Abstract
Radioimmunotherapy of non-Hodgkin lymphoma consists of a 90Y- or 131I-labeled murine anti-CD20 IgG, but both agents also include a substantial dose of unlabeled anti-CD20 IgG given immediately before the radioconjugate to reduce its uptake in the spleen (primary normal B-cell antigen sink); this extends its plasma half-life, and improves tumor visualization. Thus, these treatments combine an effective anti-CD20 radioconjugate with an unconjugated anti-CD20 antibody that is also therapeutically active, but the large anti-CD20 IgG pre-dose (~900 mg) may diminish the tumor localization of the radioimmunoconjugate (e.g., 10–35 mg). We have examined alternative approaches that enhance radionuclide targeting and improve anti-tumor responses. One uses a 90Y-labeled anti-CD22 IgG (epratuzumab) combined with an antibody therapy regimen of a humanized anti-CD20 IgG (veltuzumab). Pretargeted radionuclide therapy using a trivalent, humanized, recombinant bispecific anti-CD20 antibody with a 90Y-hapten-peptide is another highly effective method that is also less toxic than directly radiolabeled IgG. Finally, all approaches benefit from the addition of a consolidation-dosing regimen of the anti-CD20 IgG antibody. This article reviews these various options and discusses how some fundamental changes could potentially enhance the response and duration from radionuclide-targeted therapy.
Keywords: CD20, CD22, non-Hodgkin lymphoma, pretargeting, radioimmunotherapy
Introduction
Radioimmunotherapy (RAIT) is an approved approach for the treatment of follicular and transformed non-Hodgkin lymphoma (NHL) with two radiolabeled anti-CD20 IgG products.1–3 90Y-ibritumomab tiuxetan (Zevalin®; Cell Therapeutics, Seattle, Washington) and 131I-tositumomab (Bexxar®; GlaxoSmithKline, Philadelphia, PA) have shown improved objective responses over the corresponding anti-CD20 IgG used with each agent; namely, rituximab (Biogen Idec, Cambridge, MA) and tositumomab, respectively, which themselves have significant anti-tumor activity.4, 5 New clinical studies are lending increasing support for their use in frontline and consolidation settings,2, 6–22 but new laboratory investigations are providing insights into ways that could improve responses, in some cases with less toxicity.
In order to improve these treatments, we need first to understand the current treatment regimens, and how each of these agents was developed. Monoclonal antibody-based therapeutics in hematopoietic malignancies started with an examination of several naked murine monoclonal antibodies in lymphoma and leukemias. Some objective responses were observed, but overall the anti-tumor effects were minimal, in many respects limited by the amount of antibody and the number of treatments that could be given with the murine IgG.23–32 For example, in a small pilot study, Press et al. reported progressively better objective anti-tumor responses in patients given a continuous infusion of the murine anti-CD20 IgG 1F5, but it required nearly 2 grams of antibody to get a complete response. Today, this amount of antibody is commonplace for antibody therapeutics, but at that time, this was beyond the capability of most facilities to produce, and development of an anti-mouse antibody response further restricted the treatment. However, preclinical models often found that immunoconjugates (antibodies conjugated with radionuclide, drugs, or toxins) were much more active than the corresponding unlabeled antibody and required less antibody. After DeNardo et al. reported encouraging responses with a radioiodinated anti-HLA-DR antibody in B-cell malignancies, a number of other studies using antibodies to CD37, CD20 and CD22 were pursued, first in a myeloablative setting with bone marrow support, and then with non-myeloablative doses, to have more substantial anti-tumor activity than that reported with the naked antibodies.33–37 The initial studies performed with CD37 and CD20 antibodies found a more favorable biodistribution for the radioconjugates when additional unlabeled anti-CD20 IgG was mixed with the radioimmunoconjugate.34, 35 Imaging studies showed there was intense uptake of the radioimmunoconjugate in the spleen, accompanied by rapid blood clearance, when low protein doses were used.38 Since all of the above-mentioned antibodies bind to normal and malignant B-cells, the large repository B-cells in the spleen (normal and malignant B-cells, often resulting in splenomegaly) acted as a sink, taking up the small amount of radioconjugate from the blood before it had an opportunity to circulate and localize in all tumor sites. Administering additional antibody protein reduced splenic uptake and slowed the radioconjugate’s clearance from blood. Antibodies against different antigens require different amounts of antibody for this to occur.34, 35, 39–44
Adding additional antibody to an immunoconjugate raises concerns that the unlabeled antibody would compete for the binding sites in the tumor, reducing the amount of the radioimmunoconjugate localized in the tumor. However, in nude mice bearing Raji human B-cell lymphoma xenografts, Buchsbaum et al.45 confirmed that tumor localization of the radiolabeled anti-B1 antibody (murine anti-CD20 IgG2a; tositumomab) could be enhanced by first pre-dosing the animals with the unlabeled antibody before the radioimmunoconjugate. Although mouse B-cells do not have cross-reactivity with the anti-B1 antibody, nude mice have low levels of murine IgG2a.46, 47 As a consequence, when small amounts of exogenously added murine IgG2a are given to the mice, the antibody is rapidly bound by cells in the spleen having receptors for this IgG form, causing increased splenic uptake and rapid blood clearance, which subsequently reduced the amount of antibody available for tumor targeting. The pre-dose preoccupies these receptors, allowing the radioimmunoconjugate to circulate longer in the blood, thus giving it more opportunity to bind to the tumor. While this is not the same mechanism of antibody binding/clearance that occurs in humans, the end result is similar. In this model, they showed that a pre-dose of 0.1 mg reduced splenic uptake and increased the concentration of the radioimmunoconjugate in the tumor and blood, but when increased to 1.0 mg, tumor uptake decreased, a likely consequence of having too much unlabeled anti-CD20 that could compete with the radioimmunoconjugate for CD20 binding in the tumor.
