Abstract
In dental plaque α-haemolytic streptococci, including Streptococcus gordonii, are considered beneficial for oral health. These organisms produce hydrogen peroxide (H2O2) at concentrations sufficient to kill many oral bacteria. Streptococci do not produce catalase yet tolerate H2O2. We recently demonstrated that coaggregation with Actinomyces naeslundii stabilizes arginine biosynthesis in S. gordonii. Protein arginine residues are sensitive to oxidation by H2O2. Here, the ability of A. naeslundii to protect S. gordonii against self-produced H2O2 was investigated. Coaggregation with A. naeslundii enabled S. gordonii to grow in the absence of arginine, and promoted survival of S. gordonii following growth with or without added arginine. Arginine-replete S. gordonii monocultures contained 20–30 μM H2O2 throughout exponential growth. Actinomyces naeslundii did not produce H2O2 but synthesized catalase, removed H2O2 from coaggregate cultures and decreased protein oxidation in S. gordonii. On solid medium, S. gordonii inhibited growth of A. naeslundii; exogenous catalase overcame this inhibition. In coaggregate cultures, A. naeslundii cell numbers were >90% lower than in monocultures after 24 h. These results indicate that coaggregation with A. naeslundii protects S. gordonii from oxidative damage. However, high cell densities of S. gordonii inhibit A. naeslundii. Therefore, H2O2 may drive these organisms towards an ecologically balanced community in natural dental plaque.
Keywords: oral streptococci, hydrogen peroxide, Actinomyces naeslundii, metal-catalyzed oxidation, Streptococcus gordonii, catalase
Introduction
Oral viridans streptococci, including Streptococcus gordonii, Streptococcus sanguinis, Streptococcus oralis and Streptococcus mitis, are primary colonizers of human dental plaque. In the first few hours after tooth brushing, viridans streptococci may constitute 60–80% of dental plaque bacteria (Nyvad & Kilian, 1990; Diaz et al., 2006), and these organisms remain present in high numbers for at least 24 h (Nyvad & Kilian, 1987). Non-mutans viridans streptococci do not appear to contribute to oral diseases and are considered commensal organisms in the oral cavity. Evidence suggests that colonization with viridans streptococci may exclude more pathogenic bacteria and protect against caries or periodontitis. For example, a negative correlation between S. sanguinis and mutans streptococci (Streptococcus mutans and Streptococcus sobrinus) has been demonstrated in caries: in two independent studies individuals with extensive caries had significantly higher levels of mutans streptococci and significantly lower levels of S. sanguinis than those with no carious lesions (Nyvad & Kilian, 1990; Becker et al., 2002). High levels of viridans streptococci have been associated with low numbers of Tannerella for sythensis (formerly Bacteroides for-sythus) or Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans and the absence of periodontal disease (Hillman et al., 1985).
Viridans streptococci derive their name from the production of a greenish tinge (α-haemolysis) on blood agar. The α-haemolysin from S. gordonii has been identified as hydrogen peroxide (H2O2) (Barnard & Stinson, 1996), formed primarily by the action of pyruvate oxidase, SpxB (Hillman & Shivers, 1988; Spellerberg et al., 1996). Under laboratory conditions, many viridans streptococci produce sufficient quantities of H2O2 to kill S. mutans or periodontal pathogens (Holmberg & Hallander, 1973; Miyasaki et al., 1985; Kreth et al., 2005), and therefore, H2O2 may be an important mediator of competition in oral biofilms.
The deleterious effects of H2O2 on bacterial cells arise from the generation of hydroxyl radicals (•OH) in the presence of Fe(II), and the subsequent reaction of •OH with molecules in the vicinity (Imlay, 2003). This can result in cellular damage by degradation of [4Fe–4S] clusters in enzymes and enzyme inactivation (Jang & Imlay, 2007), or by oxidation of macromolecules including DNA and proteins (Imlay, 2003). Peroxidogenic streptococci avoid autotoxicity by using Mn(II) in place of Fe(II) (Jakubovics et al., 2002; Tseng et al., 2002) and by limiting their reliance on [4Fe–4S] cluster proteins, for example by having no respiratory chain. Nevertheless, accumulation of H2O2 in batch cultures reduces stationary phase survival of at least some viridans streptococci (Eisenberg, 1973; Regev-Yochay et al., 2007). In bacteria exposed to oxidative stress, loss of viability in stationary phase follows the accumulation of oxidized proteins (Dukan & Nystrom, 1999). Several different amino acid side chains in polypeptides are susceptible to oxidation, and the introduction of carbonyl groups into the side chains of arginine, proline, lysine or occasionally threonine, is perhaps the most significant outcome (Nystrom, 2005). This process is irreversible and carbonylated proteins are targeted for degradation (Grune et al., 2003; Stadtman & Levine, 2003). The oxidized amino acids cannot be recovered and must be replaced by import from the extracellular milieu or by de novo synthesis. Not all polypeptides are equally affected by carbonyl modification and different stress conditions result in oxidation of different subsets of proteins (Noda et al., 2007).
