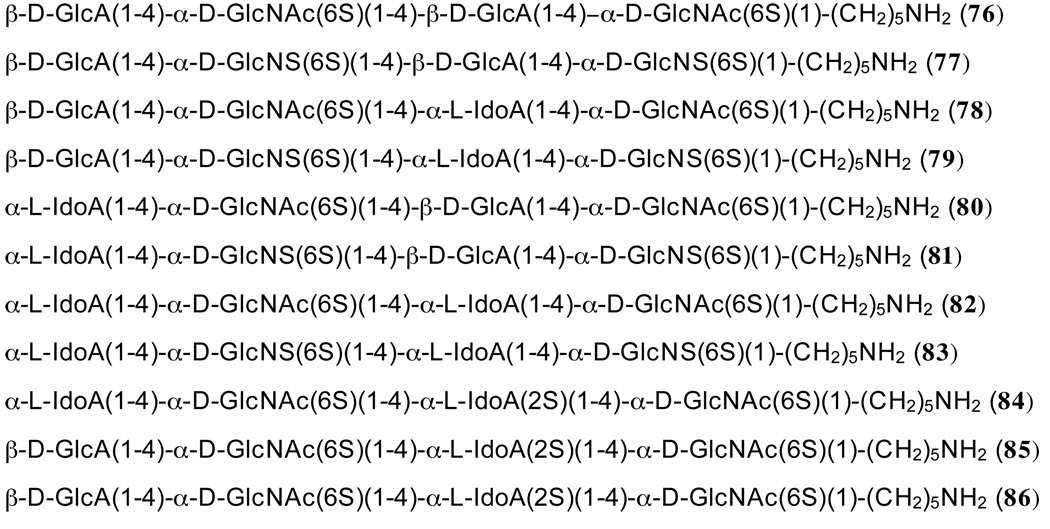Abstract
Although hundreds of heparan sulfate binding proteins have been identified, and implicated in a myriad of physiological and pathological processes, very little information is known about ligand requirements for binding and mediating biological activities by these proteins. This difficulty results from a lack of technology for establishing structure-activity-relationships, which in turn is due to the structural complexity of natural heparan sulfate (HS) and difficulties of preparing well-defined HS-oligosaccharides. To address this deficiency, we have developed a modular approach for the parallel combinatorial synthesis of HS oligosaccharides that utilizes a relatively small number of selectively protected disaccharide building blocks, which can easily be converted into glycosyl donors and acceptors. The utility of the modular building blocks has been demonstrated by the preparation of a library of twelve oligosaccharides, which has been employed to probe structural features of HS for inhibiting the protease, BACE-1. The complex variations in activity with structural changes support the view that important functional information is embedded in HS sequences. Furthermore, the most active derivative provides an attractive lead compound for the preparation of more potent compounds, which may find use as a therapeutic agent for Alzheimer's disease.
Introduction
Glycoaminoglycans (GAGs) such as heparin and heparan sulfate (HS) are naturally occurring polydisperse linear polysaccharides that are heavily O- and N-sulfated.1,2 The interaction between GAGs and proteins can have profound physiological effects on hemostasis, lipid transport and adsorption, cell growth and migration and development.3,4 Binding of GAGs can result in the immobilization of proteins at their sites of production, regulation of enzyme activity, binding of ligands to their receptors and protection of proteins against degradation. Alteration in GAG expression has been associated with disease and for example,5 significant structural changes have been reported in proteoglycans surrounding the stroma of tumors and it has been suggested that these alterations may support tumor growth and invasion.