Several groups independently examining 2 anti-CD20 IgGs confirmed the initial clinical findings supporting the use of a blocking dose of unlabeled antibody before the radioimmunoconjugate. Knox et al. found a 1.0 mg/kg pre-dose of unlabeled anti-CD20 reduced splenic uptake, improved tumor visualization, and slowed blood clearance for 90Y-ibritumomab tiuxetan, and a higher dose of 2.5 mg/kg improved the number of lesions detected by external scintigraphy.41 There was no apparent impact on the quantitative amount of the radioimmunoconjugate delivered to the tumor in patients given the 1.0 mg/kg pre-dose compared to no pre-dose, but tumor uptake was highly variable, which may have masked possible differences. Later studies examining 100 mg/m2 and 250 mg/m2 pre-dose levels also observed the desired decrease in splenic uptake and slower blood clearance, and concluded there was no apparent reduction in tumor uptake.48 Subsequently, investigators chose the pre-dose of 250 mg/m2 because clinical trials with rituximab at the time were showing encouraging anti-tumor activity, and thus the additional amounts of rituximab given as a pre-dose were considered a potential boost to the anti-tumor activity of the radioimmunoconjugate treatment. Kaminski et al. had direct evidence that a 685-mg pre-dose of the unlabeled anti-B1 given with a diagnostic injection of 131I-anti-B1 IgG caused tumor shrinkage even before the therapeutic radioimmunoconjugate was administered,35, 36 providing considerable incentive to add higher amounts of the unlabeled antibody with the radioimmunoconjugate. Interestingly, preclinical studies in mice bearing human B-cell lymphoma xenografts found better responses with the unlabeled B1 as compared to 131I-B1, lending additional support that the unlabeled antibody could benefit the overall response.49 Wahl et al. later reported other studies that examined 95-mg and 450-mg pre-dose amounts of unlabeled tositumomab, finding that the 95-mg pre-dose substantially slowed the clearance of the radioimmunoconjugate. Blood clearance was not substantially different at the 450-mg pre-dose, but the higher pre-dose was preferred for patients with larger tumor burden or splenomegaly.42, 50
Preclinical and clinical data supported the value of a pre-dose of the unlabeled anti-CD20 IgG to improve the targeting of the radioimmunoconjugates, but new preclinical data are raising questions regarding how much antibody should be given prior to the therapeutic radioimmunoconjugate doses. Currently, 2 doses of 250 mg/m2 of rituximab are given before 90Y-ibritumomab tiuxetan and 2 doses of 450 mg of tositumomab are given before 131I-tositumomab, amounting to nearly 900 mg of unlabeled antibody before the therapeutic radiolabeled antibody is administered. Indeed, the prerequisite established in the initial trials of a pre-therapy imaging study performed several days in advance of the therapeutic treatment loads the patient with considerable unlabeled antibody well before the therapeutic radioimmunoconjugate treatment is given. This pre-therapy imaging study is necessary to set the therapeutic dose of 131I-tositumomab, but the only requirement for the 111In-ibritumomab tiuxetan dose is to assess if there is an altered biodistribution, which appears to occur very rarely.51 In Europe, where the 111In-ibritumomab tiuxetan imaging study is not required before 90Y-ibritumomab tiuxetan, the pre-dose of unlabeled antibody is nevertheless still given, attesting to the belief that the unlabeled antibody is an integral and important component of this treatment regimen.19
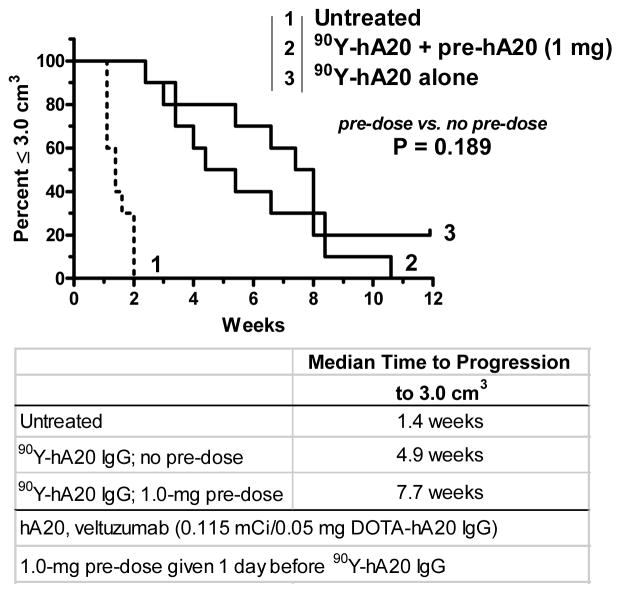
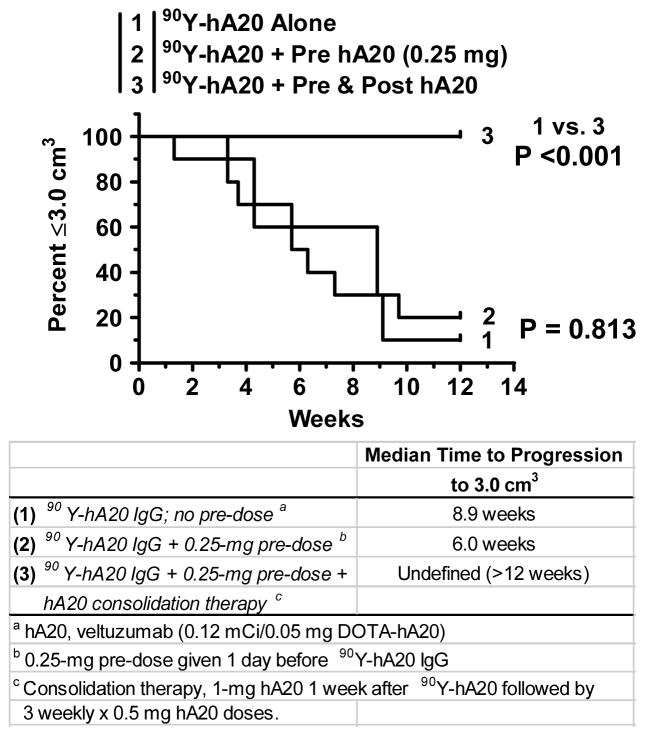
Gopal et al. were the first to highlight the possibility of compromised targeting of an anti-CD20 radioconjugate (131I-tositumomab) when an unlabeled anti-CD20 pre-dose was given.52 They reported a 55% decrease in tumor uptake when nude mice bearing human lymphoma xenografts were pre-dosed with 2 doses of 0.4 mg of unlabeled tositumomab given 24 and 6 h before 131I-tositumomab (0.2 mg 131I-tositumomab was co-injected with 0.4 mg of an irrelevant murine IgG2a to block Fc-receptor mediated binding, as discussed above), and as a consequence, anti-tumor activity was also reduced significantly. We showed subsequently similar reductions (−47%) in tumor uptake in nude mice bearing established (0.5 cm3) Ramos human B-cell lymphoma xenografts that were pre-dosed with 1.0 mg of unlabeled humanized anti-CD20 IgG1 (veltuzumab, hA20; Immunomedics, Inc., Morris Plains, NJ) given 1 day, or even just 1 hour, in advance of 111In-veltuzumab.53 While there was a trend for tumors to progress more rapidly in animals given a 1-mg pre-dose 1 day before 90Y-veltuzumab, the difference between no pre-dose and pre-dosing was not significant (median time to progress to 3.0 cm3, 7.7 vs. 4.9 weeks, respectively; P = 0.189) (Figure 1). Since 1 mg given to a 20-g mouse (i.e., 50 mg/kg) is equivalent to 4 mg/kg in a human or about 280 mg (based on a FDA-recommended conversion factor54), we examined a 0.25-mg pre-dose or 1-mg/kg human equivalent dose. This was the smallest amount of an unlabeled anti-CD20 IgG given as a pre-dose that was tested clinically, which was sufficient with both 90Y-ibritumomab tiuxetan and 131I-tositumomab to “normalize” the blood clearance of the radioconjugate. With the lower pre-dose, tumor uptake was decreased by only 25% and, as expected, the therapeutic response again was not significantly different from that of no pre-dose (e.g., median time to progress to 3.0 cm3, 8.9 vs. 6.0 weeks, no pre-dose vs. with pre-dose, respectively; P = 0.813; Figure 2). In this study, a separate group of animals was given additional amounts of unlabeled veltuzumab starting 1 week after 90Y-veltuzumab. This treatment combination resulted in 100% of the animals having no evidence of tumor after 12 weeks. Additional observations of this combination therapy are discussed below.
Figure 1.
The effect of veltuzumab pre-dosing on the therapeutic response of 90Y-veltuzumab. Groups of nude mice (n = 10) bearing s.c. Ramos human B-cell lymphoma xenografts (average 0.65 cm3) were administered 0.115 mCi of 90Y-veltuzumab (0.05 mg) alone or they were given 1.0 mg of veltuzumab 1 day prior to 90Y-veltuzumab treatment. Survival based on time for tumors to progress to 3.0 cm3.
Figure 2.
90Y-veltuzumab therapy with consolidation veltuzumab therapy. Nude mice bearing s.c. Ramos tumors were given 90Y-veltuzumab alone (0.13 mCi/0.05 mg), or a reduced veltuzumab pre-dose of 0.25 mg was given 1 day before receiving 90Y-veltuzumab, and another group received the pre-dose, as well as a veltuzumab consolidation therapy starting 1 week after the radioconjugate using 1.0 mg veltuzumab followed by 3 more weekly injections of 0.5 mg veltuzumab. Survival based on time to progression to 3.0 cm3.
