Abstract
Tea (Camellia sinensis, Theaceace), a popular beverage consumed world-wide, has been studied for its preventive effects against cancer as well as cardiovascular, neurodegenerative, and other diseases. Most of the proposed beneficial effects have been attributed to the polyphenolic compounds in tea, but the nature of these activities and the molecular mechanisms of their actions remain unclear. Tea polyphenols are known to be strong antioxidants. Prevention of oxidative stress, modulation of carcinogen metabolism, and prevention of DNA damage have been suggested as possible cancer preventive mechanisms for tea and tea polyphenols. In this chapter, we discuss these topics in the light of biotransformation and bioavailability of tea polyphenols. We also review the preventive effects of tea polyphenols in animal models of carcinogenesis and some of the possible post-initiation mechanisms of action. Finally, we discuss the effects of tea consumption on cancer risk in humans. It is our aim to raise some of the unanswered questions regarding cancer prevention by tea and to stimulate further research in this area.
Introduction
Tea (Camellia sinensis, Theaceae) is a popular beverage worldwide (Yang et al. 2002). Depending on the technology of manufacturing, tea can be classified into three major types: green tea, black tea, and Oolong tea (Balentine et al. 1997; Lambert and Yang 2003). The different production methods alter the chemical composition of the dried tea leaves. Green tea, which accounts for 20% of world tea consumption, is prepared by pan-frying or steaming the tea leaves to inactivate polyphenol oxidase. This process preserves the characteristic tea catechins, which account for 10–15% of the weight of the dried leaves. The major catechins are epigallocatechin-3-gallate (EGCG), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epicatechin (EC) (Fig. 1). Black tea, representing 78% of world tea consumption, is prepared by crushing the tea leaves and causing enzyme-catalyzed oxidation and polymerization of tea catechins in a process commonly known as “fermentation”. This process results in the formation of oligomers such as theaflavins as well as large polymeric compounds known as thearubigins. Oolong tea is made by a delicate process to crush only the rim of the tea leaf. The product retains higher levels of catechins and contains newly formed oligomers of catechins such as theasinensins. A typical cup of green tea, brewed with 2.5 g tea leaves in 250 mL hot water, contains 620–880 mg of water-extractable materials, of which about one-third are catechins. EGCG is the most abundant catechin and may account for 50–75% of the catechins.
Fig. 1.
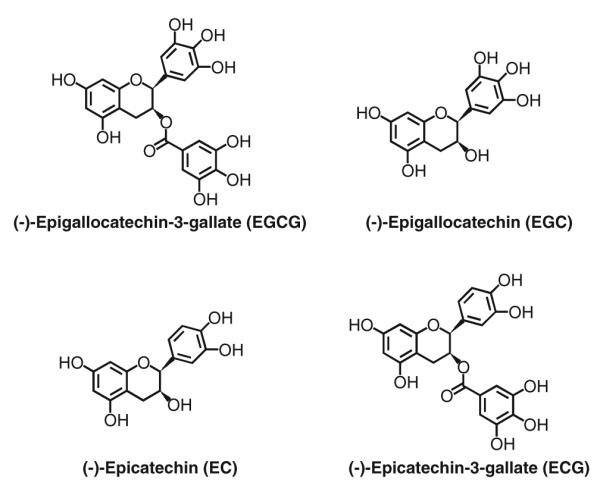
Structures of the major tea polyphenols
The possible beneficial effects of tea in the prevention of cancer as well as cardiovascular, neurodegenerative, and other diseases have been studied extensively. Most of the activities have been attributed to tea polyphenols. In this chapter, we review the antioxidative activities of tea polyphenols, their bioavailability and biotransformation, their effects on oxidative stress, and their inhibitory activities against carcinogenesis. Most of the reviewed studies are on catechins, particularly EGCG.
Redox properties and antioxidative activities of tea polyphenols
Tea catechins are characterized by the di- or tri-hydroxyl groups on the B-ring and the meta-5,7-dihydroxyl groups on the A ring. These structures provide strong antioxidative activities. The antioxidative activity is further increased by the presence of the trihydroxyl structure in the D-ring (gallate) in EGCG and ECG (Wiseman et al. 1997; Rice-Evans 1999). Tea preparations have been shown to react with reactive oxygen species (ROS), such as superoxide radical, singlet oxygen, hydroxyl radical, peroxyl radical, nitric oxide, nitrogen dioxide, and peroxynitrite. Among tea catechins, EGCG is most effective in reacting with most ROS. The B-ring appears to be the principal site of antioxidant reactions (Valcic et al. 2000; Sang et al. 2002). The polyphenolic structure allows electron delocalization, conferring high reactivity to quench free radicals. During the reaction of tea polyphenols with free radicals, several oxidation products are formed (Sang et al. 2007). Reactions of EGCG and other catechins with peroxyl radicals lead to the formation of anthocyanin-like compounds (Kondo et al. 1999), as well as seven-member B-ring anhydride dimers and ring-fission compounds (Valcic et al. 1999, 2000).
Another mechanism for the effective antioxidative activity is through metal ion chelation by the vicinal dihydroxyl and trihydroxyl structures, which prevents the generation of free radicals. Green tea can inhibit the oxidation of lipoproteins induced by Cu2+ in vitro (Hodgdon et al. 1999; Hashimoto et al. 2000). Pretreatment of macrophages or endothelial cells with green tea polyphenols reduced cellmediated low-density lipoprotein oxidation (Yoshida et al. 1999). The effects of tea and tea polyphenols on biomarkers of oxidative stress, such as DNA oxidative damage, have been demonstrated in animals after receiving carcinogenic or other types of oxidative stress; this topic will be discussed subsequently.
Administration of EGCG to rats was shown to reduce age-related increases in oxidative stress (Senthil Kumaran et al. 2008). For example, treatment of 24-month-old rats with EGCG at 100 mg/kg, i.g. decreased the levels of lipid peroxidation and protein carbonylation in the liver. The hepatic levels of antioxidants (e.g., reduced glutathione) as well as antioxidant enzymes (e.g., superoxide dismutase) were increased by the EGCG treatment. Similar effects were also observed in skeletal muscle. A second study by the same research group found that treatment of 24-month-old rats for 30 days with EGCG at 2 mg/kg, i.g. daily reduced the levels of protein carbonyls and malonyldialdehyde, and increased levels of ascorbic acid, α-tocopherol, and glutathione (reduced form) in the brain (Srividhya et al. 2008). In both of these studies, no effects were observed in young rats, suggesting that EGCG offers no protective effect in the absence of age-related increases in oxidative stress. The reasons for this age-related biological difference need to be investigated.
In humans, only transient and modest increases in total plasma antioxidant activity after tea ingestion were observed in some, but not other, experiments (Higdon and Frei 2003). Apparently, the bioavailability of tea polyphenols limits the biological activity in vivo. Supplementation of healthy human volunteers with catechins (500 mg/day) for 4 weeks resulted in an 18% decrease in plasma oxidized low density lipoprotein (LDL) compared to the control (Inami et al. 2007). Similarly, supplementation of hemodialysis patients with 455 mg/day green tea catechins for 3 months decreased plasma hydrogen peroxide, C-reactive protein and several pro-inflammatory cytokines compared to placebo-treated controls (Hsu et al. 2007). Tea catechins also blunted dialysis-induced increases in several inflammatory markers including plasma levels of Fas ligand and interleukin (IL)-6 soluble receptor.
