Abstract
Glässer’s disease is a re-emerging swine disease characterized by a severe septicaemia. Vaccination has been widely used to control the disease, although there is a lack of extended cross-protection. Trimeric autotransporters, a family of surface exposed proteins implicated in host-pathogen interactions, are good vaccine candidates. Members of this family have been described in Haemophilus parasuis and designated as virulence-associated trimeric autotransporters (VtaA). In this work, we produced 15 recombinant VtaA passenger domains and looked for the presence of antibodies directed against them in immune sera by immunoblotting. After infection with a subclinical dose of H. parasuis Nagasaki, an IgG mediated antibody response against 6 (VtaA1, 5, 6, 8, 9 and 10) of the 13 VtaA of the Nagasaki strain was detected, indicating that they are expressed in vivo. IgA production against VtaA was detected in only one animal. VtaA were more likely to be late antigens when compared to early (Omp P5 and Omp P6) and late (YaeT) defined antigens. Antibody cross-reaction with two orthologs of Nagasaki’s VtaA5 and 6, VtaA15 and 16 of strain HP1319, was also detected. No antibodies against VtaA were detected in the sera of animals immunized with a bacterin of the Nagasaki strain, suggesting poor expression in the in vitro conditions used. Taken together, these results indicate that VtaA are good candidate immunogens that could be used to improve H. parasuis vaccines. However, their capacity to confer protective immunity needs to be further studied.
Keywords: Haemophilus parasuis, VtaA, OMP, antibody, cross-reactivity
1. INTRODUCTION
Haemophilus parasuis is a small, pleomorphic, NAD-dependent member of the family Pasteurellaceae. This bacterium is the etiological agent of Glässer’s disease in swine, which is characterized by polyserositis and arthritis. Once a sporadic disease, it has increased in prevalence and severity in recent years with the adoption of new production technologies which has led to pigs being susceptible to this infection [26]. The immune status of a herd is an important determinant for the outcome of the disease, and since the 1980s, reports on the use of bacterins to control the disease by vaccination have been published [27, 30]. However, a lack of protective cross-immunity against some strains and partial cross-protection among serotypes has been reported [20, 24], pointing to the difficulty in identifying protective immunogens for a universal vaccine. Initial studies have already shown the importance of circulating antibodies in controlling H. parasuis infection [20, 24]. After experimental infection in swine, production of antibodies against outer membrane proteins (OMP) has been described [15, 21]. The production of swine antibodies against specific H. parasuis proteins has been studied and FhuA, OmpA, PalA, Omp2, D15 and HPS06257 have been described as antigenic proteins [7, 33, 34].
The trimeric autotransporter (AT-2) protein family members are characterized by a mosaic structure formed by a translocator domain that anchors the protein to the membrane and allows cell-surface exposure of the functional passenger domain, which is divided into stalk, connector, and head segments [31]. AT-2 enable high-affinity multivalent adhesive interactions with host cells and extracellular matrix components [6, 9, 28]. Some can inhibit phagocytosis or complement-mediated killing, helping the bacteria to overcome the innate immune response [12, 29]. In animal models, the protective capacities of AT-2 have been explored by both passive transfer of antibodies and by challenging animals immunized with recombinant AT-2 [1, 8, 16]. The existence of virulence-associated trimeric autotransporters (VtaA) in H. parasuis has been recently reported [25]. In tissue-invasive H. parasuis strains, they constitute a multigene family of at least 10 copies per genome, which can be subdivided into three groups on the basis of sequence similarities of the translocator domains. Interestingly, the group 1 variant is associated to pathogenic strains but not to H. parasuis non-invasive strains [25].
The objective of this study was to investigate the antigenicity of VtaA molecules expressed during an experimental infection with H. parasuis Nagasaki. All 13 VtaA passenger domains of the homologous Nagasaki strain, produced as recombinant proteins, were assayed and antibody cross-reaction was assessed with two other VtaA from a heterologous non-serotypeable strain, HP1319.
2. MATERIALS AND METHODS
2.1. Culture and bacterin production
After an in vivo passage, the highly virulent [26] H. parasuis strain Nagasaki was plated onto chocolate agar and incubated at 37 °C in an atmosphere of 5% CO2. Bacterial growth was harvested at 18 h post-inoculation and suspended in sterile PBS. A OD600 = 0.2 bacterial suspension (equivalent to approximately 108 CFU/mL) was made followed by two 10-fold dilutions to adjust the bacterial concentration to ~ 106 CFU/mL. The number of colony forming units was determined after 48 h incubation by plating 10-fold dilutions on chocolate agar plates. The bacterin used in this study was prepared by centrifuging an H. parasuis bacterial suspension containing 109 CFU/mL, discarding the supernatant, and resuspending the pellet in 0.2% formalin solution prepared in PBS. This suspension was left at 4 °C for 72 h to achieve complete inactivation and was adjuvanted using a water-in-oil emulsion (Laboratorios HIPRA, Amer, Spain).