In a recent investigation into communication between S. gordonii and Actinomyces naeslundii, we observed that the S. gordonii spxB gene, encoding pyruvate oxidase, was upregulated in coaggregate cultures containing A. naeslundii compared with S. gordonii monocultures (Jakubovics et al., 2008). In addition, the expression of arginine biosynthesis genes was stabilized by coaggregation, and S. gordonii was able to grow in coaggregates under arginine-restricted conditions that did not support monoculture growth (Jakubovics et al., 2008). Considering that H2O2 in S. gordonii will oxidize arginine residues in proteins and place a burden on arginine biosynthesis, these observations suggested a possible role of H2O2 in the interaction between S. gordonii and A. naeslundii. Here, we investigated the ability of A. naeslundii to protect S. gordonii from H2O2-induced cell damage. In addition, the role of H2O2 as a mediator of intergeneric competition was evaluated.
Materials and methods
Bacterial strains and culture conditions
Streptococcus gordonii DL1 (Challis) and A. naeslundii MG1 (ATCC43146) were maintained by subculturing anaerobically (90% N2/5% H2/5% CO2) at 37 °C in Todd–Hewitt broth (THB; Becton Dickenson, Sparks, MD) or on THB solidified with 1.5% Bacto agar. In some experiments THB medium was supplemented with 0.5% yeast extract (THBYE). Chemically defined medium (CDM) was based on Terleckyj’s FMC medium for growth of streptococci (Terleckyj et al., 1975) with modifications as follows: L-leucine and L-isoleucine were added to a final concentration of 40 mg L−1 rather than 100 mg L−1 in FMC; L-arginine and L-histidine were at 100 mg L−1 rather than 200 mg L−1 in FMC; 0.1 mM CaCl2 was included (not present in FMC) and the medium was adjusted to pH 7.3. For experiments involving coaggregate cultures, inocula were prepared by growing bacteria in an anaerobic environment for 16 h in TYEG consisting of 1% Bacto tryptone, 0.5% yeast extract, 0.3% K2HPO4 and 0.2% glucose, adjusted to pH 7.5 before autoclaving. Cells were harvested, washed twice in CDM or an identical medium lacking arginine (CDMΔarg) and resuspended in CDM or CDMΔarg. Cultures were adjusted to c. 5 × 109 CFUmL−1. For coaggregate cultures, 300 μL of each strain were combined and vortex mixed for 10 s to form robust macroscopic coaggregates. Cultures were diluted to a final volume of 15 mL (c. 1 × 108 CFUmL−1 of each strain) in CDM or CDMΔarg and incubated aerobically without shaking. Equivalent monocultures containing c. 1 × 108 CFUmL−1 of S. gordonii or A. naeslundii were also prepared. Culture turbidity was measured using a Klett–Summerson spectrophotometer (Klett Manufacturing Co., New York) fitted with a 660 nm filter.
Enumeration of S. gordonii and A. naeslundii in mixed cultures
For enumeration of bacteria, 0.5-mL samples were removed from the culture and chains, clumps or coaggregates were disrupted by sonication in a Sonopuls ultrasonic homogenizer (Bandelin Electric, Berlin) equipped with a BR30 cup booster for 1 min on 50% maximum power. Samples were serially diluted 10-fold and 20 μL aliquots were dropped onto solidified THB medium or, to enumerate A. naeslundii in mixed cultures, onto THB supplemented with 128 mg L−1 mupirocin and 2.5 mg L−1 metronidazole (Lewis et al., 1995). Plates were incubated aerobically in 5% CO2 atmosphere at 37 °C for 24 h (S. gordonii) or 48 h (A. naeslundii). Control experiments demonstrated that A. naeslundii colonies were not visible after 24 h and therefore only S. gordonii CFU from mixed cultures were counted at this point. Addition of mupirocin and metronidazole inhibited growth of S. gordonii, but had no effect on A. naeslundii colony formation (Jakubovics et al., 2008).
Quantitive determination of H2O2 concentrations
The concentration of H2O2 in culture supernatants was determined using horseradish peroxidise (HRP) and Amplex UltraRed reagent (Invitrogen, Carlsbad, CA). Cells were harvested by centrifugation in a swing-out rotor at 3200 g at 25 °C for 7 min. The supernatant was collected, filtered through a 0.22-μm pore membrane and diluted 10-fold in 50 mM sodium phosphate buffer, pH 7.4. Triplicate 50 μL samples were mixed with 50 μL sodium phosphate buffer containing 1 U HRP and 0.1 mM Amplex UltraRed and incubated at 25 °C for 30 min. Fluorescence (525 nm excitation/590 nm emission) was measured using a VICTOR3 microplate reader (Perkin Elmer, Waltham, MA). For each assay, a standard curve was prepared by diluting a 30% H2O2 solution (Sigma, St. Louis, MO).
