Abstract
We analyzed the long-term outcome of 1011 patients treated in five successive clinical trials (Total Therapy Studies 11, 12, 13A, 13B and 14) between 1984 and 1999. The event-free survival improved significantly (p=0.003) from the first two trials conducted in the 1980s to the three more recent trials conducted in the 1990s. Approximately 75% of patients treated in the 1980s and 80% in the 1990s were cured. Early intensive triple intrathecal therapy, together with more effective systemic therapy, including consolidation and reinduction treatment (Studies 13A and 13B) as well as dexamethasone (Study 13A), resulted in a very low rate of isolated central-nervous-system relapse rate (<2%), despite the reduced use of cranial irradiation. Factors consistently associated with treatment outcome were age, leukocyte count, immunophenotype, DNA index, and minimal residual disease level after remission induction treatment. Because of concerns about therapy-related secondary myeloid leukemia and brain tumors, in our current trials we reserve the use of etoposide for patients with refractory or relapsed leukemia undergoing hematopoietic stem cell transplantation, and cranial irradiation for those with CNS relapse. The next main challenge is to further increase cure rates while improving quality of life for all patients.
Keywords: B-lineage ALL, T-lineage ALL, CNS relapse, prognostic factors, leukemia, chemotherapy
Introduction
The St. Jude Total Therapy program for childhood acute lymphoblastic leukemia (ALL) has spanned over four decades. The first nine studies, conducted from 1962 to 1979, established the importance of combination systemic chemotherapy in full doses and central-nervous-system (CNS)-directed therapy.1 Total Therapy Study 10 (1979 to 1983), the first to apply risk-directed therapy, showed the efficacy of high-dose methotrexate and the feasibility of reducing the use of cranial irradiation.2 In Study 11 (1984-1988), the use of early treatment intensification improved outcome, and the schedule-dependent leukemogenic effects of epipodophyllotoxins were identified.3-5 Study 12 (1988-1991) proved the benefits of individualized therapy based on pharmacokinetics.6 In Study 13A (1991-1994), early intensification of intrathecal therapy substantially reduced CNS relapse hazard, allowing further reduction of cranial irradiation.7 This study also disclosed the pharmacodynamic basis of differences in methotrexate disposition between various phenotypic and genotypic subtypes of ALL.8-12 The long-term results of these 13 studies were reported in the December 2000 issue of Leukemia,13 together with those of 11 other groups.
In Study 13B (1994-1998), the efficacy of early intensification of intrathecal therapy was confirmed,14 paving the way to the total elimination of prophylactic cranial irradiation in subsequent Total Therapy studies.15-17 Study 13B demonstrated that host pharmacogenetics affects treatment toxicity as well as antileukemic outcome,18,19 and also represented the first leukemia trial to apply pharmacogenetics in therapy;20 that is, the dose of mercaptopurine during continuation therapy was based on polymorphisms of the gene encoding thiopurine methyltransferase to reduce the hematopoietic toxicity and the risk of secondary cancer.21-23 Study 14 (1998-1999) attempted to find optimal doses of methotrexate for various subtypes of ALL.15 Study 15 tested whether prophylactic cranial irradiation could be safely omitted in all children with newly diagnosed ALL, and its encouraging results have recently been published.17 In this report, we update the results of Studies 11 to 14.
Materials and methods
From 1984 to 1999, 1011 consecutive patients aged 18 years or younger with newly diagnosed ALL were enrolled in five successive treatment protocols (Total Therapy Studies 11, 12, 13A, 13B and 14) at St. Jude Children’s Research Hospital.3,6,7,14,15 Patients were not eligible for the studies if they had received more than 1 week of prior therapy or any treatment other than glucocorticoids, vincristine or emergency mediastinal irradiation. The diagnosis of ALL was based on immunophenotyping with panels of monoclonal antibodies directed toward lineage-associated antigens. Cytogenetic and molecular genetic studies and DNA index determination were performed with methods described previously.14 All protocols were approved by Institutional Review Board; Studies 11, 12 and 13B were also reviewed and approved by the National Cancer Institute. Written informed consent and assent were obtained from all parents and/or patients as appropriate.
Treatment
Details of Total Therapy Studies have been described in earlier publications.3,6,7,14,15 In Study 11,3 patients received 6-week remission induction with prednisone, vincristine, daunorubicin, asparaginase, teniposide and cytarabine, followed by consolidation therapy with 2 weekly doses of high-dose methotrexate at 2 g/m2. At the completion of consolidation therapy, lower-risk patients were stratified and randomized to receive antimetabolite-based therapy or four pairs of drugs (etoposide plus cyclophosphamide, mercaptopurine plus methotrexate, teniposide plus cytarabine, and prednisone plus vincristine) rotated weekly for 120 weeks, whereas higher-risk patients were stratified and randomized to receive the same four pairs of drugs in rotation weekly or every 6 weeks for 120 weeks. Triple intrathecal therapy was given on days 2, 22 and 43 of remission induction (additional doses on days 8 and 15 for patients with CNS leukemia [CNS 3] at diagnosis), and then every 8 weeks during the first year of continuation therapy. Cranial irradiation and five intrathecal treatments were added for 64% of the patients with higher-risk leukemia (18 Gy) or CNS leukemia at diagnosis (24 Gy). Overall, lower-risk cases received 9 doses of triple intrathecal therapy and higher-risk cases 13 to 15 doses.
In Study 12,6 patients received remission induction therapy identical to that in Study 11. Upon attaining complete remission, patients were stratified (by age, leukocyte count, age and DNA index) and randomized to receive either conventional doses (based on body surface area) or individualized doses (targeted to achieve an area under the plasma concentration-versus-time curve between the 50th and 90th percentiles of that in the conventional arm) of high-dose methotrexate, and teniposide plus cytarabine, given as alternating pulses for 5 courses each during the first year of 120 weeks of continuation therapy with mercaptopurine and methotrexate. Intrathecal therapy during remission induction was identical to that in Study 11, and was then given on day 1 of each of the 10 courses of pulse therapy. Cranial irradiation plus 5 intrathecal treatments were given to 37% of the patients with high-risk leukemia (18 Gy) or CNS leukemia at diagnosis (24 Gy) during weeks 59 to 61 of continuation therapy. The number of intrathecal therapy ranged from 13 to 20 doses in this study. In addition, three of the six patients with Philadelphia chromosome-positive leukemia underwent allogeneic hematopoietic stem cell transplantation.
In Study 13A,7 patients were stratified and randomized to receive high-dose (1 g/m2) or lower-dose (30 mg/m2 every 6 hours for 4 doses) methotrexate up-front, followed by 6 weeks of remission induction therapy similar to that used in the preceding two trials (except that etoposide was substituted for teniposide). All patients received 2 weeks of consolidation therapy with 2 weekly doses of high-dose methotrexate (2 g/m2) and daily mercaptopurine. Continuation therapy was risk-directed. Lower-risk patients received antimetabolite-based therapy for 120 weeks with pulses of high-dose methotrexate every 8 weeks (for the first year) and prednisone plus vincristine pulse every 4 weeks. Higher-risk cases received weekly rotation continuation therapy similar to that in Study 11, with the addition of asparaginase, high-dose methotrexate, and a reinduction phase during the first year. Recognizing the increased risk of CNS relapse in patients with CNS 2, CNS 3, or traumatic lumbar puncture with blasts had led to intensified intrathecal therapy in these patients as well as some CNS 1 cases with other high-risk features. These patients received 2 additional weekly doses of intrathecal therapy during remission induction and then every 4 weeks during the first year of continuation therapy. Cranial irradiation plus 5 intrathecal treatments were given to 22% of the patients with CNS 3 status (24 Gy) or high-risk leukemia (18 Gy). The number of intrathecal therapy doses administered was 15 for lower-risk cases and ranged from 22 to 26 for higher-risk cases. Only one of the 6 patients with Philadelphia chromosome-positive leukemia underwent transplantation.
After 2 days of treatment with mercaptopurine alone or mercaptopurine plus high-dose or lower-dose methotrexate, patients in Study 13B14 received the same 6-week remission induction and 2 weeks of consolidation as those in Study 13A. Lower-risk cases received mercaptopurine and methotrexate continuation therapy, reinforced by high-dose methotrexate every 8 weeks for the first year, dexamethasone plus vincristine pulse every 4 weeks, and reinduction therapy during weeks 16 to 21 after remission induction. Post-remission therapy for higher-risk cases was similar to that in Study 13A with some modifications (dexamethasone was substituted for prednisone, and asparaginase was used only during reinduction and omitted during continuation therapy). CNS-directed therapy was similar to that in Study 13A except that cranial irradiation plus 5 intrathecal treatments were given to only 12% of patients who have T-cell ALL and initial leukocyte count over 100 × 109/L (18 Gy), or CNS 3 status (24 Gy). The total number of intrathecal treatments ranged from 13 in lower-risk cases to 26 in high-risk cases who received cranial irradiation. Eleven patients were transplanted: 4 each for Philadelphia chromosome-positive or t(4;11) ALL and 3 for other conditions.
In Study 14,15 upfront methotrexate at various doses was given before remission induction treatment identical to that of Study 13B. Consolidation therapy consisted of 2 weekly doses of high-dose methotrexate (5 g/m2 for higher-risk and 2.5 g/m2 for lower-risk cases) together with daily mercaptopurine and triple intrathecal therapy. Post-remission therapy was similar to that in Study 13B with some modifications (high-dose methotrexate was given at 5 g/m2 for higher-risk cases and 2.5 g/m2 for lower-risk cases; polyethylene glycol-conjugated asparaginase was given at days 1, 8 and 15 and idarubicin was given at days 1 and 8 of reinduction treatment together with daily dexamethasone and weekly vincristine). The total number of triple intrathecal treatments ranged from 16 in lower-risk cases to 23 in higher-risk cases with CNS 2, CNS 3, or traumatic lumbar puncture with blasts status at diagnosis. None of the patients received prophylactic cranial irradiation, regardless of CNS status or other features at diagnosis. Two patients with poor early response were transplanted: one infant with t(4;11) ALL and the other with Philadelphia chromosome-positive ALL.