Similar to many other antioxidants, EGCG and other tea polyphenols may also act as pro-oxidants. Under cell culture conditions, EGCG is not stable, with a half-life of 0.5–2 h depending on the cultural medium (Hong et al. 2002; Hou et al. 2005). The half-life can be extended several fold by the addition of superoxide dismutase (SOD), suggesting a role for superoxide radicals in the oxidation and polymerization of EGCG. A proposed mechanism of EGCG auto-oxidation has been published previously (Hou et al. 2005). EGCG and other catechins can be oxidized to form phenolic radicals, superoxide radicals, and hydrogen peroxide. These species may trigger a variety of biochemical reactions and biological responses. For example, the radical species may contribute to the inactivation of epidermal growth factor receptor (EGFR) and telomerase, while hydrogen peroxide may contribute to cell apoptosis (Yang et al. 2007). It is not clear whether these pro-oxidation of EGCG-generated reactions occur in vivo, which usually has low oxygen partial pressure than systems in vitro. The oxygen partial pressure in a cell culture system (152 mmHg) is much higher than that in the blood or tissues (<40 mmHg) (Sherwood 2004).
The relative importance of the antioxidative and pro-oxidative activities in vivo remains to be determined. It is known that EGCG and other catechins can directly scavenge ROS and chelate free transition metals, thereby reducing oxidative stress. Tea polyphenols could also generate ROS, but the levels are relatively insignificant in the presence of existing oxidative stress. In the absence of pre-existing oxidative stress, the tea polyphenols could generate ROS that stimulate upregulation of the endogenous antioxidant systems by mechanisms through the Nrf2 signaling pathway. High concentrations of ROS generated could lead to toxicity. Some of the related studies will be discussed subsequently.
Bioavailability and biotransformation of tea polyphenols
Biotransformation of tea polyphenols
We and others have extensively studied the biotransformation of green tea polyphenols (Kohri et al. 2001; Li et al. 2001; Hu et al. 2003; Lambert and Yang 2003; Auger et al. 2008). Tea catechins are subject to methylation, glucuronidation, sulfation, and ring-fission metabolism. EGCG is readily methylated by catechol-O-methyltransferase (COMT) to form 4″-O-methyl-(-)-EGCG and 4′,4″-O-dimethyl-(-)-EGCG (Lu et al. 2003b). Studies of EGCG glucuronidation reveal that EGCG-4″-O-glucuronide is the major metabolite formed in humans, mice, and rats (Lu et al. 2003a). Human UGT1A1, 1A8, and 1A9 have high glucuronidation activity toward EGCG, with the intestinal-specific UGT1A8 being the most efficient. Our studies also suggest that mice are more similar to humans in terms of enzymatic ability to glucuronidate tea catechins than are rats (Lambert et al. 2005a). EGCG and other catechins are also sulfated by sulfotransferase in human, mouse, and rat liver cytosol (Lu 2002). Our recent results from data-dependent tandem mass spectrometric analysis of mouse urine samples, after i.p. or i.g. administration of EGCG, have shown that methylated EGCG (or glucuronidated or sulfated EGCG) can be further glucuronidated and/or sulfated (or methylated) to form related mixed EGCG metabolites (Sang et al. 2008).
At toxic doses, EGCG can form two cysteine adducts in vivo, EGCG-2″-cysteine and EGCG-2′-cysteine (Sang et al. 2005). These metabolites can be detected in the urine following administration of EGCG at 200–400 mg/kg, i.p. or 1,500 mg/kg, i.g. We hypothesize that these metabolites form as the result of oxidation of EGCG to a quinone or semi-quinone that then reacts with the sulfhydryl group of cysteine. It is possible that similar metabolites will be formed by reaction with glutathione and N-acetylcysteine, but these metabolites remain to be discovered.
In addition to phase II metabolites, our laboratory has identified several ring fission products of tea catechins in human urine and plasma after oral ingestion of decaffeinated green tea (Li et al. 2000). The compounds, 5-(3′, 4′, 5′-trihydroxyphenyl)-γ-valerolactone (M4), 5-(3′, 4′-dihydroxyphenyl)-γ-valerolactone (M6), and 5-(3′, 5′-dihydroxyphenyl)-γ-valerolactone (M6′), are believed to be derived from microbial metabolism in the colon. Indeed, anaerobic fermentation of EGC, EC, and ECG with human fecal microflora has been shown to result in the production of M4, M6, and M6′ (Meselhy et al. 1997).
Active efflux has been shown to limit the bioavailability and cellular accumulation of many compounds. The multidrug resistance-associated proteins (MRP) may play a role in limiting the bioavailability of tea catechins. We have reported that indomethacin (MRP inhibitor) increases the intracellular accumulation of EGCG; EGCG, 4″-O-methyl-EGCG, and 4′, 4″-di-O-methyl-EGCG in Madin-Darby canine kidney (MDCKII) cells overexpressing MRP-1 (Hong et al. 2003). Similarly, treatment of MRP-2 overexpressing MDCKII cells with MK-571 (an MRP-2 inhibitor) increases the intracellular levels of EGCG, 4″-O-methyl-EGCG, and 4′, 4″-di-O-methyl-EGCG, respectively. The combined effects of MRP-1 and MRP-2 on the bioavailability of the tea polyphenols remain to be determined in vivo. MRP-2, with its localization on the apical membrane of the small intestine, likely acts to limit EGCG bioavailability by actively exporting EGCG in the enterocyte back into the intestinal lumen. In contrast, MRP-1 is located on the basolateral membrane of enterocytes, hepatocytes, and other tissues. Substrates of this pump are effluxed from the interior of the cells into the blood stream or interstitial space. The role of MRP-1 would be expected to increase the bioavailability of EGCG in vivo. The influence of MRP-1 and MRP-2 on the bioavailability of EGCG in vivo is likely to depend on the tissue distribution of each efflux protein.
Pharmacokinetics of tea polyphenols
Pharmacokinetic studies of the tea catechins have been conducted in rats, mice, and humans (reviewed in Yang et al. 2008). Following i.g. administration of decaffeinated green tea (200 mg/kg) to rats, plasma levels of EGCG, EGC, and EC (conjugated and nonconjugated forms) were fit to a two-compartment model with elimination half-lives of 165, 66, and 67 min, respectively (Chen et al. 1997). The absolute bioavailability of EGCG, EGC, and EC after i.g. administration of decaffeinated green tea was 0.1, 14, and 31%, respectively. The bioavailability of EGCG in mice following oral administration of EGCG was higher (Yang et al. 2008). Whereas greater than 50% of plasma EGCG was present as the glucuronide, EGCG was present mainly as the free form in the tissues (Lambert et al. 2003). Administration of 50–2,000 mg/kg, i.g. EGCG to mice resulted in a linear increase in the plasma, liver, and prostate. In contrast, the levels of EGCG in the small intestine and colon plateaued at 500 mg/kg, i.g (Lambert et al. 2006). These results suggest that small intestinal and colonic tissues become saturated with EGCG, resulting in a plateau of the levels in these tissues.