Assessment of protein oxidation
Proteins were extracted from S. gordonii or A. naeslundii cells by enzymatic digestion of the cell wall and bead beating (Jakubovics et al., 2000). Following protein extraction, samples were divided and the protein concentration was determined in one portion using a BCA assay kit (Pierce, Rockford, IL). To the other portion, dithiothreitol (50 mM final concentration) was added and the samples were frozen immediately at − 20 °C and analyzed together when all samples were collected. Proteins containing carbonyl groups were visualized by Western blotting using the Oxyblot kit (Chemicon International, Temecula, CA) as outlined by the manufacturer. Briefly, protein extracts were denatured by mixing with an equal volume of 12% sodium dodecyl sulphate and carbonyl groups were derivatized to 2,4-dinitrophenylhydrazone (DNP) by addition of 2,4-dinitrophenylhydrazine and incubation for 15 min at 25 °C. The reaction was neutralized and proteins were separated on a 12% polyacrylamide gel. Proteins were blotted to a nitrocellulose membrane, and DNP was detected by immunostaining with a specific antibody.
H2O2 detection in bacterial colonies and agar plate competition assay
Bacterial monocultures in CDM were prepared as described above, and 10 μL portions were spotted onto solidified THB medium. In some cases, 10 U catalase (Sigma) were included in the CDM used to resuspend S. gordonii cells. For competition assays, a drop of S. gordonii culture was placed on the plate and allowed to dry. Within 5 min, A. naeslundii was spotted in close proximity but not overlapping with the S. gordonii drop. The plates were incubated aerobically in 5% CO2 atmosphere for 48 h at 37 °C. Where appropriate, H2O2 was detected by flooding the plate with 3 mL detection reagent [100 mM potassium phosphate, pH 6.0 containing 20 U mL−1 HRP and 1 mM 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS)]. Excess detection reagent was removed immediately and plates were incubated at 25 °C for 15 min before imaging. The presence of H2O2 was indicated by a purple colour.
Results
Coaggregation with A. naeslundii enhances growth and survival of S. gordonii
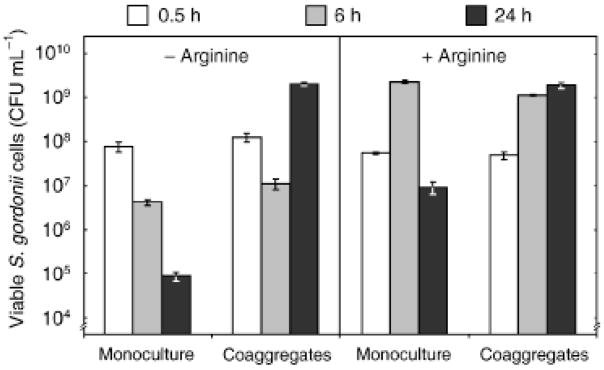
Recently, we have shown that coaggregation with A. naeslundii enables S. gordonii to initiate aerobic growth in low (0.025 mM) arginine, equivalent to the concentration of free arginine in saliva (Jakubovics et al., 2008). Under these conditions, there was a small (c. 1 log) decrease in the number of viable S. gordonii cells in monoculture after 24 h incubation (Jakubovics et al., 2008). To determine whether the extracellular arginine concentration influences the ability of S. gordonii to survive in stationary phase or to grow in coaggregation with A. naeslundii, monocultures and coaggregate cultures were set up using a CDM or a modified medium lacking arginine (CDMΔarg). Cultures were incubated aerobically for up to 24 h and viable counts of streptococci were determined. In CDMΔarg medium, S. gordonii monocultures rapidly lost viability, and viable counts decreased by c. 1 log in 6 h and c. 3 log after 24 h (Fig. 1). Coaggregation enabled S. gordonii to initiate growth in the absence of arginine and to remain viable for ≥24 h after inoculation. The onset of streptococcal growth did not occur for ≥6 h and viable counts decreased at a similar rate to monocultures during this lag phase. In CDM (0.5 mM arginine), S. gordonii grew to high cell densities within 6 h in monoculture, but rapidly lost viability in stationary phase (Fig. 1). Viable counts decreased approximately two orders of magnitude between 6 and 24 h. By contrast, in coaggregates S. gordonii CFU remained stable or even increased slightly between 6 and 24 h after inoculation.
Fig. 1.
Enhanced survival of Streptococcus gordonii by coaggregation with Actinomyces naeslundii. Monocultures or coaggregate cultures in CDMΔarg (left panel) or CDM (right panel) were incubated aerobically and viable counts of S. gordonii cells were determined at the times indicated. Data shown are mean values of triplicate measurements and SDs from triplicate measurements. Data represent one of two experiments that gave similar results.
