Abstract
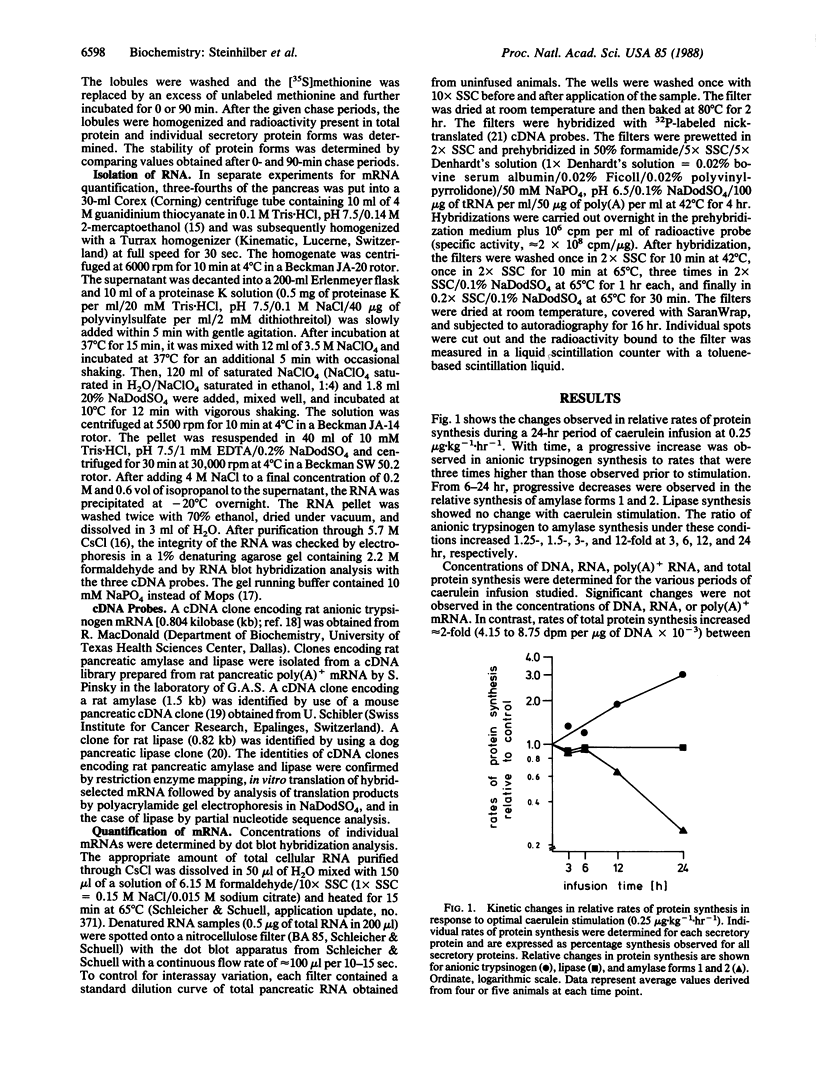
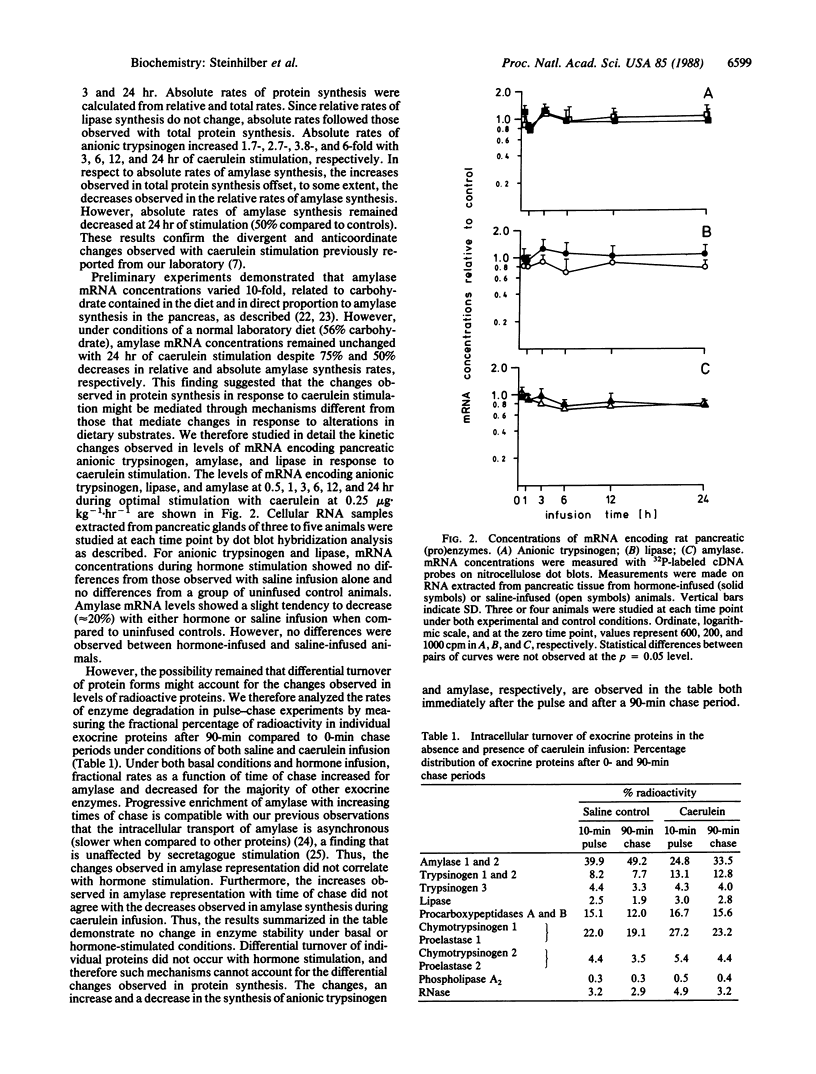
Infusion of rats with optimal doses of caerulein for up to 24 hr resulted in divergent changes in protein synthesis in the exocrine pancreas: a 3-fold increase in synthesis of anionic trypsinogen and a 75% decrease in synthesis of amylase. Lipase synthesis showed no change. Rates of total protein synthesis increased 2-fold, while DNA, RNA, and poly(A)+ mRNA concentrations were unchanged during hormonal stimulation. mRNA concentrations for anionic trypsinogen, lipase, and amylase were determined by dot blot hybridization analysis with cDNA and cRNA probes. Despite 12-fold changes in the ratio of synthesis of anionic trypsinogen to amylase at 24 hr of caerulein stimulation, changes in levels of mRNA encoding these two proteins were not observed. The slight decreases observed in amylase mRNA concentrations were found in both hormone and saline-infused animals. In vitro pulse-chase experiments after 12 hr of saline or caerulein infusion indicated that differential turnover of anionic trypsinogen and amylase did not occur during hormone stimulation. These data demonstrate that the differential regulation observed in protein synthesis that results from a single period of hormone stimulation is mediated by differential regulation of mRNA translation. The high degree of conservation observed in the 5' terminal sequences of both amylase and anionic trypsinogen mRNAs between mouse, rat, and dog suggests that sequence-specific mechanisms and secondary structure may play a role in the translational control of these two mRNAs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Kern H. F. Regulation of exocrine pancreatic secretory process by insulin in vivo. Horm Metab Res. 1975 Jul;7(4):290–296. doi: 10.1055/s-0028-1093717. [DOI] [PubMed] [Google Scholar]
- Barnard E. A. Biological function of pancreatic ribonuclease. Nature. 1969 Jan 25;221(5178):340–344. doi: 10.1038/221340a0. [DOI] [PubMed] [Google Scholar]
- Bieger W., Scheele G. Two-dimensional isoelectric focusing/sodium dodecyl sulfate gel electrophoresis of protein mixtures containing active or potentially active proteases: analysis of human exocrine pancreatic proteins. Anal Biochem. 1980 Dec;109(2):222–230. doi: 10.1016/0003-2697(80)90640-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cozzone A., Marchis-Mouren G. Messenger ribonucleic acid stability in rat pancreas and liver. Biochemistry. 1967 Dec;6(12):3911–3917. doi: 10.1021/bi00864a037. [DOI] [PubMed] [Google Scholar]
