Abstract
This paper reviews the current understanding of the vitamin D-induced differentiation of neoplastic cells, which results in the generation of cells that acquire near-normal, mature phenotype. Examples of the criteria by which differentiation is recognized in each cell type are provided, and only those effects of 1α,25-dihydroxyvitamin D3 (1,25D) on cell proliferation and survival that are associated with the differentiation process are emphasized. The existing knowledge, often fragmentary, of the signaling pathways that lead to vitamin D-induced differentiation of colon, breast, prostate, squamous cell carcinoma, osteosarcoma, and myeloid leukemia cancer cells is outlined. The important distinctions between the different mechanisms of 1,25D-induced differentiation that are cell-type and cell-context specific are pointed out where known. There is a considerable body of evidence that the principal human cancer cells can be suitable candidates for chemoprevention or differentiation therapy with vitamin D. However, further studies are needed to fully understand the underlying mechanisms in order to improve the therapeutic approaches.
Keywords: Differentiation, myeloid leukemia, signaling pathways, solid tumors, vitamin D
Introduction
In general, differentiation is a term that signifies the structural and functional changes that lead to maturation of cells during development of various lineages. Cancer cells are unable, to varying degrees, to achieve such maturation, and thus malignant neoplastic cells show a lack of, or only partial evidence of, differentiation, known as anaplasia. Since the basic underlying cause for the failure to differentiate can be attributed to structural changes in the cell’s DNA, i.e. mutations, which are essentially irreversible, it is remarkable that some compounds can induce several types of malignant cells to undergo differentiation toward the more mature phenotypes. The physiological form of vitamin D, 1α,25-dihydroxyvitamin D3 (1,25D), is one such compound, and the importance of this finding is that it offers the potential to be an alternative to, or to provide an adjunctive intervention to, the therapy, as well as to act in the prevention of neoplastic diseases.
The feasibility of differentiation therapy of cancer is supported by the early observations that some cases of neuroblastoma, a childhood malignancy, can spontaneously differentiate into tumors that are composed of normal-appearing neuronal cells, and the child’s life is spared(1,2). The reasons for this conversion have not been elucidated, but it seems reasonable to assume that, as the child matures, the endocrine and immune systems become more efficient, and one or more of such factors are able to induce differentiation of neural precursor cells to the more mature, non-invasive forms.
An example of an already successful interventional approach to differentiation therapy of a neoplastic disease is the use of all-trans retinoic acid (ATRA) for the treatment of acute promyelocytic leukemia (APL) and perhaps other leukemias (3–5). Additionally, a synthetic analog of ATRA, Fenretinide, can potentially serve as an agent that can prevent breast cancer in women(6), illustrating the fact that a demonstration of a clear clinical therapeutic effect of a differentiation agent opens up the possibility that it may also serve as a cancer chemopreventive compound.
While the role of 1,25D in cancer chemotherapy and cancer chemoprevention is only beginning to be established, there are several reasons to believe that its promise will be fulfilled. These reasons include the fact that 1,25D is a naturally occurring physiological substance and thus unlikely to cause the adverse reactions that occur when xenobiotics are administered to patients, unless it is given in very high concentrations. Second, the issue of hypercalcemia, which occurs when the concentrations of 1,25D greatly exceed the physiological range and has previously limited its clinical applications (7,8), can be addressed by the dual strategy of developing analogs of 1,25D with reduced calcium-mobilizing properties (9–12),and combining these with other compounds that enhance the differentiation-inducing actions of 1,25D or its analogs(13–15). Also, progress is being made in understanding the mechanisms responsible for 1,25D-induced differentiation, summarized later in this review, and although this understanding is by no means complete, it is likely that insights will be obtained that can be translated into clinical applications.
Differentiation of neoplastic cells induced by 1,25D and other agents rarely, if ever, results in the generation of completely normal, functioning cells. Indeed, the appearance of cells resulting from induced progenitors has been aptly described as resembling “caricatures” rather than normal cells. Such cells may exhibit, and are recognized by, some features of the normal, mature cells of the particular developmental lineage but seldom function like the mature normal cells. However, this is not the major objective of differentiation therapy of neoplastic diseases; the real benefits are due to the cessation of the proliferation of these cells, which is a consequence of cell cycle arrest associated with differentiation(16–19) and in some cases to the reduced survival of the differentiated cells. For instance, 1,25D-induced monocytic differentiation of myeloid leukemia cells can result in the G1 phase cell cycle block, resulting in cessation of cell proliferation(19), while 1,25D treatment of breast or prostate cancer cells can induce cell death by apoptosis as well as by differentiation(20–22).
An important consideration in the area of 1,25D-induced differentiation is cell type and cell context specificity. For instance, in contrast to breast and prostate cancer cells, which are induced to undergo apoptosis, in myeloid leukemia cells, 1,25D-induced differentiation is accompanied by increased cell survival(23,24). The pathways that are known to signal 1,25D-induced differentiation and the associated cell cycle and survival effects also differ, though they may overlap, in different cell types. This may be complicated further by the type of mutations that are responsible for the block of differentiation and the resulting uncontrolled proliferation of the neoplastic cells. We therefore discuss separately the principal cancer cell types known to be candidates for differentiation therapy or chemoprevention by 1,25D.
Solid tumors
Colon cancer
It is well established that colon cancer cells in culture can undergo differentiation to a more mature phenotype, and the inducing agents include the short-chain fatty acid butyrate and 1,25D. The evidence for differentiation has traditionally been the expression of the hydrolytic enzyme alkaline phosphatase (Alk Pase), which can be demonstrated on the microvilli and tubulovacuolar system of the surface “principal cells” of the colon mucosa(25,26) but is poorly expressed in proliferating colon cancer cells(27). More recently, other markers of colonic epithelial cell differentiation have been identified, and these include changes in “transepithelial electrical resistance” and ubiquitin, as based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS). The latter procedure generates specific mass spectral fingerprints characteristic of cell differentiation, and it was suggested that ubiquitin can be a marker of differentiation of the T84 human colon carcinoma cell line(28). In another colon cancer cell line, SW80, 1,25D was shown to induce easily recognizable morphological changes indicative of differentiated epithelial-like phenotype(29). These morphological changes include consequences of the adherence to the culture substratum, which make the cells look flat and polygonal, and it was demonstrated that these cells have reduced tumorigenicity when implanted into athymic mice. Thus, the epidemiological data that indicate that 1,25D has a negative effect on the incidence of human colorectal cancer(30,31) are well supported by the in vitro studies of 1,25D-induced differentiation of colon carcinoma cell lines.
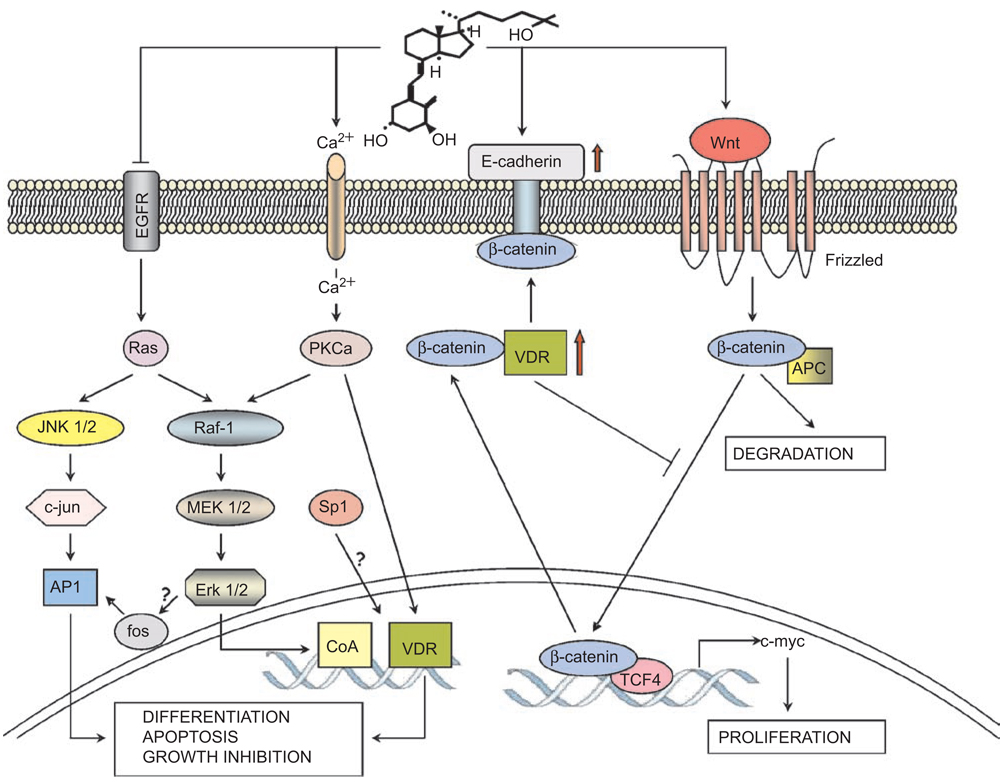
How 1,25D signals differentiation of colon cancer cells is not entirely clear, but several groups of key molecules have been identified that appear to govern this process, and an outline of their postulated interactions is integrated in Figure 1. One mechanism that can explain the reduced cell proliferation that accompanies differentiation is the marked inhibitory effect of 1,25D on the expression of epidermal growth factor receptor (EGFR), apparent at both mRNA and protein levels in CaCo-2 cells(32). The accumulated data also suggest that the central role in 1,25D-induced differentiation is played by the vitamin D receptor (VDR). An early study demonstrated that 1,25D has a protective effect on chemically induced rat colon carcinogenesis(33), and others showed that VDR can be a marker for colon cancer cell differentiation(34,35). This was followed up by Cross and colleagues in a series of experiments that showed that VDR levels increased in early stages of carcinogenesis, or in human colonic mucosa during early tumor development, but that VDR levels were low in poorly differentiated late-stage carcinomas(36,37). This suggested that VDR levels have a restraining effect on the growth of colon cells. A mechanism that can explain the increased levels of VDR in differentiated colon cells was provided by the indication that, in CaCo-2 cells, 1,25D causes an increased activity of the AP-1 transcription factor(27), which is downstream from the mitogen-activated protein kinases (MAPK) pathways and can transactivate VDR gene expression(38). The consequent up-regulation of VDR may further be increased in the presence of 1,25D by stabilization of the VDR protein(39), but the nature of the initial activation of MAPK pathways in colon cancer cells is not entirely clear. The suggested calcium-induced activation of protein kinase C alpha (PKC α) as an upstream event in MAPK activation (27,40) appears to be feasible, as an influx of calcium into the cells is known to occur after 1,25D exposure of many types of cells including colon cancer(41), but this pathway remains to be further investigated. Nonetheless, the importance of VDR in colon cancer cell differentiation is further underscored by the suggestion that butyrate-induced differentiation of CaCo-2 cells is mediated by VDR(42) and by the recent report that decreased recruitment of VDR to the vitamin D response elements (VDRE) contributes to the reduced transcriptional responsiveness of proliferating CaCo-2 cells to 1,25D(43).
Figure 1.
The suggested pathways of 1,25D-induced differentiation in colon cancer. In proliferating colon epithelial cells the β-catenin complexed with TCF-4 drives the expression of growth promoting genes such as c-myc. This is under the control of Wnt and its surface receptor Frizzled, which inactivate GSK-3β (not shown) and allow the accumulation of β-catenin and thus growth promotion. Binding of β-catenin by VDR, or by other proteins, including E-cadherin, the expression of which is induced by 1,25D (formula shown) leads to the loss of β-catenin from the transcriptional complex in the nucleus, and, as a consequence, to decreased cell proliferation. Also shown is the activation of PKCα by 1,25D-induced influx of calcium (Ca2+), which can activate by phosphorylation the transcriptional activity of VDR and repression of EGFR by 1,25D in colon-derived cells.
An emerging role for VDR, other than its function as a transcription factor that binds to VDRE in the promoter regions of 1,25D-responsive genes, is exemplified by the finding that VDR can interact with β-catenin and thereby repress its oncogenic gene-regulatory activity in colon cells(29). The transrepression of β-catenin signaling is not limited to an interaction with VDR, as such interactions can take place with other nuclear receptors, such as the retinoic acid receptor (RAR) and the androgen receptor(29,44). This interaction has been shown to involve also the co-activator p300, a histone acetyl transferase(45). The recently reported repression of the VDR gene by the transcription factor SNAIL(46) and the repression by 1,25D of the Wingless-related MMTV integration site (Wnt) antagonist DICKOPF-4(47) may also be important for the inhibition of Wnt/ β-catenin signaling by 1,25D and for its induction of differentiation in colon cancer cells.
Signaling by β-catenin can also be repressed by the 1,25D-induced up-regulation of the expression of E-cadherin(29), a transmembrane protein that plays a major role in the maintenance of the adhesive and polarized phenotype of epithelial cells(48). The presence of E-cadherin can promote nuclear export of β-catenin, and this may be augmented by direct VDR/β-catenin interaction(48). Since β-catenin/T-cell transcription factor 4 (TCF-4) complex is the nuclear effector of the Wnt growth-signaling pathway, responsible for the expression of c-myc and other growth promoting genes(49), the repressive effects of 1,25D on the growth of colon cancer cells may be explained by the ability of 1,25D to regulate the expression of VDR, E-cadherin and the activity of the β-catenin/TCF pathway, as illustrated in Figure 1.
In addition to protein-protein complex formation with β-catenin, VDR has also been reported to interact with the transcription factor-specificity protein 1 (Sp1) in SW 620 human cancer cells and thus to induce the expression of p27/Kip1 inhibitor of the cell cycle(50). However, it is not clear precisely how this is achieved given the ubiquitous nature of Sp1 binding sites in gene promoters. Nonetheless, the direct binding of VDR to other proteins, which may be ligand independent, is an area that deserves further study and has been reported to occur in cells types other than colon carcinoma, such as osteoblastic cells and myeloid leukemia, as discussed later.