2.2. Experimental infection and immunization
All procedures involving animals followed EU norms (Council Directive 86/609/EEC). To avoid maternal antibody interference and colonization by H. parasuis from the sows, snatch-farrowed colostrum-deprived pigs (Large white × Landgrace) were used in this study [3]. The six animals used were divided into two groups at 21 days after birth. The first group (animals 127, 131, 172 and 198) was intranasally inoculated with a subclinical dose (106 CFU/animal) of H. parasuis Nagasaki, whereas the second group (animals 122 and 143) was intramuscularly vaccinated with a H. parasuis Nagasaki bacterin (equivalent to 109 CFU/animal) and boosted 15 days later. Pre-immune sera were collected 7 days pre-infection or pre-immunization and immune sera were collected at 7, 14, 22, 27 and 42 days post-treatment (infection or immunization). All pigs were euthanized at 42 days post-treatment (dpt).
2.3. Recombinant expression of VtaA passenger domains
The passenger domains, excluding the translocator domain and the extended signal peptide, of the 13 vtaA genes of the serotype 5 Nagasaki strain (vtaA1 to vtaA13) and of the vtaA15 and vtaA16 genes of the heterologous serotype strain HP1319 were amplified with the primers listed in Table I using previously described procedures [25]. Strain HP1319 is a non-typable isolate from the meninges of an animal suffering polyserositis. Additionally, three fragments of the passenger domain of vtaA10 were amplified: a 3′ fragment (C-terminal or stalk), the central collagen repeats, and a 5′ fragment (N-terminal or head) (Tab. I). The amplified gene fragments were cloned using the pASK-IBA33plus plasmid system (IBA – BioTAGnologies, Göttingen, Germany) and transformed in Escherichia coli Rosetta(DE3)pLysS (Novagen – Merck Chemicals Ltd., Nottingham, UK). One colony was inoculated in 5 mL of LB broth containing carbenicillin (50 μg/mL) and cloramphenicol (34 μg/mL), incubated at 37 °C (225 rpm) to an OD600 of 0.4–0.5, and left overnight at 25 °C without shaking. After centrifugation, bacteria were used to inoculate 50 mL of the above mentioned media. Recombinant protein production was induced for 2 h at 25 °C when the culture reached an OD600 of 0.4–0.6 by adding 0.2 μg/mL of anhydrotetracycline (AHT). When necessary, the peptides were purified using the HisSpinTrap kit (GE Healthcare, Barcelona, Spain) and quantified with the BCA protein assay kit (Pierce-Thermo Scientific, Rockford, IL, USA) according to the manufacturer’s instructions.
Table I.
Primers used to amplify the vtaA passenger domains or fragments of the vtaA10 passenger domain. The size of the amplified fragment (bp) is indicated in the first column. All primers contained a restriction enzyme BsaI target except for vtaA1 which contained a restriction enzyme BsmBI target (underlined and in bold).
| Gene | Primer name | Sequence |
|---|---|---|
| vtaA Haemophilus parasuis strain Nagasaki | ||
| vtaA1 (6 792 bp) | adh7-bsm-L2(-)F | ATGGTACGTCTCAAATGGCTGCATATCTTCAAGATGGTGC |
| adh7-bsm-3′less | ATGGTACGTCTCAGCGCTAACAATATCTCTTGATAATTGATTAAATC | |
| vtaA2 (3 221 bp) | adh3-L2(-)F | ATGGTAGGTCTCAAATGGCTGCATATTTTCAAGATAGTTCTG |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
| vtaA3 (3 138 bp) | adh15-L2(-)F | ATGGTAGGTCTCAAATGGCAGCATGGTTGGTAGATGGTTC |
| adh15-y(-)R | ATGGTAGGTCTCAGCGCTAACAATATCTCTTGATAATTGATTAAATC | |
| vtaA4 (3 095 bp) | adh23-L2(-)F | ATGGTAGGTCTCAAATGTCAGCTGCATATTTTGCAGGTGG |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
| vtaA5 (2 720 bp) | adh1/21-L2(-)F | ATGGTAGGTCTCAAATGGGTGCACAACTTCACACAGGAAC |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