- Giorgi D., Bernard J. P., Lapointe R., Dagorn J. C. Regulation of amylase messenger RNA concentration in rat pancreas by food content. EMBO J. 1984 Jul;3(7):1521–1524. doi: 10.1002/j.1460-2075.1984.tb02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi D., Renaud W., Bernard J. P., Dagorn J. C. Regulation of proteolytic enzyme activities and mRNA concentrations in rat pancreas by food content. Biochem Biophys Res Commun. 1985 Mar 29;127(3):937–942. doi: 10.1016/s0006-291x(85)80034-6. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Keim V., Rohr G. Influence of secretagogues on asynchronous secretion of newly synthesized pancreatic proteins in the conscious rat. Pancreas. 1987;2(5):562–567. doi: 10.1097/00006676-198709000-00012. [DOI] [PubMed] [Google Scholar]
- Kerfelec B., LaForge K. S., Puigserver A., Scheele G. Primary structures of canine pancreatic lipase and phospholipase A2 messenger RNAs. Pancreas. 1986;1(5):430–437. doi: 10.1097/00006676-198609000-00007. [DOI] [PubMed] [Google Scholar]
- Kern H. F., Adler G., Scheele G. A. Structural and biochemical characterization of maximal and supramaximal hormonal stimulation of rat exocrine pancreas. Scand J Gastroenterol Suppl. 1985;112:20–29. doi: 10.3109/00365528509092209. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D., Moessner J., Williams J. A., Goldfine I. D. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985 Apr;100(4):1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. J., Crerar M. M., Swain W. F., Pictet R. L., Thomas G., Rutter W. J. Structure of a family of rat amylase genes. Nature. 1980 Sep 11;287(5778):117–122. doi: 10.1038/287117a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Stary S. J., Swift G. H. Two similar but nonallelic rat pancreatic trypsinogens. Nucleotide sequences of the cloned cDNAs. J Biol Chem. 1982 Aug 25;257(16):9724–9732. [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985 Oct;42(3):903–911. doi: 10.1016/0092-8674(85)90286-7. [DOI] [PubMed] [Google Scholar]
- Palla J. C., Ben Abdeljlil A., Desnuelle P. Action de l'insuline sur la biosynthèse de l'amylase et de quelques autres enzymes du pancréas de rat. Biochim Biophys Acta. 1968 Apr 16;158(1):25–35. [PubMed] [Google Scholar]
- Pinsky S. D., LaForge K. S., Scheele G. Differential regulation of trypsinogen mRNA translation: full-length mRNA sequences encoding two oppositely charged trypsinogen isoenzymes in the dog pancreas. Mol Cell Biol. 1985 Oct;5(10):2669–2676. doi: 10.1128/mcb.5.10.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch U., Rüdiger K., Vasiloudes P., Kern H., Scheele G. Lipase synthesis in the rat pancreas is regulated by secretin. Pancreas. 1986;1(6):522–528. doi: 10.1097/00006676-198611000-00010. [DOI] [PubMed] [Google Scholar]
- Rausch U., Vasiloudes P., Rüdiger K., Kern H. F. In-vivo stimulation of rat pancreatic acinar cells by infusion of secretin. II. Changes in individual rates of enzyme and isoenzyme biosynthesis. Cell Tissue Res. 1985;242(3):641–644. doi: 10.1007/BF00225431. [DOI] [PubMed] [Google Scholar]