While these human CD20− models are unable to account for how much residual unlabeled antibody might be available to compete for tumor binding with the radioconjugate had there been an antigen sink, it was interesting to find, at least in our experience, that anti-tumor responses were not more obviously affected by pre-dosing. Several reports have highlighted the ability of the naked antibody to induce apoptosis or influence the cell cycle that can enhance the sensitivity of the tumor to radiation,55–59 so these results might reflect the additive effects achieved by combining the anti-CD20 therapy with a radioimmunoconjugate Still, one would expect that there is pre-dose level that, if exceeded, the combined effect would not be able to make up any reductions in radionuclide delivery and therefore the net response would be diminished had the radioimmunoconjugates uptake been optimized. Certainly the experience of Gopal et al.52 confirmed that this can occur with a 0.4 mg pre-dose of tositumomab before its 131I-radioimmunoconjugate, and while there was trend to a significant reduction in anti-tumor uptake with a 1-mg pre-dose of veltuzumab before 90Y-veltuzumab, it did not reach a statistically significant level in the same Ramos xenograft model. While we believe both studies support the view that excessive pre-dosing can be harmful to a radioimmunoconjugate therapy, since anti-CD20 antibodies have at least 2 different mechanisms of action, it is possible that the effects might differ when using a type I or type II antibody.60, 61 However, it is difficult to predict what the potential boost in anti-tumor response provided by the unlabeled antibody might be when added to the radioimmunoconjugate, since this will undoubtedly be variable in different cell lines and in different targeting situations. Nevertheless, given the sensitivity of hematopoietic malignancies to radiation, it is reasonable to expect that responses would improve if more radiation could be selectively targeted to the tumor. This leads to a consideration of how to optimize the delivery of radiation to tumor, and how might we achieve the maximum benefit from the very effective anti-CD20 antibody immunotherapy when used in combination with a radioimmunoconjugate. Several different approaches are discussed below.
Anti-CD22 Radioconjugate Combined with Anti-CD20 Immunotherapy
While naked anti-CD20 antibodies remain the most potent for NHL, there are a number of other antigens that can be used for targeting radionuclides. Some of the more commonly expressed antigens include CD19 and CD22 expressed almost exclusively on B-cells, and importantly not on progenitor cells. Many of the other antigens are also expressed on other hematopoietic cells from different lineages, such as CD37, HLA-DR, CD74, CD138, and CD45. For a variety of reasons, it is generally better to select an immunoconjugate with a more limited cell-binding profile.
Our group has studied an anti-CD22 antibody, known as LL2 or epratuzumab (Immunomedics, Inc., Morris Plains, NJ). First used as a radioimmunoconjugate, 99mTc- and 131I-labeled murine LL2 had excellent targeting properties even at low protein doses, and one of the initial patients given a small dose of protein and radioactivity experienced a measurable anti-tumor response.37, 62, 63 Subsequent studies revealed the antibody was internalized, and therefore tumor uptake was improved by using a residualizing radiometal, such as 90Y, over tyrosine-linked 131I.64 Pre-dosing or even co-administration of unlabeled antibody did help reduce splenic uptake, but only 50 mg was sufficient, most likely because of the lower antigen density of CD22 on most malignant and non-malignant B-cells.40, 43 Objective responses have been observed in Phase I trials with 90Y-epratuzumab given as a single or fractionated dosing regimen, and anti-tumor responses were observed in lesions that were not visualized by external scintigraphy of the radioimmunoconjugate.65–67 Recently, a multi-center Phase I/II study using 2 fractionated doses given 1 week apart showed a much higher cumulative dose could be tolerated (2 × 20 mCi/m2), with an encouraging response rate in relapsed follicular NHL treated at this dose level (100% overall response, 10/11 having CR/CRu).68
Unconjugated epratuzumab was also found to have anti-tumor activity in patients, alone or when combined with rituximab,69–71 but it has not been active in animals.72 Since anti-CD20 antibodies are effective in animal models, and since there are encouraging clinical responses with 90Y-epratuzumab, we asked if 90Y-epratuzumab could be used in conjunction with anti-CD20 immunotherapy for improved activity over either agent alone. Such an approach would allow an effective radioimmunotherapeutic to be combined with a highly effective antibody therapy without concern that the radioconjugate’s targeting would be compromised by the unlabeled antibody. We reported activity of a humanized anti-CD20 IgG, veltuzumab, in animal models that was similar to rituximab,72, 73 and early results from on-going clinical studies have confirmed its activity in patients.74 Thus, the studies examined the combination of 90Y-epratuzumab with veltuzumab.
Since anti-CD20 therapy can also induce apoptosis or induce cell cycle arrest, which could enhance anti-tumor activity of a radioimmunoconjugate,55–59 there is a rationale for starting the anti-CD20 therapy in advance of RAIT. Clinically, starting treatment with the anti-CD20 antibody would reduce the B-cell sink that could potentially affect tumor uptake of the anti-CD22 radioconjugate, since it too would be targeted to normal B-cells. Although this is not a factor in mice, we decided the treatment regimen should start with the anti-CD20 IgG in advance of the anti-CD22 radioconjugate. We chose nude mice, because they are less sensitive to radiation than SCID mice, and used the Ramos human B-cell lymphoma cell line inoculated subcutaneously, since tumor growth was dependable and predictable. Once this tumor begins to grow, it progresses rapidly, and occasionally, tumors may spontaneously regress, but most often only after they have already grown to ≥2.0 cm3. While we would have preferred to initiate veltuzumab several days in advance of the 90Y-epratuzumab treatment, because the tumors grew rapidly, veltuzumab was given only 1 day in advance of the 90Y-eratuzumab, when the tumors were first visibly apparent, with 1.0 mg and then 3 subsequent doses, each of 0.5 mg, given at 1-week intervals.75
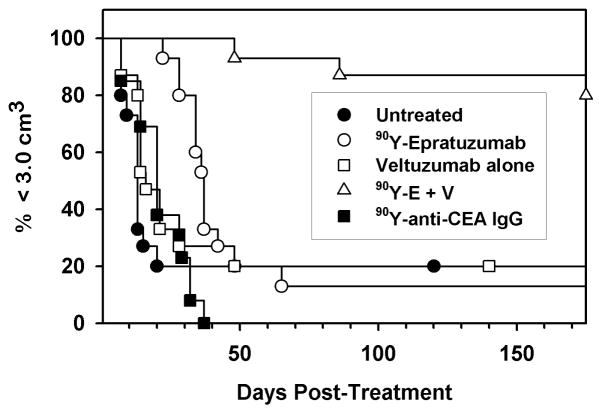
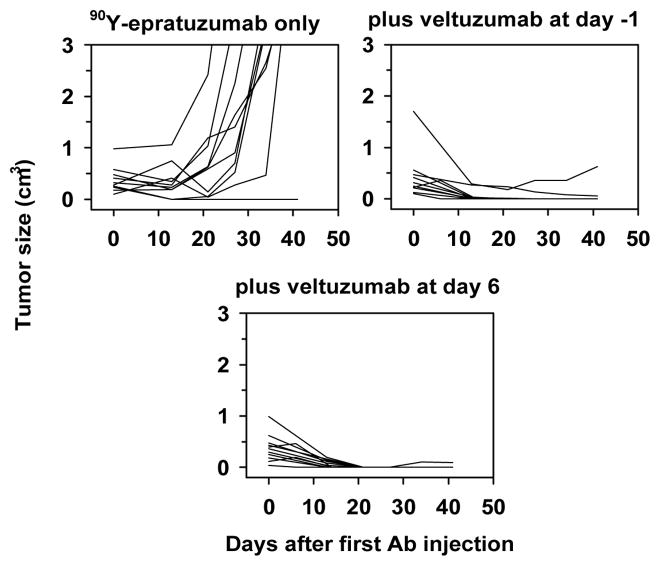
Biodistribution studies confirmed that 1 mg of veltuzumab given 1 day in advance of an 111In-epratuzumab injection had no impact on tumor or other normal tissue uptake measured 3 days after the RAIT.75 Tumors given veltuzumab alone progressed as quickly as untreated animals (Figure 3), but after an initial growth spurt over the first week, animals given a maximum dose of 90Y-epratuzumab alone experienced an initial partial (i.e., ≥50% reduction in size) or even a complete (no measurable tumor seen) response. However, this effect was short-lived, and within a few days to a few weeks, most tumors progressed rapidly. In contrast, 80% of the animals given the veltuzumab treatment with 90Y-epratuzumab had a complete response and were tumor-free for more than 5 months, when the study was terminated. Another study showed a similar result when veltuzumab was delayed for 6 days after 90Y-epratuzumab treatment (Figure 4). These results suggest that the radioimmunoconjugate was instrumental in first ablating growth of the tumor, at which point the effect of unlabeled antibody could be seen. Interestingly, the radioimmunoconjugate dose used in these studies causes severe hematologic toxicity that would require a minimum of 6 weeks before full recovery occurred, which could have compromised the number of effector cells available for antibody-dependent cellular toxicity (ADCC). While the underlying mechanism of action behind the enhanced activity with the veltuzumab consolidation dose is not known, there are a number of possibilities including: (a) cell signaling (e.g., apoptosis), (b) complement pathway, (c) effector cells capable of eliciting ADCC, perhaps in the tumor, were in sufficient number, (d) extending veltuzumab therapy 3–4 weeks after treatment may have allowed enough antibody to be present when effector cells were repopulated to exert ADCC activity.