Several studies of the systemic bioavailability of orally administered green tea and catechins in human volunteers have been conducted. We showed that oral administration of 20 mg green tea solids/kg body weight resulted in Cmax in the plasma for EGC, EC, and EGCG of 223, 124, and 77.9 ng/mL, respectively (Lee et al. 2002). Plasma EC and EGC were present mainly in the glucuronidated and sulfated form whereas 77% of the EGCG was in the free form. EGC was also methylated (to 4′-O-methyl-EGC) in humans. EGCG was also methylated: the maximum plasma concentration of 4′,4″-di-O-methyl-EGCG was 20% of that of EGCG but the cumulative excretion of 4′,4″-di-O-methyl-EGCG in the urine was 10-fold higher (140 μg) than that of EGCG (16 μg) over 24 h (Meng et al. 2002). In addition to methylated and conjugated metabolites, the ring-fission metabolites, M4, M6, and M6′, were detected in urine at 8, 4, and 8 μM, respectively, following ingestion of 200 mg EGCG (Li et al. 2000; Meng et al. 2002). Chow et al. reported that following 4 weeks of green tea polyphenol treatment of human volunteers with a dosing schedule of 800 mg once daily, there was an increase in the area under the plasma EGCG concentration-time curve from 95.6 to 145.6 min/μg min (Chow et al. 2003). No significant changes, however, were observed in the pharmacokinetics of EGCG after repeated green tea polyphenol treatment at a regimen of 400 mg twice daily. Similarly, there was no significant change in the area under the curve for EGC or EC.
Effects of tea polyphenols on carcinogen metabolism and DNA damage
Effects on carcinogen metabolism
Tea polyphenols have been shown to inhibit the expression of carcinogen activating enzymes such as cytochromes P450 (CYP) and increase the levels of enzymes which detoxify carcinogens. For example, Krishnan et al. showed that pretreatment of Swiss mice with 1% black tea polyphenols blunted the increase in expression of CYP1A1 and 1A2 induced in the liver and lung by a single oral dose of benzo[a]pyrene (B[a]P) (Krishnan et al. 2005). This is likely due to interference at the aromatic hydrocarbon receptor level. Intragastric treatment of rats with theaflavins (20 mg/kg) for 4 weeks reduced CYP1A1 activity in the intestine but not in the liver (Catterall et al. 2003). This discrepancy may be due to the limited systemic bioavailability of theaflavins. Long-term treatment of rats with green or black tea resulted in increased expression of hepatic 1A2 (Sohn et al. 1994; Xu et al. 1996). Studies from our laboratory suggested that induction of 1A2 activity is due to the caffeine present in the tea preparation (Chen et al. 1996).
Studies in animal models have also demonstrated that tea treatment can induce Phase II drug-metabolizing enzymes. Treatment of piglets with 0.2% green tea extract (45% EGCG) for 3 weeks increased the rate of formation of glutathione conjugated aflatoxin (AF)B1 by small intestinal microsomes in ex vivo studies (Tulayakul et al. 2007). Treatment of female Wistar rats with 2% green tea extract for 4 weeks was shown to increase cytosolic glutathione-S-transferase (GST) activity in the liver (Maliakal et al. 2001). However, later studies in which Wistar rats were given tea polyphenols at 833 mg/kg, i.g. once daily for 6 months, showed no effect on hepatic GST activity (Liu et al. 2003). Pretreatment of rats with 2% green tea for 6 weeks prior to a single dose of 2-amino-3-methylimididazo[4,5-f]quinoline (IQ) was found to increase excretion of glucuronide and sulfate metabolites of IQ in the urine (Embola et al. 2001). Others, however, suggested that caffeine may be the major tea component responsible for this activity (McArdle et al. 1999). Oral gavage of EGCG (200 mg/kg) to C57bl/6 J mice was shown to upregulate gene expression of γ-glutam-yltransferase, glutamate cysteine ligase, and hemeoxygenase 1 (Shen et al. 2005). This effect appeared to be via the Nrf2-antioxidant response element pathway. This topic has recently been reviewed (Na and Surh 2008).
Studies in humans have not shown the modulation of CYP expression and activity by tea and tea polyphenols. For example, 4-week treatment of human volunteers with 800 mg Polyphenon E (65% EGCG) did not significantly alter the activity of CYP1A2, CYP2D6, or CYP2C9 based on the pharmacokinetics of selected probe compounds (Chow et al. 2006). Similarly, treatment of healthy volunteers with 844 mg decaffeinated green tea extract (59% EGCG) for 14 days did not significantly affect CYP3A4 or CYP2D6 activity (Donovan et al. 2004). These results raise questions about the human relevance of the observed CYP-modulating effect in animal studies. By contrast, there is evidence suggesting that green tea preparations can modulate human Phase II metabolism. Treatment of human volunteers for 4 weeks with 800 mg/day Polyphenon E increased GST-π activity in blood lymphocytes (Chow et al. 2007). This effect was greatest in individuals with the lowest tertile baseline GST-π activity (80% increase compared to baseline). A recent intervention study in China showed that a 3-month treatment with 500 or 1,000 mg/day green tea polyphenols increased urinary excretion of the mercapturic acid conjugated of AFB1 (AFB1-NAC) by 10-fold and 8.4-fold, respectively compared to baseline (Tang et al. 2008). This result could be due to the induction of glutathione-S-transferases.
Prevention and repair of DNA damage
Tea and tea components have been shown to inhibit carcinogen-induced DNA damage in a number of cell line studies. For example, co-treatment of human leukocytes with EGCG (2 μM) and bleomycin (20 μg/mL) resulted in a 50% decrease in bleomycin-induced DNA damage compared to treatment with bleomycin alone (Glei and Pool-Zobel 2005). Green tea, black tea, and Oolong tea extract dose-dependently protected Chang liver cells from B[a]P-induced DNA damage (Yen et al. 2004). Pure EGC, EGCG, and theaflavins (10–50 μM) dose-dependently protected cells from B[a]P-induced DNA damage. At higher concentrations, however, EGC, EGCG, and theaflavins induced DNA damage by pro-oxidative mechanisms.
Studies in animal models have been consistent with the results in the cell line studies. Pretreatment of C57bl/6 Big Blue lacI transgenic mice with 2% green tea prior to a single dose of B[a]P resulted in a 54% decrease in characteristic GC to TA transversions in the liver compared to water-treated controls (Jiang et al. 2001). In contrast, green tea administration did not inhibit DNA adduct formation in the 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis model in A/J mice even though tea did reduce tumorigenesis (Shi et al. 1994). In a similar model, Xu et al. showed that green tea can prevent the formation of 8-oxo-2-deoxyguanosine (Xu et al. 1992). These results suggest that the ability of tea to prevent DNA adduct formation and oxidative DNA damage contribute to its cancer preventive activity.
C3(1) SV40 T,t antigen transgenic multiple mammary adenocarcinoma (TAg) mice spontaneously develop mammary adenocarcinoma. Treatment of mice with a solution of 0.05% green tea catechins (60% EGCG) or black tea theaflavins as the sole source of drinking fluid for 18 weeks decreased malonyldialdehyde-DNA adduct formation in the tumors (Kaur et al. 2007). This event was related to decrease mammary tumor volume and increased survival compared to water-treated controls. In another series of studies, Lin et al. reported that pre-treatment of rats with 3% green tea extract as the sole source of drinking fluid for 10 days reduced 2-amino-3-methylimididazo[4,5-f]quinoline (PhIP)-DNA adduct formation (Lin et al. 2003). PhIP-DNA adducts were reduced by 50–63% in the colon, heart, lung, and liver by green tea treatment. An interesting recent study suggested that EGCG could prevent photocarcinogenesis via an IL-12 dependent DNA repair pathway (Meeran et al. 2006). Application of EGCG (1 mg/cm2) to the backs of wildtype (C3H/HeN) mice inhibited UVB-induced photocarcinogenesis, but no inhibition was observed in IL-12-/- litter mates. The authors found that this phenomenon was related to decreased repair of pyrimidine dimers in the skin of IL-12-/-mice compared to wild-type litter mates.