Currently, more than a hundred heparan sulfate-binding proteins have been identified4 and it is to be expected that in the near future many more will be discovered. For a small number of HS binding proteins, it has been established that a specific sulfation pattern is required for mediating biological activity and the best-studied case represents the interaction of antithrombin with heparin.6 Each of the sulfates of the pentasaccharide GlcNAc6S-GlcA-GlcN3S-IdoA-GlcNS is essential for high affinity binding to antithrombin and anticoagulant activity. Interestingly, the pentasaccharide contains a rare glucosamine moiety that has a sulfate ester at C-3. The latter moiety is also required for binding of Herpes simplex gD protein to HS, which in turn is important for viral infection.7 On the other hand, it has been proposed that for some HS binding proteins, the spatial organization of clusters of negative charge in HS is an important determinant of binding and biological activity. It appears that in these cases, the HS binding proteins have a relaxed selectivity for short HS oligosaccharides, an example being thrombin, which requires a highly sulfated structure for binding.8 This diversity of interactions emphasize the need for more detailed structure-activity studies on a wider range of HS binding proteins.9
For most HS binding there is very little or no information about ligand requirements for binding and mediating biological activity.10 This difficulty is due to a lack of technology for establishing structure-activity-relations (SAR), which in turn is due to the structural complexity of natural HS and difficulties of preparing well-defined compounds.11,12 Initial approaches to establish structure-activity-relations (SAR) employ modified derivatives of heparin in which acetamido, sulfonamido, or sulfate esters are chemically modified to produce polysaccharides that have simpler compositions than the parent compound have proved useful.13 In addition, HS has been sulfated at specific positions using biosynthetic enzymes.14 Although these approaches make it possible to draw some conclusions about the requirement of particular functionalities for binding or biological activity, they do not allow determination of the structure of binding epitopes. Natural libraries of HS oligosaccharides have been generated and screened15 but sequencing of identified hits is still a technical challenge.
In principle, synthetic and chemoenzymatic approaches have the potential to provide a sufficiently large range of well-defined HS oligosaccharides for SAR or array development. Elegant synthetic approaches for heparin synthesis have been described,11,16–18 however, no efficient strategy for the synthesis of a wide range of HS structures has been reported. In order to address this problem, we have proposed a modular approach for the chemical synthesis of a wide range of HS oligosaccharides whereby a set of properly protected disaccharide building blocks that resemble the different disaccharide motifs found in HS, are assembled by a parallel combinatorial manner into larger structures.19,20 Our previous efforts suffered, however, from difficulties in preparing key mono- and disaccharide intermediates, difficulties in removing temporary protecting groups, unreliability in glycosylations and difficulties in the final deprotection. Others have attempted to develop modular approaches for HS synthesis,17,21 however, these methods provided unnatural sulfation patterns, were unable to make structures larger than disaccharides or did not demonstrate the convenient preparation of a wide range of structural motifs.
We report here a robust modular synthetic approach for the preparation of a wide range of well-defined HS oligosaccharides for SAR studies. The synthetic methodology is based on the use of a relatively small number properly protected disaccharide donors and acceptors that in a parallel combinatorial manner, using a standard set of reaction conditions, can be assembled into a large number of HS-oligosaccharides. The compounds are equipped with an anomeric aminopentyl spacer, which provides an opportunity for conjugation to a solid surface, which for example is required for microarray technology development.18 To illustrate the convenience of the modular building blocks, a library of twelve oligosaccharides has been prepared to probe the requirement for inhibition of the protease, β-secretase (or BACE-1). The cleavage of amyloid precursor protein (APP) by the protease BACE-1 is a key step in generating amyloid plaques, which are a characteristic of Alzheimer disease; synthetic compounds that inhibit this enzyme have potential as novel agents to treat this diseases.22,23
Results and Discussion
Synthetic strategy for modular HS oligosaccharide synthesis
Heparan sulfate consists of 1,4-linked disaccharide units of α-l-iduronic or β-d-glucuronic acid and either N-acetyl or N-sulfo-α-d-glucosamine. The principal positions of O-sulfation are C-2 of iduronate and C-6 of glucosamine, as well as, more rarely, C-3 of glucosamine. Variable substitution during biosynthesis results in the formation of at least twenty different disaccharide motifs. Combining these different disaccharides into larger structures potentially results in enormous structural diversity.1
A modular approach that employs a set of properly protected disaccharide building blocks that resemble the different disaccharide motives found in HS, and can easily and repeatedly be used for the preparation of multiple targets, has the potential to provide a library of HS oligosaccharides for structure activity relationship studies. A key issue of such a modular approach is the selection of a set of protecting groups that meet the following requirements: i) the C-2 hydroxyl-protecting groups of the glucuronic or iduronic acid moieties should allow the stereoselective introduction of 1,2-trans-glycosidic linkages, whereas the C-2 amino group of the glucosamine derivatives need to be derivatized in such a way that 1,2-cis-glycosidic linkages can be formed; ii) temporary protecting groups for the anomeric center and C-4 need to be selected for the preparation of glycosyl donors and acceptors; iii) a protecting group is required that can selectively be removed to reveal those hydroxyls that need sulfation; iv) the removal of the permanent protecting groups should be compatible with the presence of base and acid labile sulfate esters; v) the protecting group scheme should be applicable to synthesize each of disaccharide modules found in natural HS; and finally vi) a unified set of chemical conditions is required for the preparation of the various disaccharide modules, oligosaccharide assembly, sulfation and deprotection.