Breast cancer
The induction of differentiation of breast cancer cell lines by 1,25D and the role of 1,25D in normal development of rodent mammary tissue are well established. For instance, studies of VDR knock-out mice have shown that 1,25D participates in the growth inhibition of the normal mammary gland(51). Further, the disruption of 1,25D/VDR signaling leads to distorted morphology of murine mammary gland with duct abnormalities and increased numbers of preneoplastic lesions, suggesting that 1,25D-liganded VDR serves to maintain differentiation of normal mammary epithelium(52).
Induction of differentiation of breast cancer cells by 1,25D can be demonstrated by β-casein production(53) or by a change in overall cell size and shape, associated with changed cytoarchitecture of actin filaments and microtubules in MDA-MB-453 cells (54). Treatment of these cells with 1,25D resulted in accumulation of integrins, paxillin, and focal adhesion kinase as well as their phosphorylation. In contrast, the mesenchymal marker N-cadherin and the myoepithelial marker P-cadherin were down-regulated, suggesting that 1,25D reverses the myoepithelial features associated with the aggressive forms of human breast cancer. However, it is to be noted that not all breast cancer cell lines respond to 1,25D. In many cases this can be attributed to the lack of or low VDR expression or function(55,56), but it may also be due to alterations in 1,25D-metabolizing enzymes, which can reduce the levels of 1,25D below its effective concentration(57).
Among the breast cancer cell lines that do respond to 1,25D, a range of phenotype alterations has been reported(58), emphasizing that the mechanistic basis for the differentiating effects of 1,25D in the breast cancer cell system will be very complex. Together with the uncertainty over whether induced differentiation of breast cancer cells, per se, has potential clinical significance, mechanistic studies in this system have been largely directed to the antiproliferative effects of 1,25D on breast cancer cells. These studies revealed that induction of apoptosis and G1 cell cycle arrest result in inhibition of tumor cell growth in several types of breast cancer cells(20,57,59), but the relationship of these biological effects to differentiation is not obvious. Nonetheless, some hints did result from those studies, as detailed below.
An interesting set of candidate 1,25D-target proteins was identified by proteomic screening of a breast cancer cell line sensitive to 1,25D (MCF-7) and from a subclone of these cells derived by resistance to 1,25D (MCF-7/DRES)(60); and some of these proteins can be related to differentiation and associated phenotypic cellular changes. Examples are Rho-GDI and Rock-DI, known to participate in the formation of focal adhesions and stress fibers, which contribute to the adhesive epithelial phenotype and changes in cell shape(60). Proteins previously linked to pathways involved in 1,25D-induced differentiation, such as phospho-p38, MEK2, and RAS-GAP, were also noted in this screen(52). In a tissue culture study, the JNK pathway, also known to contribute to 1,25D-induced differentiation of colon and myeloid cells(61), was shown to cooperate with the p38 pathway to transactivate VDR in breast cancer cells, but it was proposed that this contributes to the anti-proliferative rather than the differentiation-inducing effects of 1,25D in these cells(38). The antiproliferative effects of 1,25D can also be explained by the reduction in EGFR mRNA and protein, but this is seen in only some breast cancer cell lines(62,63).
Another suggested link to differentiation in 1,25D-treated breast cancer cells is that VDR and estrogen receptor (ER) pathways converge to regulate BRCA-1, thus controlling the balance between signaling of differentiation and proliferation(64). Since ER is important for mammary gland differentiation, studies that pursue this concept would be very valuable, and it already appears that the over-expression of ER and VDR is not sufficient to make ER-negative breast cancer cells responsive to 1α,hydroxy-vitamin D5, a vitamin D analog known to mediate differentiation in a manner similar to that of 1,25D(65,66).
Prostate cancer
Similar to breast cancer, prostate cancer originates in hormone-dependent epithelial cells, and, as in breast cancer cell lines, 1,25D has anti-proliferative effects in some, but not all, established prostate cancer cell lines. The anti-proliferative action of 1,25D is, to a variable degree, due to the induction of cell death by apoptosis(67) and to cell cycle arrest(68), but to what extent these are associated with differentiation is uncertain.
The evidence of prostate cancer cell differentiation includes the release of prostate specific antigen (PSA) from cells treated with a differentiating agent, such as 1,25D(69–71). This can be useful in cultured cells, but, in patients, the increasing PSA levels suggest progressive disease, making it difficult to acquire data on the role of differentiation in clinical trials(72). A study of the role of 1,25D in the differentiation of the normal rat prostate gland was based on morphological characteristics, which included an increased abundance of cytoplasmic secretory vesicles(73). This characteristic has been used as a differentiation marker, along with the expression of keratins 8, 17, and 18, in human prostate cancer PC-3 cells(74). In other studies(75,76), the increased expression of E-cadherin was used as a marker of differentiation. However, although many reports on the effects of 1,25D on prostate cancer cells include the word “differentiation,” the documentation most often focuses on the anti-proliferative effects of 1,25D exposure, which may or may not be associated with phenotypic differentiation.
In a recent microarray analysis of 1,25D regulation of gene expression in LNCaP cells, Krishman et al.(77) reported several findings that appear relevant to 1,25D-induced differentiation. In addition to the major up-regulation of the expression of the insulin-like growth factor binding protein-3 (IGFBP-3), which functions to inhibit cell proliferation by up-regulating p21/Cip1(78), it was noted that among about a dozen genes up-regulated by 1,25D was the “prostate differentiation factor,” a member of the bone morphogenetic protein (BMP) family, which is generally involved in growth and differentiation of both embryonic and adult tissues(79). Also interesting was the finding that, in these cells, 1,25D regulates those genes that are androgen-responsive as well as the genes that encode the enzymes involved in androgen catabolism.
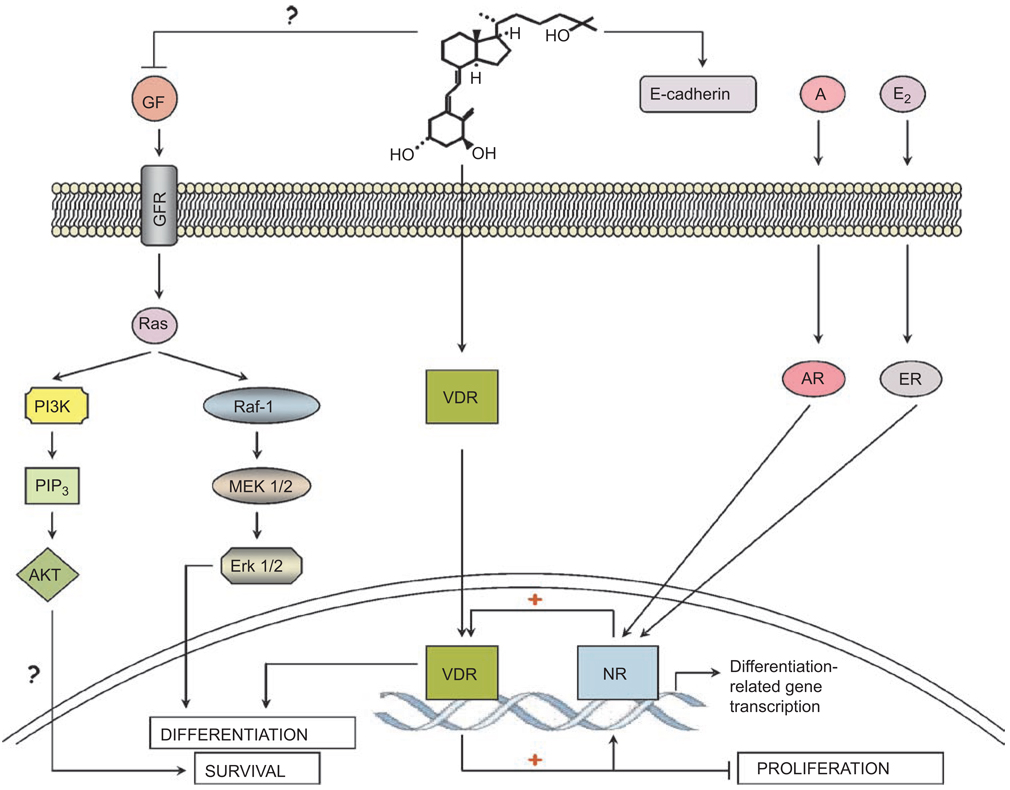
Furthermore, it has been shown that 1,25D up-regulates the expression and activity of the androgen receptor (AR)(80,81), raising the possibility that the differentiation effects of 1,25D on prostate cells are not direct but due to modifications in the level or activity of AR. Interestingly, it has also been suggested that androgens up-regulate the expression of VDR(82); thus, a positive feedback loop that includes 1,25D activation of VDR could be a factor in inducing differentiation of cancer cells derived from the hormonally regulated tissues (Figure 2), while, in normal cells, the sex hormone (androgen or estrogen) is sufficient to promote differentiation. Since 1,25D has an established anti-cancer activity in prostate cells, it can be assumed that, in this scenario, VDR selectively enhances the AR-mediated androgenic pro-differentiation but not the proliferation-enhancing activity (Figure 2). In addition, it is likely that nuclear receptors for retinoids, glucocorticoids, and PPAR affect the signaling pathways, directly or indirectly. Whether the demonstrated 1,25D-induced decrease in the expression of COX-2 and increase in 15-PGDH in prostate cancer cells(77,83) has any relationship to cell differentiation remains to be established.
Figure 2.
Signaling of differentiation by 1,25D in hormone-dependent cancer cells. This schematic illustrates the hypothesis that in normal breast or prostate cells, estrogen (E2) or androgen (A) is sufficient to induce differentiation, respectively. In cancer cells the differentiation signal provided by the hormone-liganded nuclear receptor (NR) may need to be amplified by the cooperation with 1,25D-activated VDR to induce differentiation. Since cells also receive signals from growth factors (GF), several of which activate Ras, the presence of a Ras-activated signaling pathways is exemplified by the AKT and ERK cascades, though the role of these pathways in the differentiation of hormone-dependent cells is uncertain.
Prostate cancer cells are also known to undergo “trans-differentiation” toaneuroendocrinephenotype, and when this phenotype is found in human tumors, it may indicate an aggressive form of the disease(84). Although, currently, 1,25D has no known role in this form of differentiation, this may be a promising area of future research, since recent studies point to a key role for NFκB, as well as IL-6, in this process(85,86). This suggestion is based on the finding that, in some cells, 1,25D up-regulates the expression of C/EBP β(87), which cooperates with NFκB in regulation of the secretion of the cytokine IL-6 in neuroendocrine human prostate cancer cells(85).
Keratinocytes and Squamous cell carcinoma cells
While there is extensive evidence of 1,25D-induced differentiation in normal keratinocytes, the studies of the induction of differentiation in squamous cell carcinomas (SCC), composed essentially of neoplastic keratinocytes, are less conclusive. Differentiation can be detected by the presence of various components of the keratinizing cells, such as cytokeratins K1 and K10, cornifin-beta, involucrin, and transglutaminase, considered to be a late marker of squamous cell differentiation to normal epidermal keratinocytes(88). The expression of target genes of 1,25D and analogs can also be taken as evidence that SCC cell lines can be driven to differentiation by these compounds(89). Such genes include N-cadherin, which, when over-expressed, restores the epithelial phenotype also in prostate cancer cells(90), cystatin M, protease M, type XIII collagen, and desmoglein 3(89). Bikle and colleagues have presented persuasive models for induction of keratinocyte differentiation by increased calcium levels and by calcium-1,25D interactions(91,92). The key features of calcium-induced human keratinocyte differentiation appear to include the recruitment of phosphatidylinositol 3-kinase (PI3K) to a complex at the cell plasma membrane consisting of E-cadherin, β-catenin, and p120-catenin. This complex is postulated to activate PI3K, leading to the accumulation of phosphatidylinositol 3,4,5-triphosphate (PIP3), which binds to and activates phospholipase C gamma-1 (PLC-γ1)(93,94). The activated phospholipase generates inositol triphosphate (IP3), which stimulates the release of calcium from the intracellular stores in the endoplasmic reticulum, and diacylglycerol, which, together with increased intracellular calcium, activates PKC. PKC, and perhaps calcium activation of other enzymes, then initiate signaling cascades that impinge on nuclear transcription factors such as AP-1, which lead to differentiation (95)
How much of this description applies to the 1,25D-induced differentiation is less clear, but Bikle et al.(91) presented a plausible model in which 1,25D interacts with calcium to induce keratinocyte differentiation. This model also includes a G-protein-coupled calcium-sensing surface receptor (CaR), which, when activated by 1,25D leads to the activation of PKC, with consequences described above. The associated influx of calcium, which occurs in human keratinocytes after exposure to 1,25D, has been recently shown to be mediated, at least in part, by the calcium-selective channel TRPV6 up-regulated at the mRNA and protein levels by 1,25D(96). A cohesive picture of 1,25D-induced keratinocyte differentiation is quite well, but perhaps not completely, developed. For instance, regulation of AP-1 activity in cultured human keratinocytes by 1,25D was reported to be independent of PKC(97), in contrast to the model presented by Bikle et al.(91). Takahashi et al.(98) reported that treatment of normal human keratinocytes with 1,25D increases the expression of cystatin A, a cysteine protease inhibitor, that is a component of the cornified envelope, and that it is the suppression of the Raf-1/MEK-1/ERK signaling pathway that is responsible for this effect. However, cystatin A expression is stimulated by the Ras/MEKK-1MKK7/JNK pathway(99), consistent with the schematic model of Bikle et al.(91), explaining why PKC activation may not be essential for AP-1 activation in this cell system.
An enigmatic role of caspase-14 in keratinocyte differentiation induced by 1,25D has been reported(100), and it was suggested that the absence of caspase-14 contributes to the psoriatic phenotype. Since caspase-14 is a nonapoptotic protein, it is unclear if this is related to the report that 1,25D protects keratinocytes from apoptosis(101). On the other hand, the identification of Kruppel-like factor 4 (KLF-4) and c-fos as 1,25D-responsive genes in gene expression profiling of 1,25D-treated keratinocytes(102) fits in well with the existing knowledge of differentiation signaling, as c-fos is a component of the AP-1 transcription factor, and KLF-4 is a transcription factor with a major role in cell fate decisions(103–105). Recently, it was reported that yet another transcription factor, PPAR-gamma, also has a major role in 1,25D-induced differentiation of keratinocytes(106). In these studies, dominant negative (dn) PPAR-gamma inhibited the expression of involucrin (a differentiation marker), suppressed AP-1 binding to DNA, and prevented the 1,25D-induced phosphorylation of p38. Thus, the keratinocyte system provided a wealth of interesting information on 1,25D as a differentiation-promoting and survival-regulating agent.