Loss of viability in stationary phase has been linked to H2O2 production by viridans streptococci, and can be averted in Streptococcus pneumoniae by disruption of spxB, the gene encoding the major H2O2-producing enzyme, pyruvate oxidase (Regev-Yochay et al., 2007). We tested survival of an spxB mutant of S. gordonii (kindly donated by R. J. Lamont) in aerobic CDM. In monoculture, this strain grew at a similar rate to wild type, reaching 2 × 109 CFU mL−1 after 6 h. However, unlike wild-type S. gordonii, the spxB mutant remained viable in monoculture and >1 × 109 CFU mL−1 were recovered after 24 h (data not shown).
Actinomyces naeslundii depletes H2O2 from S. gordonii cultures and protects S. gordonii from protein oxidation
To examine the role of H2O2 in S. gordonii and A. naeslundii cultures, H2O2 concentrations were measured in bacterial supernatants throughout aerobic growth of S. gordonii and A. naeslundii in monoculture and in coaggregate CDM (0.5 mM arginine) cultures. H2O2 was barely detectable in A. naeslundii cultures during incubation for 6 h. On the other hand, S. gordonii monocultures maintained a stable H2O2 concentration of c. 25 μM (Fig. 2a and b). In coaggregate cultures, H2O2 was initially low (c. 7 μM) but increased after 2 h as the streptococci outgrew the Actinomyces cells. By 4 h, the concentration of H2O2 exceeded 15 μM and it remained stable at this level until ≥6 h after inoculation.
Fig. 2.
Effects of coaggregation on H2O2 concentration and protein oxidation in Streptococcus gordonii. Monocultures or coaggregate cultures in CDM (0.5 mM arginine) were incubated aerobically. Turbidity (a) and extracellular H2O2 concentrations (b) were determined at intervals. Symbols represent S. gordonii monocultures (■), coaggregate cultures containing S. gordonii and Actinomyces naeslundii (○) and A. naeslundii monocultures (◆). After 3 h incubation, samples were removed and proteins extracted. (c) Equal amounts (1 μg) of proteins were separated using SDS-PAGE and oxidized proteins containing carbonyl groups were detected by immunostaining with the Oxyblot kit. Approximately 85–90% of proteins in the sample from coaggregate cultures originated from S. gordonii. Experiments were repeated three times with similar results.
Oxidative damage to bacterial proteins was assessed using the Oxyblot kit. This procedure derivatizes carbonyl groups, caused by oxidation of arginine, proline or lysine residues in proteins, to 2,4-dinitrophenylhydrazone and detects the modified proteins with a specific antibody. Protein oxidation was measured after 3 h growth, when the streptococci had reached late exponential phase. By this time streptococci had outgrown Actinomyces and constituted 85–90% of cells in coaggregate cultures (Fig. 2a). From measurements of proteins recovered per cell in monoculture, the efficiency of protein extraction was found to be equivalent for S. gordonii and A. naeslundii (data not shown). Therefore, it was predicted that 85–90% of proteins extracted from coaggregate cultures incubated for 3 h originated from S. gordonii. Examination of Coomassie-stained gels (not shown) confirmed that the one-dimensional protein profiles of coaggregate cultures looked very similar to those of S. gordonii monocultures. Extensive protein oxidation was observed in preparations from S. gordonii monocultures, with a particularly strong band corresponding to c. 55 kDa (Fig. 2c). In contrast, only a small number of bands from A. naeslundii protein extracts cross-reacted with the Oxyblot antibody. In coaggregate cultures, protein oxidation was markedly reduced compared with S. gordonii monocultures. A band at c. 55 kDa was apparent, but several others that were present in monoculture extracts, including a band at 48 kDa and five bands at >100 kDa, were absent from coaggregate culture extracts (Fig. 2c).
Antibacterial effects of S. gordonii -produced H2O2 against A. naeslundii
Several studies have documented the bactericidal properties of H2O2 produced by viridians streptococci against oral and extraoral bacteria (Holmberg & Hallander, 1973; LeBien & Bromel, 1975; Miyasaki et al., 1985; Kreth et al., 2005). A simple assay, based on growth on solidified THB medium, was used to determine whether A. naeslundii growth is inhibited by S. gordonii. Initially, production of H2O2 by S. gordonii and A. naeslundii on THB agar was assessed by a colorimetric reaction that produces purple staining in the presence of H2O2. This assay clearly demonstrated production of H2O2 by S. gordonii but not by A. naeslundii (Fig. 3a). Next, 10 μL drops containing 106 CFU of an exponential phase culture of each strain were spotted in close proximity on solidified THB medium and incubated aerobically under 5% CO2 for 48 h. Actinomyces naeslundii did not grow within c. 10 mm of S. gordonii (Fig. 3b). However, mixing S. gordonii cells with 10 U catalase prior to spotting on the agar reduced the zone of inhibition to < 1 mm.