Statistical analysis
The duration of event-free survival was defined as the time from diagnosis until the date of failure (induction failure, relapse, death, or the development of a second malignancy) or until the date of last contact for all event-free survivors. Patients who did not attain a complete remission were considered failures at time zero. Event-free survival and overall survival rates were estimated by the method of Kaplan Meier and were compared with the Mantel-Haenszel test.24 The Cox proportional hazards model was used to identify independent prognostic factors with respect to event-free survival and overall survival. All analyses were performed on the basis of “intent-to-treat.”
For patients who achieved complete remission, cumulative incidence functions of isolated CNS or any (isolated plus combined) CNS relapse, as well as testicular relapse, therapy-related second malignancy, and death from toxicity, were estimated by the method of Kalbfleisch and Prentice,25 and the functions were compared with Gray’s test,26 adjusting for competing events. An isolated CNS relapse was defined as one without simultaneous relapse at another site, while a combined CNS relapse was one in the CNS accompanied by relapse in the bone marrow or any other extramedullary site. The database last updated on January 23, 2009, was used for analyses.
The routine follow-up procedures for long-term survivors at our institution have been described previously.27 Briefly, after completion of therapy, remission status and late effects are comprehensively assessed at least annually. Patients who are at least 18 years old and remain in remission for at least 10 years after diagnosis are discharged from the institution and followed thereafter by the community physicians. The status of these patients is monitored by a questionnaire mailed annually by the institution’s tumor registry. No patients have been lost to follow-up during treatment. When the databases were frozen for analyses, 79%, 80%, 90%, 91% and 98% of event-free survivors in studies 11, 12, 13A, 13B and 14, respectively, had been seen within 12 months, and 8%, 8%, 2.6%, 2.6% and 2.4% had not been seen or contacted for more than 5 years.
Results
The event-free survival improved significantly from the clinical trials conducted in the 1980s (Studies 11 and 12) to those in the 1990s (Studies 13A, 13B and 14) (p=0.003, Table 1). However, the overall survival was similar between the studies (p=0.32), with the exception of Study 13B which had a better result. Late failures occurring beyond 10 years from diagnosis were uncommon (<2%).
Table 1.
Treatment Outcome According to Total Therapy Studies
| Total Therapy Studies | Total 11 | Total 12 | Total 13A | Total 13B | Total 14 | |
| Year | 1984-1988 | 1988-1991 | 1991-1994 | 1994-1998 | 1998-1999 | |
| No. of Patients | 358 | 188 | 165 | 247 | 53 | |
| Induction failure(toxic death) | 15 (8) | 6 (3) | 3 (3) | 5 (3) | 3 (2) | |
| Relapses | Hematological only | 48 | 19 | 17 | 24† | 4 |
| CNS only | 21 | 19 | 2 | 7‡ | 0 | |
| Hematological + CNS | 4 | 8 | 6 | 2 | 2 | |
| Testicular only | 1 | 2 | 0 | 1 | 0 | |
| Hematological + Testicular | 1 | 3 | 1 | 0 | 0 | |
| Hematological + CNS + Testicular | 1 | 0 | 0 | 0 | 0 | |
| Other relapses sites§ | 2 | 1 | 0 | 1 | 1 | |
| Second Cancer | 15 | 15 | 16 | 8 | 0 | |
| Infectious death in remission | 4 | 2 | 1 | 4 | 1 | |
| Other causes of remission deaths§§ | 3 | 1 | 3 | 4 | 1 | |
| 10-year cumulative risk of death in remission (%) | 1.7±0.7 | 1.6±0.9 | 1.9±1.1 | 3.3±1.2 | 4.0±2.8 | |
| 10-year event-free survival ± SE (%) | 69.6 ± 2.4 | 61.2 ± 3.6 | 71.5 ± 3.5 | 77.6 ± 2.9 | 77.4 ± 16.5 | |
| 10-year overall survival ± SE (%) | 76.5 ± 2.2 | 78.7 ± 3.0 | 78.2 ± 3.2 | 83.7 ± 2.5 | 79.2 ± 16.1 | |
Hematological relapse was associated with mediastinal relapse in one case
CNS relapse was associated with mediastinal relapse in one case and with ocular relapse in another case
One case involved the kidney and one the lymph nodes in Study 11; one involved the mediastinum in Study 12; one inbilateral eyes in Study 13B; and one in the orbit in Study 14.
One died of suicide, one of an accident, and one of a neurologic event in Study 11; one died of an accident in Study 12; two died of accidents and one of suicide in Study 13A; two died of graft-versus-host diseases, 1 of an accident and one of liver failure associated with cystic fibrosis in Study 13B; and one patient died of graft-versus-host disease in Study 14.
Protocol-specific treatment outcome
Study 11
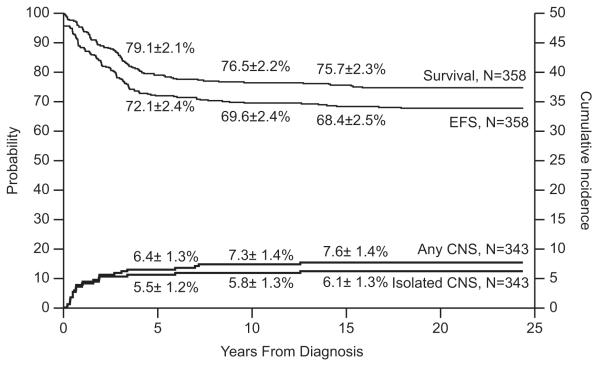
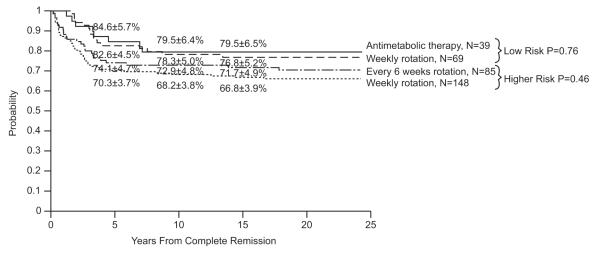
Event-free survival at 15 years for the 358 evaluable patients was 68.4±2.5%, and overall survival was 75.7±2.3% (Fig. 1). Median follow-up was 21.6 years (range, 9.5 to 24.4 years) for the 268 survivors (243 free of adverse events). The cumulative risk estimates for isolated CNS and any CNS relapses at 15 years were 6.1±1.3% and 7.6±1.4% , respectively. Of the 186 male patients, three developed testicular relapse (isolated in one patient, combined with hematologic relapse in another, and combined with hematologic and CNS relapse in the third) with a cumulative incidence of 1.7±1.0% at 10 years. The type of post-remission continuation therapy had no impact on the complete remission duration for either the lower-risk or higher-risk group (Fig. 2).
Fig. 1.
Event-free survival (EFS), survival and cumulative incidence of isolated or any CNS relapse in Total Therapy Study 11.
Fig. 2.
Duration of continuous complete remission according to postremission therapy in patients with low-risk or higher-risk ALL in Total Therapy Study 11.
Secondary neoplasms included acute myeloid leukemia (AML) in 10 patients, brain tumor in 2, and Ewing sarcoma, carcinoma, and basal cell carcinoma in the three remaining patients. The cumulative risk of any secondary neoplasms was 3.2±1.0% at 10 years and 3.8±1.0% at 15 years; that of secondary myeloid malignancies plateaued at 2.9±0.9% at 10 years. None of the patients in the lower-risk group developed secondary AML. In the higher-risk group, patients treated with drug pairs rotated every 6 weeks had a significantly higher rate of secondary AML than those who received the same drug pairs rotated weekly (p=0.03; 8.2±3.0% vs. 2.0±1.2%).
Study 12
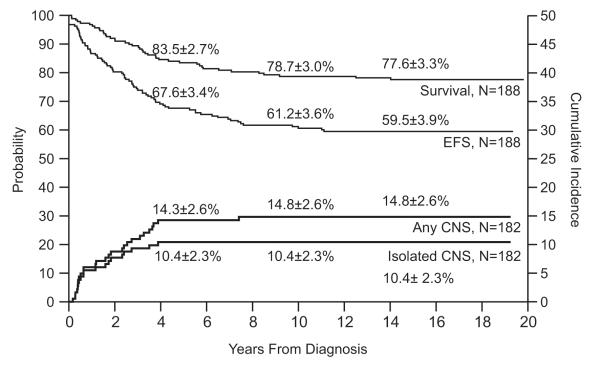
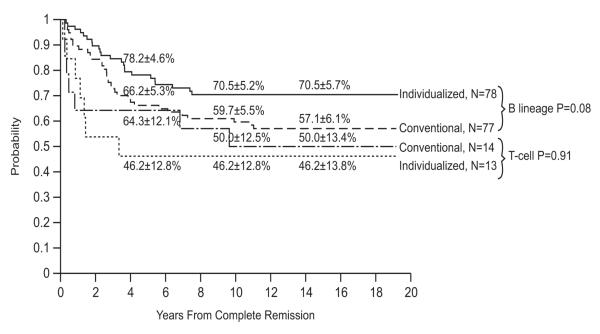
Event-free survival and overall survival estimates at 15 years for the 188 evaluable patients were 59.5±3.9% and 77.6±3.3%, respectively, (Fig. 3). Of the 146 survivors (112 free of adverse events), the median follow-up duration was 17.4 years (range, 8.4 to 19.8 years). Compared to other Total Therapy Studies, Study 12 had the highest rates of CNS relapse: 10.4±2.3% for isolated CNS relapse and 14.8±2.6% for any CNS relapse at 15 years (p<0.001). It also had the highest rate of any testicular relapse (isolated in 2 boys and combined with hematologic relapse in 3 others) with a cumulative risk of 5.1±2.2% at 10 years. Individualized doses of high-dose methotrexate tended to improve event-free survival in patients with B-lineage ALL (p=0.08) but not in those with T-lineage ALL (p=0.91, Fig. 4). Overall survival estimates did not differ between patients treated with individualized or conventional therapy for both B-lineage ALL (p=0.86; 79.3±5.1% vs. 80.5±4.7% at 15 years) and T-lineage ALL (p=0.28; 69.2±13.6% vs. 85.7±9.8% at 15 years).
Fig. 3.
Event-free survival (EFS), survival and cumulative incidence of isolated or any CNS relapse in Total Therapy Study 12.
Fig. 4.
Duration of continuous complete remission according to postremission therapy for B-lineage and T-lineage cases, respectively.