Transformed keratinocytes, which give rise to SCC, tend to be resistant to the differentiation-inducing action of 1,25D(107,108), even though apoptosis and cell cycle arrest induced by 1,25D have been demonstrated in models of SCC(109,110). While VDR expression is required for 1,25D-induced differentiation, the resistance of SCCs to 1,25D is not due to the lack of functional VDR(111). The possible explanations for the 1,25D resistance include the finding that the VDRE in the human PLCγ-1 gene is not functional(111). Another explanation for this resistance is that increased serine phosphorylation of retinoid X receptor alpha (RXRα) by the Ras/MAPK pathway leads to its degradation, and thus VDR loses its heterodimeric partner for gene transactivation(112). Yet another possibility is that VDR co-activators in SCCs are not appropriate for transactivation of differentiation-inducing genes(95). Specifically, it was suggested that the expression of differentiation markers required a complex of VDR with the Src family of co-activators(113), but in SCC the DRIP co-activator complex is over-expressed, and there is a failure of SCCs to switch from DRIP to Src, resulting in inability to express genes required for differentiation. It would be interesting to learn if this model has a wider applicability.
Osteosarcoma and osteoblasts
Differentiation as well as growth inhibition have been documented in 1,25D-treated human and rat osteosarcoma cells(114,115). The differentiation was recognized by a morphological change to the chondrocyte phenotype and by increased Alk Pase staining. The presence of Alk Pase or osteocalcin could also be detected at the mRNA level(115). In a human fetal osteoblastic cell line responsive to 1,25D, mineralized nodules were detected(116), demonstrating that an advanced degree of differentiation can be achieved in this cell system. Interestingly, 1,25D-induced differentiation in osteoblasts and osteocytes is accompanied by an increase in the potential for cell survival through enhanced anti-apoptotic signaling(117). It is possible that this is mediated by EGFR-relayed signals, as in contrast to other cell types(32,62,118), 1,25D-treated osteoblastic cells show increased levels of EGFR mRNA(119).
Recent studies suggest that the anti-apoptotic effects of 1,25D on osteoblasts and osteocytes are mediated by Src, PI3K, and JNK kinases(117). The suggested mechanisms include an association of Src with VDR, though transcriptional mechanisms were required, as shown by an inhibition of the biological effect by exposure to actinomycin D or cycloheximide. The association of VDR with other proteins may be particularly important in osteoblasts induced to differentiate by 1,25D, as another group reported that IGF-binding protein-5 (IBP-5) interacts with VDR and blocks the RXR/VDR heterodimerization in the nuclei of MG-63 and U2-OS cells, thus attenuating the expression of bone differentiation markers(120). Also, in ROS 17/28 cells the NFκB p65 subunit integrates into the VDR transcription complex and disrupts VDR binding to its co-activator Src-1(121). Although protein-protein binding between VDR and p65 subunit has not been demonstrated, this remains a possibility, further highlighting the importance of this mode of control of VDR activity.
Leukemias
Hematological malignancies are a diverse group of diseases but can be divided into two major groups, lymphocytic and myeloid leukemias. Although normal activated B and T lymphocytes express VDR, and 1,25D has antiproliferative effects on these cell types(122,123), this does not appear to alter their differentiation state, and lymphocytic leukemia cells do not respond to 1,25D. In contrast, 1,25D has been known since 1981 to induce maturation of mouse myeloid leukemia cells(124), and this can also take place in a wide variety of human myeloid leukemia cell lines, with the exception of the lines derived from the most immature acute myeloid leukemia (AML) blast cells (125–127).
Differentiation induced by 1,25D usually results in a monocyte-like phenotype, but prolonged exposure to 1,25D confers cell surface changes that result in adherence to the substratum, making the differentiated cells macrophage-like(124,128). The monocyte characteristics are recognized by changes related to phagocytosis, such as the ability to break down esters, assayed by the “non-specific esterase” (NSE) cytochemical reaction, also known as “monocytespecific esterase” (MSE) since, in hematopoietic cells, this esterase is specific for monocytes and macrophages(129). Also related to phagocytosis is the ability to generate reactive oxygen species (ROS), including superoxide, usually recognized by nitro bluetetrazolium (NBT) orcytochromereduction(130,131). The availability of flow cytometry (FC) for the recognition of surface proteins has made the study of the differentiating effects of 1,25D on myeloid leukemia cells quite simple, using CD14, a receptor for complexes of lipopolysaccarides (LPS) and LPS-binding protein(132), a near-definitive marker of the monocytic phenotype. This is usually supplemented by the FC determination of CD11b or another subunit of the human neutrophil surface protein that mediates cellular adherence(133).
In contrast to myeloid cells induced to differentiate by the phorbol ester TPA, in 1,25D-treated cells, the ability to adhere develops more slowly than the ability to phagocytose. Consequently, 1,25D treatment results in an earlier appearance of the CD14 antigen, usually accompanied in parallel by MSE positivity, than the appearance of CD11b and NBT positivity(134,135). Generally, at least two of the above parameters are measured to demonstrate monocytic differentiation, and FC methods require the use of paired isotypic IgG controls for each test sample to avoid obtaining false-positive data. Exposure of AML cells to 1,25D also results in G1 phase cell cycle arrest, which follows, rather than precedes, the phenotypic differentiation(134) and is often taken as the confirmatory evidence that differentiation has taken place. However, in contrast to cells from most solid tumors, monocytic differentiation of AML cells is accompanied by increased expression of anti-apoptotic proteins, and, consequently, 1,25D-treated myeloid cells have an increased cell survival potential(136–140).
The topic of 1,25D-induced leukemia cell differentiation has been extensively studied in many laboratories. These include several groups in Japan(141–145) and a group in Birmingham, England(146,147), who made many valuable contributions to the field. Notably, combined basic and clinical studies of 1,25D-induced leukemia cell differentiation were very comprehensively developed by Koeffler and his various collaborators(148–151). What follows in the remainder of this section is an outline of the signaling mechanisms of AML cells that have occupied the attention of the corresponding author’s laboratory.
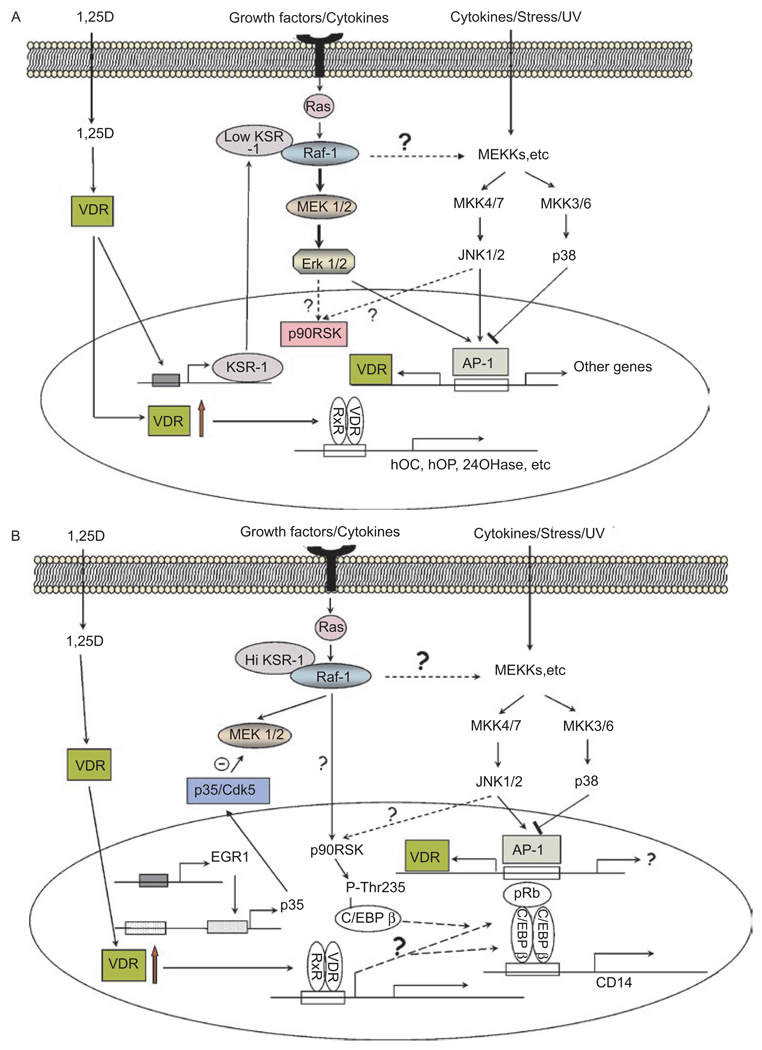
In these studies, we have focused on HL60 cells, a widely available cell line derived from a patient with promyeloblastic leukemia, with the objective of achieving with the currently available tools as clear a picture as possible of the signaling of monocytic differentiation. In this model, outlined in Figure 3(A and B), a plausible sequence of events is presented, but it is likely that other pathways are also operative but remain to be convincingly demonstrated. The details of the scheme are described below.
Figure 3.
(A) Suggested signaling of the early stages of 1,25D-induced monocytic differentiation. Binding of 1,25D to VDR stimulates its translocation to the cell nucleus where it heterodimerizes with RXR, and in myeloid precursor cells, it transactivates genes containing VDREs in their promoter regions. These include genes that encode proteins involved in calcium homeostasis and bone integrity, such as osteocalcin (hOC), osteopontin (hOP), and the 1,25D-catabolic enzyme 24-hydroxylase (24OHase). It is postulated that the regulators of signaling pathways, e.g. KSR-1, are also up-regulated in myeloid cells and alter Ras signaling from the cell membrane so that signaling by MAPKs (MEKs, ERKs, and JNKs) increases the AP-1 activity. This can have a positive feedback effect on differentiation by increasing VDR abundance. It is also suggested that a potential negative feedback mechanism is provided by p38 MAPK, as inhibition of its signaling by SB203580 enhances 1,25D-induced monocytic differentiation. (B) Later stages of 1,25D-induced differentiation. This figure illustrates that the transcription factor EGR-1, known to be up-regulated by 1,25D (189), can increase the expression of p35/Nck5a (p35) activator of Cdk5. Cdk5 activated by p35 then can phosphorylate MEK on Thr286, a site that inactivates it (200), as shown by the Θ symbol. This diminishes ERK1/2 activity downstream from MEK (not shown here), but Raf-1 can activate p90RSK directly, which, in turn, activates the transcription factor C/EBP β, perhaps bound to pRb, and increases the expression of CD14, as part of monocytic differentiation. The activation of p90RSK may also be increased by the JNK pathway, which also activates AP-1, and may lead to VDR expression. The interplay between the signaling by 1,25D, growth factor, and stress add to the overall complexity of the induction of the monocytic phenotype.
Signaling of monocytic differentiation by MAPK and parallel pathways
Early in our investigations, we recognized that 1,25D-induced monocytic differentiation is not a single continuous process but a series of events that can be divided into at least two overlapping phases. In the first phase, which lasts 24–48 h, the cells continue in the normal cell cycle while expressing markers of monocytic phenotype, such as CD14 and NSE. In the next phase, the G1 to S phase cell cycle block becomes apparent, and the expression of CD11b is also prominent, indicating a beginning of the transition to the macrophage phenotype. The first phase is characterized by high levels of ERKs activated by phosphorylation, and these levels decrease as the cells enter the second phase, while the levels of the cell cycle inhibitor p27KIp1 increase at that time. Serum-starved HL60 cells or cells treated with the MAPK inhibitor PD 98059 have a reduced growth rate and a slower rate of differentiation, but the G1 block under these conditions also coincides with decreased levels of activated ERK1/2(152). Our data suggested that the MEK/ERK pathway maintains cell proliferation during the early stages of differentiation and that the consequent G1 block leads to “terminal” differentiation. Using a different experimental design, similar results were obtained by Marcinkowska (153).
We also demonstrated that the JNK pathway, as shown by the increased phosphorylation of c-jun, plays a role in the induction of differentiation of HL60 cells by 1,25D. The data showed that 1,25D-induced differentiation of a stable clone of U937 cells transfected with a dominant negative construct of JNK-1 was reduced, as compared to cells transfected with a control construct(154), and potentiation of 1,25D-induced differentiation by the plant anti-oxidants curcumin and silibinin increased the phosphorylation of c-jun(155). This suggested that the JNK-jun pathway is involved in 1,25D-induced differentiation, which was further established in experiments that showed that the AP-1 transcription factor complex is required for this process since c-jun, together with ATF-2, is the principal component of this complex (140). This appears to be of wider significance, as c-jun expression was also reported to enhance macrophage differentiation of U937 cells(156).
However, it seems clear that the ERK and JNK MAPK pathways are not the only ones involved in signaling of 1,25D-induced differentiation. For instance, compounds SB203580 and SB2902190, reported to be specific inhibitors of the alpha and beta isoforms of signaling protein p38 MAP kinase(157), were found to markedly accelerate monocytic differentiation of HL60 cells induced by low concentrations of 1,25D(158). Paradoxically, these compounds also induced a sustained enhancement of p38 phosphorylation and of its activity in cell extracts in the absence of added inhibitor, which raised the possibility of a lack of specificity of SB compounds in this cell system or of an up-regulation of the upstream components of the p38 pathway. In addition, SB 203580 or SB 202190 treatment of HL60 cells resulted in prolonged activation of the JNK and ERK MAPK pathways(158). SB203580 treatment of HL60, HT93, and ML-1 human myeloid leukemia cell lines also increased cellular ERK activity(159). These data are consistent with the hypothesis that in HL60 cells an interruption of a negative feedback loop from a p38 target activates a common regulator of multiple MAPK pathways, but it is also possible that SB203580 has an additional unknown action.