Fig. 3.
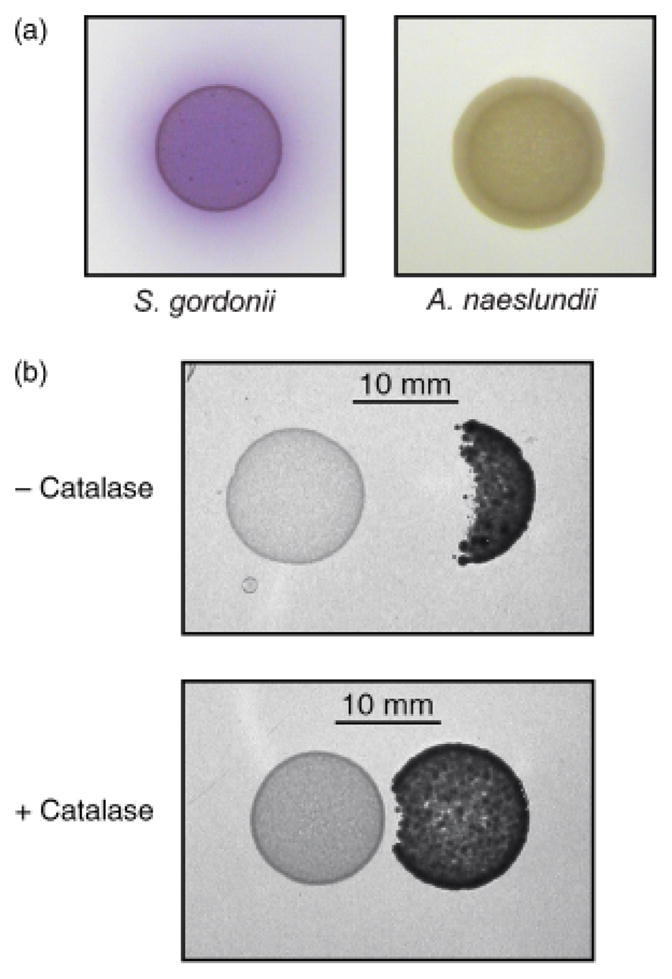
Inhibition of Actinomyces naeslundii growth by Streptococcus gordonii-produced H2O2. (a) A colorimetric assay, based on conversion of an indicator, ABTS, in the presence of HRP and H2O2 was used to detect H2O2 in cells grown on THB agar. The purple colour on and around S. gordonii cells indicates the presence of H2O2. (b) Streptococcus gordonii (left) and A. naeslundii (right) were spotted in close proximity on THB agar without (upper panel) or with (lower panel) prior addition of 10 U catalase to the S. gordonii cells.
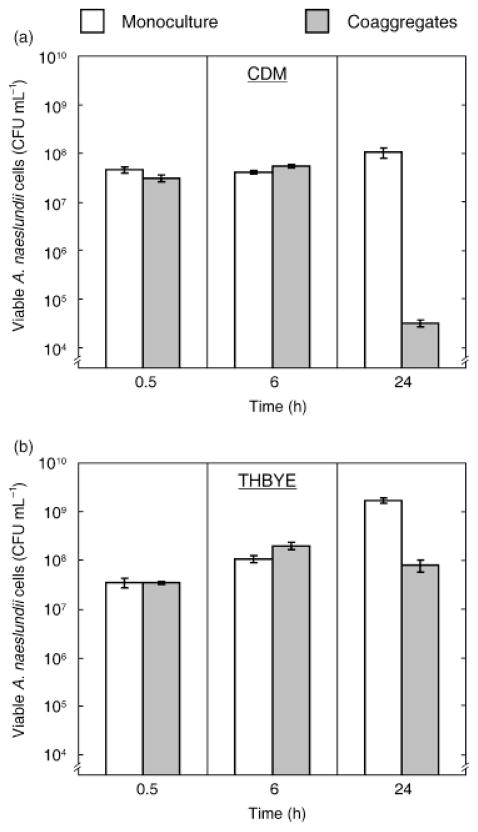
To test whether H2O2 from S. gordonii was bactericidal against A. naeslundii, monocultures and coaggregate cultures in CDM were incubated aerobically for 24 h and viable A. naeslundii cells were enumerated on selective agar. Unlike S. gordonii, A. naeslundii cells in monoculture did not lose viability within this time frame (Fig. 4a), even though the medium supported only weak growth of A. naeslundii. In coaggregate cultures, A. naeslundii CFU increased slightly over the first 6 h. Between 6 and 24 h, A. naeslundii CFU dropped by almost three orders of magnitude, indicating that these cells were efficiently killed by S. gordonii during this period. Because the growth of A. naeslundii in CDM was poor, we assessed the inhibitory properties of S. gordonii towards A. naeslundii in a rich medium (THBYE) that supported growth of A. naeslundii to high cell densities (Fig. 4b). By 6 h after inoculation, A. naeslundii cell numbers had increased greater than threefold in both monoculture and coaggregate cultures. In monoculture, A. naeslundii reached >109 CFU mL−1 by 24 h and cell numbers did not increase after prolonged (48 h) incubation (data not shown). In coaggregate culture, there was a marked (c. 2.5-fold) decrease in viable cell numbers between 6 and 24 h, indicating that S. gordonii inhibited the growth of A. naeslundii cells in this medium.