- Renaud W., Giorgi D., Iovanna J., Dagorn J. C. Regulation of concentrations of mRNA for amylase, trypsinogen I and chymotrypsinogen B in rat pancreas by secretagogues. Biochem J. 1986 Apr 1;235(1):305–308. doi: 10.1042/bj2350305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Steroid hormone regulation of gene expression. Annu Rev Pharmacol Toxicol. 1985;25:529–566. doi: 10.1146/annurev.pa.25.040185.002525. [DOI] [PubMed] [Google Scholar]
- Scheele G. A., Palade G. E. Studies on the guinea pig pancreas. Parallel discharge of exocrine enzyme activities. J Biol Chem. 1975 Apr 10;250(7):2660–2670. [PubMed] [Google Scholar]
- Scheele G., Tartakoff A. Exit of nonglycosylated secretory proteins from the rough endoplasmic reticulum is asynchronous in the exocrine pancreas. J Biol Chem. 1985 Jan 25;260(2):926–931. [PubMed] [Google Scholar]
- Schibler U., Pittet A. C., Young R. A., Hagenbüchle O., Tosi M., Gellman S., Wellauer P. K. The mouse alpha-amylase multigene family. Sequence organization of members expressed in the pancreas, salivary gland and liver. J Mol Biol. 1982 Mar 5;155(3):247–266. doi: 10.1016/0022-2836(82)90004-3. [DOI] [PubMed] [Google Scholar]
- Schick J., Kern H., Scheele G. Hormonal stimulation in the exocrine pancreas results in coordinate and anticoordinate regulation of protein synthesis. J Cell Biol. 1984 Nov;99(5):1569–1574. doi: 10.1083/jcb.99.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söling H. D., Unger K. O. The role of insulin in the regulation of -amylase synthesis in the rat pancreas. Eur J Clin Invest. 1972 Jun;2(4):199–212. doi: 10.1111/j.1365-2362.1972.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thomas G. Translational control of mRNA expression during the early mitogenic response in Swiss mouse 3T3 cells: identification of specific proteins. J Cell Biol. 1986 Dec;103(6 Pt 1):2137–2144. doi: 10.1083/jcb.103.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble E. R., Bruzzone R., Belin D. Insulin resistance is accompanied by impairment of amylase-gene expression in the exocrine pancreas of the obese Zucker rat. Biochem J. 1986 Aug 1;237(3):807–812. doi: 10.1042/bj2370807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M., Scherberg N., Gilmore R., Steiner D. F. Translational control of insulin biosynthesis. Evidence for regulation of elongation, initiation and signal-recognition-particle-mediated translational arrest by glucose. Biochem J. 1986 Apr 15;235(2):459–467. doi: 10.1042/bj2350459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker C., Puigserver A., Rausch U., Scheele G., Kern H. Multiple-level caerulein control of the gene expression of secretory proteins in the rat pancreas. Eur J Biochem. 1985 Sep 16;151(3):461–466. doi: 10.1111/j.1432-1033.1985.tb09124.x. [DOI] [PubMed] [Google Scholar]
- Wicker C., Puigserver A., Scheele G. Dietary regulation of levels of active mRNA coding for amylase and serine protease zymogens in the rat pancreas. Eur J Biochem. 1984 Mar 1;139(2):381–387. doi: 10.1111/j.1432-1033.1984.tb08017.x. [DOI] [PubMed] [Google Scholar]