Another signaling cascade known to be activated by 1,25D in human AML cells is the PI3K-AKT pathway, which is often envisaged to signal from the cell membrane to the intracellular regulators in parallel with the MAPK pathways (160). Further, monocytic leukemia cells THP-1 exposed to 1,25D in serum-free medium show a rapid and transient increase in PI3K activity, which was attributed to the formation of a VDR-PI3K protein complex (161). However, it is not clear if the lack of growth factors normally provided by the serum contributes to the observed effects. The role of the PI3K pathway in 1,25D induced differentiation was also studied by Marcinkowska and colleagues(162–164), who showed that the activation of PI3K by 1,25D can also be demonstrated in HL60 cells and that the signal is transmitted to AKT. This function of AKT may contribute to the differentiation-related increase in 1,25D-induced cell survival(139). An additional role of the PI3K, as well as of the Ras/Raf/ERK, pathway in human leukemia cells is the stimulation of steroid sulfatase, an enzyme that converts inactive estrogen and androgen precursors to the active sex hormones(147). If this is also operative in breast and/or prostate tissues, it could offer an explanation for the mutual activation of VDR and the estrogen and androgen nuclear receptors, as shown in Figure 2.
The mechanisms of the up-regulation of MAPK pathways in the initial phase of 1,25D action on leukemia cells are still unclear. The very rapid effects of 1,25D on the MAPK pathway in intestinal cells that result in rapid calcium transport (“transcaltachia”) have been attributed to a cell membrane receptor (“mVDR”)(165–167), but whether direct, non-genomic action of such mVDR can initiate or enhance the activity of MAPK pathways in leukemia cells has not been well documented. In non-starved leukemia cells, 1,25D elicits less rapid (hours rather than minutes) activation of the MAPKs. One possibility is that this is achieved by the transcriptional up-regulation of Kinase Suppressor of Ras-1 (KSR-1), a membrane-associated kinase/molecular scaffold also known as ceramide-activated protein kinase (168,169). Although a kinase activity associated with KSR-1 has been reported(170–172), the best established function of KSR-1 is to provide a platform for Raf-1 kinase to phosphorylate and thus activate its downstream targets in the MAPK pathways(173,174). Thus, since KSR-1 has been shown to have a functional DNA element regulated by VDR (VDRE)(175), the activation of the MAPKs may be a direct “genomic” action of 1,25D, as depicted in Figure 3A, rather than signaling initiation at the membrane and “non-genomic.”
Our studies(169,176), combined with those of Marcinkowska and colleagues(164,177), suggest that leukemia cell differentiation is initiated when 1,25D promotes nuclear translocation of liganded VDR, which dimerizes with RXR and transactivates several VDRE-regulated genes, including KSR-1 and KSR-2. The latter appears to play a role in increasing the survival potential of differentiating monocytic cells(24), while KSR-1 acts as a scaffold that, by simultaneously binding to Ras and Raf-1 (and perhaps to other proteins) facilitates or redirects the signaling cascade, at least initially, to MEK/ERK and thus amplifies the signal that initiates monocytic differentiation (Figure 3A).
Raf-1 participation has been shown to be required for the later stages of differentiation, when an impairment in cell cycle progression becomes apparent, and at this more advanced point in the differentiation process, MEK/ERK signaling does not appear to be involved(178,179). While this requires further study, the current model, also supported by observations in other differentiation signaling systems(180–182), suggests that Raf-1 can signal p90RSK activation independently of MEK and ERK, as outlined in Figure 3B.
A rather speculative mechanism describing how MEK/ERK signaling is diminished in the later stages of differentiation, when cell proliferation becomes arrested, is presented below.
p35/Cdk5, a protein kinase system that may interface differentiation processes with cell cycle arrest
After 24–48 h of exposure of myeloid leukemia cells to moderate concentrations of 1,25D (1– 10 nM), cell cycle progression becomes progressively arrested, principally due to a G1 to S phase block, although a G2 phase block can also be observed(183). Several mechanisms could explain these cell cycle effects, including activation of cyclin-dependent kinase 5 (Cdk5).
Cdk5 is a proline-directed serine-threonine kinase with sequence homology to the cyclin-activated kinases that regulate cell cycle progression, but its best-known function is participation in differentiation of neuronal cells(184). When combined with a “cyclin-like” neuronal Cdk5 activator (Nck5a) 35 kDa protein (p35/Nck5a, or p35), the p35/Cdk5 complex functions in monocytic cells and plays an important role in normal, and possibly abnormal, development of this hematopoietic lineage. Our initial observations were that, in HL60 cells treated with 1,25D, the monocytic phenotype and expression of Cdk5 appear in parallel. Both active and inactive Cdk5 were associated with cyclin D1 protein, and the inhibition of Cdk5 expression by an antisense oligonucleotide construct reduced the intensity of 1,25D -induced expression of the monocytic marker CD14(185). This finding demonstrated a novel (other than neuronal) cellular type for Cdk5 activity and a concomitant enhancement of monocytic differentiation.
The above study showed that protein levels and kinase activity of Cdk5 increase in HL60 cells induced to monocytic differentiation by 1,25D, but did not establish the specificity of the association of Cdk5 with the monocytic phenotype. Therefore, we showed in a subsequent study that the up-regulation of Cdk5 does not occur in granulocytic differentiation, whereas an inhibition of Cdk5 activity by the pharmacological inhibitor olomoucine, or of its expression by a plasmid construct expressing antisense Cdk5, switches the 1,25D–induced monocytic phenotype (a combination of the positive NSE reaction, the expression of the CD14 marker, and morphology) to a general myeloid phenotype (a positive NBT reaction, the CD11b marker, and morphology)(186). These findings showed that, in human myeloid cells, the up-regulation of Cdk5 is specifically associated with the monocytic phenotype.
The Nck5a 35 kDa protein has hitherto been considered to be exclusively expressed in neuronal cells, as its name implies(187). However, since we had clear evidence that Cdk5 is an active kinase in human leukemia cells HL60 and U937 induced to differentiate with 1,25D, and since the “classical” cyclins (e.g. cyclin D1, cyclin E) are not known to activate Cdk5, we investigated whether p35 can be detected in cells with active Cdk5. Indeed, we demonstrated that p35 is expressed in normal human monocytes and in leukemic cells induced to differentiate toward the monocytic lineage but not in lymphocytes, or cells induced to granulocytic differentiation by retinoic acid. The activator p35 is present in a complex with Cdk5 that has protein kinase activity, and when ectopically expressed together with Cdk5 in undifferentiated HL60 cells it induces the expression of CD14 and NSE markers of the monocytic phenotype(188). These observations not only indicate a functional relationship between Cdk5 and p35 but also support a role for this complex in monocytic differentiation.
A likely link to the diminution of ERK MAPK pathway activity at the onset of phase 2 of 1,25D-induced differentiation is provided by the EGR-1→ p35/Cdk5 ---| MEK 1/2 pathway, which was elucidated in leukemia cells by this laboratory (189). The schematic representation is shown in Figure 3B, and the supporting data can be summarized as follows.
Control of p35 expression by the EGR-1 transcription factor
The evidence in support of a role for EGR-1 in regulating the expression of p35 includes the co-ordinate expression of EGR-1 along with Cdk5, and the co-inhibition of the 1,25D –induced up-regulation of these proteins by PD 98059, an inhibitor of the MEK/ERK1/2 pathway (171,190). Further, the promoter region of human p35 has an EGR-1 binding site that overlaps with an Sp1 site, and a gel shift assay showed that a double-stranded oligonucleotide that contained this sequence bound proteins in nuclear extracts from 1,25D-treated HL60 cells. The EGR-1-site binding proteins were competed with most efficiently by an anti-EGR-1 antibody, though some competition was also observed with an anti-Sp1 antibody, but no competition was observed with an irrelevant antibody, e.g. anti-VDR. The data suggested that EGR-1, and perhaps Sp1 proteins, regulate the expression of p35 and contribute to induction of the monocytic phenotype. A “decoy” EGR-1 response element oligonucleotide inhibited both 1,25D-induced p35 expression and monocytic differentiation (189).
The Cdk5/p35 complex phosphorylates MEK
We also found that the Cdk5/p35 can phosphorylate MEK in cell extracts (189). If this can be demonstrated to occur in leukemia cells, it will provide a potential mechanism for the inhibition of the MAPK/ERK pathway seen in the later stages of differentiation (48 h after the addition of 1,25D to the cultures) since phosphorylation of MEK by p35/Cdk5 inhibits its kinase activity. Intriguingly, up-regulation of p35 (which activates Cdk5) is observed pari passu as ERK 1/2 phosphorylation is waning, consistent with a cause-effect relationship. We have thus proposed a mechanism that can shut down cell proliferation, possibly by allowing p27Kip1 to accumulate in the cell nucleus due to a decline in ERK 1/2 activity, since it has been reported that the ERK pathway can increase nuclear export of p27Kip1(191).
C/EBP β transcription factor as an effector of monocytic differentiation
One of the downstream targets of the MAPK-RSK pathway is a nuclear transcription factor, the CAAT and Enhancer Binding Protein β (C/EBP β). This transcription factor has been reported to be activated by phosphorylation both by ERK(192) and by RSK(193) and can interact directly with the promoter of CD14, one of the principal markers of monocytic differentiation(194), as illustrated in Figure 3B. We showed that the expression of C/EBP β is increased by 1,25D in parallel with markers of differentiation; conversely, the knockdown of its expression by antisense oligonucleotides, or of its transcriptional activity by “decoy” promoter competition, inhibited 1,25D-induced differentiation(195). In an additional study, the data suggested that 1,25D induced phosphorylation of C/EBP β isoforms on Thr235, and that the C/EBP β-2 isoform is one of the principal differentiation-related transcription factors in this system(87).
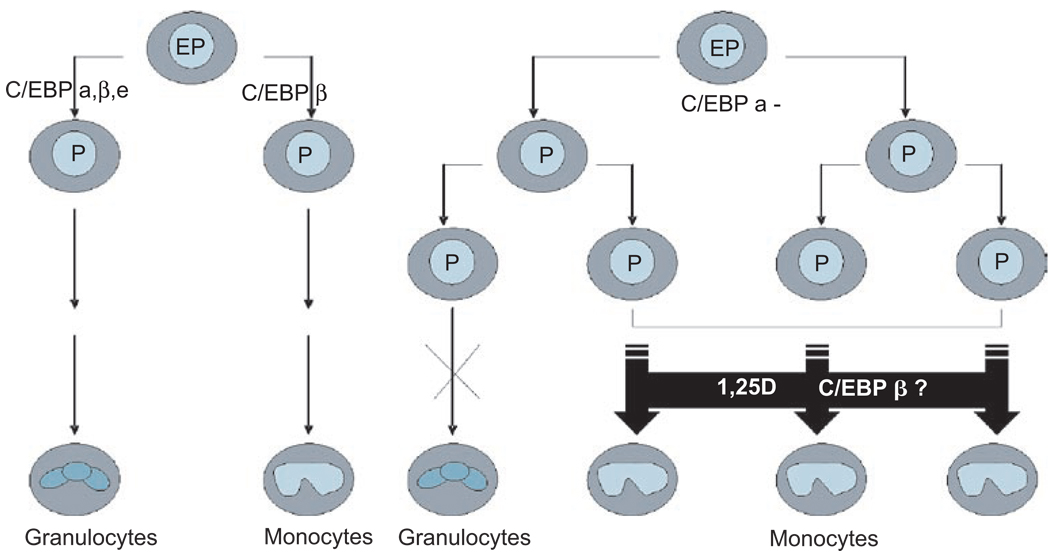
These findings suggest that 1,25D can induce leukemic progenitor cells, which have the potential to differentiate into several hematopoietic lineages, to become non-proliferating monocyte-like cells by changing the ratio of nuclear transcription factors in a manner that permits this form of differentiation(196). In this scenario, the event that initiates leukemic transformation, such as a mutation, alters the proper balance of transcription factor activity necessary for normal granulocytic cell differentiation. However, 1,25D-induced expression of C/EBP β then allows the cells to bypass this block to granulocytic differentiation by becoming monocyte-like cells instead (Figure 4).
Figure 4.
The suggested role of CAAT/Enhancer Binding Protein β in 1,25D-induced bypass of the differentiation block in leukemia cells. In this scenario, C/EBP α is indispensable for normal granulopoiesis, while C/EBP β regulates monocytic differentiation. When C/EBP α is mutated or inactivated and granulopoiesis is blocked, immature myeloid cells accumulate in the bone marrow and appear in the peripheral blood, resulting in AML. 1,25D-induced expression of C/EBP β may allow the cells to bypass this block to granulocytic differentiation by switching the lineage of cell differentiation from granulocytes to monocytes.
Interestingly, 1,25D has also been reported to have a negative effect on differentiation, as it inhibits IL-4/GM-CSF-induced differentiation of human monocytes into dendritic cells, and this contributes to 1,25D immunosuppressive activity(197,198). The data also suggested that 1,25D specifically down-regulates the expression of CSF-1 and promotes spontaneous apoptosis of mature dendritic cells, further demonstrating the pleiotropic effects of 1,25D and the cell-type specificity of the outcomes.
Conclusions
The signaling pathways presented here are shown to control the activity of several transcription factors, such as the ubiquitous AP-1 complex, the nuclear receptor VDR, and the lineage-determining C/EBP family of transcription factors. While these clearly play a role in 1,25D-induced differentiation of HL60 cells, there may be redundancy of important cellular regulators, and other pathways and transcription factors are likely to be involved. The initial steps that activate the differentiation-inducing actions of 1,25D are not entirely clear, and while cell membrane-associated events have a role, these events are not necessarily rapid, but they are sustained. It is likely that micro-RNAs will be found to further control or modulate 1,25D signaling, as retinoic acid-induced differentiation of NB4 AML cells has been shown to be associated with the up-regulation of a number of micro-RNAs, and the down-regulation of micro-RNA 181b(199). Thus, extensive additional investigations are warranted to provide a basis for the design of improved therapies of leukemia and solid tumors.
Acknowledgments
We thank Drs. Michael Danilenko and Ewa Marcinkowska for comments on the manuscript and Ms. Vivienne Lowe for expert secretarial assistance.
Declaration of interest: The authors experimental work was supported by grants from the National Cancer Institute, RO1-CA 44722-18 and RO1-CA 117942-01, and from the Polish Ministry of Science and Higher Education, grant No. 2622/P01/2006/31. The authors alone are responsible for the content and writing of the paper.