In a pilot study by Schwartz et al. heavy smokers and non-smokers were treated with green tea (400–500 mg green tea powder per cup) 5 times per day for 4 weeks (Schwartz et al. 2005). The authors found that the levels of B[a]P-deoxyguanosine (B[a]P-dG) adducts were higher in smokers than in non-smokers (two–fourfold elevation), and tea-treatment caused a 50% decrease in B[a]P-dG adducts by the end of the experiment. Tea treatment for 4 weeks also reduced the number of 8-OHdG positive cells in smokers to 50% of the pre-treatment levels. Other researchers have reported that supplementation with green tea or green tea preparations can reduce biomarkers of oxidative DNA damage. For example, Hakim et al. found in a randomized, controlled intervention study, that supplementation of heavy smokers (>10 cigarettes per day) with four cups of decaffeinated green tea (73.5 mg catechins per cup) per day for 4 months reduced urinary 8-OHdG levels by 31% compared to the control group (Hakim et al. 2003). A recent study in China found that green tea polyphenols modulated the formation of serum aflatoxin-albumin (AfA) adducts in a population at high-risk for liver cancer (Tang et al. 2008). Treatment of AfA-seropositive individuals with 500 or 1,000 mg green tea polyphenols for 3 months resulted in a dose- and time-dependent decrease in the serum levels of AfA as well as a dose-dependent decrease in urinary 8-OHdG levels compared to placebo (Tang et al. 2008).
Inhibition of carcinogenesis
Cancer prevention by tea and tea components has been studied in many different animal models of carcinogenesis (reviewed in Yang et al. 2002, 2007; Lambert et al. 2005b; Ju et al. 2007). Tea and tea constituents have been shown to inhibit the development of cancer in animal models of oral, esophageal, forestomach, stomach, intestinal, colon, skin, liver, bladder, prostate, and breast cancer. Whereas most studies have focused on the activity of the tea polyphenols, several studies have reported the importance of caffeine in the prevention of skin and lung (reviewed in Lambert et al. 2005b).
Several recent studies highlight the inhibitory activity of tea polyphenol preparations in animal models of tumorigenesis, and begin to provide some mechanistic data related to the inhibitory effect. For example, our laboratory has extensively studied the anti-tumorigenic activities of tea polyphenols in the APCmin/+ mouse model of intestinal tumorigenesis. EGCG solution, as the sole source of drinking fluid, dose-dependently (0.02–0.32% w/v) inhibited small intestinal tumorigenesis in this model (Ju et al. 2005). Inhibition of tumor multiplicity was associated with increased expression of E-cadherin and decreased levels of nuclear β-catenin, c-Myc, phospho-Akt, and phospho-extracellular regulated kinase (Erk) 1/2. We also compared the effectiveness of EGCG as a pure compound with a defined catechin mixture, Polyphenon E (PPE), containing 65% EGCG (Hao et al. 2007). Total tumor multiplicity was decreased by both dietary PPE (0.12%) or the corresponding amount of dietary EGCG (0.08%). Although PPE appeared to be more effective than EGCG at reducing total tumor multiplicity, the difference was not statistically significant. The cancer preventive activity of tea and tea polyphenol preparation has also been demonstrated in other models for colon carcinogenesis (reviewed in Ju et al. 2007). Further studies are required to more fully elucidate whether PPE or other green tea catechin preparations are more effective at inhibiting tumorigenesis than EGCG.
Whereas the colon cancer prevention activity of tea and tea polyphenol preparations has been consistently demonstrated in mouse models, results in the rat model have not been consistent (reviewed in Ju et al. 2007). We have recently examined the effect of PPE on the development of colon aberrant crypt foci (ACF) and cancer in azoxymethane (AOM)-treated rats. Treatment of rats with PPE (0.12, 0.24% in the diet) for 8 weeks, following injection with AOM, dose-dependently decreased the total number of ACF per rat by 16.3 and 36.9%, respectively. The inhibitory activity was associated with decreased levels of nuclear β-catenin and cyclin D1, and increased retinoid X receptor α staining in the ACF with high-grade dysplasia (Xiao et al. 2008). After treatment with 0.24% PPE for 34 weeks, the incidence of adenocarcinoma decreased from 57 to 23%, and the multiplicity of adenocarcinoma and adenoma was decreased by 80 and 45%, respectively (unpublished). Carter et al. examined the effect of EGCG when given during the post-initiation phase, on PhIP-induced ACF in the rat (Carter et al. 2007). Treatment with EGCG for 15 weeks reduced PhIP-induced ACF by 71% compared to water-treated controls. This decrease in ACF was associated with a 40% decrease in bromodeoxyuridine labeling in the crypt, suggesting that EGCG is inhibiting aberrant cell proliferation. The underlying mechanisms remain unclear.
Administration of green tea, black tea, EGCG, or theaflavins during initiation or promotion stage was shown to inhibit NNK-induced lung tumorigenesis in rats, mice, or hamsters (Wang et al. 1992; Xu et al. 1992; Yang et al. 1997; Chung et al. 1998; Mimoto et al. 2000; Zhang et al. 2000; Liao et al. 2004; Schuller et al. 2004; Lu et al. 2006). Treatment with green or black tea for 60 weeks also inhibited the spontaneous formation of lung tumors in A/J mice (Landau et al. 1998). In our recent study, the oral administration of 0.5% PPE or 0.044% caffeine in the drinking fluid for 32 weeks was found to inhibit the progression of lung adenomas to adenocarcinomas in A/J mice that had been treated with a single dose of NNK 20 weeks earlier (Lu et al. 2006). PPE and caffeine treatment inhibited cell proliferation in adenocarcinomas, enhanced apoptosis in adenocarcinomas and adenomas, and decreased levels of c-Jun and phospho-Erk1/2. In the normal lung tissues, neither agent had a significant effect on cell proliferation or apoptosis. Oral administration of green tea infusion reduced the number of lung colonies of mouse Lewis lung carcinoma cells in a metastasis system (Yang et al. 2005). These results suggest that tea preparations may be preventive agents for all stages of lung carcinogenesis.
Because of the low blood and tissue levels of tea polyphenols achievable through oral administration, their effectiveness in cancer prevention is limited. An approach that has been explored is to use polyphenols in combination with other agents. For example, we recently demonstrated the synergistic inhibitory action of a combination of PPE and atorvastatin against NNK-induced lung carcinogenesis in A/J mice (Lu et al. 2008). The synergistic action of this combination against human lung H1299 and H460 cells was also demonstrated. In both the cell lines and mouse model, down-regulation of the anti-apoptotic protein Mcl-1 and induction of apoptosis were shown to be associated with the synergistic inhibitory action (Lu et al. 2008).