| vtaA6 (2 828 bp) | adh19-L2(-)F | ATGGTAGGTCTCAAATGTCAGCTACTCACTCAGGAACACC |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
| vtaA7 (2 618 bp) | adh25-L2(-)F | ATGGTAGGTCTCAAATGGCAGATCGTCATAAAGGAACTCC |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
| vtaA8 (2 756 bp) | adh9-L2(-)F | ATGGTAGGTCTCAAATGGCAAGGCTTTTTCTAGATGAGGC |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
| vtaA9 (2 405 bp) | adh1/21-L2(-)F | ATGGTAGGTCTCAAATGGGTGCACAACTTCACACAGGAAC |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
| vtaA10 (4 488 bp) | adh11-L2(-)F | ATGGTAGGTCTCAAATGGCATATCTTGAATATGGTGCTCGA |
| adh5-3′less | ATGGTAGGTCTCAGCGCTACGGTTGATCTTATTGTGAATATCG | |
| vtaA11 (4 047 bp) | adh5-L2(-)F | ATGGTAGGTCTCAAATGTCAATTACTGATTCATCATATTTTCAAC |
| adh5-3′less | ATGGTAGGTCTCAGCGCTACGGTTGATCTTATTGTGAATATCG | |
| vtaA12 (4 013 bp) | adh17-L2(-)F | ATGGTAGGTCTCAAATGGCAAGGCTTTTTCTAGATGGTGC |
| G3-3′less | ATGGTAGGTCTCAGCGCTTCTCTCTAACTTCGCACCCAATC | |
| vtaA13 (3 752 bp) | adh13-L2(-)F | ATGGTAGGTCTCAAATGGCTAACACAACAAATACCGCAAAAC |
| G3-3′less | ATGGTAGGTCTCAGCGCTTCTCTCTAACTTCGCACCCAATC | |
| vtaA10 Haemophilus parasuis strain Nagasaki partial constructions | ||
| vtaA10 5′ (1 562 bp) | adh11-L2(-)F | ATGGTAGGTCTCAAATGGCATATCTTGAATATGGTGCTCGA |
| adh11parcial_5′R | ATGGTAGGTCTCAGCGCTCATAGCGGCAATCGTATCAATTTTA | |
| vtaA10 central (1 004 bp) | adh11parcial_CF | ATGGTAGGTCTCAAATGGATAAAATTGATACGATTGCCGCTA |
| adh11parcial_CR | ATGGTAGGTCTCAGCGCTTTTATCCGTTTGATTTTCGTCTTTCG | |
| vtaA10 3′ (1 980 bp) | adh11parcial_3′F | ATGGTAGGTCTCAAATGGTTGGTCCGAAAGACGAAAATCAA |
| adh11parcial_3′R | ATGGTAGGTCTCAGCGCTACGGTTGATCTTATTGTGAATATCG | |
| vtaA Haemophilus parasuis strain HP1319 | ||
| vtaA15 (2 828 bp) | adh19-L2(-)F | ATGGTAGGTCTCAAATGTCAGCTACTCACTCAGGAACACC |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
| vtaA16 (2 765 bp) | adh9HP1319L2(-)F | ATGGTAGGTCTCAAATGTCAGCTGCTGCTAACACAACAAAT |
| G1-3′less | ATGGTAGGTCTCAGCGCTACGACCAATATCTCTTGATAATTGAT | |
2.4. Immunoblotting of OMP from H. parasuis
The antibody response was evaluated by immunoblotting. Briefly, bacterial cultures were grown to OD600 = 0.7 in PPLO supplemented with 1% of IsoVitaleX (BD, Madrid, Spain). OMP were purified using the sodium N-lauroyl sarcosinate method [4] and quantified with the EZQ protein quantification kit (Invitrogen, Barcelona, Spain). Twenty-five μg of the individual OMP were suspended in 4× SDS buffer, denaturated at 95 °C for 15 min and separated by molecular size in a NuPAGE® Novex 4–12% Bis-Tris Gel (Invitrogen). Proteins were transferred to a Hybond™ ECL™ nitrocellulose membrane (GE Healthcare) by electrodiffusion. All of the following steps were performed according to the instructions provided in the ECL Advance Western Blotting Detection Kit (GE Healthcare). After blocking, the membranes were cut into strips, incubated with different pig sera diluted 1:2000 in blocking solution (GE Healthcare) for 1 h, and washed with TBS-tween20. The presence of IgG was detected by chemiluminescense with a mouse anti-porcine IgG (AbD Serotec, Oxford, UK) conjugated with Horse Radish Peroxidase (HRP) diluted 1:250000. Imaging was performed with a Fluorochem HD2 chemiluminescent workstation (Alpha Innotech, San Leandro, CA, USA). To monitor the maturation of the immune response, early antigens Omp P5, Omp P6 and late antigen YeaT (also called D15) [35] were identified in the OMP preparations of the Nagasaki strain by MALDI-TOFF using MASCOTT software analysis at the Institut de Biotecnologia i de Biomedicina of the Universitat Autònoma de Barcelona.