Secondary neoplasms included 10 cases of brain tumor, 3 AML, and 2 myelodysplastic syndrome, with a cumulative risk of 8.3±2.1% at 15 years. Notably, this study had the highest rate of secondary brain tumor: 5.5±1.7% at 15 years. Not surprisingly, all brain tumors developed among the 71 patients who had received cranial irradiation, resulting in a cumulative risk of 14.1±4.2% at 15 years; none of the 113 non-irradiated patients had this complication (p<0.0001). The risk was especially high among the 7 irradiated patients with thiopurine methyltransferase deficiency: 42.9±20.6%.
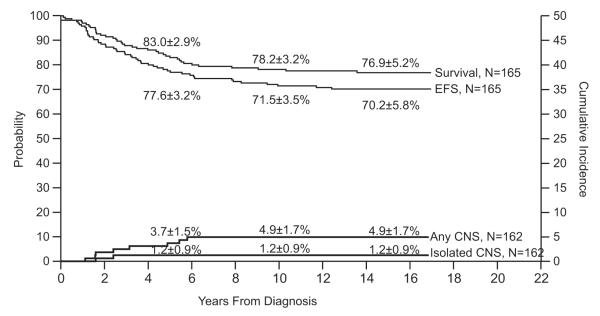
Study 13A
Among 165 evaluable patients, the event-free survival was 70.2±5.8% and overall survival 76.9±5.2% at 15 years (Fig. 5). The median follow-up duration of the 127 survivors (116 free of events) was 14.6 years (range, 7.4 to 17.0 years). Isolated and any CNS relapse rates were only 1.2±0.9% and 4.9±1.7% at 15 years. Only 1 of 92 boys developed a combined testicular and hematologic relapse (cumulative risk, 1.1±1.1% at 10 years).
Fig. 5.
Event-free survival (EFS), survival and cumulative incidence of isolated or any CNS relapse in Total Therapy Study 13A.
Secondary leukemias (10 AML, 2 ALL and 1 myelodysplastic syndrome) occurred in 13 patients, brain tumors in 2 and osteosarcoma in 1. The cumulative risk of any second neoplasm was 9.9±2.4% at 10 years and 15 years. The cumulative risk of secondary myeloid malignancy was 6.8±2.0% at 10 years, the highest rate among all Total Therapy Studies.
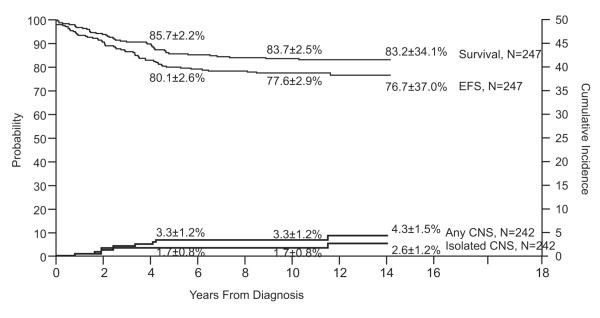
Study 13B
Event-free survival at 10 years for the 247 evaluable patients was 77.6±2.9%, and overall survival was 83.7±2.5% (Fig. 6). Median follow-up was 11.4 years (range, 2.4 to 14.2 years) for the 206 survivors (191 event-free). The cumulative risk of isolated CNS relapse at 10 years was only 1.7±0.8% and that of any CNS relapse was 3.3±1.2%. Of the 144 boys, only one developed an isolated testicular relapse (cumulative risk at 10 years, 0.7±0.7%).
Fig. 6.
Event-free survival (EFS), survival and cumulative incidence of isolated or any CNS relapse in Total Therapy Study 13B.
Eight patients have developed secondary myeloid malignancies (4 AML, 3 myelodysplastic syndrome and 1 chronic myeloid leukemia) with a cumulative risk of 3.3±1.2% at 10 years. To date, none of the patients has developed other types of second neoplasms.
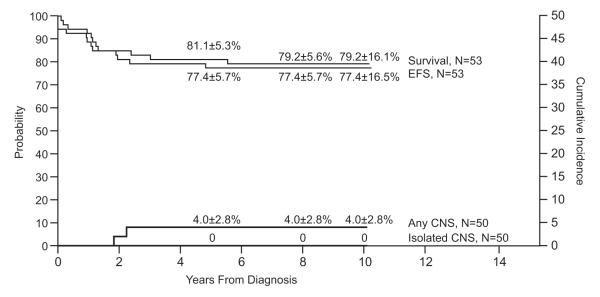
Study 14
Because of excessive toxicities encountered during remission induction, the protocol was terminated early, after enrolling 53 patients. Event-free survival was 77.4±5.7% at 5 years and 77.4±16.5% at 10 years; the overall survival was 81.1±5.3% and 79.2±16.1% , respectively (Fig. 7). Two patients developed a combined CNS and hematologic relapse (cumulative risk, 4.0±2.8% at 10 years). To date, none of the patients has developed an isolated CNS relapse, any testicular relapse, or a secondary neoplasm.
Fig. 7.
Event-free survival (EFS), survival and cumulative incidence of isolated or any CNS relapse in Total Therapy Study 14.
Treatment results according to presenting features
Prognostic factors were analyzed for all studies except Study 14 because of its limited enrollment. High-risk B-lineage according to the NCI/Rome criteria (age <1 or >10 years with leukocyte count >50×109/L)28 and T-cell phenotype were consistently adverse prognostic factors, whereas DNA index ≥1.16 was a favorable factor for event-free survival in all 4 studies analyzed (Tables 2 to 5). Initial leukocyte count and Philadelphia chromosome had prognostic significance in most studies, and age was consistently associated with overall survival in the 4 studies. The use of intensified intrathecal treatment in Studies 13A and 13B abolished the adverse prognostic significance of CNS 2 and of traumatic lumbar puncture with blasts observed in previous studies. With the improvement in event-free survival for B-lineage cases in Studies 13A and 13B, the favorable prognostic impact of ETV6-RUNX1 (also known as TEL-AML1) fusion was less noticeable. Notably, systematic minimal residual disease measurement was initiated in Study 13B, and this factor had independent prognostic impact for both event-free survival and overall survival.
Table 2.
Treatment results according to presenting features in patients treated in Study 11
| Event-free survival ± SE (%) | Overall survival ± SE (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | No. of patients |
Year 5 | Year 10 | Year 15 | p-value | Year 5 | Year 10 | Year 15 | p-value |
| B-lineage | |||||||||
| NCI Standard | 183 | 85.2 ± 2.6 | 82.0 ± 2.8 | 81.4 ± 2.9 | <.0001 | 89.1 ± 2.3 | 86.9 ± 2.5 | 85.8 ± 2.6 | 0.0001 |
| NCI High | 113 | 62.8 ± 4.5 | 61.1 ± 4.6 | 60.2 ± 4.7 | 73.5 ± 4.1 | 69.9 ± 4.3 | 69.0 ± 4.5 | ||
| T-lineage | |||||||||
| NCI Standard | 10 | 40.0 ± 13.9 | 40.0 ± 15.5 | 40.0 ± 15.5 | 0.69 | 40.0 ± 13.9 |
40.0 ± 15.5 | 40.0 ± 15.5 | 0.21 |
| NCI High | 52 | 51.9 ± 6.8 | 50.0 ± 6.8 | 46.0 ± 6.9 | 63.5 ± 6.6 | 61.5 ± 6.6 | 61.5 ± 6.9 | ||
| Sex | |||||||||
| Male | 186 | 68.3 ± 3.4 | 64.5 ± 3.5 | 63.4 ± 3.6 | 0.06 | 74.7 ± 3.2 | 71.5 ± 3.3 | 70.4 ± 3.4 | 0.03 |
| Female | 172 | 76.2 ± 3.2 | 75.0 ± 3.3 | 73.8 ± 3.4 | 83.7 ± 2.8 | 82.0 ± 2.9 | 81.4 ± 3.0 | ||
|
Age at diagnosis
(yrs.) |
|||||||||
| <1 | 11 | 45.5 ± 13.7 | 45.5 ± 13.7 | 36.4 ± 13.0 | 0.0007 | 63.6 ± 13.6 |
63.6 ± 13.6 | 63.6 ± 13.6 | 0.006 |
| 1-9 | 257 | 76.3 ± 2.6 | 73.5 ± 2.8 | 72.7 ± 2.8 | (0.01)* | 82.5 ± 2.4 | 80.2 ± 2.5 | 79.4 ± 2.6 | |
| >10 | 90 | 63.3 ± 5.0 | 61.1 ± 5.1 | 60.0 ± 5.3 | 71.1 ± 4.7 | 67.8 ± 4.9 | 66.6 ± 5.1 | ||
| Race | |||||||||
| White | 314 | 72.9 ± 2.5 | 70.4 ± 2.6 | 69.4 ± 2.6 | 0.26 | 79.9 ± 2.3 | 77.7 ± 2.4 | 76.7 ± 2.4 | 0.20 |
| Black | 43 | 65.1 ± 7.1 | 62.8 ± 7.2 | 60.4 ± 7.5 | 72.1 ± 6.7 | 67.4 ± 7.0 | 67.4 ± 7.3 | ||
| Other | 1 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | ||
| WBC×109/L | |||||||||
| <10 | 154 | 80.5 ± 3.2 | 78.6 ± 3.3 | 77.9 ± 3.4 | <.0001 | 84.4 ± 2.9 | 83.1 ± 3.0 | 82.5 ± 3.1 | 0.0001 |
