, No. (%) | 61 (12.2) | ---------- | ------------- |
Apolipoprotein E genotype data were unavailable for 36 participants.
The Mini-Mental State Exam (MMSE) was included in the clinical assessment battery beginning October 1996. Thus, these data were only available for a subsample of participants (N=303).
(5.1) Unadjusted analyses
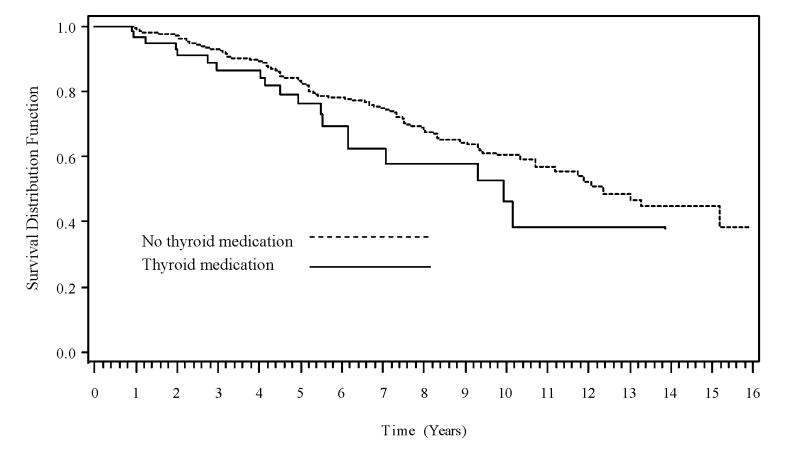
Although the survival curves suggest a faster rate of DAT with time for participants taking thyroid medication compared to those not taking thyroid medication (Figure 1) and for participants with thyroid disease compared to those without the disease (not shown), the difference between the survival curves was not significant when stratified by either medication (p=.120) or disease (p=.136). Likewise, there was no statistically significant difference between the survival curves for the 3 diagnosis/medication groups (p=.163). No significant differences in time to CDR>0 were found for the medication (p=.220), disease (p=.442), and combined disease/medication (p=.373) groups in the unadjusted analyses.
Figure 1.
Time to DAT for medicated and non-medicated participants. p=0.120 (log-rank test on difference between curves)
(5.2) Adjusted analyses
The results of the adjusted analyses are presented in Table 2. Only 463 participants were included in the Cox proportional hazards analyses because APOE genotype data were missing for 36 participants. Participants taking a thyroid medication at baseline had a faster rate of DAT diagnosis with time (HR=1.67, 95% CI=.99–2.78, p=.054). The relationship between thyroid disease at baseline (p=.093), or disease/medication group (p=.121), and rate of DAT diagnosis with time did not reach the criterion for statistical significance. No association was found between medication (p=.321), disease (p=.502), or the combined disease/medication (p=.645) grouping variable and time to a CDR>0. Older age, minority race, and having one or more apolipoprotein E ε4 (APOE) alleles predicted a significantly faster rate of both DAT diagnosis and CDR>0 with time in all models.
Table 2.
Hazard ratios from the adjusted analyses.