Figure 3.
Combination RAIT and antibody therapy using 90Y-epratuzumab and unlabeled veltuzumab. Treatment was initiated once the s.c. Ramos xenographs were visible in BALB/c nude mice. Treatment consisted of 90Y-epratuzumab (90Y-E) alone, veltuzumab (V) alone (1 mg followed 1 week later with 3 more weekly injections of 0.5 mg), or 90Y-E combined with V therapy that was started 1 day before the radioconjugate. An irrelevant 90Y-anti-CEA IgG was given to assess non-specific radiation effects.
Figure 4.
Veltuzumab antibody therapy enhances the response of 90Y-epratuzumab even when started 1 week after the radioconjugate treatment. Growth curves of the individual animals with s.c. Ramos xenografts treated with 90Y-epratuzumab alone or with veltuzumab therapy initiated either 1 day before (day-1) or 6 days after the radioconjugate dose.
Anti-CD20 Radioconjugate Therapy with Unlabeled anti-CD20 Consolidation Therapy
With evidence that immunotherapy with veltuzumab anti-CD20 IgG can contribute to the anti-tumor response of radiolabeled anti-CD22 IgG, it was important to assess whether the addition of an anti-CD20 immunotherapy combined with an anti-CD20 radioconjugate enhances anti-tumor activity. Borrowing from the same treatment regimen used in combination with 90Y-epratuzumab, veltuzumab was initially configured for administration at 1.0 mg 1 day in advance of 90Y-veltuzumab, continuing with 3 sequential weekly doses of 0.5 mg. As shown previously in Figure 1, animals receiving a 1.0-mg pre-dose before 90Y-veltuzumab, with no additional treatments, had no improvement in response over animals given only 90Y-veltuzumab. In this study, when the veltuzumab treatment regimen started with 1 mg given as a pre-dose before the 90Y-veltuzumab therapy, there was no improvement in response over that seen with 90Y-veltuzumab alone, despite the continuation of additional veltuzumab injections. Thus, continued consolidation therapy with veltuzumab had no impact on response. However, if this same dosing regimen was shifted so that veltuzumab therapy started 1 week after the 90Y-veltuzumab dose (i.e., no veltuzumab given before the 90Y-veltuzumab), a significantly improved median time to progression and a number of cures were observed (Figure 5). These results suggest that the 1.0-mg pre-dose may have reduced the radioimmunoconjugate uptake just enough to impede the ability of the consolidation therapy to “drive the therapeutic response to completion.” As shown earlier in Figure 2, if the veltuzumab pre-dose was reduced to 0.25 mg, and the animals received a full consolidation treatment of veltuzumab (4 weekly doses; 1.0 mg + 3 × 0.5 mg) starting 1 week after the 90Y-veltuzumab treatment, we again observed a significant enhancement in anti-tumor activity. Thus, consolidation antibody therapy could be a very effective way to improve the objective response achieved with a radioimmunoconjugate therapy, as well as to extend the response duration. In order to achieve the best response, the radioimmunoconjugate must be given an opportunity to reach its highest possible concentration in the tumor, another reason to moderate the amount of an unlabeled antibody that can compete with the radioimmunoconjugate’s binding to the tumor.
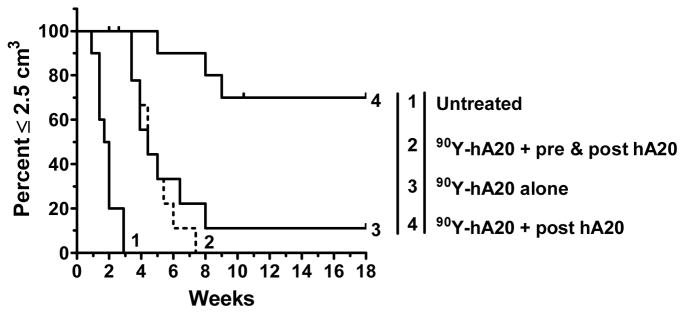
Figure 5.
Consolidation veltuzumab therapy improves response of 90Y-veltuzumab, but only when given in the absence of a pre-dose, or as in Figure 2, when the pre-dose is minimized. Ramos-bearing nude mice were given 0.13 mCi of 90Y-veltuzumab alone or animals received 1.0 mg of veltuzumab prior to the radioconjugate, which was then followed with 3 additional weekly injections of 0.5 mg of veltuzumab (pre & post hA20). Other animals received the same veltuzumab regimen, but it was started 1 week after the radioconjugate was given.
In retrospect, the initial clinical data that showed pre-doses of about 1.0 to 1.5 mg/kg would have been sufficient to reduce splenic uptake and increase the radioimmunoconjugate’s residence time in the blood, at least in patients without excessive tumor burden or splenomegaly. Instead, with both of the currently-approved RAIT products, patients now receive 2 doses of unlabeled anti-CD20 IgG amounting to about 6.5 mg/kg (a total of 13 mg/kg) before the therapeutic radioimmunoconjugate is given. Perhaps in patients with large tumor burdens or with enlarged spleens, a substantial portion of this pre-dose amount might be consumed by the antigen sink, leaving relatively small amounts of residual antibody in the blood to compete with the radioimmunoconjugate for tumor binding. However, given the current interest in moving RAIT to a frontline setting, and particularly in the situation now where 90Y-ibrituzumab is being used in a consolidation therapy after patients have already achieved a complete response,18 one needs to rethink how to best deliver the radioimmunoconjugate portion of this regimen. Minimizing the pre-dose would be an important first step, but consideration should be given to examining other radioimmunoconjugates that do not compete with anti-CD20 antibody therapeutic responses, allowing each treatment to build a better response. While animal data suggest that a consolidation treatment with an anti-CD20 IgG would be useful, adding consolidation therapy to the current treatment regimens would not likely bring much additional benefit since, as our data suggest, when a higher pre-dose is given, additional consolidation treatments did not improve responses. Although we did not test more aggressive consolidation treatment regimens (e.g., higher doses) in the animals given the 1.0 mg pre-dose, under the current clinical procedures, patients already have a considerable load of unlabeled antibody, so that consolidation therapy would likely be best given several weeks later.
Pretargeted radioimmunotherapy
Thanks in large part to the pre-dose of the unlabeled anti-CD20 antibody, the radioimmunoconjugate has an extended half-life in the blood, which increases radiation exposure of the red marrow that contributes the dose-limiting hematological toxicity, and this in turn reduces the amount of the radioimmunoconjugate that can be given, thus reducing the amount of radiation delivered to tumor. Fortunately, NHL is very sensitive to radiation and impressive responses at the maximum dose allowed for each of the approved radioimmunoconjugates have been achieved. However, there is another approach that could be used to target radiation selectively, and which can deliver more radiation to the tumor with much less severe hematological toxicity.
Pretargeting procedures have been shown in many tumor models to outperform a directly radiolabeled antibody.76 Several pretargeting methods have been investigated, but each system is based on the fundamental principle that the slow antibody-targeting step should be separated from radionuclide targeting, which should be able to localize quickly in the tumor and then just as quickly be removed from the body.77 Our group has focused on a pretargeting system based on recombinant, humanized bispecific antibodies (bsMAb) that subsequently bind a radiolabeled hapten-peptide, primarily to avoid the use of foreign immunogenic proteins, such as streptavidin, which effectively pretargets radiolabeled biotin.76 All of these pretargeting systems are so efficient at trapping the radionuclide that in some model systems, the radiolabeled effector is taken up by the tumor at a maximum level within an hour, and the percent uptake of pretargeted hapten-peptide or other small molecules delivered to the tumor can equal that of a directly targeted IgG, yet the rapid clearance of the radiolabeled effector molecule from the blood and body greatly reduces radiation exposure.78–82
We showed a bsMAb-pretargeting system based on veltuzumab and paired with a 90Y-labeled hapten-peptide had significantly enhanced anti-tumor responses as compared to 90Y-veltuzumab (Figure 6).82 With pretargeting, not only did we increase the time to progression, but we were able to completely ablate a large number of the tumors, even at doses well below the maximum tolerated dose for this procedure; yet with the 90Y-veltuzumab direct conjugate, only a short temporary response could be achieved.53, 82 Because the recombinant humanized bsMAb lacks the Fc-portion of the immunoglobulin, we next asked whether this pretargeting system could benefit from the addition of the veltuzumab IgG immunotherapy. Since it might also be necessary clinically to pre-dose patients with unlabeled anti-CD20 IgG to allow better pretargeting with the bsMAb, we also determined how a pre-dose given 1 day in advance of the bsMAb impacted this pretargeting procedure.