Abbreviations and Glossary
- 1,25D
1a,25-dihydroxyvitamin D3
- 24OHase
24-hydroxylase
- A
androgen
- AKT
serine/threonine-specific, protein kinase B
- Alk-Pase
alkaline phosphatase
- AML
acute myeloid leukemia
- AP-1
activating protein-1
- APC
adenomatous polyposis coli
- APL
acute promyelocytic leukemia
- AR
androgen receptor
- ATRA
all-trans retinoic acid
- BMP
bone morphogenetic protein
- BRCA-1
(Breast Cancer-1)-breast cancer tumor suppressor gene
- CaCo-2
human epithelial colorectal adenocarcinoma cell line
- CaR
calcium-sensing surface receptor
- Cdk5
cyclin-dependent kinase 5
- C/EBP
CCAAT/enhancer binding protein
- CoA
co-activator
- COX
Cyclooxygenase
- DRIP
Vitamin D Receptor-Interacting Protein
- E2
estrogen
- EGFR
epidermal growth factor receptor
- EGR-1
early growth response protein-1
- EP
early progenitor
- ER
estrogen receptor
- ERK
extracellular-signal regulated kinase
- FC
flow cytometry
- GF
growth factor
- GFR
growth factor receptor
- GM-CSF
granulocyte macrophage-colony stimulating factor
- hOC
human osteocalcin
- hOP
human osteopontin
- IBP-5
IGF binding protein-5
- IGF
Insulin-like Growth Factor
- IGFBP-3
insulin-like growth factor binding protein-3
- IL-4
Interleukin-4
- IP3
inositol triphosphate
- JNK
Jun N-terminal kinase
- KLF-4
Kruppel-like factor 4
- KSR-1
kinase suppressor of Ras-1
- LPS
lipopolysaccharides
- MALDI-TOFMS
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MAPK
mitogen-activated protein kinase
- MG-63
Human osteosarcoma cell line
- MSE
monocyte-specific esterase (“non-specific” esterase)
- NBT
nitroblue tetrazolium
- Nck5a
“cyclin-like” neuronal Cdk5 activator
- NFkappaB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NR
nuclear receptor
- NSE
non-specific esterase
- P
progenitor
- PGDH
Prostaglandin Dehydrogenase
- p90RSK
ribosomal S6 kinase (MAPK-activated protein kinase-1)
- PI3K
phosphatidylinositol 3-kinase
- PIP3
phosphatidylinositol 3, 4, 5-triphosphate
- PKC
protein kinase C
- PLC-g1
phospholipase C gamma-1
- PPAR
Peroxisome Proliferator-Activated Receptors
- Rb
retinoblastoma protein
- PSA
prostate-specific antigen
- RAR
retinoic acid receptor
- ROS
reactive oxygen species
- RXRa
retinoid X receptor alpha
- SCC
squamous cell carcinoma
- Sp-1
specificity protein-1
- Src
Non-Receptor Protein Tyrosine Kinase
- TCF4
T-cell transcription factor-4
- Wnt
Wingless-related MMTV integration site
- TPA
12-O-tetradecanoylphorbol-13-acetate
- U2-OS
Human osteosarcoma cell line expressing wild type p53 and Rb, but lacking p16
- VDR
vitamin D receptor
- VDRE
vitamin D3 response element
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Smithers DW. Maturation in human tumours. Lancet. 1969;2:949–952. doi: 10.1016/s0140-6736(69)90603-5. [DOI] [PubMed] [Google Scholar]
- 2.Walton JD, Kattan DR, Thomas SK, Spengler BA, Guo HF, Biedler JL, Cheung NK, Ross RA. Characteristics of stem cells from human neuroblastoma cell lines and in tumors. Neoplasia. 2004;6:838–845. doi: 10.1593/neo.04310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen SJ, Zhu YJ, Tong JH, Dong S, Huang W, Chen Y, Xiang WM, Zhang L, Li XS, Qian GQ. Rearrangements in the second intron of the RARalfa gene are present in a large majority of patients with acute promyelocytic leukemia and are used as molecular marker for retinoic acid-induced leukemic cell differentiation. Blood. 1991;78:2696–2701. [PubMed] [Google Scholar]
- 4.Degos L, Wang ZY. All trans retinoic acid in acute promyelocytic leukemia. Oncogene. 2001;20:7140–7145. doi: 10.1038/sj.onc.1204763. [DOI] [PubMed] [Google Scholar]
- 5.Schlenk RF, Frohling S, Hartmann F, Fischer JT, Glasmacher A, del Valle F, Grimminger W, Gotze K, Waterhouse C, Schoch R, Pralle H, Mergenthaler HG, Hensel M, Koller E, Kirchen H, Preiss J, Salwender H, Biedermann HG, Kremers S, Griesinger F, Benner A, Addamo B, Dohner K, Haas R, Dohner H. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia. 2004;18:1798–1803. doi: 10.1038/sj.leu.2403528. [DOI] [PubMed] [Google Scholar]
- 6.Camerini T, Mariani L, De Palo G, Marubini E, Di Mauro MG, Decensi A, Costa A, Veronesi U. Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol. 2001;19:1664–1670. doi: 10.1200/JCO.2001.19.6.1664. [DOI] [PubMed] [Google Scholar]
- 7.Jung SJ, Lee YY, Pakkala S, de Vos S, Elstner E, Norman AW, Green J, Uskokovic M, Koeffler HP. 1,25(OH)2-16ene-vitamin D3 is a potent antileukemic agent with low potential to cause hypercalcemia. Leuk Res. 1994;18:453–463. doi: 10.1016/0145-2126(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 8.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):S582–S586. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 9.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 10.Ji Y, Wang X, Donnelly RJ, Uskokovic MR, Studzinski GP. Signaling of monocytic differentiation by a non-hypercalcemic analog of vitamin D3, 1,25(OH)2-5,6 trans-16-ene-vitamin D3, involves nuclear vitamin D receptor (nVDR) and non-nVDR-mediated pathways. J Cell Physiol. 2002;191:198–207. doi: 10.1002/jcp.10091. [DOI] [PubMed] [Google Scholar]
- 11.Collins ED, Bishop JE, Bula CM, Acevedo A, Okamura WH, Norman AW. Effect of 25-hydroxyl group orientation on biological activity and binding to the 1alpha,25-dihydroxy vitamin D3 receptor. J Steroid Biochem Mol Biol. 2005;94:279–288. doi: 10.1016/j.jsbmb.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Aparna R, Subhashini J, Roy KR, Reddy GS, Robinson M, Uskokovic MR, Venkateswara Reddy G, Reddanna P. Selective inhibition of cyclooxygenase-2 (COX-2) by 1alpha,25-dihydroxy- 16-ene-23-yne-vitamin D3, a less calcemic vitamin D analog. J Cell Biochem. 2008;104:1832–1842. doi: 10.1002/jcb.21749. [DOI] [PubMed] [Google Scholar]
- 13.Danilenko M, Wang X, Studzinski GP. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J Natl Cancer Inst. 2001;93:1224–1233. doi: 10.1093/jnci/93.16.1224. [DOI] [PubMed] [Google Scholar]
- 14.Danilenko M, Wang Q, Wang X, Levy J, Sharoni Y, Studzinski GP. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1alpha,25-dihydroxyvitamin D3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Res. 2003;63:1325–1332. [PubMed] [Google Scholar]
- 15.Danilenko M, Studzinski GP. Enhancement by other compounds of the anti-cancer activity of vitamin D3 and its analogs. Exp Cell Res. 2004;298:339–358. doi: 10.1016/j.yexcr.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Coffman FD, Studzinski GP. Differentiation-related mechanisms which suppress DNA replication. Exp Cell Res. 1999;248:58–73. doi: 10.1006/excr.1999.4457. [DOI] [PubMed] [Google Scholar]
- 17.Harrison LE, Wang QM, Studzinski GP. Butyrate-induced G2/M block in CaCo-2 colon cancer cells is associated with decreased p34cdc2 activity. Proc Soc Exp Biol Med. 1999;222:150–156. doi: 10.1046/j.1525-1373.1999.d01-125.x. [DOI] [PubMed] [Google Scholar]
- 18.Harrison LE, Wang QM, Studzinski GP. 1,25-dihydroxyvitamin D3-induced retardation of the G(2)/M traverse is associated with decreased levels of p34(cdc2) in HL60 cells. J Cell Biochem. 1999;75:226–234. doi: 10.1002/(sici)1097-4644(19991101)75:2<226::aid-jcb5>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Wang QM, Studzinski GP, Chen F, Coffman FD, Harrison LE. p53/56(lyn) antisense shifts the 1,25-dihydroxyvitamin D3-induced G1/S block in HL60 cells to S phase. J Cell Physiol. 2000;183:238–246. doi: 10.1002/(SICI)1097-4652(200005)183:2<238::AID-JCP10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Welsh J, Simboli-Campbell M, Narvaez CJ, Tenniswood M. Role of apoptosis in the growth inhibitory effects of vitamin D in MCF-7 cells. Adv Exp Med Biol. 1995;375:45–52. doi: 10.1007/978-1-4899-0949-7_4. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Ling X, Huang H, Brattain L, Apontes P, Wu J, Binderup L, Brattain MG. Differential regulation of survivin expression and apoptosis by vitamin D3 compounds in two isogenic MCF-7 breast cancer cell sublines. Oncogene. 2005;24:1385–1395. doi: 10.1038/sj.onc.1208330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myrthue A, Rademacher BL, Pittsenbarger J, Kutyba-Brooks B, Gantner M, Qian DZ, Beer TM. The iroquois homeobox gene 5 is regulated by 1,25-dihydroxyvitamin D3 in human prostate cancer and regulates apoptosis and the cell cycle in LNCaP prostate cancer cells. Clin Cancer Res. 2008;14:3562–3570. doi: 10.1158/1078-0432.CCR-07-4649. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Studzinski GP. Antiapoptotic action of 1,25-dihydroxyvitamin D3 is associated with increased mitochondrial MCL-1 and RAF-1 proteins and reduced release of cytochrome c. Exp Cell Res. 1997;235:210–217. doi: 10.1006/excr.1997.3667. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Patel R, Studzinski GP. hKSR-2, a vitamin D-regulated gene, inhibits apoptosis in arabinocytosine-treated HL60 leukemia cells. Mol Cancer Ther. 2008;7:2798–2806. doi: 10.1158/1535-7163.MCT-08-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helander HF. Enzyme patterns and protein absorption in rat colon during development. Acta Anat (Basel) 1975;91:330–349. doi: 10.1159/000144395. [DOI] [PubMed] [Google Scholar]
- 26.Ono K. Alkaline phosphatase activity of the large intestinal principal cells in postnatal developing rats. Acta Histochem. 1976;57:312–319. [PubMed] [Google Scholar]
- 27.Chen A, Davis BH, Bissonnette M, Scaglione-Sewell B, Brasitus TA. 1,25-Dihydroxyvitamin D3 stimulates activator protein-1-dependent CaCo-2 cell differentiation. J Biol Chem. 1999;274:35505–35513. doi: 10.1074/jbc.274.50.35505. [DOI] [PubMed] [Google Scholar]
- 28.Marvin-Guy LF, Duncan P, Wagniere S, Antille N, Porta N, Affolter M, Kussmann M. Rapid identification of differentiation markers from whole epithelial cells by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry and statistical analysis. Rapid Commun Mass Spectrom. 2008;22:1099–1108. doi: 10.1002/rcm.3479. [DOI] [PubMed] [Google Scholar]
- 29.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 32.Tong WM, Hofer H, Ellinger A, Peterlik M, Cross HS. Mechanism of antimitogenic action of vitamin D in human colon carcinoma cells: relevance for suppression of epidermal growth factor-stimulated cell growth. Oncol Res. 1999;11:77–84. [PubMed] [Google Scholar]
- 33.Belleli A, Shany S, Levy J, Guberman R, Lamprecht SA. A protective role of 1,25-dihydroxyvitamin D3 in chemically induced rat colon carcinogenesis. Carcinogenesis. 1992;13:2293–2298. doi: 10.1093/carcin/13.12.2293. [DOI] [PubMed] [Google Scholar]
- 34.Shabahang M, Buras RR, Davoodi F, Schumaker LM, Nauta RJ, Evans SR. 1,25-Dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res. 1993;53:3712–3718. [PubMed] [Google Scholar]
- 35.Vandewalle B, Adenis A, Hornez L, Revillion F, Lefebvre J. 1,25-dihydroxyvitamin D3 receptors in normal and malignant human colorectal tissues. Cancer Lett. 1994;86:67–73. doi: 10.1016/0304-3835(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 36.Sheinin Y, Kaserer K, Wrba F, Wenzl E, Kriwanek S, Peterlik M, Cross HS. In situ mRNA hybridization analysis and immunolocalization of the vitamin D receptor in normal and carcinomatous human colonic mucosa: relation to epidermal growth factor receptor expression. Virchows Arch. 2000;437:501–507. doi: 10.1007/s004280000275. [DOI] [PubMed] [Google Scholar]
- 37.Cross HS, Bareis P, Hofer H, Bischof MG, Bajna E, Kriwanek S, Bonner E, Peterlik M. 25-Hydroxyvitamin D3-1alpha-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids. 2001;66:287–292. doi: 10.1016/s0039-128x(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 38.Qi X, Pramanik R, Wang J, Schultz RM, Maitra RK, Han J, DeLuca HF, Chen G. The p38 and JNK pathways cooperate to trans-activate vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to vitamin D3-induced growth inhibition. J Biol Chem. 2002;277:25884–25892. doi: 10.1074/jbc.M203039200. [DOI] [PubMed] [Google Scholar]
- 39.Wiese RJ, Uhland-Smith A, Ross TK, Prahl JM, DeLuca HF. Up-regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization. J Biol Chem. 1992;267:20082–20086. [PubMed] [Google Scholar]
- 40.Alrefai WA, Scaglione-Sewell B, Tyagi S, Wartman L, Brasitus TA, Ramaswamy K, Dudeja PK. Differential regulation of the expression of Na+/H+ exchanger isoform NHE3 by PKC-alpha in CaCo-2 cells. Am J Physiol Cell Physiol. 2001;281:C1551–C1558. doi: 10.1152/ajpcell.2001.281.5.C1551. [DOI] [PubMed] [Google Scholar]