Fig. 4.
Inhibitory properties of Streptococcus gordonii towards Actinomyces naeslundii in CDM (0.5 mM arginine) (a) and THBYE medium (b). Viable A. naeslundii cells in aerobic monocultures or coaggregate cultures containing S. gordonii were enumerated on selective agar. Experiments were performed twice with similar results. Data are means and SDs from triplicate measurements of one representative experiment.
Discussion
Dental plaque is a complex microbial ecosystem in which viridans streptococci, including S. gordonii, and A. naeslundii are often found in high numbers from the earliest stages. Several factors may enhance the coexistence of these species. For example, efficient degradation of host carbohydrates often requires combinations of enzymes from more than one bacterial species (Bradshaw et al., 1994). Streptococcus gordonii can utilize free sialic acids, but does not produce sialidase and therefore cannot release sialic acids from host glycoproteins (Bradshaw et al., 1994; Byers et al., 1996). Actinomyces naeslundii has sialidase activity (Costello et al., 1979; Bradshaw et al., 1994) and could potentially supply nutrients for S. gordonii. On the other hand, A. naeslundii lacks several glycolytic and proteolytic activities that are produced by S. gordonii, including N-acetyl-glucosaminidase, α-fucosidase, gly-pro diamino peptidase and trypsin-like protease (Bradshaw et al., 1994). In addition, there is evidence that the ability of A. naeslundii to bind surface receptors on S. gordonii contributes to its retention in biofilms under flowing saliva (Palmer et al., 2001). Here, we demonstrate that H2O2 production results in S. gordonii obtaining a benefit from A. naeslundii in the form of protection against oxidative stress. When S. gordonii cells significantly outnumber A. naeslundii, H2O2 production overwhelms the Actinomyces cells and kills them as well as itself. Therefore, H2O2 may be an additional factor that drives coexistence and modulates the A. naeslundii population while selecting against overgrowth of S. gordonii.
In an attempt to identify the key gene functions of S. gordonii that are involved in interactions with A. naeslundii, we recently applied DNA microarray analysis to S. gordonii monocultures and coaggregate cultures containing A. naeslundii (Jakubovics et al., 2008). These experiments demonstrated that S. gordonii arginine biosynthesis is a major pathway affected by coaggregation. Further investigation demonstrated that aerobic arginine biosynthesis is inefficient in S. gordonii and only occurs when ≥0.1 mM arginine is supplied initially (Jakubovics et al., 2008). However, coaggregation with A. naeslundii can overcome this arginine requirement and enable growth of S. gordonii in the absence of arginine. Arginine is a well-recognized nutritional antioxidant (Fang et al., 2002) and can react directly with free radicals to neutralize them (Lass et al., 2002). Furthermore, arginine residues in proteins are sensitive to an irreversible oxidation reaction in the presence of free radical generating systems such as H2O2/Fe2+ (Stadtman & Levine, 2003). Therefore, H2O2 produced by S. gordonii is likely to deplete the intracellular arginine pool and increase the requirement for arginine. Bacterial cell membranes are largely permeable to H2O2 (Seaver & Imlay, 2001), and consequently removal of H2O2 from the bulk medium by A. naeslundii will reduce the H2O2 concentration inside S. gordonii cells. In control experiments, we found that addition of exogenous catalase (10 U mL−1) produced higher growth yields of S. gordonii in low arginine concentrations (0.016 or 0.032 mM arginine) compared with cultures that did not contain catalase (data not shown). Coaggregation may also provide a local microenvironment that protects S. gordonii, for example by producing anaerobic pockets.
Not all bacterial proteins are equally sensitive to oxidation and it is thought that proteins that bind metal ions or interact with metals transiently are especially prone to metal-catalyzed oxidation (Nyström, 2005). In Escherichia coli, arginine biosynthesis enzymes are highly represented within the carbonylation-sensitive proteins that have been identified to date. Thus, in a recent study of proteins that were oxidized in response to carbon, nitrogen or phosphate starvation, 62 carbonyl-containing proteins were identified of which three were arginine biosynthesis enzymes (Noda et al., 2007). These were acetylornithinase (ArgJ) and arginosuccinate synthase (ArgG), oxidized during nitrogen limitation, and carbamoyl phosphate synthase large subunit (CarA) that was oxidized upon carbon starvation (Noda et al., 2007). Carbonyl modification of proteins can result in loss of enzyme activity, a phenomenon that was first demonstrated with glutamine synthetase (Levine et al., 1981). Further studies will be required to determine whether arginine biosynthesis enzymes in S. gordonii are specific targets of protein oxidation in the presence of H2O2.