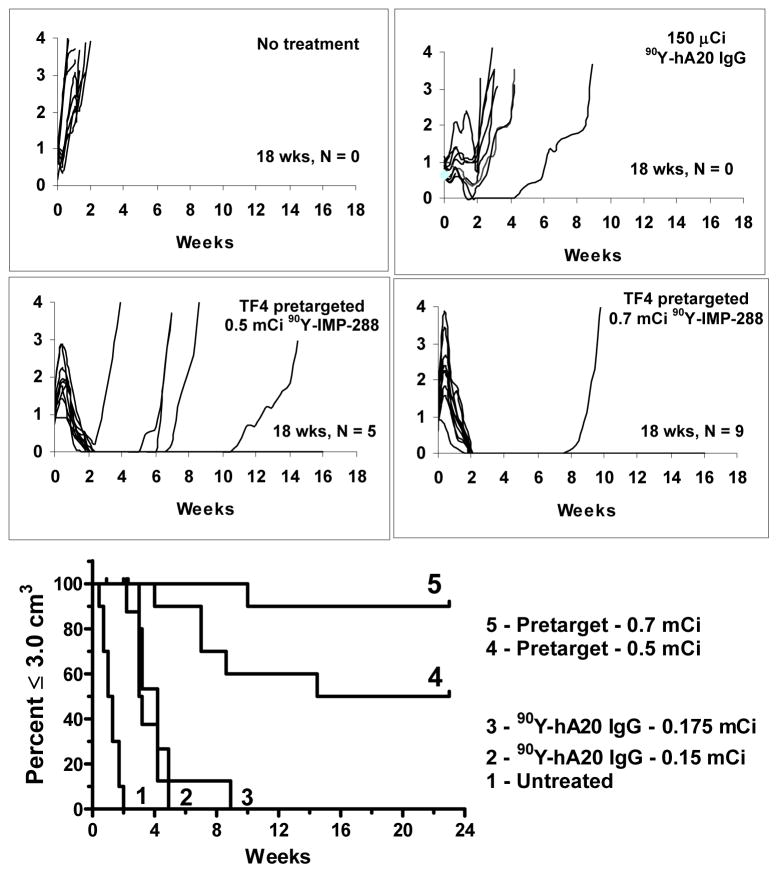
Figure 6.
Veltuzumab bsMAb pretargeted 90Y-hapten-peptide improves therapeutic response compared to directly radiolabeled 90Y-veltuzumab. Nude mice bearing s.c. Ramos tumors were treated with the MTD of 90Y-veltuzumab (0.13 mCi/0.05 mg) or a pretargeting procedure using a humanized, recombinant bispecific antibody containing 2 veltuzumab Fab fragments and one anti-hapten Fab fragment capable of binding the 90Y-hapten peptide. 0.5 or 0.7 mCi of the pretargeted 90Y-hapten-peptide only result in ~50–70% loss in WBC that recovers in several weeks, whereas 0.13 mCi of 90Y-veltuzumab causes >90% loss in WBC that requires 7 weeks to recover. Growth curves and the subsequent survival curve shows the improved responses in the pretargeted animals with the ability to completely ablate 9/10 tumors at the 0.7 mCi 90Y-hapten-peptide dose.
Unlike the 90Y-veltuzumab direct conjugate whose anti-tumor activity was not affected significantly by a 1.0-mg veltuzumab pre-dose, anti-tumor responses with the pretargeting procedure were seriously impaired.53 However, biodistribution studies indicated a higher loss (~75%) in the tumor uptake of the radiolabeled hapten-peptide under these conditions. Additional consolidation therapy (3 × 0.5 mg) after the 1-mg pre-dose had no effect, because without adequate uptake of the pretargeted 90Y-hapten peptide, tumors progressed rapidly (Figure 7A). When the pre-dose was reduced to 0.25 mg, tumor uptake was 50% lower than when no pre-dose was given, but anti-tumor responses showed some evidence of improvement over the pretargeting procedure in the absence of a pre-dose (Figure 7B and C). Consolidation anti-CD20 veltuzumab therapy further enhanced the therapeutic effects when a 0.25-mg pre-dose was given (Figure 7C).
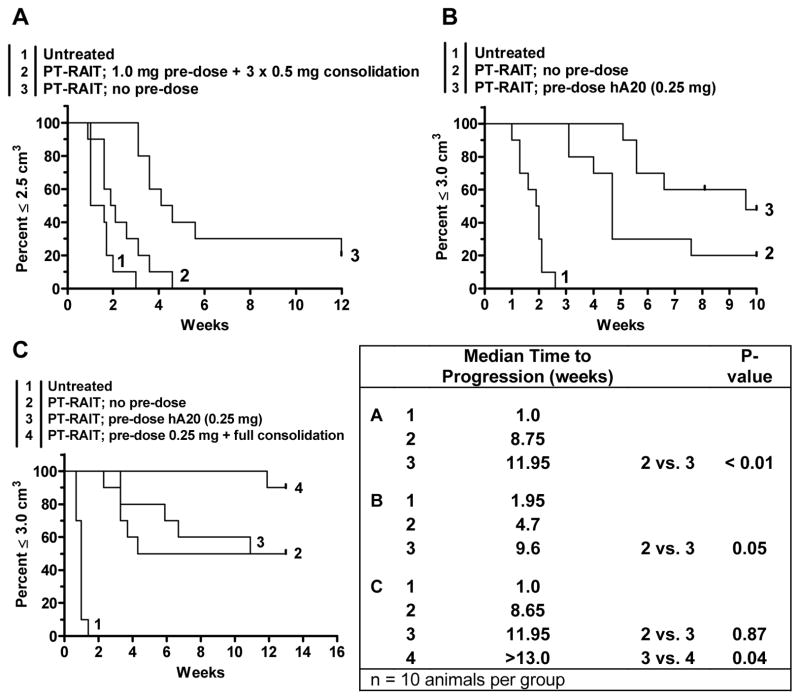
Figure 7.
The effect of veltuzumab pre-dosing and consolidation therapy on a veltuzumab-bsMAb pretargeting procedure. (A) Administering 1.0 mg of veltuzumab 1 day before the injection of the veltuzumab bsMAb reduces efficacy of the pretargeting procedure using 0.25 mCi of the 90Y-hapten-peptide, despite the use of additional veltuzumab therapy after the pretargeted 90Y-hapten peptide was given. (B) minimizing the veltuzumab pre-dose to 0.25 mg restores the anti-tumor effects of the pretargeting procedure, even improving it in this study. (C) Adding a full veltuzumab consolidation therapy after the pretargeted therapy (i.e., 1.0 mg veltuzumab starting 1 week after the 90Y-hapten peptide, followed by 3 weekly doses of 0.5 mg veltuzumab) improves response, even when animals were pre-dosed with 0.25 mg veltuzumab.
Efforts to assess the broader applicability of pretargeted radioimmunotherapy/veltuzumab consolidation therapy have been hampered by the difficulty to get a wide range of lymphoma cell lines to grow consistently in unconditioned nude mice. While SCID mice are more receptive to tumor xenograft growth, they are far more sensitive to radiation than nude mice, requiring extensive reduction in the administered activity to avoid fatal events. SCID mice are often able to tolerate a pretargeting treatment of just 0.125 mCi. Animals bearing RL xenografts (0.6 cm3) experience a significant, yet modest improvement in survival over the untreated animals, but when combined with the veltuzumab consolidation therapy, the response is substantially and significantly enhanced (unpublished results).