- 41.Wali RK, Baum CL, Sitrin MD, Brasitus TA. 1,25(OH)2 vitamin D3 stimulates membrane phosphoinositide turnover, activates protein kinase C, and increases cytosolic calcium in rat colonic epithelium. J Clin Invest. 1990;85:1296–1303. doi: 10.1172/JCI114567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaschott T, Werz O, Steinmeyer A, Steinhilber D, Stein J. Butyrate-induced differentiation of CaCo-2 cells is mediated by vitamin D receptor. Biochem Biophys Res Commun. 2001;288:690–696. doi: 10.1006/bbrc.2001.5832. [DOI] [PubMed] [Google Scholar]
- 43.Cui M, Klopot A, Jiang Y, Fleet JC. The effect of differentiation on 1,25 dihydroxyvitamin D-mediated gene expression in the enterocyte-like cell line, Caco-2. J Cell Physiol. 2009;218:113–121. doi: 10.1002/jcp.21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Easwaran V, Pishvaian M, Salimuddin, Byers S. Crossregulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415–1418. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]
- 45.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 46.Larriba MJ, Valle N, Palmer HG, Ordonez-Moran P, Alvarez-Diaz S, Becker KF, Gamallo C, de Herreros AG, Gonzalez-Sancho JM, Munoz A. The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr Relat Cancer. 2007;14:141–151. doi: 10.1677/ERC-06-0028. [DOI] [PubMed] [Google Scholar]
- 47.Pendas-Franco N, Garcia JM, Pena C, Valle N, Palmer HG, Heinaniemi M, Carlberg C, Jimenez B, Bonilla F, Munoz A, Gonzalez-Sancho JM. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 48.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 49.Wilson AJ, Velcich A, Arango D, Kurland AR, Shenoy SM, Pezo RC, Levsky JM, Singer RH, Augenlicht LH. Novel detection and differential utilization of a c-myc transcriptional block in colon cancer chemoprevention. Cancer Res. 2002;62:6006–6010. [PubMed] [Google Scholar]
- 50.Huang YC, Chen JY, Hung WC. Vitamin D3 receptor/Sp1 complex is required for the induction of p27Kip1 expression by vitamin D3. Oncogene. 2004;23:4856–4861. doi: 10.1038/sj.onc.1207621. [DOI] [PubMed] [Google Scholar]
- 51.Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97:153–164. doi: 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004;80 Suppl 6:S1721–S1724. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- 53.Escaleira MT, Brentani MM. Vitamin D3 receptor (VDR) expression in HC-11 mammary cells: regulation by growthmodulatory agents, differentiation, and Ha-Ras transformation. Breast Cancer Res Treat. 1999;54:123–133. doi: 10.1023/a:1006198107805. [DOI] [PubMed] [Google Scholar]
- 54.Pendas-Franco N, Gonzalez-Sancho JM, Suarez Y, Aguilera O, Steinmeyer A, Gamallo C, Berciano MT, Lafarga M, Munoz A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75:193–207. doi: 10.1111/j.1432-0436.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 55.Agadir A, Lazzaro G, Zheng Y, Zhang XK, Mehta R. Resistance of HBL100 human breast epithelial cells to vitamin D action. Carcinogenesis. 1999;20:577–582. doi: 10.1093/carcin/20.4.577. [DOI] [PubMed] [Google Scholar]
- 56.Valrance ME, Brunet AH, Welsh J. Vitamin D receptor-dependent inhibition of mammary tumor growth by EB1089 and ultraviolet radiation in vivo. Endocrinology. 2007;148:4887–4894. doi: 10.1210/en.2007-0267. [DOI] [PubMed] [Google Scholar]
- 57.Byrne B, Welsh J. Identification of novel mediators ofVitamin D signaling and 1,25(OH)2D3 resistance in mammary cells. J Steroid Biochem Mol Biol. 2007;103:703–707. doi: 10.1016/j.jsbmb.2006.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q, Lee D, Sysounthone V, Chandraratna RAS, Christakos S, Korah R, Wieder R. 1,25-dihydroxyvitamin D3 and retinoic acid analogues induce differentiation in breast cancer cells with function- and cell-specific additive effects. Breast Cancer Res Treat. 2001;67:157–168. doi: 10.1023/a:1010643323268. [DOI] [PubMed] [Google Scholar]
- 59.Simboli-Campbell M, Narvaez CJ, van Weelden K, Tenniswood M, Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat. 1997;42:31–41. doi: 10.1023/a:1005772432465. [DOI] [PubMed] [Google Scholar]
- 60.Byrne BM, Welsh J. Altered thioredoxin subcellular localization and redox status in MCF-7 cells following 1,25-dihydroxyvitamin D3 treatment. J Steroid Biochem Mol Biol. 2005;97:57–64. doi: 10.1016/j.jsbmb.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 61.Li QP, Qi X, Pramanik R, Pohl NM, Loesch M, Chen G. Stressinduced c-Jun-dependent Vitamin D receptor (VDR) activation dissects the non-classical VDR pathway from the classical VDR activity. J Biol Chem. 2007;282:1544–1551. doi: 10.1074/jbc.M604052200. [DOI] [PubMed] [Google Scholar]
- 62.McGaffin KR, Acktinson LE, Chrysogelos SA. Growth and EGFR regulation in breast cancer cells by vitamin D and retinoid compounds. Breast Cancer Res Treat. 2004;86:55–73. doi: 10.1023/B:BREA.0000032923.66250.92. [DOI] [PubMed] [Google Scholar]
- 63.McGaffin KR, Chrysogelos SA. Identification and characterization of a response element in the EGFR promoter that mediates transcriptional repression by 1,25-dihydroxyvitamin D3 in breast cancer cells. J Mol Endocrinol. 2005;35:117–133. doi: 10.1677/jme.1.01813. [DOI] [PubMed] [Google Scholar]
- 64.Campbell MJ, Gombart AF, Kwok SH, Park S, Koeffler HP. The anti-proliferative effects of 1alpha,25(OH)2D3 on breast and prostate cancer cells are associated with induction of BRCA1 gene expression. Oncogene. 2000;19:5091–5097. doi: 10.1038/sj.onc.1203888. [DOI] [PubMed] [Google Scholar]
- 65.Lazzaro G, Agadir A, Qing W, Poria M, Mehta RR, Moriarty RM, Das Gupta TK, Zhang XK, Mehta RG. Induction of differentiation by 1alpha-hydroxyvitamin D5 in T47D human breast cancer cells and its interaction with vitamin D receptors. Eur J Cancer. 2000;36:780–786. doi: 10.1016/s0959-8049(00)00016-2. [DOI] [PubMed] [Google Scholar]
- 66.Peng X, Jhaveri P, Hussain-Hakimjee EA, Mehta RG. Overexpression of ER and VDR is not sufficient to make ER-negative MDA-MB231 breast cancer cells responsive to 1alpha-hydroxyvitamin D5. Carcinogenesis. 2007;28:1000–1007. doi: 10.1093/carcin/bgl230. [DOI] [PubMed] [Google Scholar]
- 67.Campbell MJ, Reddy GS, Koeffler HP. Vitamin D3 analogs and their 24-oxo metabolites equally inhibit clonal proliferation of a variety of cancer cells but have differing molecular effects. J Cell Biochem. 1997;66:413–425. [PubMed] [Google Scholar]
- 68.Getzenberg RH, Light BW, Lapco PE, Konety BR, Nangia AK, Acierno JS, Dhir R, Shurin Z, Day RS, Trump DL, Johnson CS. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology. 1997;50:999–1006. doi: 10.1016/S0090-4295(97)00408-1. [DOI] [PubMed] [Google Scholar]
- 69.Zhao XY, Feldman D. The role of vitamin D in prostate cancer. Steroids. 2001;66:293–300. doi: 10.1016/s0039-128x(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 70.Krishnan AV, Peehl DM, Feldman D. Inhibition of prostate cancer growth by vitamin D: regulation of target gene expression. J Cell Biochem. 2003;88:363–371. doi: 10.1002/jcb.10334. [DOI] [PubMed] [Google Scholar]
- 71.Beer TM, Garzotto M, Park B, Mori M, Myrthue A, Janeba N, Sauer D, Eilers K. Effect of calcitriol on prostate-specific antigen in vitro and in humans. Clin Cancer Res. 2006;12:2812–2816. doi: 10.1158/1078-0432.CCR-05-2310. [DOI] [PubMed] [Google Scholar]
- 72.Reiter W. The clinical value of the Enzymun-Test for total and free PSA—a multicentre evaluation. Anticancer Res. 1999;19:5559–5562. [PubMed] [Google Scholar]
- 73.Konety BR, Schwartz GG, Acierno JS, Jr, Becich MJ, Getzenberg RH. The role of vitamin D in normal prostate growth and differentiation. Cell Growth Differ. 1996;7:1563–1570. [PubMed] [Google Scholar]
- 74.Floryk D, Tollaksen SL, Giometti CS, Huberman E. Differentiation of human prostate cancer PC-3 cells induced by inhibitors of inosine 5’-monophosphate dehydrogenase. Cancer Res. 2004;64:9049–9056. doi: 10.1158/0008-5472.CAN-04-1553. [DOI] [PubMed] [Google Scholar]
- 75.Campbell MJ, Elstner E, Holden S, Uskokovic M, Koeffler HP. Inhibition of proliferation of prostate cancer cells by a 19-norhexafluoride vitamin D3 analogue involves the induction of p21waf1, p27kip1 and E-cadherin. J Mol Endocrinol. 1997;19:15–27. doi: 10.1677/jme.0.0190015. [DOI] [PubMed] [Google Scholar]
- 76.Ortel B, Sharlin D, O’Donnell D, Sinha AK, Maytin EV, Hasan T. Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br J Cancer. 2002;87:1321–1327. doi: 10.1038/sj.bjc.6600575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krishnan AV, Shinghal R, Raghavachari N, Brooks JD, Peehl DM, Feldman D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate. 2004;59:243–251. doi: 10.1002/pros.20006. [DOI] [PubMed] [Google Scholar]
- 78.Boyle BJ, Zhao XY, Cohen P, Feldman D. Insulin-like growth factor binding protein-3 mediates 1 alpha,25-dihydroxyvitamin D3 growth inhibition in the LNCaP prostate cancer cell line through p21/WAF1. J Urol. 2001;165:1319–1324. [PubMed] [Google Scholar]
- 79.Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, Ke HZ, Qi H, Owen TA, Thompson DD. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998;273:13760–13767. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- 80.Zhao XY, Ly LH, Peehl DM, Feldman D. Induction of androgen receptor by 1alpha,25-dihydroxyvitamin D3 and 9-cis retinoic acid in LNCaP human prostate cancer cells. Endocrinology. 1999;140:1205–1212. doi: 10.1210/endo.140.3.6561. [DOI] [PubMed] [Google Scholar]
- 81.Zhao XY, Peehl DM, Navone NM, Feldman D. 1alpha,25-dihydroxyvitamin D3 inhibits prostate cancer cell growth by androgen-dependent and androgen-independent mechanisms. Endocrinology. 2000;141:2548–2556. doi: 10.1210/endo.141.7.7549. [DOI] [PubMed] [Google Scholar]
- 82.Tuohimaa P, Lyakhovich A, Aksenov N, Pennanen P, Syvala H, Lou YR, Ahonen M, Hasan T, Pasanen P, Blauer M, Manninen T, Miettinen S, Vilja P, Ylikomi T. Vitamin D and prostate cancer. J Steroid Biochem Mol Biol. 2001;76:125–134. doi: 10.1016/s0960-0760(00)00141-2. [DOI] [PubMed] [Google Scholar]
- 83.Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65:7917–7925. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 84.Cussenot O, Villette JM, Cochand-Priollet B, Berthon P. Evaluation and clinical value of neuroendocrine differentiation in human prostatic tumors. Prostate Suppl. 1998;8:43–51. [PubMed] [Google Scholar]
- 85.Xiao W, Hodge DR, Wang L, Yang X, Zhang X, Farrar WL. Co-operative functions between nuclear factors NFkB and CCAT/enhancer-binding protein-beta (C/EBP-beta) regulate the IL-6 promoter in autocrine human prostate cancer cells. Prostate. 2004;61:354–370. doi: 10.1002/pros.20113. [DOI] [PubMed] [Google Scholar]
- 86.Mori R, Xiong S, Wang Q, Tarabolous C, Shimada H, Panteris E, Danenberg KD, Danenberg PV, Pinski JK. Gene profiling and pathway analysis of neuroendocrine transdifferentiated prostate cancer cells. Prostate. 2009;69:12–23. doi: 10.1002/pros.20851. [DOI] [PubMed] [Google Scholar]
- 87.Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP. Regulation of C/EBPbeta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2006;312:2054–2065. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abban G, Yildirim NB, Jetten AM. Regulation of the vitamin D receptor and cornifin beta expression in vaginal epithelium of the rats through vitamin D3. Eur J Histochem. 2008;52:107–114. doi: 10.4081/1200. [DOI] [PubMed] [Google Scholar]
- 89.White JH. Profiling 1,25-dihydroxyvitamin D3-regulated gene expression by microarray analysis. J Steroid Biochem Mol Biol. 2004;89–90:239–244. doi: 10.1016/j.jsbmb.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 90.Tomita K, van Bokhoven A, van Leenders GJ, Ruijter ET, Jansen CF, Bussemakers MJ, Schalken JA. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–3654. [PubMed] [Google Scholar]
- 91.Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 92.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 93.Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires srcand fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16:3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bikle DD, Oda Y, Xie Z. Vitamin D and skin cancer: a problem in gene regulation. J Steroid Biochem Mol Biol. 2005;97:83–91. doi: 10.1016/j.jsbmb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 96.Lehen’kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, Prevarskaya N. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem. 2007;282:22582–22591. doi: 10.1074/jbc.M611398200. [DOI] [PubMed] [Google Scholar]
- 97.Johansen C, Iversen L, Ryborg A, Kragballe K. 1alpha,25-dihydroxyvitamin D3 induced differentiation of cultured human keratinocytes is accompanied by a PKC-independent regulation of AP-1 DNA binding activity. J Invest Dermatol. 2000;114:1174–1179. doi: 10.1046/j.1523-1747.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi H, Ibe M, Honma M, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. 1,25-dihydroxyvitamin D3 increases human cystatin A expression by inhibiting the Raf-1/MEK1/ERK signaling pathway of keratinocytes. Arch Dermatol Res. 2003;295:80–87. doi: 10.1007/s00403-003-0396-5. [DOI] [PubMed] [Google Scholar]