2.5. Immunoblotting of recombinant VtaA
Recombinant E. coli pellets were solubilized by adding 50 μL 4× SDS, Urea 8 M buffer pH = 7.6 (4% SDS, 8 M Urea, 0.2 M DTT, 10 mM NaHPO4, 60 mM NaCl, and 10 mM pefabloc) to a pellet from a culture of OD600 = 1. The same buffer was used to adjust the concentration of purified proteins to 0.3 μg/μL. These suspensions were denatured at 94 °C for 15 min, 5 μL mixed with 5 μL of a loading buffer (2× LDS (Invitrogen), 5% β-mercaptoethanol and 1.2× SDS, Urea 2.4 M buffer) and incubated at 70 °C for 10 min. The production of recombinant protein was confirmed in a NuPAGE® Novex 4–12% Bis-Tris Gel gel stained with SYPRO Tangerine (Invitrogen). Immunoblotting was performed as described above. For IgA detection an anti-porcine IgA conjugated with HRP diluted 1:100000 (AbD Serotec) was used. Negative controls for immunoblotting assays included pre-immune sera collected prior to infection or vaccination and, for non-purified recombinant VtaA, E. coli Rosetta(DE3)pLysS transformed with an empty pASK-IBA33plus plasmid. As positive controls, the 6× His C-terminal residues of the recombinant VtaA were detected using an Anti-His(C-term)-HRP Antibody (Invitrogen).
3. RESULTS
3.1. Kinetics of the antibody response against whole OMP
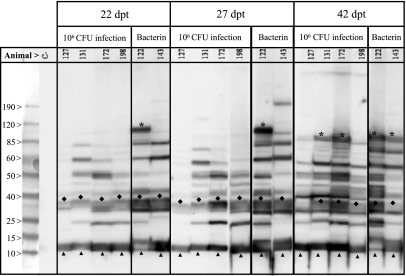
Following H. parasuis infection or bacterin immunization, all animals progressively developed a specific IgG response varying in intensity and in the number of detected OMP for each individual piglet (Fig. 1). By 42 dpt, all antiserum recognized nearly the same antigenic repertoire. Sera collected at 42 dpt from animals infected with live bacteria (127, 131, 172 and 198) and vaccinated (122 and 143) were comparable, although they had different chemiluminescence intensities. No reactivity was detected with the pre-immune sera (data not shown). An immune response to early (Omp P5 and Omp P6) and late (YaeT) antigens [35] was identified (Fig. 1).
Figure 1.
Detection by immunoblot of IgG against OMP from the Nagasaki strain of H. parasuis. Sera collected 22, 27 and 42 dpt from animals subclinically infected (127, 131, 172 and 198) or immunized with a bacterin (122 and 143) were tested by immunoblotting with H. parasuis Nagasaki OMP. The positions of two early antigens, Omp P5 and Omp P6, and a late antigen, YaeT are indicated. Molecular size standards in kDa are shown on the left. C−: Pooled pre-immune sera; * YaeT: Outer membrane protein assembly factor; ♦ Omp P5: Outer membrane protein P5; ▲ Omp P6: Outer membrane protein P6.
3.2. Antigenicity of the VtaA passenger domains of the H. parasuis Nagasaki strain
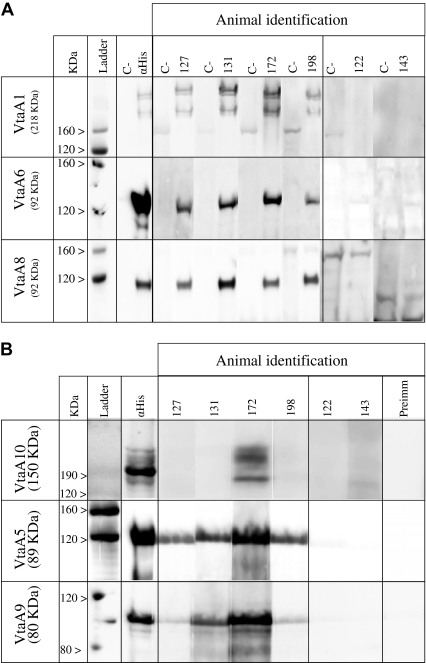
None of the animals showed clinical signs post-inoculation or post-vaccination. Since normal colonization of animals with E. coli is unavoidable, immunoblot interpretation was difficult when recombinant and E. coli immunoreactive proteins had similar molecular weights. Therefore, recombinant VtaA5, VtaA9 and VtaA10 had to be purified. Specific porcine IgG to 6 (VtaA1, 5, 6, 8, 9 and 10) of the 13 VtaA passenger domains of the Nagasaki strain were detected in sera from piglets infected subclinically (see Fig. 2 for an example). Multiple banding was observed with the highest molecular weight VtaA, probably corresponding to residual mutimeric forms of the native protein.
Figure 2.
Immunoblot screening for the presence of IgG toward recombinant VtaA passenger domains. Sera from animals undergoing a subclinical infection (127, 131, 172 and 198) or bacterin immunization (122, 143) at 42 dpt were tested by immunoblotting for the presence of specific IgG against VtaA passenger domains; an example for each positive VtaA is given. The in silico calculated sizes of the Vta-reactive material are indicated in column 1. Molecular weight standards are on the left of the figure. (A) For VtaA1, VtaA6 and VtaA8 a lysate of recombinant E. coli Rosetta was used for the screening. An E. coli Rosetta strain transformed with an empty plasmid was used as the negative control (C−). (B) For VtaA5, VtaA10 and VtaA9 purified proteins were used. Pre-immune sera were used as the negative control.