| 10-49 | 109 | 76.1 ± 4.1 | 72.5 ± 4.3 | 71.5 ± 4.3 | 81.7 ± 3.7 | 78.0 ± 3.9 | 76.1 ± 4.1 | ||
| 50-99 | 30 | 66.7 ± 8.4 | 63.3 ± 8.6 | 63.3 ± 8.8 | 83.3 ± 6.7 | 76.7 ± 7.6 | 76.7 ± 7.7 | ||
| >100 | 65 | 47.7 ± 6.1 | 46.2 ± 6.1 | 42.9 ± 6.2 | 60.0 ± 6.0 | 58.5 ± 6.0 | 58.5 ± 6.4 | ||
| Cell lineage | |||||||||
| B | 296 | 76.7 ± 2.5 | 74.0 ± 2.5 | 73.3 ± 2.6 | <.0001 | 83.1 ± 2.2 | 80.4 ± 2.3 | 79.4 ± 2.4 | <.0001 |
| T | 62 | 50.0 ± 6.3 | 48.4 ± 6.3 | 44.9 ± 6.4 | (0.04)* | 59.7 ± 6.1 | 58.1 ± 6.3 | 58.1 ± 6.4 | (0.03)* |
| CNS status | |||||||||
| CNS 1 | 260 | 78.8 ± 2.5 | 76.2 ± 2.6 | 75.0 ± 2.8 | <.0001 | 83.8 ± 2.3 | 81.5 ± 2.4 | 80.3 ± 2.5 | 0.007 |
| CNS 2 | 48 | 56.2 ± 7.0 | 56.2 ± 7.0 | 54.2 ± 7.1 | (0.03)* | 68.7 ± 6.6 | 64.6 ± 6.8 | 64.6 ± 6.8 | |
| CNS 3 | 14 | 35.7 ± 11.7 | 35.7 ± 11.7 | 35.7 ± 11.7 | 64.3 ± 12.1 |
64.3 ± 12.1 | 64.3 ± 12.8 | ||
| Traumatic with blasts |
36 | 58.3 ± 8.0 | 52.8 ± 8.1 | 52.8 ± 8.1 | 63.9 ± 7.8 | 61.1 ± 7.9 | 61.1 ± 7.9 | ||
| DNA index | |||||||||
| 1.16-1.60 | 66 | 87.9 ± 4.0 | 84.8 ± 4.4 | 84.8 ± 4.5 | 0.007 | 89.4 ± 3.8 | 84.8 ± 4.4 | 84.8 ± 4.5 | 0.03 |
| Other | 292 | 68.5 ± 2.7 | 66.1 ± 2.8 | 64.7 ± 2.8 | 76.7 ± 2.5 | 74.7 ± 2.5 | 73.6 ± 2.6 | ||
| t(9;22) | |||||||||
| Present | 12 | 41.7 ± 13.0 | 41.7 ± 13.0 | 41.7 ± 13.0 | 0.01 | 41.7 ± 13.0 |
41.7 ± 13.0 | 41.7 ± 13.0 | 0.002 |
| Absent | 346 | 73.1 ± 2.4 | 70.5 ± 2.5 | 69.3 ± 2.5 | (0.01)* | 80.3 ± 2.1 | 77.7 ± 2.2 | 76.9 ± 2.3 | (0.002)* |
| t(1;19) | |||||||||
| Present | 13 | 69.2 ± 12.1 | 69.2 ± 12.1 | 69.2 ± 12.1 | 0.95 | 76.9 ± 11.1 |
69.2 ± 12.1 | 69.2 ± 12.1 | 0.59 |
| Absent | 345 | 72.2 ± 2.4 | 69.6 ± 2.5 | 68.4 ± 2.6 | 79.1 ± 2.2 | 76.8 ± 2.3 | 75.9 ± 2.4 | ||
| ETV6-RUNX1 | |||||||||
| Present | 14 | 100 ± 0.0 | 92.9 ± 6.6 | 92.9 ± 6.9 | 0.05 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 0.04 |
| Absent | 344 | 70.9 ± 2.4 | 68.6 ± 2.5 | 67.4 ± 2.6 | 78.2 ± 2.2 | 75.6 ± 2.3 | 74.7 ± 2.4 | ||
| t(4;11) | |||||||||
| Present | 3 | 33.3 ± 19.2 | 33.3 ± 19.2 | 33.3 ± 19.2 | 0.23 | 66.7 ± 22.2 |
33.3 ± 19.2 | 33.3 ± 19.2 | 0.10 |
| Absent | 355 | 72.4 ± 2.4 | 69.9 ± 2.4 | 68.7 ± 2.5 | 79.2 ± 2.2 | 76.9 ± 2.2 | 76.0 ± 2.3 | ||
Significant difference in multivariate analysis
Table 5.
Treatment results according to presenting features in patients treated in Study 13B
| Event-free survival ± SE (%) | Overall survival ± SE (%) | ||||||
|---|---|---|---|---|---|---|---|
| Factors | No. of patients |
Year 5 | Year 10 | *p-value | Year 5 | Year 10 | *p-value |
| B-lineage | |||||||
| NCI Standard | 113 | 87.5 ± 3.1 | 85.7 ± 3.5 | 0.01 | 91.1 ± 2.7 | 89.2 ± 3.1 | 0.04 |
| NCI High | 91 | 75.8 ± 4.5 | 73.6 ± 5.0 | 81.3 ± 4.1 | 79.0 ± 4.6 | ||
| T-lineage | |||||||
| NCI Standard | 6 | 83.3 ± 13.9 | 83.3 ± 17.0 | 0.33 | 100 ± 0.0 | 100 ± 0.0 | 0.18 |
| NCI High | 37 | 67.4 ± 7.7 | 61.6 ± 8.3 | 78.2 ± 6.8 | 75.3 ± 7.3 | ||
| Sex | |||||||
| Male | 144 | 78.4 ± 3.4 | 74.8 ± 3.9 | 0.21 | 85.4 ± 3.0 | 83.9 ± 3.2 | 0.77 |
| Female | 103 | 82.4 ± 3.8 | 81.4 ± 4.1 | 86.3 ± 3.4 | 83.3 ± 4.0 | ||
| Age at diagnosis (yrs.) | |||||||
| <1 | 10 | 70.0 ± 13.6 | 70.0 ± 13.6 | 0.08 | 70.0 ± 13.6 | 70.0 ± 13.6 | 0.004 |
| 1-9 | 161 | 83.8 ± 2.9 | 81.2 ± 3.3 | 91.2 ± 2.2 | 89.3 ± 2.6 | (0.006)* | |
| >10 | 76 | 73.6 ± 5.1 | 70.9 ± 5.6 | 76.2 ± 4.9 | 73.5 ± 5.5 | ||
| Race | |||||||
| White | 172 | 79.1 ± 3.1 | 77.3 ± 3.3 | 0.79 | 84.3 ± 2.8 | 83.1 ± 3.0 | 0.54 |
| Black | 45 | 86.5 ± 5.1 | 81.7 ± 6.5 | 90.9 ± 4.3 | 88.4 ± 5.3 | ||
| Other | 30 | 76.0 ± 7.8 | 72.6 ± 9.0 | 86.3 ± 6.3 | 79.3 ± 8.1 | ||
| WBC×109/L | |||||||
| <10 | 111 | 81.9 ± 3.7 | 80.9 ± 4.0 | 0.004 | 84.5 ± 3.4 | 82.6 ± 3.8 | 0.002 |
| 10-49 | 70 | 88.6 ± 3.8 | 84.3 ± 4.6 | (0.03)* | 94.3 ± 2.8 | 91.4 ± 3.5 | |
| 50-99 | 28 | 78.6 ± 7.8 | 74.8 ± 8.6 | 92.9 ± 4.9 | 89.1 ± 6.1 | ||
| >100 | 38 | 60.4 ± 7.9 | 57.5 ± 8.4 | 68.1 ± 7.5 | 68.1 ± 7.9 | ||
| Cell lineage | |||||||
| B | 204 | 82.3 ± 2.7 | 80.3 ± 3.0 | 0.03 | 86.7 ± 2.4 | 84.7 ± 2.7 | 0.18 |
| T | 43 | 69.6 ± 7.0 | 64.7 ± 7.8 | 81.3 ± 5.9 | 78.8 ± 6.6 | ||
| CD 10 ** | |||||||
| Positive | 198 | 84.8 ± 2.6 | 83.2 ± 2.9 | 0.10 | 90.3 ± 2.1 | 88.8 ± 2.4 | 0.03 |
| Negative | 32 | 75.0 ± 7.5 | 71.7 ± 8.1 | 75.0 ± 7.5 | 75.0 ± 7.8 | ||
| CNS status | |||||||
| CNS 1 | 145 | 80.6 ± 3.3 | 77.1 ± 3.7 | 0.96 | 85.4 ± 2.9 | 82.6 ± 3.4 | 0.82 |
| CNS 2 | 78 | 79.4 ± 4.6 | 78.0 ± 5.0 | 85.8 ± 4.0 | 85.8 ± 4.2 | ||
| CNS 3 | 7 | 71.4 ± 15.6 | 71.4 ± 15.6 | 71.4 ± 15.6 | 71.4 ± 15.6 | ||
| Traumatic with blasts |
17 | 82.4 ± 8.9 | 82.4 ± 9.6 | 94.1 ± 5.5 | 88.2 ± 8.1 | ||
| DNA index | |||||||
| 1.16-1.60 | 46 | 91.3 ± 4.1 | 91.3 ± 4.3 | 0.01 | 93.5 ± 3.6 | 91.3 ± 4.3 | 0.11 |
| Other | 201 | 77.5 ± 3.0 | 74.4 ± 3.3 | 84.0 ± 2.6 | 81.9 ± 2.9 | ||
| t(9;22) | |||||||
| Present | 7 | 28.6 ± 13.9 | 28.6 ± 13.9 | <.0001 | 42.9 ± 16.2 | 42.9 ± 16.2 | 0.0005 |
| Absent | 240 | 81.6 ± 2.5 | 79.0 ± 2.8 | (<0.001)* | 87.0 ± 2.2 | 84.8 ± 2.5 | (<0.001)* |
| t(1;19) | |||||||
| Present | 10 | 80.0 ± 11.9 | 80.0 ± 14.6 | 0.80 | 90.0 ± 9.0 | 90.0 ± 10.8 | 0.56 |
| Absent | 237 | 80.1 ± 2.6 | 77.5 ± 2.9 | 85.6 ± 2.3 | 83.4 ± 2.6 | ||
| ETV6-RUNX1 | |||||||
| Present | 39 | 84.5 ± 5.9 | 81.7 ± 6.6 | 0.64 | 86.8 ± 5.5 | 86.8 ± 5.8 | 0.49 |
| Absent | 208 | 79.3 ± 2.8 | 76.8 ± 3.1 | 85.5 ± 2.4 | 83.1 ± 2.8 | ||
| t(4;11) | |||||||
| Present | 7 | 42.9 ± 16.2 | 42.9 ± 16.2 | 0.002 | 42.9 ± 16.2 | 42.9 ± 16.2 | <.0001 |
| Absent | 240 | 81.2 ± 2.5 | 78.6 ± 2.8 | (0.007)* | 87.0 ± 2.2 | 84.8 ± 2.5 | (0.001)* |
| DAY 19 MRD | |||||||
| <0.01% | 62 | 87.1 ± 4.3 | 85.5 ± 4.8 | 0.003 | 95.2 ± 2.7 | 93.5 ± 3.3 | 0.001 |
| 0.01 to <1% | 37 | 70.3 ± 7.4 | 64.9 ± 8.4 | 81.1 ± 6.3 | 75.7 ± 7.5 | ||
| ≥1% | 28 | 60.7 ± 9.0 | 57.1 ± 9.7 | 67.9 ± 8.6 | 64.3 ± 9.3 | ||
| DAY 46 MRD | |||||||
| <0.01% | 102 | 86.3 ± 3.4 | 85.3 ± 3.8 | <.0001 | 94.1 ± 2.3 | 92.1 ± 2.9 | <.0001 |
| 0.01 to <1% | 29 | 75.7 ± 8.0 | 64.7 ± 9.3 | 82.6 ± 7.0 | 78.9 ± 7.9 | ||
| ≥1% | 7 | 14.3 ± 9.4 | 14.3 ± 9.4 | 28.6 ± 13.9 | 14.3 ± 9.4 | ||
Significant difference in multivariate analysis.