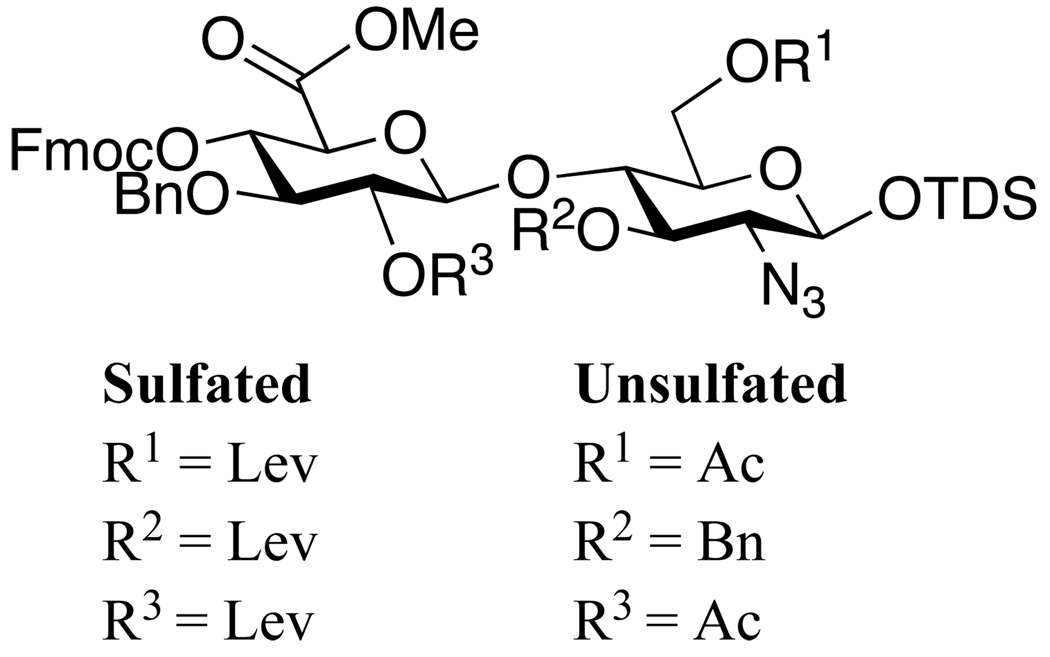
The protecting group strategy that we have developed for the disaccharide modules is summarized in Figure 1. Thus, levulinoyl esters (Lev)24 will be employed for those hydroxyls that need sulfation. In HS, the C-3 and C-6 of glucosamine and C-2 hydroxyls of hexuronic acid moiety can be sulfated and therefore depending on the sulfation pattern of a targeted disaccharide module, one or more of these positions will be protected as Lev esters. An important feature of the Lev ester is that when present at the C-2’ position, it will be able to direct the formation of 1,2-trans-glycosides by neighboring group participation.25 In case that the C-2‘ position of a disaccharide module does not need sulfation, an acetyl group is employed as a permanent protecting group. This ester can also perform neighboring group participation but is stable under the conditions used for the removal of Lev esters. An azido group will be used as an amino-masking functionality.26 This derivative does not perform neighboring group participation and therefore allows the introduction of α-glucosides. An azido-group can easily be reduced to an amine, which can either be acetylated or sulfated. The C-4’ hydroxyl, which is required for extension, will be protected as 9-fluorenylmethyl carbonate (Fmoc). The Fmoc group can be removed with Et3N in dichloromethane or DMF without affecting the Lev ester whereas the Lev group can be cleaved with hydrazine buffered with acetic acid and these conditions do not affect the Fmoc carbonate.24 The anomeric center of the disaccharides will be protected as thexyldimethyl silyl (TDS) glycosides and this functionality can easily be removed by treatment with HF in pyridine without affecting the other protecting groups. The resulting lactol can then be converted into a trichloroacetimidate by employing K2CO3 and trichloroacetonitrile in DCM.20 Finally, benzyl ethers are used as permanent protecting groups for the other hydroxyls.