Removal of high concentrations of H2O2 by bacteria is associated with catalase activity. Streptococci do not make catalase; production of catalase within the genus Actinomyces is strain dependent (Collins et al., 2000). The strain used here, A. naeslundii MG1, generated bubbles on immersion in 3% H2O2, indicating that catalase was produced. This is consistent with the annotated genome sequence of A. naeslundii MG1, which includes a single catalase, encoded by hktE (http://cmr.tigr.org/cgi-bin/CMR/GenomePage.cgi?org=gan). In coaggregate cultures in CDM, A. naeslundii initially removed H2O2 from the medium, presumably due to catalase activity. However, after prolonged incubation (>6 h), this activity was insufficient to protect A. naeslundii cells from being killed. Similarly, on solidified medium and in nutrient-rich planktonic cultures, S. gordonii H2O2 inhibited growth of A. naeslundii. These findings are in line with studies on several oral and extra-oral bacteria, which have demonstrated that production of catalase is not sufficient for resistance to H2O2 from viridans streptococci (Miyasaki et al., 1985; Uehara et al., 2001; Regev-Yochay et al., 2006).
In summary, the data presented here support the hypothesis that H2O2 drives the changes in S. gordonii arginine homeostasis that occur upon coaggregation with A. naeslundii (Jakubovics et al., 2008). In addition, H2O2 secreted by S. gordonii is a key factor influencing the dynamics of interactions between S. gordonii and A. naeslundii in vitro. It will be important to determine how H2O2 influences the ecology of mixed species communities in open systems where nutrients are constantly replenished and waste products removed, as occurs in the oral cavity. Further, the role of saliva in interbacterial interactions needs to be addressed, because salivary components including secretory IgA and lactoperoxidase are known to affect the antibacterial properties of H2O2 (Carlsson, 1980; Adamson & Carlsson, 1982; Carlsson et al., 1983; Uehara et al., 2006). Such interactions are fundamental to the formation of stable dental plaque communities.
Acknowledgments
The authors thank R.J. Levine for advice and E. Ko for assistance with experimental procedures. We gratefully acknowledge the gift of the S. gordonii spxB mutant strain from R.J. Lamont. This research was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health and by Public Health Service grant DE11090 from the National Institute of Dental and Craniofacial Research awarded to M.M.V.
References
- Adamson M, Carlsson J. Lactoperoxidase and thiocyanate protect bacteria from hydrogen peroxide. Infect Immun. 1982;35:20–24. doi: 10.1128/iai.35.1.20-24.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard JP, Stinson MW. The alpha-hemolysin of Streptococcus gordonii is hydrogen peroxide. Infect Immun. 1996;64:3853–3857. doi: 10.1128/iai.64.9.3853-3857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw DJ, Homer KA, Marsh PD, Beighton D. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology. 1994;140:3407–3412. doi: 10.1099/13500872-140-12-3407. [DOI] [PubMed] [Google Scholar]
- Byers HL, Homer KA, Beighton D. Utilization of sialic acid by viridans streptococci. J Dent Res. 1996;75:1564–1571. doi: 10.1177/00220345960750080701. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Bactericidal effect of hydrogen peroxide is prevented by the lactoperoxidase-thiocyanate system under anaerobic conditions. Infect Immun. 1980;29:1190–1192. doi: 10.1128/iai.29.3.1190-1192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J, Iwami Y, Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983;40:70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MD, Hoyles L, Kalfas S, Sundquist G, Monsen T, Nikolaitchouk N, Falsen E. Characterization of Actinomyces isolates from infected root canals of teeth: description of Actinomyces radicidentis sp. nov. J Clin Microbiol. 2000;38:3399–3403. doi: 10.1128/jcm.38.9.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello AH, Cisar JO, Kolenbrander PE, Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979;26:563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz PI, Chalmers NI, Rickard AH, Kong C, Milburn CL, Palmer RJ, Jr, Kolenbrander PE. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol. 2006;72:2837–2848. doi: 10.1128/AEM.72.4.2837-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S, Nyström T. Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J Biol Chem. 1999;274:26027–26032. doi: 10.1074/jbc.274.37.26027. [DOI] [PubMed] [Google Scholar]
- Eisenberg RJ. Induction of unbalanced growth and death of Streptococcus sanguis by oxygen. J Bacteriol. 1973;116:183–191. doi: 10.1128/jb.116.1.183-191.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305:709–718. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- Hillman JD, Shivers M. Interaction between wild-type, mutant and revertant forms of the bacterium Streptococcus sanguis and the bacterium Actinobacillus actinomycetemcomitans in vitro and in the gnotobiotic rat. Arch Oral Biol. 1988;33:395–401. doi: 10.1016/0003-9969(88)90196-3. [DOI] [PubMed] [Google Scholar]