Data available in only a subset of patients.
Independent risk factors for isolated CNS relapse in Study 11 were high leukocyte counts, the presence of Philadelphia chromosome and the presence of blasts in cerebrospinal fluid (non-CNS1 status); in Study 12, non-CNS1 status was the only factor. There were too few events in Studies 13A and 13B for analysis.
Discussion
Our single-institution experience reported here shows that risk-directed therapy cured approximately 75% of children with ALL in the 1980s and up to 80% in the 1990s. The rarity of late relapses observed in this analysis supports our previous working definition of cure of childhood ALL, i.e., 10 or more years of event-free survival.27 With advances in therapy, testicular and other extramedullary relapse outside of CNS has become very rare. However, CNS control remains a therapeutic challenge. The cumulative risk of any CNS relapse was 7.6% in Study 11 and 14.8% in Study 12. The high rate of CNS relapse in Study 12 can be attributed not only to inadequate intrathecal therapy during remission induction and early postremission therapy, but also to the relatively low intensity of systemic therapy (e.g., lack of consolidation and reinduction therapy as well as a lack of glucocorticoid pulses). Study 12 yielded a reasonably good long-term survival rate owing, at least in part, to the high retrieval rate of CNS relapse. However, patients who received salvage therapy including cranial or craniospinal irradiation for CNS relapse would have been more prone to late treatment-related sequelae than those remaining in first remission.
Starting from Study 13A, early intensive intrathecal therapy has been administered to patients at high risk of CNS relapse, including those with any amount of blasts in cerebrospinal fluid at diagnosis (even from traumatic lumbar puncture),29-31 and reinduction treatment has become an integral component of therapy. With these treatment modifications, and in spite of reductions in cranial irradiation, 10-year rates of isolated and any CNS relapse have decreased to 1.2% and 4.9% in Study 13A, and to 1.7% and 3.3% in Study 13B. In turn, this has boosted 10-year event-free survival to 71.5% and 77.6%, respectively. Because of the improvements in CNS control and the high salvage rate of CNS relapse, together with the risk of devastating radiation-associated late effects (second cancers in particular)27,32 we decided to omit the use of prophylactic CNS irradiation in all patients. Instead, we have relied on effective intrathecal and systemic therapy (such as high-dose methotrexate, intensive asparaginase, dexamethasone) for CNS control since 1998, beginning with Study 14. More recently, special precautions have also been taken to decrease the rate of traumatic lumbar puncture.33 The results of Studies 14 and 1517 showed that with effective risk-adjusted chemotherapy, prophylactic cranial irradiation can be safely omitted in all patients because CNS relapse rate remained low and those who developed isolated CNS relapse have a very high salvage rate. For patients who are at risk of CNS relapse with contemporary therapy including those with any CNS involvement, T-cell immunophenotype and t(1;19)[TCF3-PBX1],17,34 we further intensify early intrathecal treatment in our current clinical trial (Study 16).
The development of secondary neoplasm was one of the major causes of treatment failure in our clinical trials with a 10-year cumulative risk ranging from 3.2% to 9.9%, albeit none occurred in Study 14 thus far. Because the latency period of radiation-induced second neoplasm is generally over 10 years,27,32 we expect that this could occur in additional patients, especially among those enrolled in Studies 11 and 12, where two thirds and one third of the patients received CNS irradiation, respectively. We identified several risk factors that potentiate the development of second malignancy. Weekly administration of etoposide or teniposide for an extended period increased the risk of secondary AML.5 Concomitant intensive antimetabolite and asparaginase treatment, as well as the use of granulocyte colony-stimulating factor therapy, can potentiate the risk of epipodophylloxin-induced secondary AML.35-37 Intensive use of antimetabolites prior to and during cranial radiation can increase the risk of radiation-related brain tumor, especially among patients with thiopurine methyltransferase deficiency.21 In this regard, patients with a deficiency in this enzyme have a higher risk of developing second cancer than those with wild type activity.38 In our recently Study 15 (2000-2007), we prospectively analyzed genetic polymorphism and activity of this enzyme, decreasing the starting dose of mercaptopurine to 60 mg/m2 in patients with heterozygous deficiency; limited the use of epipodophyllotoxins only in those who will undergo allogeneic transplantation for very high-risk ALL; and totally omitted the use of prophylactic cranial irradiation.17 With a median follow-up time of 4.0 years (range, 1.2 to 8.4), the 5-year survival rate for the 498 evaluable patients was 93.5% (95% confidence interval, 89.8 to 97.2), and thus far only one patient developed a myelodysplastic syndrome.
Given the overriding prognostic impact of treatment,39,40 it is not surprising that there are only a few presenting features consistently predictive of outcome: age, leukocyte count, T-lineage and DNA index for event-free survival, and only age for overall survival. In contrast to the results in collaborative study groups, race has no prognostic significance in our studies, a result that we attribute to the access to effective treatment offered at our institution irrespective of the patients’ insurance status.41 Minimal residual disease measurement is one of the most important predictors of treatment outcome because it accounts for leukemic genetic abnormality, host pharmacogenetics and pharmacodynamics, and treatment compliance.17,42-49 We have been using this measure for risk-directed therapy not only to improve cure rates but also in efforts to avert excessive toxicities. The recent application of genome-wide microarray and high-throughput sequencing methodologies has identified new high-risk subgroups, such as early T-cell precursor leukemia50 and B-cell precursor ALL with genetic alteration of IKZF1,51-53 a gene that encodes the lymphoid transcription factor IKAROS. These patients typically also have high levels of minimal residual disease at the end of remission induction. For these high-risk patients, innovative treatment strategies are needed to improve their outcome.54,55 Finally, while the acquired genetic abnormalities of leukemic cells play a critical role in drug responsiveness, it is also important to study inherited genetic variations which are associated not only with host drug disposition but also with leukemic cell biology.56
Table 3.