| 95% CI | ||||
|---|---|---|---|---|
| HR | Lower | Upper | P-value | |
| Time to DAT diagnosis | ||||
| Thyroid disease diagnosis | 1.49 | .94 | 2.38 | .0925 |
| Older age | 3.93 | 2.62 | 5.89 | <.0001 |
| Male | .95 | .64 | 1.39 | .7707 |
| More education | .82 | .56 | 1.20 | .3016 |
| Minority race | 2.69 | 1.33 | 5.46 | .0060 |
| ≥1 apolipoprotein E ε4 allele | 1.70 | 1.15 | 2.52 | .0080 |
| Use of at least one thyroid | ||||
| medication | 1.67 | .99 | 2.78 | .0539 |
| Older age | 3.93 | 2.62 | 5.89 | <.0001 |
| Male | .95 | .64 | 1.39 | .7707 |
| More education | .82 | .56 | 1.20 | .3016 |
| Minority race | 2.69 | 1.33 | 5.46 | .0060 |
| ≥1 apolipoprotein E ε4 allele | 1.70 | 1.15 | 2.52 | .0080 |
| Thyroid disease/thyroid medication group | .1205 | |||
| dx/no rx vs. no dx/no rx | .99 | .39 | 2.49 | |
| dx/rx vs. no dx/no rx | 1.72 | 1.02 | 2.87 | |
| Older age | 3.86 | 2.57 | 5.79 | <.0001 |
| Male | .95 | .64 | 1.39 | .7752 |
| More education | .83 | .57 | 1.21 | .3215 |
| Minority race | 2.68 | 1.32 | 5.44 | .0062 |
| ≥1 apolipoprotein E ε4 allele | 1.68 | 1.14 | 2.50 | .0094 |
| Time to Clinical Dementia Rating > 0 | ||||
| Thyroid disease diagnosis | 1.14 | .78 | 1.67 | .5017 |
| Older age | 3.04 | 2.23 | 4.14 | <.0001 |
| Male | 1.17 | .87 | 1.58 | .2963 |
| More education | .93 | .69 | 1.24 | .6131 |
| Minority race | 2.24 | 1.28 | 3.93 | .0048 |
| ≥1 apolipoprotein E ε4 allele | 1.51 | 1.10 | 2.08 | .0102 |
| Use of at least one thyroid | ||||
| medication | 1.24 | .81 | 1.91 | .3213 |
| Older age | 3.02 | 2.22 | 4.12 | <.0001 |
| Male | 1.17 | .87 | 1.57 | .3098 |
| More education | .91 | .68 | 1.22 | .5354 |
| Minority race | 2.25 | 1.28 | 3.94 | .0047 |
| ≥1 apolipoprotein E ε4 allele | 1.51 | 1.10 | 2.07 | .0104 |
| Thyroid disease/thyroid medication group | .6446 | |||
| dx/no rx vs. no dx/no rx | .96 | .48 | 1.91 | |
| dx/rx vs. no dx/no rx | 1.23 | .79 | 1.90 | |
| Older age | 3.03 | 2.22 | 4.15 | <.0001 |
| Male | 1.14 | .85 | 1.55 | .3793 |
| More education | .91 | .68 | 1.22 | .5267 |
| Minority race | 2.25 | 1.28 | 3.95 | .0046 |
| ≥1 apolipoprotein E ε4 allele | 1.51 | 1.10 | 2.07 | .0105 |
6. DISCUSSION
Results suggest that taking thyroid medication at baseline may be associated with a faster rate of DAT diagnosis with time. Interpretation of the Cox proportional hazards models indicates that the hazard rate of developing DAT with time for participants who reported use of at least one thyroid medication at baseline was increased 67% compared to their non-medicated counterparts.
The association between the use of thyroid medication at baseline and a faster time to any dementia diagnosis was not significant. The CDR > 0 group contains participants with diagnoses of mixed dementia, with features of both AD and vascular dementia, as well as other non-AD diagnoses such as pure vascular dementia, frontotemporal dementia, and primary progressive aphasia; all of which lack a β-amyloid plaque component in their pathologies (Arnold et al., 2000; Erkinjuntti, 2007; Kertesz et al., 1994; Oslin et al., 1998). Since previous work suggests that it is, most likely, the amyloid component of AD pathology on which thyroid hormone has its effects, we would expect disease states lacking this component to show a lesser association with thyroid medication use.
We found a nonsignificant trend suggesting that baseline thyroid dysfunction may also be related in some way to more rapid time to diagnosis of DAT. Although some researchers have reported a lack of association between thyroid disorders and AD (Lindeman et al., 1999; Luboshitzky et al., 1996; van der Cammen et al., 2003), the findings of others suggest a link between these two disease states (de Jong et al., 2007; Folstein et al., 1975; Kalmijn et al., 2000; Tan et al., 2008; van Boxtel et al., 2004; Wahlin et al., 2005; Yoshimasu et al., 1991). All referenced reports that found no association relied on cross-sectional analysis, eventually citing a lack of extensive follow-up as a weakness. Our longitudinal model addresses that concern and may, therefore, add support to their conclusions. However, unlike these studies, ours relied on self-reported diagnosis of thyroid disease instead of more objective diagnostic methods, and is therefore not directly comparable. Our findings, coupled with the uncertainty of past research, make the relationship between thyroid dysfunction and AD one of continuing interest for future study.