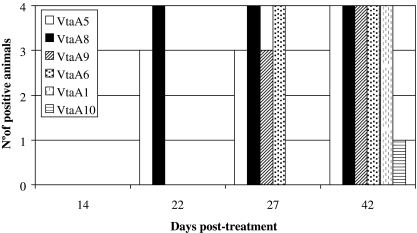
To monitor the appearance of antibodies during the course of infection, sera collected at different dpt were tested by immunoblotting. The VtaA first detected by IgG antibodies were VtaA5 and VtaA8 (22 dpt), followed 5 days later by VtaA6 and VtaA9. Finally, at the end of the experiment (42 dpt), VtaA1 and VtaA10 were also revealed by sera from infected animals, although VtaA10 was detected only by the sera of animal 172 (Fig. 3). The intensity of the antibody response against VtaA increased over time. IgA production was detected only at 42 dpt against one of the VtaA (VtaA10) and, only in one animal (172) infected with a subclinical dose (result not shown). No specific IgG or IgA antibodies against the VtaA were detected with pooled pre-immune sera of the 4 animals. Sera from bacterin-immunized piglets were consistently negative for VtaA during the course of the experiment despite a strong antibody response against OMP (Fig. 1).
Figure 3.
Kinetics of VtaA-specific IgG in H. parasuis infected piglets. The accumulated number of animals (4 in total) producing specific IgG to VtaA at different dpt is presented. The figure summarizes all results obtained by immunoblotting.
3.3. Antibody reactivity against the different fragments of the VtaA10 passenger domain
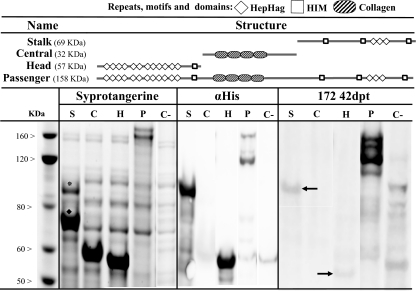
VtaA10 fragments (stalk, collagen repeats and head) were used to study antibody reactivity along VtaA structural domains, but they also revealed their respective importance for VtaA multimerization. After molecular size separation by SDS-PAGE, the central collagen repeats showed an apparent molecular weight of 60 kDa instead of the expected 32 kDa (Fig. 4). This reflected a strong interaction between monomers. Similar findings were observed with the stalk segment when the SDS and urea concentrations were not sufficient to disrupt entirely the multimeric structure of this protein fragment. Aberrant protein tertiary structure folding was further demonstrated by the weak reactivity of the anti-histidine monoclonal antibody against the collagen domains and stronger recognition of the multimeric stalk. The head domain migrated at the expected rate for a domain of this size with exposed histidine tails. Using pig immune serum (animal 172 at 42 dpt) none of the three VtaA10 fragments studied yielded a good antibody detection signal compared to the whole passenger domain, although they were more efficiently produced by the transformed E. coli. The central collagen repeats were not detected while the multimeric stalk and the head showed some reactivity with immune sera 172 (42 dpt).
Figure 4.
Detection and antigenic properties of three fragments of the VtaA10 passenger domain. The stalk, collagen repeats and head of the entire VtaA10 passenger domain were produced in E. coli Rosetta. Size separated proteins were detected by the Sypro tangerine stain. In a parallel SDS-PAGE, recombinant proteins were immunodetected using a monoclonal antibody against the histidine C-terminal tails (αHis) of the recombinant polypeptides or serum of animal 172 collected at 42 dpt. E. coli Rosetta transformed with an empty pASK-IBA33 plasmid was used as a negative control (C−). Molecular size standards are shown on the left of the figure. Arrows indicate weak specific bands for the head and multimeric stalk domains of VtaA10. * Indicates multimeric and ♦ monomeric forms of the stalk.
3.4. Antibody cross-reactivity with VtaA from a heterologous strain
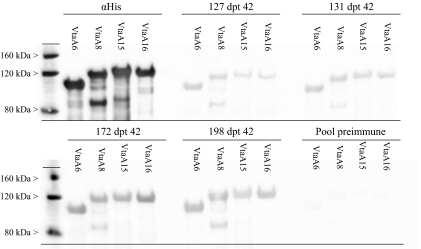
Pre-immune sera and sera at 42 dpt of the 4 animals (127, 131, 172 and 198) were used to evaluate the degree of cross-reaction with purified VtaA15 and VtaA16 (group 1 VtaA) passenger domains belonging to strain HP1319. Purified orthologs of VtaA15 and 16 in the Nagasaki strain, VtaA6 and VtaA8, were used as positive controls. Amino acid identities between orthologs ranged between 72 and 95% for their head while the stalks were more than 90% identical [25]. The number of collagen repeats varied from two to four. Overall, the same IgG binding patterns (and intensity of binding) were obtained with the heterologous and homologous VtaA. No reaction was detected with the corresponding pre-immune sera (Fig. 5).