- Hillman JD, Socransky SS, Shivers M. The relationships between streptococcal species and periodontopathic bacteria in human dental plaque. Arch Oral Biol. 1985;30:791–795. doi: 10.1016/0003-9969(85)90133-5. [DOI] [PubMed] [Google Scholar]
- Holmberg K, Hallander HO. Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch Oral Biol. 1973;18:423–434. doi: 10.1016/0003-9969(73)90167-2. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS, Smith AW, Jenkinson HF. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol Microbiol. 2000;38:140–153. doi: 10.1046/j.1365-2958.2000.02122.x. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS, Smith AW, Jenkinson HF. Oxidative stress tolerance is manganese (Mn2+) regulated in Streptococcus gordonii. Microbiology. 2002;148:3255–3263. doi: 10.1099/00221287-148-10-3255. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS, Gill SR, Iobst SE, Vickerman MM, Kolenbrander PE. Regulation of gene expression in a mixed-genus community: stabilized arginine biosynthesis in Streptococcus gordonii by coaggregation with Actinomyces naeslundii. J Bacteriol. 2008;190:3646–3657. doi: 10.1128/JB.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron–sulfur enzymes. J Biol Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Shi W, Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Suessenbacher A, Wolkart G, Mayer B, Brunner F. Functional and analytical evidence for scavenging of oxygen radicals by L-arginine. Mol Pharmacol. 2002;61:1081–1088. doi: 10.1124/mol.61.5.1081. [DOI] [PubMed] [Google Scholar]
- LeBien TW, Bromel MC. Antibacterial properties of a peroxidogenic strain of Streptococcus mitior (mitis) Can J Microbiol. 1975;21:101–103. doi: 10.1139/m75-015. [DOI] [PubMed] [Google Scholar]
- Levine RL, Oliver CN, Fulks RM, Stadtman ER. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci USA. 1981;78:2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R, McKenzie D, Bagg J, Dickie A. Experience with a novel selective medium for isolation of Actinomyces spp. from medical and dental specimens. J Clin Microbiol. 1995;33:1613–1616. doi: 10.1128/jcm.33.6.1613-1616.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki KT, Wilson ME, Zambon JJ, Genco RJ. Influence of endogenous catalase activity on the sensitivity of the oral bacterium Actinobacillus actinomycetemcomitans and the oral haemophili to the bactericidal properties of hydrogen peroxide. Arch Oral Biol. 1985;30:843–848. doi: 10.1016/0003-9969(85)90141-4. [DOI] [PubMed] [Google Scholar]
- Noda Y, Berlett BS, Stadtman ER, Aponte A, Morgan M, Shen RF. Identification of enzymes and regulatory proteins in Escherichia coli that are oxidized under nitrogen, carbon, or phosphate starvation. Proc Natl Acad Sci USA. 2007;104:18456–18460. doi: 10.1073/pnas.0709368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95:369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Nyvad B, Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24:267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- Palmer RJ, Jr, Kazmerzak K, Hansen MC, Kolenbrander PE. Mutualism versus independence: strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. Infect Immun. 2001;69:5794–5804. doi: 10.1128/IAI.69.9.5794-5804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol. 2006;188:4996–5001. doi: 10.1128/JB.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Yochay G, Trzcinski K, Thompson CM, Lipsitch M, Malley R. SpxB is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J Bacteriol. 2007;189:6532–6539. doi: 10.1128/JB.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellerberg B, Cundell DR, Sandros J, Pearce BJ, Idänpään-Heikkilä I, Rosenow C, Masure HR. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Terleckyj B, Willett NP, Shockman GD. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975;11:649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HJ, McEwan AG, Paton JC, Jennings MP. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun. 2002;70:1635–1639. doi: 10.1128/IAI.70.3.1635-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Kikuchi K, Nakamura T, Nakama H, Agematsu K, Kawakami Y, Maruchi N, Totsuka K. H2O2 produced by viridans group streptococci may contribute to inhibition of methicillin-resistant Staphylococcus aureus colonization of oral cavities in newborns. Clin Infect Dis. 2001;32:1408–1413. doi: 10.1086/320179. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Agematsu K, Kikuchi K, et al. Secretory IgA, salivary peroxidase, and catalase-mediated microbicidal activity during hydrogen peroxide catabolism in viridans streptococci: pathogen coaggregation. J Infect Dis. 2006;194:98–107. doi: 10.1086/504439. [DOI] [PubMed] [Google Scholar]