Consistent with prior research, we also found that increasing age, minority race, and presence of at least one APOE ε4 allele each independently contribute to an increased risk of developing DAT or more rapid disease progression once diagnosed (Mortimer and Borenstein, 2006).
Our study had limitations. First, the demographics of our volunteer-based sample were not precisely representative of the regional population as a whole. Our subjects were older, contained a higher percentage of females, and were more homogenous concerning race and education level (U.S. Census Bureau, 2008). In our sample, 17.4% of participants reported having a thyroid disorder, and 12.2% reported use of thyroid medications, at baseline. Recent national reports on the epidemiology of thyroid dysfunction state that approximately 4.5% of Americans are affected (CDC, 2006). The National Center for Health Statistics reports that 2.2% of the American population utilizes prescription thyroid medications (CDC, 1998). However, these national point estimates include Americans of all ages. Recent research shows that the prevalence of thyroid dysfunction increases with age (Hollowell, 2002), and therefore, one would expect a larger proportion of individuals in our sample, comprised of older adults, to have thyroid dysfunction. The Colorado Thyroid Disease Prevalence Study reported thyroid disease and medication usage rates at 11.7% and 5.9%, respectively (Gay, 2000). Although the N for that study was larger, the study population was similar to ours in that participants were older, more educated, and larger proportions were Caucasian and female compared to the general population of the area (Gay, 2000).
Second, our reliance on self-reported thyroid disorder from participants is potentially problematic. This was the only method to identify thyroid malfunction because of lack of information from laboratory assays of thyroid function. However, all participants were nondemented at baseline, and their reports of thyroid disease were verified by collateral source interview. Additional longitudinal studies utilizing standardized thyroid function assays for diagnosis of thyroid dysfunction are needed.
Finally, we examined use of thyroid medication at baseline and information on the dosage of thyroid medications was unavailable. Thyroid medication doses often undergo significant titration in order to stabilize patients. Metabolic changes, initiation of new medications, and diet change can all cause changes in serum thyroid hormone levels that require dose adjustments. Lack of information on patient compliance complicates matters further. Future research using large datasets of linked clinical and pharmacy claim data, which includes information on dose titrations and yields some indication of patient compliance, may help to more accurately trace the effect that thyroid hormone medication may have on AD development.
In conclusion, the results of our study suggest that use of thyroid hormone supplementation at baseline may be associated with an increased rate of progression to a DAT diagnosis when compared to non-medicated control participants, after adjustment for demographic characteristics and the presence of thyroid disease. Speculatively, thyroid hormone supplementation may alter neurologic physiology in a way that lends itself towards more rapid development and progression of AD pathologies. However, further studies with more specific thyroid disease diagnostic methods and detailed medication tracking are needed.
Acknowledgments
This research was made possible by Washington University School of Medicine’s Alzheimer’s Disease Research Center and its Memory and Aging Project, supported by the National Institute on Aging (P50AG005681 and P01AG003991), the Charles and Joanne Knight Alzheimer Research Initiative of Washington University’s Alzheimer’s Disease Research Center, the Postdoctoral Program of 1UL1RR024992-01 from the National Center for Research Resources, and the Novartis Undergraduate Research Fellowship through the Office for Research on Aging at St. Louis College of Pharmacy.
The authors thank Dr. Patrick E. Fontane, PhD of St. Louis College of Pharmacy and Dr. John C. Morris, MD of the Alzheimer’s Disease Research Center and Department of Neurology, Washington University School of Medicine, St. Louis, Missouri for comments on earlier versions of this manuscript.
Footnotes
(7.1) Disclosure statement
The authors declare no conflicts of interest or financial interests in any product or service mentioned in this article including grants, employment, gifts, stock holdings, or honoraria.
The study protocol was approved by the Washington University School of Medicine Human Research Protection Office.