Figure 5.
Cross-reactivity of antibodies generated against VtaA of the H. parasuis Nagasaki strain with orthologues of strain HP1319. Sera from animals undergoing a subclinical infection (127, 131, 172 and 198) were used in immunoblotting experiments with recombinant VtaA15 and VtaA16 passenger domains cloned from H. parasuis strain HP1319. All VtaA were purified, and 1.5 μg of each were used in the immunoblots. Positive controls were VtaA6 and VtaA8 while the negative control was pooled pre-immune sera. αHis: Monoclonal antibody against histidine C-terminal tails.
4. DISCUSSION
AT-2 are thought to be good universal vaccine candidates for the human pathogen serogroup B meningococcus. They are surface exposed molecules that participate in host-pathogen interactions associated with virulence and can induce a good antibody bactericidal response [2, 10]. The immune response against AT-2 has been evaluated in human pathogens using animal models or convalescent sera; here we report the antibody response against AT-2 after a controlled exposure of a pathogen to its natural host. Furthermore, VtaA are the first AT-2 described in H. parasuis [25] and their capacity to induce a specific antibody response after an infection with this bacterium will help understand host-pathogen interactions in Glässer’s disease.
Sera from all pigs exposed to a subclinical dose of H. parasuis recognized a similar OMP antigenic repertoire, but to different degrees. A genetic difference among animals is the most likely reason for this observation. IgG antibodies against VtaA were produced by animals inoculated with live bacteria, but not by the piglets immunized with a bacterin although they had an efficient qualitative and quantitative antibody response against OMP. These results indicate that VtaA are expressed in vivo, but not, or very little, in the in vitro growth conditions used. However, it can not be ruled out that they will be expressed using other culture media or growth conditions. Also, the results obtained with bacterin-immunized piglets should be interpreted carefully since only 2 animals were used, and bacterial cultures for bacterin production were grown in a different media than for OMP purification. Supporting these results, and in agreement with the proteome map of H. parasuis strain SHO165 [35], our proteomic analysis of high molecular weight OMP preparations from the Nagasaki strain grown in vitro never revealed VtaA. Furthermore, VtaA transcription was detected in a study using samples from necrotic swine lung, but not in several in vitro studies using cultures mimicking the host conditions [11, 18, 19].
Following inoculation of the Nagasaki strain, IgG against VtaA started to be detected at 22 dpt and, progressively, more VtaA were detected. VtaA are more likely to be late antigens when compared with well described early (Omp P5 and Omp P6) and late antigens (YaeT). This pattern of detection could be due to a sequential expression of VtaA during the course of infection together with the production of antibodies specific for each VtaA. This could be indicative of a surface antigen switching to escape the host immune response. The lack of antibodies against the remaining VtaA of virulence-associated group 1 (VtaA2, VtaA3 and VtaA7) and group 2 (VtaA11) pointed to the existence of VtaA specific antibodies therefore supporting this hypothesis. Also, no reactivity was found with the more divergent passenger domains of group 3 VtaA12 and VtaA13 [25]. Alternatively, the strong cross-reactivity with VtaA15 and VtaA16, the orthologues of Nagasaki VtaA5 and VtaA6, strongly suggests the presence of some shared epitopes among group 1 VtaA. This would indicate that only a limited number of VtaA are expressed and the maturation of the immune response is progressively driven toward epitopes common to different VtaA. The relationship between amino acid sequence variability and cross-reactivity is of special interest for AT-2 based vaccines in order to elucidate the VtaA repertoire necessary to induce good protection.
Immunoblots of the head, the collagen repeats and the stalk fragments of VtaA10 were weakly antigenic compared to the whole passenger domain. The need for the whole passenger domain for antibody reactivity indicates that antibodies against VtaA10 are mostly directed against conformational epitopes, which can be partially recovered after denaturation. Furthermore, it seems that the complete passenger domain has a greater resistance to denaturation and is better recognized by the antibodies. In other bacterial species, AT-2 like NadA are known to maintain secondary and tertiary structure after denaturation conditions [5, 14]. However, convalescent sera from patients with meningitis react against the NadA passenger domains and all sub-fragments derived from it [13].
No serological IgA response was detected against the VtaA of the Nagasaki strain albeit with only one exception. With the human respiratory pathogen Moraxella catarrhalis, a mucosal IgA and a serological IgG response against AT-2 (UspA or Hag) can be detected in convalescent sera, and both responses correlate with clearance of pathogenic strains [17, 22, 23]. In contrast, with the human systemic pathogen Neisseria meningitidis, NadA induces only IgG responses [13]. Interestingly, the H. parasuis Nagasaki strain is also highly invasive and causes meningitis and can be detected in the lung only for a short period of time after experimental inoculation [32]. Further studies will be necessary to establish a protective role for antibodies against VtaA.