- 99.Takahashi H, Honma M, Ishida-Yamamoto A, Namikawa K, Kiyama H, Iizuka H. Expression of human cystatin A by keratinocytes is positively regulated via the Ras/MEKK1/MKK7/JNK signal transduction pathway but negatively regulated via the Ras/Raf-1/MEK1/ERK pathway. J Biol Chem. 2001;276:36632–36638. doi: 10.1074/jbc.M102021200. [DOI] [PubMed] [Google Scholar]
- 100.Lippens S, Kockx M, Denecker G, Knaapen M, Verheyen A, Christiaen R, Tschachler E, Vandenabeele P, Declercq W. Vitamin D3 induces caspase-14 expression in psoriatic lesions and enhances caspase-14 processing in organotypic skin cultures. Am J Pathol. 2004;165:833–841. doi: 10.1016/S0002-9440(10)63346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schafer-Korting M, Kleuser B. 1alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001;117:1241–1249. doi: 10.1046/j.0022-202x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- 102.Lu J, Goldstein KM, Chen P, Huang S, Gelbert LM, Nagpal S. Transcriptional profiling of keratinocytes reveals a vitamin D-regulated epidermal differentiation network. J Invest Dermatol. 2005;124:778–785. doi: 10.1111/j.0022-202X.2005.23641.x. [DOI] [PubMed] [Google Scholar]
- 103.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Autieri MV. Kruppel-like factor 4: transcriptional regulator of proliferation, or inflammation, or differentiation, or all three? Circ Res. 2008;102:1455–1457. doi: 10.1161/CIRCRESAHA.108.178954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alder JK, Georgantas RW, 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dai X, Sayama K, Shirakata Y, Tokumaru S, Yang L, Tohyama M, Hirakawa S, Hanakawa Y, Hashimoto K. PPAR gamma is an important transcription factor in 1 alpha,25-dihydroxyvitamin D3-induced involucrin expression. J Dermatol Sci. 2008;50:53–60. doi: 10.1016/j.jdermsci.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 107.Bikle DD, Pillai S, Gee E. Squamous carcinoma cell lines produce 1,25 dihydroxyvitamin D, but fail to respond to its prodifferentiating effect. J Invest Dermatol. 1991;97:435–441. doi: 10.1111/1523-1747.ep12481267. [DOI] [PubMed] [Google Scholar]
- 108.Solomon C, White JH, Kremer R. Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha. J Clin Invest. 1999;103:1729–1735. doi: 10.1172/JCI6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McGuire TF, Trump DL, Johnson CS. Vitamin D3-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J Biol Chem. 2001;276:26365–26373. doi: 10.1074/jbc.M010101200. [DOI] [PubMed] [Google Scholar]
- 110.Ma Y, Yu WD, Kong RX, Trump DL, Johnson CS. Role of nongenomic activation of phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase 1/2 pathways in 1,25D3-mediated apoptosis in squamous cell carcinoma cells. Cancer Res. 2006;66:8131–8138. doi: 10.1158/0008-5472.CAN-06-1333. [DOI] [PubMed] [Google Scholar]
- 111.Xie Z, Bikle DD. Differential regulation of vitamin D responsive elements in normal and transformed keratinocytes. J Invest Dermatol. 1998;110:730–733. doi: 10.1046/j.1523-1747.1998.00175.x. [DOI] [PubMed] [Google Scholar]
- 112.Goltzman D, White J, Kremer R. Studies of the effects of 1,25-dihydroxyvitamin D on skeletal and calcium homeostasis and on inhibition of tumor cell growth. J Steroid Biochem Mol Biol. 2001;76:43–47. doi: 10.1016/s0960-0760(00)00146-1. [DOI] [PubMed] [Google Scholar]
- 113.Oda Y, Sihlbom C, Chalkley RJ, Huang L, Rachez C, Chang CP, Burlingame AL, Freedman LP, Bikle DD. Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Mol Endocrinol. 2003;17:2329–2339. doi: 10.1210/me.2003-0063. [DOI] [PubMed] [Google Scholar]
- 114.Tokuumi Y. Correlation between the concentration of 1,25 alpha dihydroxyvitamin D3 receptors and growth inhibition, and differentiation of human osteosarcoma cells induced by vitamin D3. Nippon Seikeigeka Gakkai Zasshi. 1995;69:181–190. [PubMed] [Google Scholar]
- 115.Van Auken M, Buckley D, Ray R, Holick MF, Baran DT. Effects of the vitamin D3 analog 1 alpha, 25-dihydroxyvitamin D3-3 beta-bromoacetate on rat osteosarcoma cells: comparison with 1 alpha, 25-dihydroxyvitamin D3. J Cell Biochem. 1996;63:302–310. doi: 10.1002/(sici)1097-4644(19961201)63:3<302::aid-jcb5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 116.Harris SA, Enger RJ, Riggs BL, Spelsberg TC. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10:178–186. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- 117.Vertino AM, Bula CM, Chen JR, Almeida M, Han L, Bellido T, Kousteni S, Norman AW, Manolagas SC. Nongenotropic anti-apoptotic signaling of 1alpha 25(OH)2-vitamin D3 and analogs through the ligand binding domain of the vitamin D receptor in osteoblasts andandosteocytes. Mediation by Src, phosphatidylinositol 3-, and JNK kinases. J Biol Chem. 2005;280:14130–14137. doi: 10.1074/jbc.M410720200. [DOI] [PubMed] [Google Scholar]
- 118.Cordero JB, Cozzolino M, Lu Y, Vidal M, Slatopolsky E, Stahl PD, Barbieri MA, Dusso A. 1,25-Dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. J Biol Chem. 2002;277:38965–38971. doi: 10.1074/jbc.M203736200. [DOI] [PubMed] [Google Scholar]
- 119.Gonzalez EA, Disthabanchong S, Kowalewski R, Martin KJ. Mechanisms of the regulation of EGF receptor gene expression by calcitriol and parathyroid hormone in UMR 106-01 cells. Kidney Int. 2002;61:1627–1634. doi: 10.1046/j.1523-1755.2002.00327.x. [DOI] [PubMed] [Google Scholar]
- 120.Schedlich LJ, Muthukaruppan A, O’Han MK, Baxter RC. Insulin-like growth factor binding protein-5 interacts with the vitamin D receptor and modulates the vitamin D response in osteoblasts. Mol Endocrinol. 2007;21:2378–2390. doi: 10.1210/me.2006-0558. [DOI] [PubMed] [Google Scholar]
- 121.Lu X, Farmer P, Rubin J, Nanes MS. Integration of the NFkappaB p65 subunit into the vitamin D receptor transcriptional complex: identification of p65 domains that inhibit 1,25-dihydroxyvitamin D3-stimulated transcription. J Cell Biochem. 2004;92:833–848. doi: 10.1002/jcb.20143. [DOI] [PubMed] [Google Scholar]
- 122.Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsoukas CD, Provvedini DM, Manolagas SC. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984;224:1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- 124.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishii Y, Suda T. 1 alpha,25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60) Biochem J. 1982;204:713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Studzinski GP, Bhandal AK, Brelvi ZS. A system for monocytic differentiation of leukemic cells HL 60 by a short exposure to 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med. 1985;179:288–295. doi: 10.3181/00379727-179-42098. [DOI] [PubMed] [Google Scholar]
- 127.Munker R, Norman A, Koeffler HP. Vitamin D compounds. Effect on clonal proliferation and differentiation of human myeloid cells. J Clin Invest. 1986;78:424–430. doi: 10.1172/JCI112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brackman D, Lund-Johansen F, Aarskog D. Expression of cell surface antigens during the differentiation of HL-60 cells induced by 1,25-dihydroxyvitamin D3, retinoic acid and DMSO. Leuk Res. 1995;19:57–64. doi: 10.1016/0145-2126(94)00061-e. [DOI] [PubMed] [Google Scholar]
- 129.Uphoff CC, Drexler HG. Biology of monocyte-specific esterase. Leuk Lymphoma. 2000;39:257–270. doi: 10.3109/10428190009065825. [DOI] [PubMed] [Google Scholar]
- 130.Steiner M, Priel I, Giat J, Levy J, Sharoni Y, Danilenko M. Carnosic acid inhibits proliferation and augments differentiation of human leukemic cells induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Nutr Cancer. 2001;41:135–144. doi: 10.1080/01635581.2001.9680624. [DOI] [PubMed] [Google Scholar]
- 131.Sharabani H, Izumchenko E, Wang Q, Kreinin R, Steiner M, Barvish Z, Kafka M, Sharoni Y, Levy J, Uskokovic M, Studzinski GP, Danilenko M. Cooperative anti-tumor effects of vitamin D3 derivatives and rosemary preparations in a mouse model of myeloid leukemia. Int J Cancer. 2006;118:3012–3021. doi: 10.1002/ijc.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 133.Hickstein DD, Ozols J, Williams SA, Baenziger JU, Locksley RM, Roth GJ. Isolation and characterization of the receptor on human neutrophils that mediates cellular adherence. J Biol Chem. 1987;262:5576–5580. [PubMed] [Google Scholar]
- 134.Studzinski GP, Rathod B, Wang QM, Rao J, Zhang F. Uncoupling of cell cycle arrest from the expression of monocytic differentiation markers in HL60 cell variants. Exp Cell Res. 1997;232:376–387. doi: 10.1006/excr.1997.3484. [DOI] [PubMed] [Google Scholar]
- 135.Ji Y, Kutner A, Verstuyf A, Verlinden L, Studzinski GP. Derivatives of vitamins D2 and D3 activate three MAPK pathways and upregulate pRb expression in differentiating HL60 cells. Cell Cycle. 2002;1:410–415. doi: 10.4161/cc.1.6.269. [DOI] [PubMed] [Google Scholar]
- 136.Sikora E, Grassilli E, Bellesia E, Troiano L, Franceschi C. Studies of the relationship between cell proliferation and cell death. III.AP-1 DNA-binding activity during concanavalin A-induced proliferation or dexamethasone-induced apoptosis of rat thymocytes. Biochem Biophys Res Commun. 1993;192:386–391. doi: 10.1006/bbrc.1993.1427. [DOI] [PubMed] [Google Scholar]
- 137.Hewison M, Dabrowski M, Vadher S, Faulkner L, Cockerill FJ, Brickell PM, O’Riordan JL, Katz DR. Antisense inhibition of vitamin D receptor expression induces apoptosis in monoblastoid U937 cells. J Immunol. 1996;156:4391–4400. [PubMed] [Google Scholar]
- 138.Marcinkowska E, Chrobak A, Wiedlocha A. Evading apoptosis by calcitriol-differentiated human leukemic HL-60 cells is not mediated by changes in CD95 receptor system but by increased sensitivity of these cells to insulin. Exp Cell Res. 2001;270:119–127. doi: 10.1006/excr.2001.5335. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y, Zhang J, Studzinski GP. AKT pathway is activated by 1, 25-dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle. 2006;5:447–451. doi: 10.4161/cc.5.4.2467. [DOI] [PubMed] [Google Scholar]
- 140.Wang X, Studzinski GP. The requirement for and changing composition of the activating protein-1 transcription factor during differentiation of human leukemia HL60 cells induced by 1,25-dihydroxyvitamin D3. Cancer Res. 2006;66:4402–4409. doi: 10.1158/0008-5472.CAN-05-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakagawa K, Kurobe M, Konno K, Fujishima T, Takayama H, Okano T. Structure-specific control of differentiation and apoptosis of human promyelocytic leukemia (HL-60) cells by A-ring diastereomers of 2-methyl-1alpha,25-dihydroxyvitamin D3 and its 20-epimer. Biochem Pharmacol. 2000;60:1937–1947. doi: 10.1016/s0006-2952(00)00486-x. [DOI] [PubMed] [Google Scholar]
- 142.Urahama N, Ito M, Sada A, Yakushijin K, Yamamoto K, Okamura A, Minagawa K, Hato A, Chihara K, Roeder RG, Matsui T. The role of transcriptional coactivator TRAP220 in myelomonocytic differentiation. Genes Cells. 2005;10:1127–1137. doi: 10.1111/j.1365-2443.2005.00906.x. [DOI] [PubMed] [Google Scholar]
- 143.Ikezoe T, Bandobashi K, Yang Y, Takeuchi S, Sekiguchi N, Sakai S, Koeffler HP, Taguchi H. HIV-1 protease inhibitor ritonavir potentiates the effect of 1,25-dihydroxyvitamin D3 to induce growth arrest and differentiation of human myeloid leukemia cells via down-regulation of CYP24. Leuk Res. 2006;30:1005–1011. doi: 10.1016/j.leukres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 144.Takahashi E, Nakagawa K, Suhara Y, Kittaka A, Nihei K, Konno K, Takayama H, Ozono K, Okano T. Biological activities of 2alpha-substituted analogues of 1alpha,25-dihydroxyvitamin D3 in transcriptional regulation and human promyelocytic leukemia (HL-60) cell proliferation and differentiation. Biol Pharm Bull. 2006;29:2246–2250. doi: 10.1248/bpb.29.2246. [DOI] [PubMed] [Google Scholar]
- 145.Suzuki T, Tazoe H, Taguchi K, Koyama Y, Ichikawa H, Hayakawa S, Munakata H, Isemura M. DNA microarray analysis of changes in gene expression induced by 1,25-dihydroxyvitamin D3 in human promyelocytic leukemia HL-60 cells. Biomed Res. 2006;27:99–109. doi: 10.2220/biomedres.27.99. [DOI] [PubMed] [Google Scholar]
- 146.Hughes PJ, Brown G. 1Alpha,25-dihydroxyvitamin D3-mediated stimulation of steroid sulphatase activity in myeloid leukaemic cell lines requires VDRnuc-mediated activation of the RAS/RAF/ERK-MAP kinase signalling pathway. J Cell Biochem. 2006;98:590–617. doi: 10.1002/jcb.20787. [DOI] [PubMed] [Google Scholar]