Treatment results according to presenting features in patients treated in Study 12
| Event-free survival ± SE (%) | Overall survival ± SE (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | No. of patients |
Year 5 | Year 10 | Year 15 | p-value | Year 5 | Year 10 | Year 15 | p-value |
| B-lineage | |||||||||
| NCI Standard | 99 | 78.8 ± 4.1 | 71.7 ± 4.5 | 70.7 ± 4.9 | 0.002 | 89.9 ± 3.0 | 84.8 ± 3.6 | 82.7 ± 4.1 | 0.06 |
| NCI High | 60 | 56.7 ± 6.3 | 51.7 ± 6.5 | 48.2 ± 7.1 | 75.0 ± 5.5 | 71.6 ± 5.8 | 71.6 ± 6.4 | ||
| T-lineage | |||||||||
| NCI Standard | 5 | 60.0 ± 19.0 | 60.0 ± 19.0 | 60.0 ± 19.0 | 0.51 | 80.0 ± 16.0 | 80.0 ± 16.0 | 80.0 ± 17.9 | 0.68 |
| NCI High | 24 | 50.0 ± 9.8 | 41.7 ± 9.6 | 41.7 ± 10.6 | 79.2 ± 8.1 | 70.8 ± 9.0 | 70.8 ± 9.9 | ||
| Sex | |||||||||
| Male | 102 | 62.7 ± 4.8 | 55.9 ± 4.9 | 54.9 ± 5.4 | 0.13 | 81.4 ± 3.8 | 77.5 ± 4.1 | 76.3 ± 4.6 | 0.61 |
| Female | 86 | 73.3 ± 4.7 | 67.4 ± 5.0 | 65.0 ± 5.5 | 86.0 ± 3.7 | 80.2 ± 4.3 | 79.0 ± 4.7 | ||
|
Age at diagnosis
(yrs.) |
|||||||||
| <1 | 8 | 25.0 ± 12.5 | 25.0 ± 12.5 | 25.0 ± 12.5 | 0.001 | 50.0 ± 15.8 | 50.0 ± 15.8 | 50.0 ± 17.7 | 0.003 |
| 1-9 | 128 | 75.0 ± 3.8 | 68.8 ± 4.1 | 66.4 ± 4.5 | 89.8 ± 2.7 | 85.2 ± 3.1 | 83.5 ± 3.5 | (0.05)* | |
| >10 | 52 | 55.8 ± 6.8 | 48.0 ± 6.9 | 48.0 ± 7.6 | 73.1 ± 6.1 | 67.2 ± 6.5 | 67.2 ± 7.1 | ||
| Race | |||||||||
| White | 170 | 68.8 ± 3.5 | 61.8 ± 3.7 | 59.9 ± 4.0 | 0.52 | 82.4 ± 2.9 | 78.2 ± 3.2 | 77.0 ± 3.5 | 0.77 |
| Black | 17 | 52.9 ± 11.5 | 52.9 ± 11.5 | 52.9 ± 13.7 | 94.1 ± 5.5 | 82.4 ± 8.9 | 82.4 ± 10.4 | ||
| Other | 1 | 100 ± 0.0 | 100 ± 0.0 | No Data | 100 ± 0.0 | 100 ± 0.0 | No Data | ||
| WBC×109/L | |||||||||
| <10 | 88 | 75.0 ± 4.6 | 71.6 ± 4.8 | 71.6 ± 5.1 | 0.0002 | 87.5 ± 3.5 | 86.4 ± 3.7 | 85.1 ± 4.0 | 0.09 |
| 10-49 | 58 | 67.2 ± 6.1 | 56.9 ± 6.4 | 55.2 ± 7.1 | (0.002)* | 84.5 ± 4.7 | 74.1 ± 5.7 | 72.4 ± 6.3 | (0.03)* |
| 50-99 | 16 | 68.8 ± 11.1 | 62.5 ± 11.5 | 56.2 ± 13.2 | 81.2 ± 9.4 | 75.0 ± 10.4 | 75.0 ± 11.3 | ||
| >100 | 26 | 42.3 ± 9.3 | 34.6 ± 8.9 | 30.8 ± 9.7 | 69.2 ± 8.8 | 65.4 ± 9.1 | 65.4 ± 10.3 | ||
| Cell lineage | |||||||||
| B | 159 | 70.4 ± 3.6 | 64.2 ± 3.8 | 62.2 ± 4.2 | 0.02 | 84.3 ± 2.9 | 79.9 ± 3.2 | 78.5 ± 3.5 | 0.39 |
| T | 29 | 51.7 ± 9.0 | 44.8 ± 8.9 | 44.8 ± 9.6 | 79.3 ± 7.4 | 72.4 ± 8.1 | 72.4 ± 9.0 | ||
| CNS status | |||||||||
| CNS 1 | 125 | 73.6 ± 3.9 | 67.2 ± 4.2 | 64.7 ± 4.6 | 0.03 | 87.2 ± 3.0 | 83.2 ± 3.3 | 81.5 ± 3.7 | 0.22 |
| CNS 2 | 34 | 52.9 ± 8.3 | 41.2 ± 8.2 | 41.2 ± 9.5 | 79.4 ± 6.8 | 67.6 ± 7.9 | 67.6 ± 9.3 | ||
| CNS 3 | 10 | 50.0 ± 14.4 | 50.0 ± 14.4 | 50.0 ± 15.8 | 80.0 ± 11.9 | 80.0 ± 11.9 | 80.0 ± 13.5 | ||
| Traumatic with blasts |
19 | 63.2 ± 10.6 | 63.2 ± 10.6 | 63.2 ± 11.1 | 68.4 ± 10.3 | 68.4 ± 10.3 | 68.4 ± 10.7 | ||
| DNA index | |||||||||
| 1.16-1.60 | 35 | 80.0 ± 6.6 | 77.1 ± 7.0 | 73.9 ± 8.7 | 0.05 | 91.4 ± 4.7 | 88.6 ± 5.3 | 85.3 ± 6.8 | 0.20 |
| Other | 153 | 64.7 ± 3.8 | 57.5 ± 4.0 | 56.2 ± 4.3 | 81.7 ± 3.1 | 76.5 ± 3.4 | 75.7 ± 3.7 | ||
| t(9;22) | |||||||||
| Present | 6 | 66.7 ± 17.2 | 66.7 ± 19.2 | 66.7 ± 27.2 | 0.81 | 83.3 ± 13.9 | 66.7 ± 19.2 | 66.7 ± 27.2 | 0.45 |
| Absent | 182 | 67.6 ± 3.5 | 61.0 ± 3.6 | 59.3 ± 3.9 | 83.5 ± 2.7 | 79.1 ± 3.0 | 77.9 ± 3.3 | ||
| t(1;19) | |||||||||
| Present | 7 | 71.4 ± 15.6 | 71.4 ± 15.6 | 57.1 ± 18.7 | 0.90 | 71.4 ± 15.6 | 71.4 ± 15.6 | 71.4 ± 17.1 | 0.63 |
| Absent | 181 | 67.4 ± 3.5 | 60.8 ± 3.6 | 59.6 ± 4.0 | 84.0 ± 2.7 | 79.0 ± 3.0 | 77.8 ± 3.3 | ||
| ETV6-RUNX1 | |||||||||
| Present | 35 | 88.6 ± 5.3 | 77.1 ± 7.0 | 77.1 ± 7.2 | 0.01 | 91.4 ± 4.7 | 85.7 ± 5.8 | 85.7 ± 6.0 | 0.19 |
| Absent | 153 | 62.7 ± 3.9 | 57.5 ± 4.0 | 55.5 ± 4.5 | (0.01)* | 81.7 ± 3.1 | 77.1 ± 3.4 | 75.7 ± 3.8 | |
| t(4;11) | |||||||||
| Present | 3 | 33.3 ± 19.2 | 33.3 ± 19.2 | 33.3 ± 19.2 | 0.31 | 66.7 ± 22.2 | 66.7 ± 22.2 | 66.7 ± 27.2 | 0.67 |
| Absent | 185 | 68.1 ± 3.4 | 61.6 ± 3.6 | 59.9 ± 3.9 | 83.8 ± 2.7 | 78.9 ± 3.0 | 77.7 ± 3.3 | ||
Significant difference in multivariate analysis
Table 4.
Treatment results according to presenting features in patients treated in Study 13A
| Event-free survival ± SE (%) | Overall survival ± SE (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | No. of patients |
Year 5 | Year 10 | Year 15 | *p-value | Year 5 | Year 10 | Year 15 |
*p- value |
| B-lineage | |||||||||
| NCI Standard | 84 | 88.1 ± 3.5 | 83.3 ± 4.1 | 82.1 ± 6.6 | 0.003 | 89.3 ± 3.4 | 86.9 ± 3.7 | 86.9 ± 5.7 | 0.02 |
| NCI High | 58 | 69.0 ± 6.0 | 62.0 ± 6.4 | 60.1 ± 10.5 | 81.0 ± 5.1 | 72.4 ± 5.9 | 70.3 ± 9.3 | ||
| T-lineage | |||||||||
| NCI Standard | 2 | 50.0 ± 25.0 | 50.0 ± 25.0 | 50.0 ± 35.4 | 0.91 | 100 ± 0.0 | 50.0 ± 25.0 | 50.0 ± 35.4 | 0.97 |
| NCI High | 21 | 61.9 ± 10.2 | 52.4 ± 10.4 | 52.4 ± 16.2 | 61.9 ± 10.2 | 61.9 ± 10.2 | 57.1 ± 16.7 | ||
| Sex | |||||||||
| Male | 92 | 71.7 ± 4.7 | 68.5 ± 4.8 | 66.2 ± 7.7 | 0.20 | 79.3 ± 4.2 | 75.0 ± 4.5 | 73.7 ± 6.9 | 0.29 |
| Female | 73 | 84.9 ± 4.2 | 75.2 ± 5.0 | 75.2 ± 8.4 | 87.7 ± 3.8 | 82.1 ± 4.5 | 80.8 ± 7.6 | ||
|
Age at diagnosis
(yrs.) |
|||||||||
| <1 | 5 | 20.0 ± 12.6 | 20.0 ± 12.6 | 20.0 ± 17.9 | <.0001 | 40.0 ± 17.9 | 40.0 ± 17.9 | 40.0 ± 21.9 | <.0001 |
| 1-9 | 117 | 87.2 ± 3.1 | 82.0 ± 3.6 | 81.1 ± 6.0 | (<0.001) * |
89.7 ± 2.8 | 86.3 ± 3.2 | 85.4 ± 5.3 | (0.002) * |
| >10 | 43 | 58.1 ± 7.4 | 48.8 ± 7.4 | 46.4 ± 10.7 | 69.8 ± 6.9 | 60.5 ± 7.3 | 57.7 ± 10.4 | ||
| Race | |||||||||
| White | 139 | 79.9 ± 3.4 | 73.3 ± 3.7 | 71.8 ± 6.1 | 0.18 | 85.6 ± 3.0 | 79.8 ± 3.4 | 78.3 ± 5.4 | 0.07 |
| Black | 23 | 69.6 ± 9.3 | 65.2 ± 9.9 | 65.2 ± 15.7 | 73.9 ± 8.9 | 73.9 ± 9.2 | 73.9 ± 14.3 | ||
| Other | 3 | 33.3 ± 19.2 | 33.3 ± 19.2 | 33.3 ± 27.2 | 33.3 ± 19.2 | 33.3 ± 19.2 | 33.3 ± 27.2 | ||
| WBC×109/L | |||||||||
| <10 | 71 | 80.3 ± 4.7 | 74.6 ± 5.2 | 71.7 ± 8.1 | 0.06 | 83.1 ± 4.4 | 80.3 ± 4.7 | 78.7 ± 7.3 | 0.15 |
| 10-49 | 50 | 78.0 ± 5.8 | 72.0 ± 6.3 | 72.0 ± 10.2 | 82.0 ± 5.4 | 76.0 ± 6.0 | 76.0 ± 9.3 | ||
| 50-99 | 20 | 90.0 ± 6.5 | 85.0 ± 8.0 | 85.0 ± 14.7 | 95.0 ± 4.8 | 90.0 ± 6.7 | 90.0 ± 11.6 | ||
| >100 | 24 | 58.3 ± 9.7 | 50.0 ± 9.8 | 50.0 ± 14.4 | 75.0 ± 8.6 | 66.7 ± 9.3 | 62.5 ± 14.5 | ||
| Cell lineage | |||||||||
| B | 142 | 80.3 ± 3.3 | 74.6 ± 3.7 | 73.1 ± 6.0 | 0.02 | 85.9 ± 2.9 | 81.0 ± 3.3 | 80.2 ± 5.2 | 0.004 |
| T | 23 | 60.9 ± 9.8 | 52.2 ± 10.0 | 52.2 ± 16.1 | 65.2 ± 9.6 | 60.9 ± 9.8 | 56.5 ± 16.7 | (0.02)* | |
| CD 10 ** | |||||||||
| Positive | 127 | 89.0 ± 2.8 | 82.6 ± 3.4 | 81.8 ± 5.6 | <.0001 | 92.1 ± 2.4 | 89.0 ± 2.8 | 87.3 ± 4.7 | <.0001 |
| Negative | 22 | 40.9 ± 9.9 | 36.4 ± 9.7 | 36.4 ± 13.0 | 54.5 ± 10.2 | 45.5 ± 10.1 | 45.5 ± 13.7 | ||
| CNS status | |||||||||
| CNS 1 | 101 | 83.2 ± 3.7 | 75.2 ± 4.3 | 73.1 ± 7.2 | 0.34 | 85.1 ± 3.5 | 79.2 ± 4.0 | 77.0 ± 6.6 | 0.89 |
| CNS 2 | 42 | 71.4 ± 6.9 | 69.0 ± 7.0 | 69.0 ± 10.7 | 83.3 ± 5.7 | 78.6 ± 6.2 | 78.6 ± 9.4 | ||
| CNS 3 | 6 | 50.0 ± 17.7 | 50.0 ± 17.7 | 50.0 ± 20.4 | 66.7 ± 17.2 | 66.7 ± 17.2 | 66.7 ± 19.2 | ||
| Traumatic with blasts |
16 | 68.8 ± 11.1 | 61.9 ± 12.1 | 61.9 ± 22.1 | 75.0 ± 10.4 | 75.0 ± 10.8 | 75.0 ± 18.8 | ||
| DNA index | |||||||||
| 1.16-1.60 | 32 | 93.8 ± 4.2 | 93.8 ± 4.3 | 93.8 ± 7.4 | 0.003 | 96.9 ± 3.0 | 96.9 ± 3.1 | 96.9 ± 5.4 | 0.005 |
| Other | 133 | 73.7 ± 3.8 | 66.1 ± 4.1 | 64.6 ± 6.5 | (0.009)* | 79.7 ± 3.5 | 73.7 ± 3.8 | 72.1 ± 5.9 | (0.03)* |
| t(9;22) | |||||||||
| Present | 6 | 33.3 ± 15.7 | 16.7 ± 10.8 | No Data | 0.0002 | 50.0 ± 17.7 | 50.0 ± 17.7 | 50.0 ± 20.4 | 0.05 |
| Absent | 159 | 79.2 ± 3.2 | 73.5 ± 3.5 | 72.2 ± 5.7 | (0.008)* | 84.3 ± 2.9 | 79.2 ± 3.2 | 77.9 ± 5.2 | |
| t(1;19) | |||||||||
| Present | 8 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 0.09 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 0.14 |
| Absent | 157 | 76.4 ± 3.4 | 70.0 ± 3.7 | 68.7 ± 5.9 | 82.2 ± 3.0 | 77.1 ± 3.4 | 75.7 ± 5.3 | ||
| ETV6-RUNX1 | |||||||||
| Present | 42 | 88.1 ± 4.9 | 83.3 ± 5.7 | 81.0 ± 10.2 | 0.06 | 90.5 ± 4.5 | 85.7 ± 5.3 | 85.7 ± 9.0 | 0.11 |