Conclusions
Radioimmunotherapy, an extremely effective treatment for follicular non-Hodgkin lymphoma, is currently under-utilized. Data from new clinical trials may eventually overcome the reluctance for its use, and preclinical data are now revealing ways that this procedure can be improved, which could provide additional incentives for wider acceptance. Cancer is rarely treated with a single agent or administration, but usually involves combined modalities or agents given repeatedly. Since tumors rarely have unique signatures that allow exclusive uptake when using targeted therapies, in moving forward, when using a more toxic antibody conjugate, we need to learn how to best combine different strategies that foster the highest localization ratio of the immunoconjugate, and to ensure that they do so in concert, not in competition when both target the same antigen. Using antibodies that react with different antigens can induce responses by different mechanisms of action, thus providing additive or synergistic effects. Furthermore, pretargeting methods are now being recognized for their ability to improve radionuclide targeting with reduced hematological toxicity, making these procedures very promising in an environment where the current chemotherapeutic agents are associated with major hematopoietic toxicity. Since pretargeting can deliver the same radiation doses to tumor as direct RAIT while not having a commensurate level of myelotoxicity, this lends itself to combination therapies with cytotoxic drugs. Other issues of importance, and discussed elsewhere,83 include fractionating RAIT and the use of multiple treatment cycles. If radioimmunotherapy is to be used in a consolidation setting, we will need to ensure if the radionuclide is well matched for the disease setting, involving either bulky tumors or minimal disease. Thus, targeting (or pretargeting) of radionuclides for the treatment of hematological malignancies remains a very promising modality, with a number of new opportunities to enhance efficacy.
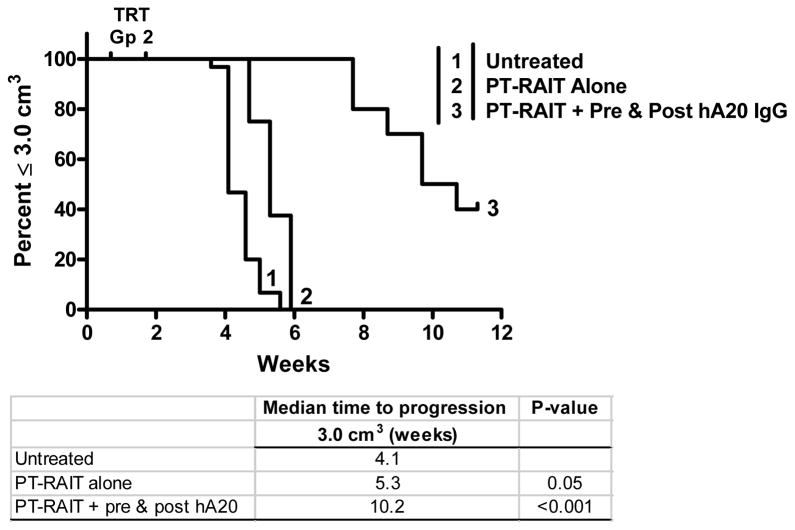
Figure 8.
Veltuzumab consolidation therapy enhances anti-tumor response in SCID mice bearing RL human lymphoma xenografts. SCID mice received 0.125 mCi of veltuzumab bsMAb pretargeted 90Y-hapten-peptide alone, or they were given a 0.25 mg pre-dose of veltuzumab 1 day before the bsMAb injection, as well as receiving a full veltuzumab consolidation therapy (1.0 mg + 3 × 0.5 mg) starting 1 week after the 90Y-hapten-peptide. (TRT, treatment-related toxicity in pretargeting alone group).
Acknowledgments
We thank M. Jules Mattes, PhD (Center for Molecular Medicine and Immunology), Edmund A. Rossi, PhD (IBC Pharmaceuticals, Inc.), William J. McBride, PhD (Immunomedics, Inc.), and Chien-Hsing Chang, PhD (Immunomedics, Inc.), for collaborations and provision of certain critical reagents.
Grant Support: This work was supported in part by USPHS grant P01 CA103985 from the National Cancer Institute, NIH (DM Goldenberg).
Financial Disclosures: DM Goldenberg is an officer and shareholder of Immunomedics, Inc., and IBC Pharmaceuticals, Inc. RM Sharkey and H Karacay declare no financial conflicts.
Footnotes
Presented in part at the Twelfth Conference on Cancer Therapy with Antibodies and Immunoconjugates, Parsippany, NJ, October 16–18, 2008.
References
- 1.Davies AJ. Radioimmunotherapy for B-cell lymphoma: Y90 ibritumomab tiuxetan and I131 tositumomab. Oncogene. 2007;26:3614–3628. doi: 10.1038/sj.onc.1210378. [DOI] [PubMed] [Google Scholar]
- 2.Press OW. Evidence mounts for the efficacy of radioimmunotherapy for B-cell lymphomas. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.18.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchegger F, Press OW, Delaloye AB, Ketterer N. Radiolabeled and native antibodies and the prospect of cure of follicular lymphoma. Oncologist. 2008;13:657–667. doi: 10.1634/theoncologist.2008-0020. [DOI] [PubMed] [Google Scholar]
- 4.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 5.Davis TA, Kaminski MS, Leonard JP, et al. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res. 2004;10:7792–7798. doi: 10.1158/1078-0432.CCR-04-0756. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RI, Kaminski MS, Wahl RL, et al. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin’s lymphomas. J Clin Oncol. 2005;23:7565–7573. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 7.Kaminski MS, Radford JA, Gregory SA, et al. Re-treatment with I-131 tositumomab in patients with non-Hodgkin’s lymphoma who had previously responded to I-131 tositumomab. J Clin Oncol. 2005;23:7985–7993. doi: 10.1200/JCO.2005.01.0892. [DOI] [PubMed] [Google Scholar]
- 8.Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441–449. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 9.Press OW, Unger JM, Braziel RM, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24:4143–4149. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 10.Emmanouilides C, Witzig TE, Gordon LI, et al. Treatment with yttrium 90 ibritumomab tiuxetan at early relapse is safe and effective in patients with previously treated B-cell non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006;47:629–636. doi: 10.1080/10428190500376076. [DOI] [PubMed] [Google Scholar]
- 11.Peyrade F, Italiano A, Fontana X, Peyrottes I, Thyss A. Retreatment with 90Y-labelled ibritumomab tiuxetan in a patient with follicular lymphoma who had previously responded to treatment. Lancet Oncol. 2007;8:849–850. doi: 10.1016/S1470-2045(07)70277-2. [DOI] [PubMed] [Google Scholar]
- 12.Shah J, Wang W, Harrough VD, et al. Retreatment with yttrium-90 ibritumomab tiuxetan in patients with B-cell non-Hodgkin’s lymphoma. Leuk Lymphoma. 2007;48:1736–1744. doi: 10.1080/10428190701528517. [DOI] [PubMed] [Google Scholar]
- 13.Witzig TE, Molina A, Gordon LI, et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109:1804–1810. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 14.Morschhauser F, Illidge T, Huglo D, et al. Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood. 2007;110:54–58. doi: 10.1182/blood-2007-01-068056. [DOI] [PubMed] [Google Scholar]
- 15.Gopal AK, Metcalfe TL, Gooley TA, et al. High-dose therapy and autologous stem cell transplantation for chemoresistant Hodgkin lymphoma: the Seattle experience. Cancer. 2008;113:1344–1350. doi: 10.1002/cncr.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs SA, Sewerdlow SH, Kant J, et al. Phase II trial of short-course CHOP-R followed by 90Y-britumomab tiuxetan and extended rituximab in previously untreated follicular lymphoma. Clin Cancer Res. 2008;14:7088–7094. doi: 10.1158/1078-0432.CCR-08-0529. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan A, Nademanee A, Fung HC, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:90–95. doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 18.Morschhauser F, Radford J, Van Hoof A, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 19.Zinzani PL, d’Amore F, Bombardieri E, et al. Consensus conference: implementing treatment recommendations on yttrium-90 immunotherapy in clinical practice - report of a European workshop. Eur J Cancer. 2008;44:366–373. doi: 10.1016/j.ejca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Zinzani PL, Tani M, Fanti S, et al. A phase II trial of CHOP chemotherapy followed by yttrium 90 ibritumomab tiuxetan (Zevalin) for previously untreated elderly diffuse large B-cell lymphoma patients. Ann Oncol. 2008;19:769–773. doi: 10.1093/annonc/mdm560. [DOI] [PubMed] [Google Scholar]