- 147.Hughes PJ, Lee JS, Reiner NE, Brown G. The vitamin D receptor-mediated activation of phosphatidylinositol 3-kinase (PI3Kalpha) plays a role in the 1alpha,25-dihydroxyvitamin D3-stimulated increase in steroid sulphatase activity in myeloid leukaemic cell lines. J Cell Biochem. 2008;103:1551–1572. doi: 10.1002/jcb.21545. [DOI] [PubMed] [Google Scholar]
- 148.Munker R, Kobayashi T, Elstner E, Norman AW, Uskokovic M, Zhang W, Andreeff M, Koeffler HP. A new series of vitamin D analogs is highly active for clonal inhibition, differentiation, and induction of WAF1 in myeloid leukemia. Blood. 1996;88:2201–2209. [PubMed] [Google Scholar]
- 149.Muto A, Kizaki M, Yamato K, Kawai Y, Kamata-Matsushita M, Ueno H, Ohguchi M, Nishihara T, Koeffler HP, Ikeda Y. 1,25-Dihydroxyvitamin D3 induces differentiation of a retinoic acid-resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1) Blood. 1999;93:2225–2233. [PubMed] [Google Scholar]
- 150.Shiohara M, Uskokovic M, Hisatake J, Hisatake Y, Koike K, Komiyama A, Koeffler HP. 24-Oxo metabolites of vitamin D3 analogues: disassociation of their prominent antileukemic effects from their lack of calcium modulation. Cancer Res. 2001;61:3361–3368. [PubMed] [Google Scholar]
- 151.O’Kelly J, Uskokovic M, Lemp N, Vadgama J, Koeffler HP. Novel Gemini-vitamin D3 analog inhibits tumor cell growth and modulates the Akt/mTOR signaling pathway. J Steroid Biochem Mol Biol. 2006;100:107–116. doi: 10.1016/j.jsbmb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 152.Wang X, Studzinski GP. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J Cell Biochem. 2001;80:471–482. doi: 10.1002/1097-4644(20010315)80:4<471::aid-jcb1001>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 153.Marcinkowska E. Evidence that activation of MEK1,2/Erk1,2 signal transduction pathway is necessary for calcitriol-induced differentiation of HL-60 cells. Anticancer Res. 2001;21:499–504. [PubMed] [Google Scholar]
- 154.Wang Q, Wang X, Studzinski GP. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1,25-dihydroxyvitamin D3. J Cell Biochem. 2003;89:1087–1101. doi: 10.1002/jcb.10595. [DOI] [PubMed] [Google Scholar]
- 155.Wang Q, Salman H, Danilenko M, Studzinski GP. Cooperation between antioxidants and 1,25-dihydroxyvitamin D3 in induction of leukemia HL60 cell differentiation through the JNK/AP-1/Egr-1 pathway. J Cell Physiol. 2005;204:964–974. doi: 10.1002/jcp.20355. [DOI] [PubMed] [Google Scholar]
- 156.Szabo E, Preis LH, Birrer MJ. Constitutive cJun expression induces partial macrophage differentiation in U-937 cells. Cell Growth Differ. 1994;5:439–446. [PubMed] [Google Scholar]
- 157.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 158.Wang X, Rao J, Studzinski GP. Inhibition of p38 MAP kinase activity up-regulates multiple MAP kinase pathways and potentiates 1,25-dihydroxyvitamin D3-induced differentiation of human leukemia HL60 cells. Exp Cell Res. 2000;258:425–437. doi: 10.1006/excr.2000.4939. [DOI] [PubMed] [Google Scholar]
- 159.Ishii Y, Sakai S, Honma Y. Pyridinyl imidazole inhibitor SB203580 activates p44/42 mitogen-activated protein kinase and induces the differentiation of human myeloid leukemia cells. Leuk Res. 2001;25:813–820. doi: 10.1016/s0145-2126(01)00026-1. [DOI] [PubMed] [Google Scholar]
- 160.Weinberg RA. The Biology of Cancer. London: Garland Science; 2006. pp. 173–183. [Google Scholar]
- 161.Hmama Z, Nandan D, Sly L, Knutson KL, Herrera-Velit P, Reiner NE. 1alpha,25-dihydroxyvitamin D3-induced myeloid cell differentiation is regulated by a vitamin D receptor-phosphatidylinositol 3-kinase signaling complex. J Exp Med. 1999;190:1583–1594. doi: 10.1084/jem.190.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marcinkowska E, Kutner A. Side-chain modified vitamin D analogs require activation of both PI 3-K and erk1,2 signal transduction pathways to induce differentiation of human promyelocytic leukemia cells. Acta Biochim Pol. 2002;49:393–406. [PubMed] [Google Scholar]
- 163.Marcinkowska E, Wiedlocha A. Phosphatidylinositol-3 kinase-dependent activation of Akt does not correlate with either high mitogenicity or cell migration induced by FGF-1. Anticancer Res. 2003;23:4071–4077. [PubMed] [Google Scholar]
- 164.Gocek E, Kielbinski M, Marcinkowska E. Activation of intracellular signaling pathways is necessary for an increase in VDR expression and its nuclear translocation. FEBS Lett. 2007;581:1751–1757. doi: 10.1016/j.febslet.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 165.Berry DM, Antochi R, Bhatia M, Meckling-Gill KA. 1,25-Dihydroxyvitamin D3 stimulates expression and translocation of protein kinase Calpha and Cdelta via a nongenomic mechanism and rapidly induces phosphorylation of a 33-kDa protein in acute promyelocytic NB4 cells. J Biol Chem. 1996;271:16090–16096. doi: 10.1074/jbc.271.27.16090. [DOI] [PubMed] [Google Scholar]
- 166.Zanello SB, Collins ED, Marinissen MJ, Norman AW, Boland RL. Vitamin D receptor expression in chicken muscle tissue and cultured myoblasts. Horm Metab Res. 1997;29:231–236. doi: 10.1055/s-2007-979027. [DOI] [PubMed] [Google Scholar]
- 167.Marcinkowska E. A run for a membrane vitamin D receptor. Biol Signals Recept. 2001;10:341–349. doi: 10.1159/000046902. [DOI] [PubMed] [Google Scholar]
- 168.Okazaki T, Bielawska A, Bell RM, Hannun YA. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 169.Wang X, Studzinski GP. Kinase suppressor of RAS (KSR) amplifies the differentiation signal provided by low concentrations 1,25-dihydroxyvitamin D3. J Cell Physiol. 2004;198:333–342. doi: 10.1002/jcp.10443. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Y, Yao B, Delikat S, Bayoumy S, Lin XH, Basu S, McGinley M, Chan-Hui PY, Lichenstein H, Kolesnick R. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 171.Wang X, Studzinski GP. Phosphorylation of raf-1 by kinase suppressor of ras is inhibited by “MEK-specific” inhibitors PD 098059 and U0126 in differentiating HL60 cells. Exp Cell Res. 2001;268:294–300. doi: 10.1006/excr.2001.5292. [DOI] [PubMed] [Google Scholar]
- 172.Xing HR, Kolesnick R. Kinase suppressor of Ras signals through Thr269 of c-Raf-1. J Biol Chem. 2001;276:9733–9741. doi: 10.1074/jbc.M008096200. [DOI] [PubMed] [Google Scholar]
- 173.Michaud NR, Therrien M, Cacace A, Edsall LC, Spiegel S, Rubin GM, Morrison DK. KSR stimulates Raf-1 activity in a kinase-independent manner. Proc Natl Acad Sci USA. 1997;94:12792–12796. doi: 10.1073/pnas.94.24.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morrison DK. KSR: a MAPK scaffold of the Ras pathway? J Cell Sci. 2001;114:1609–1612. doi: 10.1242/jcs.114.9.1609. [DOI] [PubMed] [Google Scholar]
- 175.Wang X, Wang TT, White JH, Studzinski GP. Induction of kinase suppressor of RAS-1(KSR-1) gene by 1,alpha25-dihydroxyvitamin D3 in human leukemia HL60 cells through a vitamin D response element in the 5’-flanking region. Oncogene. 2006;25:7078–7085. doi: 10.1038/sj.onc.1209697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Garay E, Donnelly R, Wang X, Studzinski GP. Resistance to 1,25D-induced differentiation in human acute myeloid leukemia HL60-40AF cells is associated with reduced transcriptional activity and nuclear localization of the vitamin D receptor. J Cell Physiol. 2007;213:816–825. doi: 10.1002/jcp.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gocek E, Kielbinski M, Wylob P, Kutner A, Marcinkowska E. Side-chain modified vitamin D analogs induce rapid accumulation of VDR in the cell nuclei proportionately to their differentiation-inducing potential. Steroids. 2008;73:1359–1366. doi: 10.1016/j.steroids.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 178.Wang X, Studzinski GP. Raf-1 signaling is required for the later stages of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells but is not mediated by the MEK/ERK module. J Cell Physiol. 2006;209:253–260. doi: 10.1002/jcp.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jamshidi F, Zhang J, Harrison JS, Wang X, Studzinski GP. Induction of differentiation of human leukemia cells by combinations of COX inhibitors and 1,25-dihydroxyvitamin D3 involves Raf1 but not Erk 1/2 signaling. Cell Cycle. 2008;7:917–924. doi: 10.4161/cc.7.7.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Porras A, Muszynski K, Rapp UR, Santos E. Dissociation between activation of Raf-1 kinase and the 42-kDa mitogen-activated protein kinase/90-kDa S6 kinase (MAPK/RSK) cascade in the insulin/Ras pathway of adipocytic differentiation of 3T3 L1 cells. J Biol Chem. 1994;269:12741–12748. [PubMed] [Google Scholar]
- 181.Kuo WL, Abe M, Rhee J, Eves EM, McCarthy SA, Yan M, Templeton DJ, McMahon M, Rosner MR. Raf, but not MEK or ERK, is sufficient for differentiation of hippocampal neuronal cells. Mol Cell Biol. 1996;16:1458–1470. doi: 10.1128/mcb.16.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol. 2002;22:3237–3246. doi: 10.1128/MCB.22.10.3237-3246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Godyn JJ, Xu H, Zhang F, Kolla S, Studzinski GP. A dual block to cell cycle progression in HL60 cells exposed to analogues of vitamin D3. Cell Prolif. 1994;27:37–46. doi: 10.1111/j.1365-2184.1994.tb01404.x. [DOI] [PubMed] [Google Scholar]
- 184.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen F, Studzinski GP. Cyclin-dependent kinase 5 activity enhances monocytic phenotype and cell cycle traverse in 1,25-dihydroxyvitamin D3-treated HL60 cells. Exp Cell Res. 1999;249:422–428. doi: 10.1006/excr.1999.4522. [DOI] [PubMed] [Google Scholar]
- 186.Chen F, Rao J, Studzinski GP. Specific association of increased cyclin-dependent kinase 5 expression with monocytic lineage of differentiation of human leukemia HL60 cells. J Leukoc Biol. 2000;67:559–566. doi: 10.1002/jlb.67.4.559. [DOI] [PubMed] [Google Scholar]
- 187.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 188.Chen F, Studzinski GP. Expression of the neuronal cyclin-dependent kinase 5 activator p35Nck5a in human monocytic cells is associated with differentiation. Blood. 2001;97:3763–3767. doi: 10.1182/blood.v97.12.3763. [DOI] [PubMed] [Google Scholar]
- 189.Chen F, Wang Q, Wang X, Studzinski GP. Up-regulation of Egr1 by 1,25-dihydroxyvitamin D3 contributes to increased expression of p35 activator of cyclin-dependent kinase 5 and consequent onset of the terminal phase of HL60 cell differentiation. Cancer Res. 2004;64:5425–5433. doi: 10.1158/0008-5472.CAN-04-0806. [DOI] [PubMed] [Google Scholar]
- 190.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Foster JS, Fernando RI, Ishida N, Nakayama KI, Wimalasena J. Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J Biol Chem. 2003;278:41355–41366. doi: 10.1074/jbc.M302830200. [DOI] [PubMed] [Google Scholar]
- 192.Park BH, Qiang L, Farmer SR. Phosphorylation of C/EBPbeta at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Buck M, Poli V, Hunter T, Chojkier M. C/EBPbeta phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell. 2001;8:807–816. doi: 10.1016/s1097-2765(01)00374-4. [DOI] [PubMed] [Google Scholar]
- 194.Pan Z, Hetherington CJ, Zhang DE. CCAAT/enhancer-binding protein activates the CD14 promoter and mediates transforming growth factor beta signaling in monocyte development. J Biol Chem. 1999;274:23242–23248. doi: 10.1074/jbc.274.33.23242. [DOI] [PubMed] [Google Scholar]
- 195.Ji Y, Studzinski GP. Retinoblastoma protein and CCAAT/enhancer-binding protein beta are required for 1,25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004;64:370–377. doi: 10.1158/0008-5472.can-03-3029. [DOI] [PubMed] [Google Scholar]
- 196.Studzinski GP, Wang X, Ji Y, Wang Q, Zhang Y, Kutner A, Harrison JS. The rationale for deltanoids in therapy for myeloid leukemia: role of KSR-MAPK-C/EBP pathway. J Steroid Biochem Mol Biol. 2005;97:47–55. doi: 10.1016/j.jsbmb.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 198.Zhu K, Glaser R, Mrowietz U. Vitamin D3 and analogues modulate the expression of CSF-1 and its receptor in human dendritic cells. Biochem Biophys Res Commun. 2002;297:1211–1217. doi: 10.1016/s0006-291x(02)02357-4. [DOI] [PubMed] [Google Scholar]
- 199.Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder H, Calin GA, Liu CG, Andreeff M, Croce CM. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 200.Sharma P, Veeranna, Sharma M, Amin ND, Sihag RK, Grant P, Ahn N, Kulkarni AB, Pant HC. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J Biol Chem. 2002;277:528–534. doi: 10.1074/jbc.M109324200. [DOI] [PubMed] [Google Scholar]