Mechanisms for the anti-cancer activities of tea polyphenols
In addition to the ability of tea polyphenols to inhibit the level of activated carcinogens, oxidative stress-induced cellular damage, carcinogen-DNA adduct formation, and possibly the initiation of carcinogenesis, the inhibition of post-initiation events has also been studied extensively. Most of the studies on the anti-cancer activities have focused on the effects of EGCG on signal transduction pathways in cell lines, and numerous mechanisms for the action of tea polyphenols have been proposed (reviewed in Hou et al. 2004; Lambert et al. 2005b; Khan et al. 2006; Yang et al. 2007). These include inhibition of MAP kinases and the PI3K/AKT pathway, inhibition of NFκB- and AP-1-mediated transcription, inhibition of growth factor-mediated signaling, inhibition of aberrant arachidonic acid metabolism, and other activities. The end result of these effects may be the inhibition of tumor cell growth, induction of apoptosis, or the inhibition of angiogenesis. Some of these activities have been demonstrated to be associated with the inhibition of carcinogenesis in animal models. In most cases, however, the concentrations of EGCG required to observe these biological effects in vitro exceed the concentrations achievable in plasma and tissues by 10–100-fold, and questions remain as to the relevance of these in vitro observations to the mechanisms of the cancer-preventive activities in vivo (reviewed in Hou et al. 2004; Yang et al. 2007).
In general, if an effect can be observed in vitro at concentrations lower or similar to those observed in vivo, then the event may occur in vivo. For example, EGCG was reported to bind to the 67-kDa laminin receptor with a Kd of 0.04 μM, to vimentin with a Kd of 3.3 nM, and interact with Bcl-2 with a Ki of 0.33 μM (Leone et al. 2003; Ermakova et al. 2005). In all these studies, there were experiments demonstrating the biological relevance of the effects in their specific experimental systems, but it required much higher concentrations of EGCG to cause growth inhibition and induce apoptosis. The general applicability of these mechanisms for cancer prevention remains to be investigated. The differences between the effective concentrations determined with pure enzymes and those in cell lines or tissues are probably due to the non-specific binding of EGCG to many proteins and the limited amount of EGCG that can enter the cells. When a small amount of pure enzyme is used in an enzymatic assay, inhibition may be observed with nanomolar concentrations of EGCG, but it may take much higher concentrations of EGCG to inhibit the activity in cell lines or tissues. This point is illustrated in the inhibition of 20 s proteasome chymotryptic activities by EGCG; i.e., the IC50 observed in a cell-free system was 0.1–0.2 μM, but it was 1–40 μM in tumor cell lines (Nam et al. 2001). Based on the above considerations, our understanding of the mechanisms of the cancer preventive action of EGCG, and perhaps other phenolic compounds, are as follows: (1) different mechanisms are likely to be involved in different experimental systems; (2) the possible involvement of multiple targets in one system and their possible synergistic actions remain to be studied; (3) some of the proposed mechanisms based on studies in cancer cell lines may not be relevant to cancer prevention because of the high concentration of agents used; (4) many of the observed effects are probably secondary events or downstream events and it is important to identify the direct targets of EGCG action; and (5) mechanisms of cancer prevention need to be demonstrated in relevant models or human tissues.
Possible cancer prevention by tea in humans
Many case-control studies have shown that individuals who frequently consume tea had lower cancer risk; for example, lower risk of gastric and esophageal cancer was observed among green tea consumers in Japan and China (reviewed by Ju et al. 2007). Gao et al. reported that green tea consumption was associated with a reduced risk of esophageal cancer (Gao et al. 1994). From the Shanghai Cancer Registry, 1,016 eligible cases of esophageal cancer were matched with controls, and patient interviews were conducted. After adjustment for known confounders, a protective effect was observed in nonsmokers, mostly women. For women consuming ≥ 15 g of dry green tea leaves per month (one cup of tea typically contains 2 g tea leaves), the odds ratio (OR) was 0.34. Among men (mostly smokers), the OR was 0.80, but not statistically significant. In a more recent study, we and our collaborators investigated the association between pre-diagnostic urinary tea polyphenols, and the risk of developing gastric and esophageal cancers. Using a nested case-control design, we compared 190 cases of gastric cancer and 46 cases of esophageal cancer with 772 control subjects from the Shanghai Cohort. Urinary EGC positivity showed a statistically significant inverse association with gastric cancer (OR = 0.52) after adjustment for confounders. The protective effect was primarily seen among subjects with below population median levels of serum carotenoids. Similar tea polyphenol-cancer risk associations were observed for combined risk of gastric cancer and esophageal cancer (Sun et al. 2002). In the same cohort, a similar result was also observed between urinary levels of tea catechins (and their metabolites) and colon cancer risk (Yuan et al. 2007).
Tea consumption has also been associated with the reduced risk of other types of cancer. For example, a population-based case-control study of women of Asian descent living in Los Angeles, found that green tea drinkers had a significantly reduced risk of breast cancer (OR = 0.71 and 0.53 for consumption of 0–85.7 mL and >85.7 mL of tea per day, respectively) (Wu et al. 2003b). Among women who carried at least one low-activity COMT allele (Wu et al. 2003a). The authors concluded that individuals with a low-activity COMT allele have a reduced risk of breast cancer because they metabolize tea polyphenols less efficiently and, therefore, had prolonged exposure to the active parent compound. The Japanese Public Health Center-based Prospective Study (JPHC) reported an association between green tea consumption and decreased risk of advanced prostate cancer (Kurahashi et al. 2008). In a cohort of 49,920 men, a dose-dependent decrease in the risk of advanced prostate cancer was observed (P for trend = 0.01). There was, however, no association between tea consumption and the risk of localized prostate cancer.
Not all epidemiological studies have observed an inverse relationship between tea consumption and cancer risk (reviewed in Ju et al. 2007). Several recent prospective studies in Japan found no association between green tea intake and decreased breast cancer risk (Hoshiyama et al. 2005). In the Ohsaki National Health Insurance Cohort Study in Japan, after an 11-year follow-up, although green tea consumption was associated with decreased mortality due to cardiovascular diseases, it had no association with cancer deaths (Kuriyama et al. 2006).
The inconsistent results of epidemiological studies of tea and cancer prevention could be due to a variety of reasons. Case-control studies are generally less powerful than prospective studies, because of confounding factors. For example, individuals with a stomach problem, which increases the risk for gastric cancer, may refrain from drinking green tea because of stomach irritation. Genetic factors in different populations may affect the results; for example, a greater protective effect of tea against breast cancer was observed in women with at least one low activity allele of COMT (Wu et al. 2003b). Nutritional factors could also play a role in affecting the results; for example, the protective effect against colon cancer was more clearly seen in subjects with lower serum levels of carotenoids (Sun et al. 2002). The quantity and quality of the tea consumed will definitely affect the outcome of epidemiological studies. Reliance on questions of “number of cups of tea consumed per day” represents a potential weakness in many epidemiological studies. In future studies, more precise quantitative and qualitative information of tea consumption should be a goal in the study design. Objective measurements of exposure biomarkers, such as urinary catechins and their metabolites (Yuan et al. 2007), could be very useful and should be explored further.