- 21.Zinzani PL, Tani M, Fanti S, et al. A phase 2 trial of fludarabine and mitoxantrone chemotherapy followed by yttrium-90 ibritumomab tiuxetan for patients with previously untreated, indolent, nonfollicular, non-Hodgkin lymphoma. Cancer. 2008;112:856–862. doi: 10.1002/cncr.23236. [DOI] [PubMed] [Google Scholar]
- 22.Zinzani PL, Tani M, Pulsoni A, et al. Fludarabine and mitoxantrone followed by yttrium-90 ibritumomab tiuxetan in previously untreated patients with follicular non-Hodgkin lymphoma trial: a phase II non-randomised trial (FLUMIZ) Lancet Oncol. 2008;9:352–358. doi: 10.1016/S1470-2045(08)70039-1. [DOI] [PubMed] [Google Scholar]
- 23.Nadler LM, Stashenko P, Hardy R, et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40:3147–3154. [PubMed] [Google Scholar]
- 24.Ritz J, Pesando JM, Sallan SE, et al. Serotherapy of acute lymphoblastic leukemia with monoclonal antibody. Blood. 1981;58:141–152. [PubMed] [Google Scholar]
- 25.Ritz J, Pesando JM, Notis-McConarty J, Clavell LA, Sallan SE, Schlossman SF. Use of monoclonal antibodies as diagnostic and therapeutic reagents in acute lymphoblastic leukemia. Cancer Res. 1981;41:4771–4775. [PubMed] [Google Scholar]
- 26.Miller RA, Maloney DG, McKillop J, Levy R. In vivo effects of murine hybridoma monoclonal antibody in a patient with T-cell leukemia. Blood. 1981;58:78–86. [PubMed] [Google Scholar]
- 27.Miller RA, Maloney DG, Warnke R, Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;306:517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 28.Dillman RO, Shawler DL, Sobol RE, et al. Murine monoclonal antibody therapy in two patients with chronic lymphocytic leukemia. Blood. 1982;59:1036–1045. [PubMed] [Google Scholar]
- 29.Foon KA. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;307:686–687. doi: 10.1056/NEJM198209093071118. [DOI] [PubMed] [Google Scholar]
- 30.Dillman RO, Shawler DL, Dillman JB, Royston I. Therapy of chronic lymphocytic leukemia and cutaneous T-cell lymphoma with T101 monoclonal antibody. J Clin Oncol. 1984;2:881–891. doi: 10.1200/JCO.1984.2.8.881. [DOI] [PubMed] [Google Scholar]
- 31.Meeker TC, Lowder J, Maloney DG, et al. A clinical trial of anti-idiotype therapy for B cell malignancy. Blood. 1985;65:1349–1363. [PubMed] [Google Scholar]
- 32.Press OW, Appelbaum F, Ledbetter JA, et al. Monoclonal antibody 1F5 (anti-CD20) serotherapy of human B cell lymphomas. Blood. 1987;69:584–591. [PubMed] [Google Scholar]
- 33.Press OW, Eary JF, Badger CC, et al. Treatment of refractory non-Hodgkin’s lymphoma with radiolabeled MB-1 (anti-CD37) antibody. J Clin Oncol. 1989;7:1027–1038. doi: 10.1200/JCO.1989.7.8.1027. [DOI] [PubMed] [Google Scholar]
- 34.Press OW, Eary JF, Appelbaum FR, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med. 1993;329:1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 35.Kaminski MS, Zasadny KR, Francis IR, et al. Radioimmunotherapy of B-cell lymphoma with [131I]anti-B1 (anti-CD20) antibody. N Engl J Med. 1993;329:459–465. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- 36.Kaminski MS, Zasadny KR, Francis IR, et al. Iodine-131-anti-B1 radioimmunotherapy for B-cell lymphoma. J Clin Oncol. 1996;14:1974–1981. doi: 10.1200/JCO.1996.14.7.1974. [DOI] [PubMed] [Google Scholar]
- 37.Goldenberg DM, Horowitz JA, Sharkey RM, et al. Targeting, dosimetry, and radioimmunotherapy of B-cell lymphomas with iodine-131-labeled LL2 monoclonal antibody. J Clin Oncol. 1991;9:548–564. doi: 10.1200/JCO.1991.9.4.548. [DOI] [PubMed] [Google Scholar]
- 38.Eary JF, Press OW, Badger CC, et al. Imaging and treatment of B-cell lymphoma. J Nucl Med. 1990;31:1257–1268. [PubMed] [Google Scholar]
- 39.Kaminski MS, Fig LM, Zasadny KR, et al. Imaging, dosimetry, and radioimmunotherapy with iodine 131-labeled anti-CD37 antibody in B-cell lymphoma. J Clin Oncol. 1992;10:1696–1711. doi: 10.1200/JCO.1992.10.11.1696. [DOI] [PubMed] [Google Scholar]
- 40.Juweid M, Sharkey RM, Markowitz A, et al. Treatment of non-Hodgkin’s lymphoma with radiolabeled murine, chimeric, or humanized LL2, an anti-CD22 monoclonal antibody. Cancer Res. 1995;55:5899s–5907s. [PubMed] [Google Scholar]
- 41.Knox SJ, Goris ML, Trisler K, et al. Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin Cancer Res. 1996;2:457–470. [PubMed] [Google Scholar]
- 42.Wahl RL, Zasadny KR, MacFarlane D, et al. Iodine-131 anti-B1 antibody for B-cell lymphoma: an update on the Michigan Phase I experience. J Nucl Med. 1998;39:21S–27S. [PubMed] [Google Scholar]
- 43.Juweid ME, Stadtmauer E, Hajjar G, et al. Pharmacokinetics, dosimetry, and initial therapeutic results with 131I- and 111In-/90Y-labeled humanized LL2 anti-CD22 monoclonal antibody in patients with relapsed, refractory non-Hodgkin’s lymphoma. Clin Cancer Res. 1999;5:3292s–3303s. [PubMed] [Google Scholar]
- 44.DeNardo GL, DeNardo SJ, Shen S, et al. Factors affecting 131I-Lym-1 pharmacokinetics and radiation dosimetry in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. J Nucl Med. 1999;40:1317–1326. [PubMed] [Google Scholar]
- 45.Buchsbaum DJ, Wahl RL, Glenn SD, Normolle DP, Kaminski MS. Improved delivery of radiolabeled anti-B1 monoclonal antibody to Raji lymphoma xenografts by predosing with unlabeled anti-B1 monoclonal antibody. Cancer Res. 1992;52:637–642. [PubMed] [Google Scholar]
- 46.Sharkey RM, Natale A, Goldenberg DM, Mattes MJ. Rapid blood clearance of immunoglobulin G2a and immunoglobulin G2b in nude mice. Cancer Res. 1991;51:3102–3107. [PubMed] [Google Scholar]
- 47.Reddy N, Ong GL, Behr TM, Sharkey RM, Goldenberg DM, Mattes MJ. Rapid blood clearance of mouse IgG2a and human IgG1 in many nude and nu/+ mouse strains is due to low IgG2a serum concentrations. Cancer Immunol Immunother. 1998;46:25–33. doi: 10.1007/s002620050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witzig TE, White CA, Wiseman GA, et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20(+) B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:3793–3803. doi: 10.1200/JCO.1999.17.12.3793. [DOI] [PubMed] [Google Scholar]
- 49.Buchsbaum DJ, Wahl RL, Normolle DP, Kaminski MS. Therapy with unlabeled and 131I-labeled pan-B-cell monoclonal antibodies in nude mice bearing Raji Burkitt’s lymphoma xenografts. Cancer Res. 1992;52:6476–6481. [PubMed] [Google Scholar]
- 50.Wahl RL. Tositumomab and 131I therapy in non-Hodgkin’s lymphoma. J Nucl Med. 2005;46(Suppl 1):128S–140S. [PubMed] [Google Scholar]
- 51.Conti PS, White C, Pieslor P, Molina A, Aussie J, Foster P. The role of imaging with 111In-ibritumomab tiuxetan in the ibritumomab tiuxetan (zevalin) regimen: results from a Zevalin Imaging Registry. J Nucl Med. 2005;46:1812–1818. [PubMed] [Google Scholar]