References
- Arnold SE, Han LY, Clark CM, Grossman M, Trojanowski JQ. Quantitative neurohistological features of frontotemprol degeneration. Neurobiol Aging. 2000;21(6):913–919. doi: 10.1016/s0197-4580(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Berg L. Clinical Dementia Rating. Psychopharmacology Bulletin. 1988;24:637–639. [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer disease: Relation of histologic markers of dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Burmeister LA, Ganguli M, Dodge HH, Toczek T, DeKosky ST, Nebes RD. Hypothyroidism and cognition: preliminary evidence for a specific defect in memory. Thyroid. 2001;11(12):1177–1185. doi: 10.1089/10507250152741037. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) Centers for Disease Control and Prevention. Hyattsville, MD: U.S. Department of Health and Human Services; 1998. National Health and Nutrition Examination Survey III Data. < http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm.>. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) Centers for Disease Control and Prevention. Hyattsville, MD: U.S. Department of Health and Human Services; 2006. National Health and Nutrition Examination Survey 2005–2006 Data. < http://www.cdc.gov/nchs/about/major/nhanes/nhanes2005-2006/lab05_06.htm>. [Google Scholar]
- Choi AR, Manning P. Overshooting the mark: subclinical hyperthyroidism secondary to excess thyroid hormone treatment may be more prevalent that we realise. NZ Med J. 2009;122(1289):93–94. [PubMed] [Google Scholar]
- de Jong FJ, Masaki K, Chen H, Remaley AT, Breteler MM, Petrovitch H, White LR, Launer LJ. Thyroid function, the risk of dementia and neruopathologic changes: The Honolulu-Asia Study. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkinjuntti T. Vascular cognitive deterioration and stroke. Cerebrovasc Dis. 2007;24(S1):189–194. doi: 10.1159/000107395. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gay J, Manowitz N, Mayor G, Ridgway C. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- Gold Standard, Inc. Levothyroxine. [Accessed: 6 Aug 2008];Clinical Pharmacology [database online] Available at: http://www.clinicalpharmacology.com.
- Hayden KM, Zandi PP, Khachaturian AS, Norton MC, Tzchanz JT, Pieper CF, Breitner JCS, Welsh-Bohmer KA. Statin use does not reduce cognitive decline in the elderly. The Cache County study Alzheimer’s and Dementia. 2005;1(1):S97. [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Toran-Allerand CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem. 1994;269(18):13065–13068. [PubMed] [Google Scholar]
- Kalmijn S, Mehta KM, Pols HA, Hofman A, Drexhage HA, Breteler MM. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam Study Clin Endocrinol. 2000;53(6):733–737. doi: 10.1046/j.1365-2265.2000.01146.x. [DOI] [PubMed] [Google Scholar]
- Katzman R. The Prevalence and Malignancy of Alzheimer Disease A Major Killer. Alzheimers Dement. 2008 doi: 10.1016/j.jalz.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Hudson L, Mackenzie IR, Munoz DG. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44:2065. doi: 10.1212/wnl.44.11.2065. [DOI] [PubMed] [Google Scholar]
- Latasa MH, Belandia B, Pascual A. Thyroid hormones regulated beta-amyloid gene splicing and protein secretion in neuroblastoma cells. Endocrinology. 1998;139(6):2692–2698. doi: 10.1210/endo.139.6.6033. [DOI] [PubMed] [Google Scholar]
- Lima FR, Gervais A, Colin C, Izembart M, Neto VM, Mallat M. Regulation of microglial development: a novel role for thyroid hormone. J Neurosci. 2001;21(6):2028–2038. doi: 10.1523/JNEUROSCI.21-06-02028.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman RD, Schade DS, LaRue A, Romero LJ, Liang HC, Baumgartner RN, Koehler KM, Garry PJ. Subclinical hypothyroidism in a biethnic urban community. J Am Geriatr Soc. 1999;47:703–709. doi: 10.1111/j.1532-5415.1999.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Luboshitzky R, Oberman AS, Kaufman N, Reichman N, Flatau E. Prevelance of cognitive dysfunction and hypothyroidism in an elderly community population. Isr J Med Sci. 1996;32:60–65. [PubMed] [Google Scholar]