Figure 1.
Orthogonal protecting groups for disaccharide building blocks
Preparation of modular building blocks
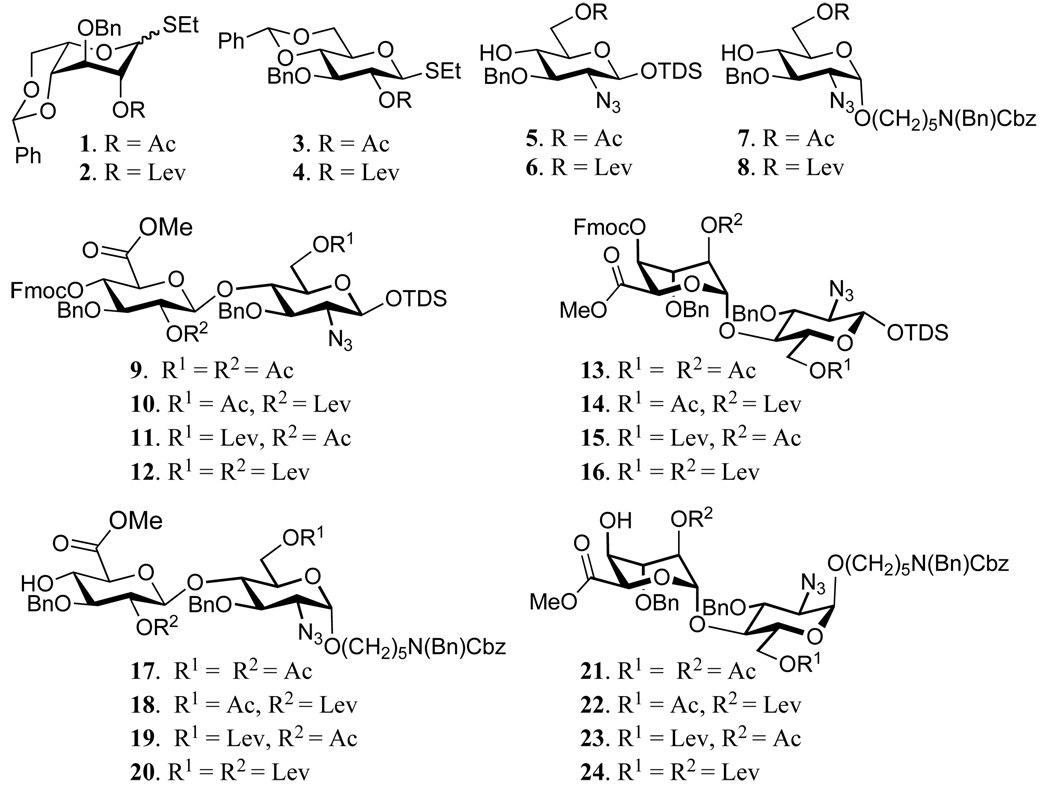
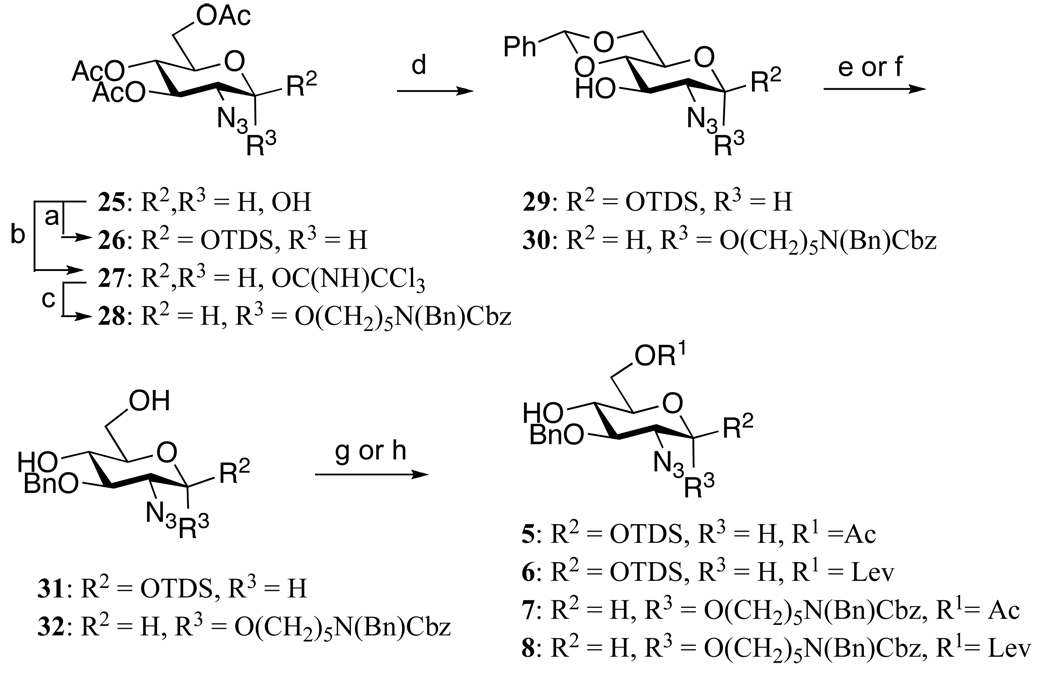
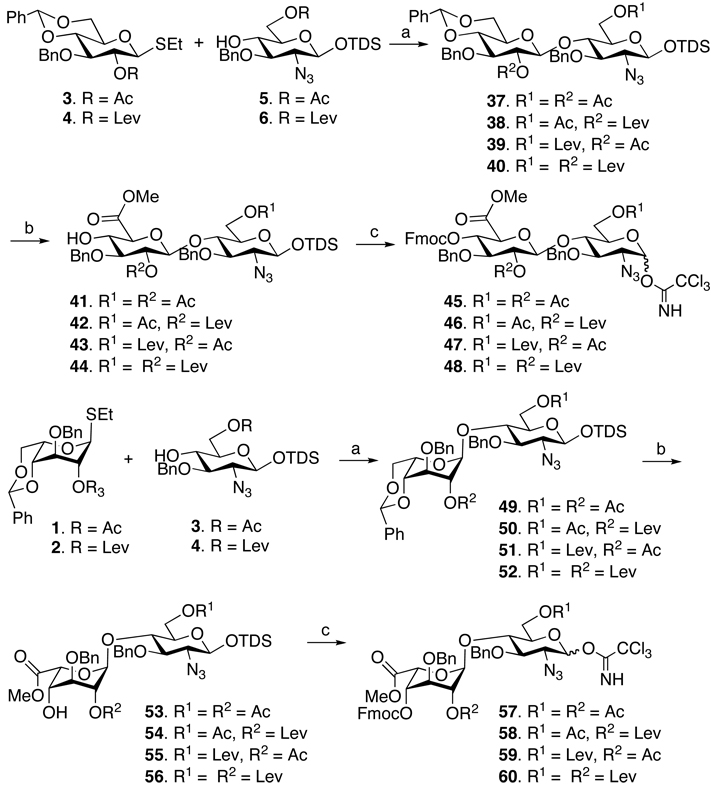
Based on these considerations, the six strategically chosen monosaccharide building blocks 1–6 were prepared, which were employed for the preparation of the eight disaccharide modules 9–16 (Figure 2). It was observed that installment of an aminopentyl linker at the disaccharide stage led to mixtures of anomers, which were difficult to separate by traditional silica column chromatography and therefore the spacer containing glycosyl acceptors 7 and 8 were employed for the preparation of disaccharide modules 17–24. Glycosyl acceptors 5–8 could be prepared form readily available lactol 2527 (Scheme 1). Thus, for the preparation of 5 and 6, the anomeric center of 25 was protected as a TDS ether by reaction with TDSCl in the presence of imidazole in DCM to give 26 in an excellent yield of 90%. The chemical synthesis of compounds 7 and 8 required the installation of an α-anomeric N-(benzyl)benzyloxycarbonyl protected aminopentenyl spacer, which was achieved by conversion of lactol 25 into a