In conclusion, these results show the production of an IgG response against VtaA in piglets infected with H. parasuis, indicating that they are expressed in vivo and might play a role in the interaction between the host and pathogen in animals exposed to the bacteria. Accordingly, they could be good candidates to be used as immunogens for vaccine development. However, whether this IgG response against the VtaA confers protection against H. parasuis infection and Glässer’s disease onset has to be further studied.
Acknowledgments
The authors would like to thank the Institut de Biotecnologia i de Biomedicina of the Universitat Autònoma de Barcelona for its collaboration. We thank Dr Virginia Aragon and Mark Felice for critical review of the manuscript. This work was founded by the Spanish Ministerio de Ciencia e Innovación (AGL2007-60432).
References
- 1.Aebi C., Maciver I., Latimer J.L., Cope L.D., Stevens M.K., Thomas S.E., et al. , A protective epitope of Moraxella catarrhalis is encoded by two different genes, Infect. Immun. (1997) 65:4367–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bambini S., Muzzi A., Olcen P., Rappuoli R., Pizza M., Comanducci M., Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus, Vaccine (2009) 27:2794–2803 [DOI] [PubMed] [Google Scholar]
- 3.Blanco I., Galina-Pantoja L., Oliveira S., Pijoan C., Sanchez C., Canals A., Comparison between Haemophilus parasuis infection in colostrums-deprived and sow-reared piglets, Vet. Microbiol. (2004) 103:21–27 [DOI] [PubMed] [Google Scholar]
- 4.Carlone G.M., Thomas M.L., Rumschlag H.S., Sottnek F.O., Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species, J. Clin. Microbiol. (1986) 24:330–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comanducci M., Bambini S., Brunelli B., Adu-Bobie J., Arico B., Capecchi B., et al. , NadA, a novel vaccine candidate of Neisseria meningitidis, J. Exp. Med. (2002) 195:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter S.E., Surana N.K., St. Geme J.W. 3rd, Trimeric autotransporters: a distinct subfamily of autotransporter proteins, Trends Microbiol. (2005) 13:199–205 [DOI] [PubMed] [Google Scholar]
- 7.Del Rio M.L., Navas J., Martin A.J., Gutierrez C.B., Rodriguez-Barbosa J.I., Rodriguez Ferri E.F., Molecular characterization of Haemophilus parasuis ferric hydroxamate uptake (fhu) genes and constitutive expression of the FhuA receptor, Vet. Res. (2006) 37:49–59 [DOI] [PubMed] [Google Scholar]
- 8.Forsgren A., Brant M., Riesbeck K., Immunization with the truncated adhesin Moraxella catarrhalis immunoglobulin D-binding protein (MID764-913) is protective against M. catarrhalis in a mouse model of pulmonary clearance, J. Infect. Dis. (2004) 190:352–355 [DOI] [PubMed] [Google Scholar]
- 9.Girard V., Mourez M., Adhesion mediated by autotransporters of Gram-negative bacteria: structural and functional features, Res. Microbiol. (2006) 157:407–416 [DOI] [PubMed] [Google Scholar]
- 10.Giuliani M.M., Adu-Bobie J., Comanducci M., Arico B., Savino S., Santini L., et al. , A universal vaccine for serogroup B meningococcus, Proc. Natl. Acad. Sci. USA (2006) 103:10834–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill C.E., Metcalf D.S., MacInnes J.I., A search for virulence genes of Haemophilus parasuis using differential display RT-PCR, Vet. Microbiol. (2003) 96:189–202 [DOI] [PubMed] [Google Scholar]
- 12.Leduc I., Olsen B., Elkins C., Localization of the domains of the Haemophilus ducreyi trimeric autotransporter DsrA involved in serum resistance and binding to the extracellular matrix proteins fibronectin and vitronectin, Infect. Immun. (2009) 77:657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litt D.J., Savino S., Beddek A., Comanducci M., Sandiford C., Stevens J., et al. , Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease, J. Infect. Dis. (2004) 190:1488–1497 [DOI] [PubMed] [Google Scholar]
- 14.Magagnoli C., Bardotti A., De Conciliis G., Galasso R., Tomei M., Campa C., et al. , Structural organization of NadADelta(351–405), a recombinant MenB vaccine component, by its physico-chemical characterization at drug substance level, Vaccine (2009) 27:2156–2170 [DOI] [PubMed] [Google Scholar]
- 15.Martin de la Fuente A.J., Rodriguez-Ferri E.F., Frandoloso R., Martinez S., Tejerina F., Gutierrez-Martin C.B., Systemic antibody response in colostrum-deprived pigs experimentally infected with Haemophilus parasuis, Res. Vet. Sci. (2009) 86:248–253 [DOI] [PubMed] [Google Scholar]