- 52.Gopal AK, Press OW, Wilbur SM, Maloney DG, Pagel JM. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but no radiolabeled anti-CD45-Ab. Blood. 2008;112:830–835. doi: 10.1182/blood-2008-01-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharkey RM, Karacay H, Johnson CR, et al. Pretargeted vs. directly targeted radioimmunotherapy with anti-CD20 antibody consolidation of non-Hodgkin lymphoma in a nude mouse model. J Nucl Med. 2009;50:444–453. doi: 10.2967/jnumed.108.058602. [DOI] [PubMed] [Google Scholar]
- 54.Food and Drug Administration Guidance for Industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers: Center for Drug Evaluation and Research (CDER) Pharmacology and Toxicology. 2005 July;:1–27. [Google Scholar]
- 55.Hernandez MC, Knox SJ. Radiobiology of radioimmunotherapy: targeting CD20 B-cell antigen in non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2004;59:1274–1287. doi: 10.1016/j.ijrobp.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 56.Skvortsova I, Popper BA, Skvortsov S, et al. Pretreatment with rituximab enhances radiosensitivity of non-Hodgkin’s lymphoma cells. J Radiat Res (Tokyo) 2005;46:241–248. doi: 10.1269/jrr.46.241. [DOI] [PubMed] [Google Scholar]
- 57.Skvortsova I, Skvortsov S, Popper BA, et al. Rituximab enhances radiation-triggered apoptosis in non-Hodgkin’s lymphoma cells via caspase-dependent and -independent mechanisms. J Radiat Res (Tokyo) 2006;47:183–196. doi: 10.1269/jrr.47.183. [DOI] [PubMed] [Google Scholar]
- 58.Kapadia NS, Engles JM, Wahl RL. In vitro evaluation of radioprotective and radiosensitizing effects of rituximab. J Nucl Med. 2008;49:674–678. doi: 10.2967/jnumed.107.043752. [DOI] [PubMed] [Google Scholar]
- 59.Ivanov A, Swann R, Illidge T. New insights into the mechanisms of action of radioimmunotherapy in lymphoma. J Pharm Pharmacol. 2008;60:987–998. doi: 10.1211/jpp.60.8.0006. [DOI] [PubMed] [Google Scholar]
- 60.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 61.Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Wang Y, Chang CH. Novel designs of multivalent anti-CD20 humanized antibodies as improved lymphoma therapeutics. Cancer Res. 2008;68:8384–8392. doi: 10.1158/0008-5472.CAN-08-2033. [DOI] [PubMed] [Google Scholar]
- 62.Murthy S, Sharkey RM, Goldenberg DM, et al. Lymphoma imaging with a new technetium-99m labelled antibody, LL2. Eur J Nucl Med. 1992;19:394–401. doi: 10.1007/BF00177365. [DOI] [PubMed] [Google Scholar]
- 63.Blend MJ, Hyun H, Kozloff M, et al. Improved staging of B-cell non-Hodgkin’s lymphoma patients with 99mTc-labeled LL2 monoclonal antibody fragment. Cancer Res. 1995;55:5764s–5770s. [PubMed] [Google Scholar]
- 64.Sharkey RM, Behr TM, Mattes MJ, et al. Advantage of residualizing radiolabels for an internalizing antibody against the B-cell lymphoma antigen, CD22. Cancer Immunol Immunother. 1997;44:179–188. doi: 10.1007/s002620050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vose JM, Colcher D, Gobar L, et al. Phase I/II trial of multiple dose 131Iodine-MAb LL2 (CD22) in patients with recurrent non-Hodgkin’s lymphoma. Leuk Lymphoma. 2000;38:91–101. doi: 10.3109/10428190009060322. [DOI] [PubMed] [Google Scholar]
- 66.Sharkey RM, Brenner A, Burton J, et al. Radioimmunotherapy of non-Hodgkin’s lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-Epratuzumab): do tumor targeting and dosimetry predict therapeutic response? J Nucl Med. 2003;44:2000–2018. [PubMed] [Google Scholar]
- 67.Linden O, Hindorf C, Cavallin-Stahl E, et al. Dose-fractionated radioimmunotherapy in non-Hodgkin’s lymphoma using DOTA-conjugated, 90Y-radiolabeled, humanized anti-CD22 monoclonal antibody, epratuzumab. Clin Cancer Res. 2005;11:5215–5222. doi: 10.1158/1078-0432.CCR-05-0172. [DOI] [PubMed] [Google Scholar]
- 68.Kraeber-Bodere F, Morschhauser F, Huglo D, et al. Fractionated radioimmunotherapy in NHL with DOTA-conjugated, humanized anti-CD22 IgG, epratuzumab: Results at high cumulative doses of 90Y. J Clin Oncol. 2008;26(15S):454S. (abstr 8502) [Google Scholar]
- 69.Leonard JP, Schuster SJ, Emmanouilides C, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 70.Leonard JP, Coleman M, Ketas JC, et al. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21:3051–3059. doi: 10.1200/JCO.2003.01.082. [DOI] [PubMed] [Google Scholar]
- 71.Leonard JP, Coleman M, Ketas JC, et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin’s lymphoma: phase I/II clinical trial results. Clin Cancer Res. 2004;10:5327–5334. doi: 10.1158/1078-0432.CCR-04-0294. [DOI] [PubMed] [Google Scholar]
- 72.Stein R, Qu Z, Chen S, et al. Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and Its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin’s lymphoma. Clin Cancer Res. 2004;10:2868–2878. doi: 10.1158/1078-0432.ccr-03-0493. [DOI] [PubMed] [Google Scholar]
- 73.Goldenberg DM, Rossi EA, Stein R, et al. Properties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody. Blood. 2009;113:1062–1070. doi: 10.1182/blood-2008-07-168146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morschhauser F, Leonard JP, Fayad LE, et al. Humanized ani-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: Phase I/II results. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.1219.9117. in press (ahead of print, May 18, 2009) [DOI] [PubMed] [Google Scholar]
- 75.Mattes MJ, Sharkey RM, Karacay H, Czuczman MS, Goldenberg DM. Therapy of advanced B-lymphoma xenografts with a combination of 90Y-anti-CD22 IgG (epratuzumab) and unlabeled anti-CD20 IgG (veltuzumab) Clin Cancer Res. 2008;14:6154–6160. doi: 10.1158/1078-0432.CCR-08-0404. [DOI] [PubMed] [Google Scholar]
- 76.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 77.Sharkey RM, Karacay H, Cardillo TM, et al. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin Cancer Res. 2005;11:7109s–7121s. doi: 10.1158/1078-0432.CCR-1004-0009. [DOI] [PubMed] [Google Scholar]
- 78.Axworthy DB, Reno JM, Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci U S A. 2000;97:1802–1807. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boerman OC, Kranenborg MH, Oosterwijk E, et al. Pretargeting of renal cell carcinoma: improved tumor targeting with a bivalent chelate. Cancer Res. 1999;59:4400–4405. [PubMed] [Google Scholar]
- 80.Gautherot E, Rouvier E, Daniel L, et al. Pretargeted radioimmunotherapy of human colorectal xenografts with bispecific antibody and 131I-labeled bivalent hapten. J Nucl Med. 2000;41:480–487. [PubMed] [Google Scholar]
- 81.Karacay H, Brard PY, Sharkey RM, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879–7885. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 82.Sharkey RM, Karacay H, Litwin S, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin’s lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res. 2008;68:5282–5290. doi: 10.1158/0008-5472.CAN-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharkey RM, Press OW, Goldenberg DM. A re-examination of radioimmunotherapy in the treatment of non-Hodgkin lymphoma: Prospects for dual-targeted antibody/radioantibody therapy. Blood. 2009;113:3891–3895. doi: 10.1182/blood-2008-11-188896. [DOI] [PMC free article] [PubMed] [Google Scholar]