trichloroacetimidate 27, which was coupled with N-(benzyl)benzyloxycarbonyl aminopentanol in the presence of TMSOTf in a mixture of dichloromethane and diethyl ether to give 28 as a mixture of anomers (α/β = 3/1), which could be separated by silica gel column chromatography to afford the individual anomers. A number of other anomeric spacers was examined and for example, lower yields in subsequent glycosylations was obtained when benzyloxycarbonyl amino propanol was employed. Furthermore, the use of N-(benzyl)benzyloxycarbonyl propanol gave compounds having complex 1H-NMR due to E/Z isomerism of the amino-protecting groups.18 Fortunately, the latter problem could be partially alleviated by employing N-(benzyl)benzyloxycarbonyl protected aminopentanol.
Figure 2.
Modular mono- and disaccharide building blocks
Scheme 1.
Synthesis of 2-azido-2-deoxy-α-D-glucopyranoside acceptors (a) TDSCl, imidazole, DCM (90%); (b) CCl3CN, DBU, DCM (90%); (c) HO(CH2)5N(Bn)Cbz, DCM:Et2O, TMSOTf, molecular sieves, −20°C (α-anomer, 62%) (d) i) NaOMe, MeOH; ii) PhCH(OMe)2, CSA, DMF (29: 92% and 30: 76%, 2 steps); (e) i) BnBr, Ag2O, molecular sieves, DCM; ii) DCM:TFA:H2O (31: 95%, 2 steps); (f) NaH, BnBr, DMF; ii) DCM:TFA:H2O (32: 93%, 2 steps); (g) AcOH, 2-chloro-1-methyl-1-pyridinium iodide, DABCO, DCM (5: 65%, 7: 68%), (h) LevOH, 2-chloro-1-methyl-1-pyridinium iodide, DABCO, DCM (6: 86%, 8: 82%)
The acetyl esters of 26 and 28 were cleaved by treatment with sodium methoxide in methanol and the 4,6-diol of the resulting compound was protected as a benzylidene acetal by treatment with PhCH(OMe)2 in the presence of a catalytic amount of camphorsulfonic acid in DMF to give 29 and 30, respectively. The C-3 hydroxyl of 29 was benzylated with benzyl bromide in the presence of Ag2O and the benzylidene acetal of the resulting compound was hydrolyzed by treatment with TFA in DCM and water to afford diol 31. The more robust compound 30 was benzylated with benzyl bromide and NaH in DMF and the benzylidene acetal of the resulting compound was removed by TFA in DCM and water to give 32. The C-6 hydroxyl of 31 and 32 were regioselectively protected as an acetyl ester by reaction with acetic acid and the activator 2-chloro-1-methyl-1-pyridinium iodide in the presence of DABCO in DCM to give the target compounds 5 and 7, respectively. Compounds 6 and 8, which have a Lev ester at C-6, were prepared by a similar procedure using levulinic acid instead of acetic acid.