- 16.Mason K.W., Zhu D., Scheuer C.A., McMichael J.C., Zlotnick G.W., Green B.A., Reduction of nasal colonization of nontypeable Haemophilus influenzae following intranasal immunization with rLP4/rLP6/UspA2 proteins combined with aqueous formulation of RC529, Vaccine (2004) 22:3449–3456 [DOI] [PubMed] [Google Scholar]
- 17.Meier P.S., Freiburghaus S., Martin A., Heiniger N., Troller R., Aebi C., Mucosal immune response to specific outer membrane proteins of Moraxella catarrhalis in young children, Pediatr. Infect. Dis. J. (2003) 22:256–262 [DOI] [PubMed] [Google Scholar]
- 18.Melnikow E., Dornan S., Sargent C., Duszenko M., Evans G., Gunkel N., et al. , Microarray analysis of Haemophilus parasuis gene expression under in vitro growth conditions mimicking the in vivo environment, Vet. Microbiol. (2005) 110:255–263 [DOI] [PubMed] [Google Scholar]
- 19.Metcalf D.S., MacInnes J.I., Differential expression of Haemophilus parasuis genes in response to iron restriction and cerebrospinal fluid, Can. J. Vet. Res. (2007) 71:181–188 [PMC free article] [PubMed] [Google Scholar]
- 20.Miniats O.P., Smart N.L., Ewert E., Vaccination of gnotobiotic primary specific pathogen-free pigs against Haemophilus parasuis, Can. J. Vet. Res. (1991) 55:33–36 [PMC free article] [PubMed] [Google Scholar]
- 21.Miniats O.P., Smart N.L., Rosendal S., Cross protection among Haemophilus parasuis strains in immunized gnotobiotic pigs, Can. J. Vet. Res. (1991) 55:37–41 [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy T.F., Brauer A.L., Aebi C., Sethi S., Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease, Infect. Immun. (2005) 73:8161–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy T.F., Brauer A.L., Aebi C., Sethi S., Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease, Infect. Immun. (2005) 73:3471–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen R., Pathogenicity and immunity studies of Haemophilus parasuis serotypes, Acta Vet. Scand. (1993) 34:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pina S., Olvera A., Barcelo A., Bensaid A., Trimeric autotransporters of Haemophilus parasuis: generation of an extensive passenger domain repertoire specific for pathogenic strains, J. Bacteriol. (2009) 191:576–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapp-Gabrielson V., Oliveira S., Pijoan C., Haemophilus parasuis, in: Straw B.E., Zimmerman J.J., D’Allaire S., Taylor D.J.(Eds.), Diseases of swine, Iowa State University Press, Iowa, 2006, p. 1153 [Google Scholar]
- 27.Riising H.J., Prevention of Glässer’s disease through immunity to Haemophilus parasuis, Zentralbl. Veterinarmed. B (1981) 28:630–638 [DOI] [PubMed] [Google Scholar]
- 28.Scarselli M., Serruto D., Montanari P., Capecchi B., Adu-Bobie J., Veggi D., et al. , Neisseria meningitidis NhhA is a multifunctional trimeric autotransporter adhesin, Mol. Microbiol. (2006) 61:631–644 [DOI] [PubMed] [Google Scholar]
- 29.Sjolinder H., Eriksson J., Maudsdotter L., Aro H., Jonsson A.B., Meningococcal outer membrane protein NhhA is essential for colonization and disease by preventing phagocytosis and complement attack, Infect. Immun. (2008) 76:5412–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smart N.L., Miniats O.P., Preliminary assessment of a Haemophilus parasuis bacterin for use in specific pathogen free swine, Can. J. Vet. Res. (1989) 53:390–393 [PMC free article] [PubMed] [Google Scholar]
- 31.Szczesny P., Lupas A., Domain annotation of trimeric autotransporter adhesins – daTAA, Bioinformatics (2008) 24:1251–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vahle J.L., Haynes J.S., Andrews J.J., Experimental reproduction of Haemophilus parasuis infection in swine: clinical, bacteriological, and morphologic findings, J. Vet. Diagn. Invest. (1995) 7:476–480 [DOI] [PubMed] [Google Scholar]
- 33.Zhang B., Tang C., Yang F.L., Yue H., Molecular cloning, sequencing and expression of the outer membrane protein A gene from Haemophilus parasuis, Vet. Microbiol. (2009) 136:408–410 [DOI] [PubMed] [Google Scholar]
- 34.Zhou M., Guo Y., Zhao J., Hu Q., Hu Y., Zhang A., et al. , Identification and characterization of novel immunogenic outer membrane proteins of Haemophilus parasuis serovar 5, Vaccine (2009) 27:5271–5277 [DOI] [PubMed] [Google Scholar]
- 35.Zhou M., Zhang A., Guo Y., Liao Y., Chen H., Jin M., A comprehensive proteome map of the Haemophilus parasuis serovar 5, Proteomics (2009) 9:2722–2739 [DOI] [PubMed] [Google Scholar]