| Absent | 123 | 74.0 ± 3.9 | 67.4 ± 4.2 | 66.5 ± 6.7 | 80.5 ± 3.6 | 75.6 ± 3.9 | 73.8 ± 6.0 | ||
| t(4;11) | |||||||||
| Present | 4 | 25.0 ± 15.3 | 25.0 ± 15.3 | 25.0 ± 15.3 | 0.006 | 75.0 ± 18.8 | 50.0 ± 20.4 | 50.0 ± 20.4 | 0.18 |
| Absent | 161 | 78.9 ± 3.2 | 72.6 ± 3.5 | 71.3 ± 5.8 | 83.2 ± 2.9 | 78.9 ± 3.2 | 77.5 ± 5.3 | ||
Significant difference in multivariate analysis.
Data available in only a subset of patients.
Acknowledgments
This work was supported by grants (CA21765, CA51001, CA60419, CA78224, CA36401, and GM61393) from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Rivera G, Pinkel D, Simone JV, Hancock ML, Crist WM. Treatment of acute lymphoblastic leukemia. 30 years’ experience at St. Jude Children’s Research Hospital. N Engl N Med. 1993;329:1289–1295. doi: 10.1056/NEJM199310283291801. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Simone JV, Hancock ML, Evans WE, Williams DL, Bowman WP, Dahl GV, Dodge RK, Ochs J, Abromowitch M, Rivera GK. Impact of three methods of treatment intensification on acute lymphoblastic leukemia in children: long-term results of St. Jude Total Therapy Study X. Leukemia. 1992;6:150–157. [PubMed] [Google Scholar]
- 3.Rivera GK, Raimondi SC, Hancock ML, Behm FG, Pui CH, Abromowitch M, Mirro J, Jr, Ochs JS, Look AT, Williams DL, Murphy SB, Dahl GV, Kalwinsky DK, Evans WE, Kun LE, Simone JV, Crist WM. Improved outcome in childhood acute lymphoblastic leukaemia with reinforced early treatment and rotational combination chemotherapy. Lancet. 1991;337:61–66. doi: 10.1016/0140-6736(91)90733-6. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Behm FG, Raimondi SC, Dodge RK, George SL, Rivera GK, Mirro J, Jr, Kalwinsky DK, Dahl GV, Murphy SB. Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. N Engl J Med. 1989;321:136–142. doi: 10.1056/NEJM198907203210302. [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Ribeiro RC, Hancock ML, Rivera GK, Evans WE, Raimondi SC, Head DR, Behm FG, Mahmoud MH, Sandlund JT, Crist WM. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med. 1991;325:1682–1687. doi: 10.1056/NEJM199112123252402. [DOI] [PubMed] [Google Scholar]
- 6.Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998;338:499–505. doi: 10.1056/NEJM199802193380803. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Mahmoud HH, Rivera GK, Hancock ML, Sandlund JT, Behm FG, Head DR, Relling MV, Ribeiro RC, Rubnitz JE, Kun LE, Evans WE. Early intensification of intrathecal chemotherapy virtually eliminates central nervous system relapse in children with acute lymphoblastic leukemia. Blood. 1998;92:411–415. [PubMed] [Google Scholar]
- 8.Barredo JC, Synold TW, Laver J, Relling MV, Pui CH, Priest DG, Evans W. Differences in constitutive and post-methotrexate folypolyglutamate synthetase activity in B-lineage and T-lineage leukemia. Blood. 1994;84:564–569. [PubMed] [Google Scholar]
- 9.Synold TW, Relling MV, Boyett JM, Rivera GK, Sandlund JT, Mahmoud H, Crist WM, Pui CH, Evans WE. Blast cell methotrexate-polyglutamate accumulation in vivo differs by lineage, ploidy and methotrexate dose in acute lymphoblastic leukemia. J Clin Invest. 1994;94:1996–2001. doi: 10.1172/JCI117552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masson E, Relling MV, Synold TW, Liu Q, Schuetz JD, Sandlund JT, Pui CH, Evans WE. Accumulation of methotrexate polyglutamates in lymphoblasts is a determinant of antileukemic effects in vivo: a rationale for high-dose methotrexate. J Clin Invest. 1996;97:73–80. doi: 10.1172/JCI118409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galpin AJ, Schuetz JD, Masson E, Yanishevski Y, Synold TW, Barredo JC, Pui CH, Relling MV, Evans WE. Differences in folypolyglutamate synthetase and dihydrofolate reductase expression in human B-lineage versus T-lineage leukemic lymphoblasts: mechanisms for lineage differences in methotrexate polyglutamylation and cytotoxicity. Mol Pharmacol. 1997;52:155–163. doi: 10.1124/mol.52.1.155. [DOI] [PubMed] [Google Scholar]
- 12.Belkov VM, Krynetski EY, Schuetz JD, Yanishevski Y, Masson E, Mathew S, Raimondi S, Pui CH, Relling MV, Evans WE. Reduced folate carrier expression in acute lymphoblastic leukemia: a mechanism for ploidy but not lineage differences in methotrexate accumulation. Blood. 1999;93:1643–1650. [PubMed] [Google Scholar]
- 13.Pui CH, Boyett JM, Rivera GK, Hancock ML, Sandlund JT, Ribeiro RC, Rubnitz JE, Behm FG, Raimondi SC, Gajjar A, Razzouk B, Campana D, Kun LE, Relling MV, Evans WE. Long-term results of total therapy studies 11, 12, and 13A for childhood acute lymphoblastic leukemia at St. Jude Children’s Research Hospital. Leukemia. 2000;14:2286–2294. doi: 10.1038/sj.leu.2401938. [DOI] [PubMed] [Google Scholar]
- 14.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, Rubnitz JE, Razzouk BI, Howard SC, Hudson MM, Cheng C, Kun LE, Raimondi SC, Behm FG, Downing JR, Relling MV, Evans WE. Improved outcome for children with acute lymphoblastic leukemia: results of total therapy study XIIIB at St. Jude Children’s Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 15.Kishi S, Griener J, Cheng C, Das S, Cook EH, Pei D, Hudson MM, Rubnitz JE, Sandlund JT, Pui CH, Relling MV. Homocysteine, pharmacogenetics, and neurotoxicity in children with leukemia. J Clin Oncol. 2003;21:3084–3091. doi: 10.1200/JCO.2003.07.056. [DOI] [PubMed] [Google Scholar]
- 16.Pui CH, Relling MV, Sandlund JT, Downing JR, Campana D, Evans WE. Total Therapy study XV for newly diagnosed childhood acute lymphoblastic leukemia: study design and preliminary results. Ann Hematol. 2006;85(suppl 1):88–91. [Google Scholar]
- 17.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W, Handgretinger R, Downing JR, Evans WE, Relling MV. Treating childhood acute lymphoblastic leukemia without prophylactic cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishi S, Cheng C, French D, Pei D, Das S, Cook EH, Hijiya N, Rizzari C, Rosner GL, Frudakis T, Pui CH, Evans WE, Relling MV. Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood. 2007;109:4151–4157. doi: 10.1182/blood-2006-10-054528. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocha JC, Cheng C, Liu W, Kishi S, Das S, Cook EH, Sandlund JT, Rubnitz JE, Ribeiro R, Campana D, Pui CH, Evans WE, Relling MV. Pharmacogenetics of outcome in children with acute lymphoblastic leukemia. Blood. 2005;105:4752–4758. doi: 10.1182/blood-2004-11-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Relling MV, Pui CH, Cheng C, Evans WE. Thiopurine methyltransferase in acute lymphoblastic leukemia. Blood. 2006;107:843–844. doi: 10.1182/blood-2005-08-3379. [DOI] [PubMed] [Google Scholar]
- 21.Relling MV, Rubnitz JE, Rivera GK, Boyett JM, Hancock ML, Felix CA, Kun LE, Walter AW, Evans WE, Pui CH. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999;354:34–39. doi: 10.1016/S0140-6736(98)11079-6. [DOI] [PubMed] [Google Scholar]
- 22.Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, Pui CH, Evans WE. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91:2001–2008. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- 23.Relling MV, Yanishevski Y, Nemec J, Evans WE, Boyett JM, Behm FG, Pui CH. Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia. Leukemia. 1998;12:346–352. doi: 10.1038/sj.leu.2400928. [DOI] [PubMed] [Google Scholar]
- 24.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1996;50:163–170. [PubMed] [Google Scholar]
- 25.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Wiley; New York, NY: 2002. [Google Scholar]
- 26.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1998;16:1141–1154. [Google Scholar]