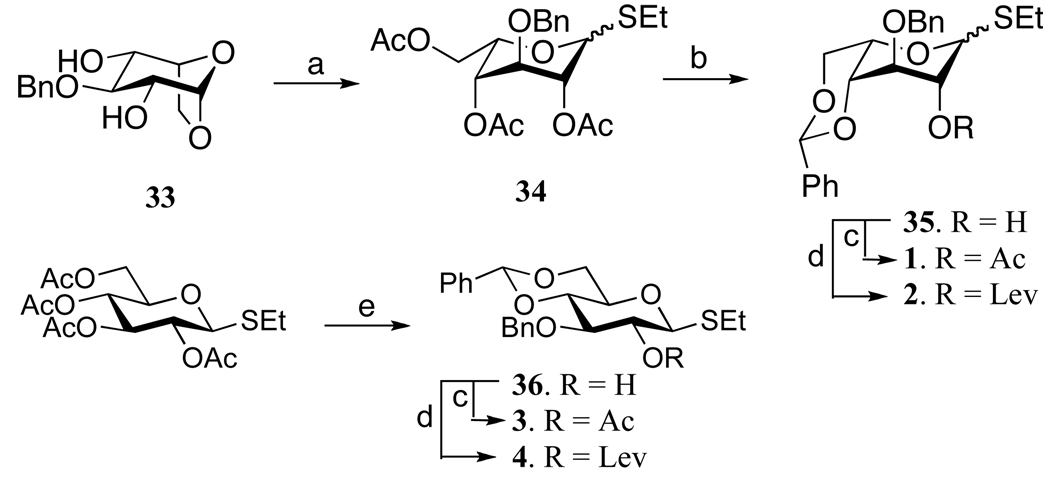
Idosyl donors 1 and 2 were easily prepared by starting from readily available 1,6-anhydro-idose 33 (Scheme 2).17,28 Thus, the treatment of 3317 with ethanethiol and BF3 Et2O in DCM29 gave thio-idoside 34, which was deacetylated to give a triol, which was protected as 4,6-O-benzyldine acetal to give 35. The C-2 hydroxyl of 35 was protected as an acetyl or Lev ester by reaction with acetic anhydride in pryidine or levulinic acid in the presence of the activator DCC to provide the required idosyl donors 1 and 2.Glucosyl donors 3 and 4 were readily obtained by saponification of the acetyl ester of ethyl 2,3,4-tri-O-acetyl thioglucoside using standard conditions followed by selective protection of the 4,6-diol of the resulting compound as a benzylidene acetal by treatment with PhCH(OMe)2 and camphorsulfonic acid in DMF and then regioselective benzylation of the C-3 hydroxyl by first preparing a stannylidene acetal by treatment with dibutyl tin oxide followed by reaction with benzyl bromide in the presence of CsF in DMF to give 36. The latter compound was a convenient starting material for the preparation of glucosyl donors 3 and 4 by protecting of the C-2 hydroxyl as an acetyl- or Lev esters, respectively, using standard conditions.
Scheme 2.