- Mallet M, Lima FR, Gervais A, Colin C, Neto VM. New insight into the role of thyroid hormone in the CNS: the microglial track. Molecular Pscychiatry. 2002;7(1):7–8. doi: 10.1038/sj.mp.4000988. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Sano M. Treatment of Alzheimer’s Disease. N Engl J Med. 1999;341(22):1670–1679. doi: 10.1056/NEJM199911253412207. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Borenstein AR. Tools of the epidemiologist. Alzheimer Dis Assoc Disord. 2006;20:S35–41. doi: 10.1097/00002093-200607001-00004. [DOI] [PubMed] [Google Scholar]
- O’Barr SA, Oh JS, Ma C, Brent GA, Schultz JJ. Thyroid hormone regulates endogenous amyloid beta precursor protein gene expression and processing in both in vitro and in vivo models. Thyroid. 2006;16(12):1207–1213. doi: 10.1089/thy.2006.16.1207. [DOI] [PubMed] [Google Scholar]
- Oslin D, Atkinson RM, Smith DM, Hendrie H. Alcohol related dementia: proposed clinical criteria. Intl J Geriatric Psych. 1998;13(4):203–212. doi: 10.1002/(sici)1099-1166(199804)13:4<203::aid-gps734>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Rocca WA. Frequency, distribution, and risk factors for Alzheimer’s disease. Nurs Clin North Am. 1994;29(1):101–111. [PubMed] [Google Scholar]
- Slooter AJ, Bronzova J, Witteman JC, Van Broeckhoven C, Hofman A, van Duijn CM. Estrogen use and early onset Alzheimer’s disease: a population-based study. J Neurol Neurosurg Psychiatry. 1999;67:779–781. doi: 10.1136/jnnp.67.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhanov AV, Pilipenko PI, Korczyn AD, Hofman A, Voevoda MI, Shishkin SV, Simonova GI, Nikitin YP, Felgin VL. Risk factors for Alzheimer’s disease in Russia: a case-control study. Eur J Neurol. 2006;13(9):990–995. doi: 10.1111/j.1468-1331.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Keil DP, Wolf PA, Seshardi S. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med. 2008;168(14):1514–1520. doi: 10.1001/archinte.168.14.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J. A vaccine for Alzheimer’s Disease? Science News. 1999;156(2):20. [Google Scholar]
- U.S. Census Bureau. American Community Survey, 2007. 2008 < http://factfinder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=&_lang=en&_ts=>.
- van Boxtel MP, Menheere PP, Bekers O, Hogervorst E, Jolles J. Thyroid function, depressed mood, and cognitive performance in older individuals: the Mastricht Aging Study. Psychoneuroendocrinology. 2004;29(7):891–898. doi: 10.1016/j.psyneuen.2003.08.002. [DOI] [PubMed] [Google Scholar]
- van der Cammen TJ, Mattace-Raso F, van Harskamp F, de Jager MC. Lack of association between thyroid disorders and Alzheimer’s disease in older persons: a cross-sectional observational study in a geriatric outpatient population. J Am Geriatr Soc. 2003;51(6):884. doi: 10.1046/j.1365-2389.2003.51278.x. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Grant E, Miller JP, et al. Clinical outcomes of possible versus probably Alzheimer’s disease. Neurology. 2003;61:661–667. doi: 10.1212/wnl.61.5.661. [DOI] [PubMed] [Google Scholar]
- Wahlin A, Bunce D, Wahlin TB. Longitudinal evidence of the impact of normal thyroid stimulating hormone variations on cognitive functioning in very old age. Psychoneuroendocrinology. 2005;30(7):625–637. doi: 10.1016/j.psyneuen.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Yasar S, Zhou J, Xue Q, Fried LP, Carlson M. The effect of antihypertensive medication use on age-related cognitive decline. Alzheimer’s and Dementia. 2006;2(3):S415. [Google Scholar]
- Yoshimasu F, Kokmen E, Hay ID, Beard CM, Offoard KP, Kurualnd LT. The association between Alzheimer’s disease and thyroid disease in Rochester, Minnesota. Neurology. 1991;41(11):1745–1747. doi: 10.1212/wnl.41.11.1745. [DOI] [PubMed] [Google Scholar]