- 27.Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, Ribeiro RC, Relling MV, Kun LE, Evans WE, Hudson MM. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Eng J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 28.Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, Murphy S, Pui CH, Pullen J, Reaman G, Sallan SE, Sather H, Shuster J, Simon R, Trigg M, Tubergen D, Uckun F, Ungerleider R. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 29.Mahmoud HH, Rivera GK, Hancock ML, Krance RA, Kun LE, Behm FG, Ribeiro RC, Sandlund JT, Crist WM, Pui CH. Low leukocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia. N Engl J Med. 1993;329:314–319. doi: 10.1056/NEJM199307293290504. [DOI] [PubMed] [Google Scholar]
- 30.Gajjar A, Harrison PL, Sandlund JT, Rivera GK, Ribeiro RC, Rubnitz JE, Razzouk B, Relling MV, Evans WE, Boyett JM, Pui CH. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood. 2000;96:3381–3384. [PubMed] [Google Scholar]
- 31.Bürger B, Zimmermann M, Mann G, Kühl J, Löning L, Riehm H, Reiter A, Schrappe M. Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol. 2003;21:184–188. doi: 10.1200/JCO.2003.04.096. [DOI] [PubMed] [Google Scholar]
- 32.Hijiya N, Hudson M, Lensing S, Zacher M, Onciu M, Behm FG, Razzouk BI, Ribeiro RC, Rubnitz JE, Sandlund JT, Rivera GK, Evans WE, Relling MV, Pui CH. Cumulative incidence of secondary neoplasms as a first event after treatment of childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 33.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 34.Jeha S, Pei D, Raimondi SC, Onciu M, Campana D, Cheng C, Sandlund JT, Ribeiro RC, Rubnitz JE, Howard SC, Downing JR, Evans WE, Relling MV, Pui CH. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. doi: 10.1038/leu.2009.42. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pui CH, Relling MV, Rivera GK, Hancock ML, Raimondi SC, Heslop HE, Santana VM, Ribeiro RC, Sandlund JT, Mahmoud HH, Evans WE, Crist WM, Krance RA. Epipodophyllotoxin-related acute myeloid leukemia - a study of 35 cases. Leukemia. 1995;9:1990–1996. [PubMed] [Google Scholar]
- 36.Pui CH, Relling MV, Behm FG, Hancock ML, Boyett JM, Raimondi SC, Krance RA, Mahmoud HH, Ribeiro RC, Sandlund JT, Head DR, Evans WE, Crist WM, Rivera GK. L-asparaginase may potentiate the leukemogenic effect of the epipodophyllotoxins. Leukemia. 1995;9:1680–1684. [PubMed] [Google Scholar]
- 37.Relling MV, Boyett JM, Blanco JG, Raimondi S, Behm FG, Sandlund JT, Rivera GK, Kun LE, Evans WE, Pui CH. Granulocyte colony stimulating factor and the risk of secondary myeloid malignancy after etoposide treatment. Blood. 2003;101:3862–3867. doi: 10.1182/blood-2002-08-2405. [DOI] [PubMed] [Google Scholar]
- 38.Schmiegelow K, Forestier E, Kristinsson J, Söderhäll S, Vettenranta K, Weinshilboum R, Wesenberg F. Thiopurine methyltransferase activity is related to the risk of relapse of childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Leukemia. 2009;23:557–564. doi: 10.1038/leu.2008.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pui CH, Evans WE. Treatment for acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 40.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 41.Pui CH, Sandlund JT, Pei D, Rivera GK, Howard SC, Ribeiro RC, Rubnitz JE, Razzouk BI, Hudson MM, Cheng C, Raimondi SC, Behm FG, Downing JR, Relling MV, Evans WE. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA. 2003;290:2001–2007. doi: 10.1001/jama.290.15.2001. [DOI] [PubMed] [Google Scholar]
- 42.Coustan-Smith E, Behm FG, Sanchez J, Boyett JM, Hancock ML, Raimondi SC, Rubnitz JE, Rivera GK, Sandlund JT, Pui CH, Campana D. Immunological detection of minimal residual disease in children with acute lymphoblastic leukemia. Lancet. 1998;351:550–554. doi: 10.1016/S0140-6736(97)10295-1. [DOI] [PubMed] [Google Scholar]
- 43.Coustan-Smith E, Sancho J, Hancock ML, Boyett JM, Behm FG, Raimondi SC, Sandlund JT, Rivera GK, Rubnitz JE, Ribeiro RC, Pui CH, Campana D. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96:2691–2696. [PubMed] [Google Scholar]
- 44.Coustan-Smith E, Sancho J, Behm FG, Hancock ML, Razzouk BI, Ribeiro RC, Rivera GK, Rubnitz JE, Sandlund JT, Pui CH, Campana D. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002;100:52–58. doi: 10.1182/blood-2002-01-0006. [DOI] [PubMed] [Google Scholar]
- 45.Coustan-Smith E, Ribeiro RC, Stow P, Zhou Y, Pui CH, Rivera GK, Pedrosa F, Campana D. A simplified flow cytometric assay identifies children with acute lymphoblastic leukemia who have a superior clinical outcome. Blood. 2006;108:97–102. doi: 10.1182/blood-2006-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fronkova E, Mejstrikova E, Avigad S, Chik KW, Castillo L, Manor S, Reznickova L, Valova T, Zdrahalova K, Hrusak O, Jabali Y, Schrappe M, Conter V, Izraeli S, Li CK, Stark B, Stary J, Trka J. Minimal residual disease (MRD) analysis in the non-MRD-based ALL IC-BFM 2002 protocol for childhood ALL: is it possible to avoid MRD testing? Leukemia. 2008;22:989–997. doi: 10.1038/leu.2008.22. [DOI] [PubMed] [Google Scholar]
- 47.Flohr T, Schrauder A, Cazzaniga G, Panzer-Grümayer R, van der Velden V, Fischer S, Stanulla M, Basso G, Niggli FK, Schäfer BW, Sutton R, Koehler R, Zimmermann M, Valsecchi MG, Gadner H, Masera G, Schrappe M, van Dongen JJ, Biondi A, Bartram CR, International BFM Study Group (I-BFM-SG) Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22:771–782. doi: 10.1038/leu.2008.5. [DOI] [PubMed] [Google Scholar]
- 48.Ratei R, Basso G, Dworzak M, Gaipa G, Veltroni M, Rhein P, Biondi A, Schrappe M, Ludwig WD, Karawajew L. Monitoring treatment response of childhood precursor B-cell acute lymphoblastic leukemia in the AIEOP-BFM-ALL 2000 protocol with multiparameter flow cytometry: predictive impact of early blast reduction on the remission status after induction. Leukemia. 2009;23:528–534. doi: 10.1038/leu.2008.324. [DOI] [PubMed] [Google Scholar]
- 49.Van der Velden VHJ, Corral L, Valsecchi MGMG, Jansen MWJC, De Lorenzo P, Cazzaniga G, Panzer-Grümayer ER, Schrappe M, Schrauder A, Meyer C, Marschalek R, Nigro LL, Metzler M, Basso G, Mann G, Den Boer ML, Biondi A, Pieters R, Van Dongen JJM. Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia. 2009;23:1073–1079. doi: 10.1038/leu.2009.17. [DOI] [PubMed] [Google Scholar]
- 50.Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, Cheng C, Su X, Rubnitz JE, Basso G, Biondi A, Pui CH, Downing JR, Campana D. Early T-cell precursor leukaemia; a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mullighan CG, Su X, Zhang J, Radtke I, Phillips LAA, Miller CB, Ma J, Liu W, Cheng C, Schulman BA, Harvey RC, Chen IM, Clifford RJ, Carroll WL, Reaman G, Bowman WP, Devidas M, Gerhard DS, Yang W, Relling MV, Shurtleff SA, Campana D, Borowitz MJ, Pui CH, Smith M, Hunger SP, Willman CL, Downing JR. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.den Boer ML, van Slegtenhorst M, de Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, van Zutven LJ, Beverloo HB, van der Spek PJ, Escherich G, Horstmann MA, Janka-Schaub GE, Kamps WA, Evans WE, Pieters R. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10:125–134. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang JJ, Bhojwani D, Yang W, Cai X, Stocco G, Crews K, Wang J, Morrison D, Devidas M, Hunger SP, Willman CL, Raetz EA, Pui CH, Evans WE, Relling MV, Carroll WL. Genome-wide copy number profiling reveals molecular evolution from diagnosis to relapse in childhood acute lymphoblastic leukemia. Blood. 2008;112:4178–4183. doi: 10.1182/blood-2008-06-165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukaemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 55.Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukemia. Nat Rev Drug Discov. 2007;6:149–165. doi: 10.1038/nrd2240. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Cheng C, Yang W, Pei D, Cao X, Fan Y, Pounds SB, Neale G, Trevino LR, French D, Campana D, Downing JR, Evans WE, Pui CH, Devidas M, Bowman WP, Camitta BM, Willman CL, Davies SM, Borowitz MJ, Carroll WL, Hunger SP, Relling MV. Genome-wide interrogation of germline genetic variations associated with treatment response in childhood acute lymphoblastic leukemia. JAMA. 2009;301:393–403. doi: 10.1001/jama.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]