Even though the results of epidemiological studies have not yielded a clear conclusion between tea consumption and cancer risk, tea or tea polyphenol preparations could be used for the prevention of certain types of cancer, if such an activity could be demonstrated in well-designed intervention studies. A recent double-blind study by Bettuzzi et al. followed 200 individuals with high-grade prostate intraepithelial neoplasia (PIN) receiving either 600 mg of green tea catechins daily or placebo (100 individuals in each group) for 12 months. Only 3% of the patients in the catechin treatment group developed prostate cancer, whereas the rate of cancer development on the placebo group was 30% (Bettuzzi et al. 2006). No adverse effect was associated with the treatment. These results are very exciting, and the impact would be tremendous if the results could be reproduced in similar trials with larger numbers of subjects. Several human trials with tea polyphenol preparations for the prevention of cancer of the prostate, breast, colorectum, oral cavity and as well as leukemia are ongoing or being planned. It is hoped that these studies will yield clear conclusions.
Concluding remarks
The antioxidative and anti-carcinogenic activities of tea polyphenols have been clearly demonstrated in animal models. Nevertheless, the relative importance of the antioxidative mechanisms in comparison to other proposed mechanisms for cancer prevention remains unclear. In theory, tea polyphenols should be able to quench ROS generated during the metabolism of environmental carcinogenesis or during inflammation and carcinogenesis. Nevertheless, the effectiveness of this mechanism is limited by the low blood and tissue concentrations of polyphenols derived from oral administration or consumption of tea or tea polyphenols. The antioxidative activity may be prominent when high doses of tea are ingested to counteract the effect of high levels of exogenous or endogenous ROS. On this topic, the results in the literature have not been consistent, and more studies are needed. Even when the antioxidative activity can be measured by the commonly used biomarkers, it does not mean that this is the mechanism by which tea polyphenols block carcinogenesis; other anti-carcinogenesis mechanisms as described above may be just as or even more important. Inhibition of oxidative DNA damage by the consumption of tea polyphenols has been demonstrated in heavy smokers. The possibility of using tea polyphenols to protect against cellular damage caused by environmental pollutants remains to be investigated.
In spite of the strong evidence for the cancer preventive activity of tea polyphenols in animal models, such an activity has not been consistently observed in studies on humans. This may be due to the relatively low levels of tea consumption by humans and the confounding factors in epidemiological studies involving different populations. More definitive information on the cancer preventive or anti-carcinogenic activity of tea polyphenols will come from well-designed cohort studies as well as human intervention trials. Knowledge gained from the above reviewed studies on the biological properties and activities of tea polyphenols will help in the design of these studies.
Acknowledgments
Grant support: NIH grants CA120915, CA122474, and CA133021.
Contributor Information
Chung S. Yang, Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 164 Frelinghuysen Road, Piscataway, NJ 08854, USA
Joshua D. Lambert, Department of Food Science, The Pennsylvania State University, 332 Food Science Bldg, University Park, PA 16802, USA
Shengmin Sang, Human Nutrition Research Program, Biomedical, Biotechnology Research Institute, North Carolina Central University, North Carolina Research Campus, 500 Laureate Way, Kannapolis, NC 28081, USA.
References
- Auger C, Mullen W, Hara Y, Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J Nutr. 2008;138:1535S–1542S. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with highgrade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Carter O, Dashwood RH, Wang R, Dashwood WM, Orner GA, Fischer KA, Lohr CV, Pereira CB, Bailey GS, Williams DE. Comparison of white tea, green tea, epigallocatechin-3-gallate, and caffeine as inhibitors of PhIP-induced colonic aberrant crypts. Nutr Cancer. 2007;58:60–65. doi: 10.1080/01635580701308182. [DOI] [PubMed] [Google Scholar]
- Catterall F, McArdle NJ, Mitchell L, Papayanni A, Clifford MN, Ioannides C. Hepatic and intestinal cytochrome P450 and conjugase activities in rats treated with black tea theafulvins and theaflavins. Food Chem Toxicol. 2003;41:1141–1147. doi: 10.1016/s0278-6915(03)00073-5. [DOI] [PubMed] [Google Scholar]
- Chen L, Bondoc FY, Lee MJ, Hussin AH, Thomas PE, Yang CS. Caffeine induces cytochrome P4501A2: induction of CYP1A2 by tea in rats. Drug Metab Dispos. 1996;24:529–533. [PubMed] [Google Scholar]
- Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- Chow HH, Hakim IA, Vining DR, Crowell JA, Cordova CA, Chew WM, Xu MJ, Hsu CH, Ranger-Moore J, Alberts DS. Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol Biomarkers Prev. 2006;15:2473–2476. doi: 10.1158/1055-9965.EPI-06-0365. [DOI] [PubMed] [Google Scholar]
- Chow HH, Hakim IA, Vining DR, Crowell JA, Tome ME, Ranger-Moore J, Cordova CA, Mikhael DM, Briehl MM, Alberts DS. Modulation of human glutathione s-transferases by polyphenon e intervention. Cancer Epidemiol Biomarkers Prev. 2007;16:1662–1666. doi: 10.1158/1055-9965.EPI-06-0830. [DOI] [PubMed] [Google Scholar]
- Chung FL, Wang M, Rivenson A, Iatropoulos MJ, Reinhardt JC, Pittman B, Ho CT, Amin SG. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: caffeine as an important constituent. Cancer Res. 1998;58:4096–4101. [PubMed] [Google Scholar]
- Donovan JL, Chavin KD, Devane CL, Taylor RM, Wang JS, Ruan Y, Markowitz JS. Green tea (Camellia sinensis) extract does not alter cytochrome p450 3A4 or 2D6 activity in healthy volunteers. Drug Metab Dispos. 2004;32:906–908. doi: 10.1124/dmd.104.000083. [DOI] [PubMed] [Google Scholar]
- Embola CW, Weisburger JH, Weisburger MC. Urinary excretion of N-OH–2-amino-3-methylimidazo[4, 5-f]quinoline-N-glucuronide in F344 rats is enhanced by green tea. Carcinogenesis. 2001;22:1095–1098. doi: 10.1093/carcin/22.7.1095. [DOI] [PubMed] [Google Scholar]
- Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem. 2005;280:16882–16890. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- Glei M, Pool-Zobel BL. The main catechin of green tea, (-)-epigallocatechin-3-gallate (EGCG), reduces bleomycin-induced DNA damage in human leucocytes. Toxicology In Vitro. 2005 doi: 10.1016/j.tiv.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Hakim IA, Harris RB, Brown S, Chow HH, Wiseman S, Agarwal S, Talbot W. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. J Nutr. 2003;133:3303S–3309S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- Hao X, Bose M, Lambert JD, Ju J, Lu G, Lee MJ, Park S, Husain A, Wang S, Sun Y, Yang CS. Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr Cancer. 2007;59:62–69. doi: 10.1080/01635580701365050. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Yaita M, Tanaka K, Hara Y, Kojo S. Inhibition of radical reaction of apolipoprotein B-100 and alpha-tocopherol in human plasma by green tea catechins. J Agric Food Chem. 2000;48:6380–6383. doi: 10.1021/jf000973i. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Hodgdon JM, Proudfoot JM, Croft KD, Pudde IB, Mori TA, Berllin LJ. Comparison of the effects of black and green tea on invitro lipoprotein oxidation in human serum. J Sci Food Agric. 1999;79:561–566. [Google Scholar]
- Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003;310:222–227. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, Yatsuya H, Sakata K, Kondo T, Kikuchi S, Toyoshima H, Hayakawa N, Tamakoshi A, Yoshimura T. Green tea and stomach cancer-a short review of prospective studies. J Epidemiol. 2005;15(Suppl 2):S109–S112. doi: 10.2188/jea.15.S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Lambert JD, Chin KV, Yang CS. Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat Res. 2004;555:3–19. doi: 10.1016/j.mrfmmm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of Action of (-)-Epigallocatechin-3-Gallate: Autooxidation-Dependent Inactivation of Epidermal Growth Factor Receptor and Direct effects on Growth Inhibition in Human Esophageal Cancer KYSE 150 Cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- Hsu SP, Wu MS, Yang CC, Huang KC, Liou SY, Hsu SM, Chien CT. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am J Clin Nutr. 2007;86:1539–1547. doi: 10.1093/ajcn/86.5.1539. [DOI] [PubMed] [Google Scholar]
- Hu M, Chen J, Lin H. Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther. 2003;307:314–321. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- Inami S, Takano M, Yamamoto M, Murakami D, Tajika K, Yodogawa K, Yokoyama S, Ohno N, Ohba T, Sano J, Ibuki C, Seino Y, Mizuno K. Tea catechin consumption reduces circulating oxidized low-density lipoprotein. Int Heart J. 2007;48:725–732. doi: 10.1536/ihj.48.725. [DOI] [PubMed] [Google Scholar]
- Jiang T, Glickman BW, de Boer JG. Protective effect of green tea against benzo[a]pyrene-induced mutations in the liver of Big Blue transgenic mice. Mutat Res. 2001;480–481:147–151. doi: 10.1016/s0027-5107(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, Yang GY, Liu YY, Hou Z, Lin Y, Ma J, Shih WJ, Carothers AM, Yang CS. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. 2007;17:395–402. doi: 10.1016/j.semcancer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Greaves P, Cooke DN, Edwards R, Steward WP, Gescher AJ, Marczylo TH. Breast cancer prevention by green tea catechins and black tea theaflavins in the C3(1) SV40 T, t antigen transgenic mouse model is accompanied by increased apoptosis and a decrease in oxidative DNA adducts. J Agric Food Chem. 2007;55:3378–3385. doi: 10.1021/jf0633342. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Kohri T, Nanjo F, Suzuki M, Seto R, Matsumoto N, Yamakawa M, Hojo H, Hara Y, Desai D, Amin S, Conaway CC, Chung FL. Synthesis of (-)-[4–3H]epigallocatechin gallate and its metabolic fate in rats after intravenous administration. J Agric Food Chem. 2001;49:1042–1048. doi: 10.1021/jf0011236. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kurihara M, Miyata N, Suzuki T, Toyoda M. Scavenging mechanisms of (-)-epigallocatechin gallate and (-)-epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action. Free Radic Biol Med. 1999;27:855–863. doi: 10.1016/s0891-5849(99)00133-1. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Raghunathan R, Maru GB. Effect of polymeric black tea polyphenols on benzo(a)pyrene [B(a)P]-induced cytochrome P4501A1 and 1A2 in mice. Xenobiotica. 2005;35:671–682. doi: 10.1080/00498250500202155. [DOI] [PubMed] [Google Scholar]
- Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523–524:201–208. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133:4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Hong J, Lee MJ, Sang S, Meng XF, Lu H, Yang CS. Biotransformation and bioavailability of tea polyphenols: implications for cancer prevention research. In: Shahidi F, Ho C-T, editors. Phenolic compounds in foods and natural health products. Washington: 2005a. pp. 212–224. (ACS Symposium Series 909). [Google Scholar]
- Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005b;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, Newmark HL, Yang CS. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab Dispos. 2006;34:8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- Landau JM, Wang ZY, Yang GY, Ding W, Yang CS. Inhibition of spontaneous formation of lung tumors and rhabdomyosarcomas in A/J mice by black and green tea. Carcinogenesis. 1998;19:501–507. doi: 10.1093/carcin/19.3.501. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–8121. [PubMed] [Google Scholar]
- Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, Huang B, Chung JY, Yan S, Ho CT, Yang CS. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol. 2000;13:177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- Li C, Meng X, Winnik B, Lee MJ, Lu H, Sheng S, Buckley B, Yang CS. Analysis of urinary metabolites of tea catechins by liquid chromatography/electrospray ionization mass spectrometry. Chem Res Toxicol. 2001;14:702–707. doi: 10.1021/tx0002536. [DOI] [PubMed] [Google Scholar]
- Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, Seril DN, Yang CS. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- Lin DX, Thompson PA, Teitel C, Chen JS, Kadlubar FF. Direct reduction of N-acetoxy-PhIP by tea polyphenols: a possible mechanism for chemoprevention against PhIP-DNA adduct formation. Mutat Res. 2003;523–524:193–200. doi: 10.1016/s0027-5107(02)00335-4. [DOI] [PubMed] [Google Scholar]
- Liu TT, Liang NS, Li Y, Yang F, Lu Y, Meng ZQ, Zhang LS. Effects of long-term tea polyphenols consumption on hepatic microsomal drug-metabolizing enzymes and liver function in Wistar rats. World J Gastroenterol. 2003;9:2742–2744. doi: 10.3748/wjg.v9.i12.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Toxicology, Rutgers. The State University of New Jersey; New Brunswick: 2002. Mechanistic studies on the phase II metabolism and absorption of tea catechins; p. 160. [Google Scholar]
- Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003a;31:452–461. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab Dispos. 2003b;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–11501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- Lu G, Xiao H, You H, Lin Y, Jin H, Snagaski B, Yang CS. Synergistic inhibition of lung tumorigenesis by a combination of green tea polyphenols and atorvastatin. Clin Cancer Res. 2008;14:4981–4988. doi: 10.1158/1078-0432.CCR-07-1860. [DOI] [PubMed] [Google Scholar]
- Maliakal PP, Coville PF, Wanwimolruk S. Tea consumption modulates hepatic drug metabolizing enzymes in Wistar rats. J Pharm Pharmacol. 2001;53:569–577. doi: 10.1211/0022357011775695. [DOI] [PubMed] [Google Scholar]
- McArdle NJ, Clifford MN, Ioannides C. Consumption of tea modulates the urinary excretion of mutagens in rats treated with IQ. Role of caffeine. Mutat Res. 1999;441:191–203. doi: 10.1016/s1383-5718(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Mantena SK, Elmets CA, Katiyar SK. (-)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- Meng X, Sang S, Zhu N, Lu H, Sheng S, Lee MJ, Ho CT, Yang CS. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem Res Toxicol. 2002;15:1042–1050. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- Meselhy MR, Nakamura N, Hattori M. Biotransformation of (-)-epicatechin 3-O-gallate by human intestinal bacteria. Chem Pharm Bull (Tokyo) 1997;45:888–893. doi: 10.1248/cpb.45.888. [DOI] [PubMed] [Google Scholar]
- Mimoto J, Kiura K, Matsuo K, Yoshino T, Takata I, Ueoka H, Kataoka M, Harada M. (-)-Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J mice. Carcinogenesis. 2000;21:915–919. doi: 10.1093/carcin/21.5.915. [DOI] [PubMed] [Google Scholar]
- Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46:1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. Implications of the mechanisms of action of tea polyphenols as antioxidants in vitro for chemoprevention in humans. Proc Soc Exp Biol Med. 1999;220:262–266. doi: 10.1046/j.1525-1373.1999.d01-45.x. [DOI] [PubMed] [Google Scholar]
- Sang SM, Tian S, Meng X, Stark RE, Rosen RT, Yang CS, Ho C-T. Theadibenzotropolone A, a new type pigment from enzymatic oxidation of (-)-epicatechin and (-)-epigallocatechin gallate and characterized from black tea using LC/MS/MS. Tetrahedron Lett. 2002;43:7129–7133. [Google Scholar]
- Sang S, Lambert JD, Hong J, Tian S, Lee MJ, Stark RE, Ho CT, Yang CS. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem Res Toxicol. 2005;18:1762–1769. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- Sang S, Yang I, Buckley B, Ho CT, Yang CS. Autoxidative quinone formation in vitro and metabolite formation in vivo from tea polyphenol (-)-epigallocatechin-3-gallate: studied by real-time mass spectrometry combined with tandem mass ion mapping. Free Radic Biol Med. 2007;43:362–371. doi: 10.1016/j.freeradbiomed.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang S, Lee MJ, Yang I, Buckley B, Yang CS. Human urinary metabolite profile of tea polyphenols analyzed by liquid chromatography/electrospray ionization tandem mass spectrometry with data-dependent acquisition. Rapid Commun Mass Spectrom. 2008;22:1567–1578. doi: 10.1002/rcm.3546. [DOI] [PubMed] [Google Scholar]
- Schuller HM, Porter B, Riechert A, Walker K, Schmoyer R. Neuroendocrine lung carcinogenesis in hamsters is inhibited by green tea or theophylline while the development of adenocarcinomas is promoted: implications for chemoprevention in smokers. Lung Cancer. 2004;45:11–18. doi: 10.1016/j.lungcan.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schwartz JL, Baker V, Larios E, Chung FL. Molecular and cellular effects of green tea on oral cells of smokers: a pilot study. Mol Nutr Food Res. 2005;49:43–51. doi: 10.1002/mnfr.200400031. [DOI] [PubMed] [Google Scholar]
- Senthil Kumaran V, Arulmathi K, Srividhya R, Kalaiselvi P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp Gerontol. 2008;43:176–183. doi: 10.1016/j.exger.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong AN. Comparison of (-)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6 J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22:1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- Sherwood L. Human physiology: from cells to systems. Wadsworth Publishing Co.; Belmont: 2004. [Google Scholar]
- Shi ST, Wang ZY, Smith TJ, Hong JY, Chen WF, Ho CT, Yang CS. Effects of green tea and black tea on 4-(methylnitrosamino)-1-(3- pyridyl)-1-butanone bioactivation, DNA methylation, and lung tumorigenesis in A/J mice. Cancer Res. 1994;54:4641–4647. [PubMed] [Google Scholar]
- Sohn OS, Surace A, Fiala ES, Richie JP, Jr, Colosimo S, Zang E, Weisburger JH. Effects of green and black tea on hepatic xenobiotic metabolizing systems in the male F344 rat. Xenobiotica. 1994;24:119–127. doi: 10.3109/00498259409043226. [DOI] [PubMed] [Google Scholar]
- Srividhya R, Jyothilakshmi V, Arulmathi K, Senthilkumaran V, Kalaiselvi P. Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (-)-epigallocatechin-3-gallate. Int J Dev Neurosci. 2008;26:217–223. doi: 10.1016/j.ijdevneu.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Sun CL, Yuan JM, Lee MJ, Yang CS, Gao YT, Ross RK, Yu MC. Urinary tea polyphenols in relation to gastric and esophageal cancers: a prospective study of men in Shanghai, China. Carcinogenesis. 2002;23:1497–1503. doi: 10.1093/carcin/23.9.1497. [DOI] [PubMed] [Google Scholar]
- Tang L, Tang M, Xu L, Luo H, Huang T, Yu J, Zhang L, Gao W, Cox SB, Wang JS. Modulation of aflatoxin biomarkers in human blood and urine by green tea polyphenols intervention. Carcinogenesis. 2008;29:411–417. doi: 10.1093/carcin/bgn008. [DOI] [PubMed] [Google Scholar]
- Tulayakul P, Dong KS, Li JY, Manabe N, Kumagai S. The effect of feeding piglets with the diet containing green tea extracts or coumarin on in vitro metabolism of aflatoxin B1 by their tissues. Toxicon. 2007;50:339–348. doi: 10.1016/j.toxicon.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Valcic S, Muders A, Jacobsen NE, Liebler DC, Timmermann BN. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (-)-epigallocatechin gallate with peroxyl radicals. Chem Res Toxicol. 1999;12:382–386. doi: 10.1021/tx990003t. [DOI] [PubMed] [Google Scholar]
- Valcic S, Burr JA, Timmermann BN, Liebler DC. Antioxidant chemistry of green tea catechins. New oxidation products of (-)-epigallocatechin gallate and (-)-epigallocatechin from their reactions with peroxyl radicals. Chem Res Toxicol. 2000;13:801–810. doi: 10.1021/tx000080k. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, Yang CS. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3- pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Res. 1992;52:1943–1947. [PubMed] [Google Scholar]
- Wiseman SA, Balentine DA, Frei B. Antioxidants in tea. Crit Rev Food Sci Nutr. 1997;37:705–718. doi: 10.1080/10408399709527798. [DOI] [PubMed] [Google Scholar]
- Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003a;63:7526–7529. [PubMed] [Google Scholar]
- Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003b;106:574–579. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- Xiao H, Hao X, Simi B, Ju J, Jiang H, Reddy BS, Yang CS. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis. 2008;29:113–119. doi: 10.1093/carcin/bgm204. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- Xu M, Bailey AC, Hernaez JF, Taoka CR, Schut HA, Dashwood RH. Protection by green tea, black tea, and indole-3-carbinol against 2-amino-3-methylimidazo[4, 5-f]quinoline-induced DNA adducts and colonic aberrant crypts in the F344 rat. Carcinogenesis. 1996;17:1429–1434. doi: 10.1093/carcin/17.7.1429. [DOI] [PubMed] [Google Scholar]
- Yang GY, Liu Z, Seril DN, Liao J, Ding W, Kim S, Bondoc F, Yang CS. Black tea constituents, theaflavins, inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice. Carcinogenesis. 1997;18:2361–2365. doi: 10.1093/carcin/18.12.2361. [DOI] [PubMed] [Google Scholar]
- Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- Yang CS, Liao J, Yang GY, Lu G. Inhibition of lung tumorigenesis by tea. Exp Lung Res. 2005;31:135–144. doi: 10.1080/01902140490495525. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–S151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- Yen GC, Ju JW, Wu CH. Modulation of tea and tea polyphenols on benzo(a)pyrene-induced DNA damage in Chang liver cells. Free Radic Res. 2004;38:193–200. doi: 10.1080/10715760310001638001. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Ishikawa T, Hosoai H, Suzukawa M, Ayaori M, Hisada T, Sawada S, Yonemura A, Higashi K, Ito T, Nakajima K, Yamashita T, Tomiyasu K, Nishiwaki M, Ohsuzu F, Nakamura H. Inhibitory effect of tea flavonoids on the ability of cells to oxidize low density lipoprotein. Biochem Pharmacol. 1999;58:1695–1703. doi: 10.1016/s0006-2952(99)00256-7. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int J Cancer. 2007;120:1344–1350. doi: 10.1002/ijc.22460. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu Q, Lantry LE, Wang Y, Kelloff GJ, Anderson MW, Wiseman RW, Lubet RA, You M. A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53- independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents taxol or adriamycin. Cancer Res. 2000;60:901–907. [PubMed] [Google Scholar]