Synthesis of thioethyl gluco- and idosyl donor. (a) i) AcOH, Ac2O, TFA, r.t; ii) EtSH, BF3.Et2O, DCM, 0°C to room temperature; (b) i) NaOMe, MeOH; ii) PhCH(OMe)2, CSA, DMF, 70%; (c) Ac2O, Py, r.t, 85%; (d) LevOH, DCC, DMAP, DCM, 72%; (e) (i) NaOMe, MeOH; ii) PhCH(OMe)2, p-TsOH, DMF, 61% (2 steps); iii) (Bu2Sn)O, MeOH, 75–80°C, BnBr, CsF, DMF, 16 hr, r.t, 61%; (c) Ac2O, Py, 60%: 1: 93%, 3: 85%; (d) LevOH, DCC, DMAP, DCM, r.t, 2: 89% 4: 70%
Having glycosyl donors 1–4 and acceptors 5–8 in hand, attention was focused on the parallel combinatorial synthesis of a range of disaccharide modules (Scheme 3). Thus, a NIS/TMSOTf mediated coupling30 of each of the thioglycosyl donors 1–4 with each the glycosyl acceptors 5 and 6 gave eight different disaccharides (37–40 and 49–52) having either a glucoside or idoside at the non reducing end and a Lev ester at C-6 or C-2’ or at both hydroxyls. In each glycosylation, only a 1,2-trans-glycoside was formed due to neighboring group participation by the C-2 acetyl or Lev ester of the glycosyl donors giving the disaccharides in excellent yields ranging from 70%–95%. Next, the disaccharides 37–40 and 49–52 were converted into glycosyl acceptors 41–44 and 53–56 and glycosyl donors 45–48 and 57–60 by a unified set of reaction conditions. Thus, the benzylidene acetals of 37–40 and 49–52 was removed by treatment with p-toluenesulfonic acid in the presence of ethanethiol or by a mixture of TFA, DCM and water to give the corresponding diols, which were oxidized with 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) in the presence of iodobenzene diacetate (BAIB) as the cooxidant.31,32 The resulting carboxylic acids were protected as methyl esters by treatment with diazomethane to give compounds 41–44 and 53–56 in an overall yield ranging from 65–90%. Interestingly, the use of sodium hypochloride as the co-oxidant in the TEMPO oxidation31 led to a lower yield of product due to partial oxidation of the secondary hydroxyl. The disaccharides were also the starting material for the preparation of glycosyl donors 45–48 and 57–60 by protection of the C-4’ alcohols as an Fmoc carbonate by treatment with FmocCl in pyridine in the presence of DMAP followed by removal of the anomeric TDS with HF in pyridine and installation of the anomeric trichloroacetimidate using trichloroacetonitrile and K2CO3 in DCM. The latter reaction conditions did not affect the base labile Fmoc protecting group. Each reaction was high yielding regardless of the chemical composition of the disaccharide. The spacer containing disaccharide acceptors 17–24 (Figure 2) were prepared by a similar strategy by coupling glycosyl donors 1–4 with glycosyl acceptors 7 and 8 followed by benzylidene acetal removal, selective oxidation of the C-6’ hydroxyl of the resulting compound and protection of the resulting carboxylic acid as a methyl ester (for details see supporting information).
Scheme 3.
Synthesis of glucuronyl and idouronyl disaccharides. (a) NIS, TMSOTf, 0°C, DCM (37: 81%, 38: 75%, 39: 92%, 40: 75%. 49: 90% 50: 66%, 51: 95%, 52: 80%); (b) i) EtSH, p-TsOH, DCM or DCM:TFA:H2O; ii) TEMPO, BAIB, DCM, H2O, 1 h; iii) CH2N2, THF (3 steps 65–85%); (e) i) FmocCl, Py,DMAP, 0°C to room temperature; (ii)! HF.Py,18 h; iii) K2CO3, CCl3CN, DCM (70–90%)
In principle, parallel combinatorial coupling of the eight glycosyl donors 45–48 and 57–60 with the eight glycosyl acceptors 41–44 and 53–56 (or the corresponding spacer containing acceptors 17–24) will give sixty four different tetrasaccharides. These compounds can easily be converted into glycosyl acceptors by removal of the Fmoc carbonate and each of the resulting compounds can then again be coupled with the eight glycosyl donors to provide five hundred and twelve different hexasaccharides. After the assembly of the oligosaccharides, the azido moieties can be converted into acetamido or N-sulfate derivatives further increasing the structural diversity of synthetic compounds. It may also be possible to obtain additional compounds by converting the azido moiety of the disaccharides into NHAlloc and then employ it in a glycosylation with an azido-containing disaccharide. The Alloc and azido offer a convenient set of orthogonal aminoprotecting groups that allow selective modification of each function.33
Preparation of a hexasaccharide using modular building blocks
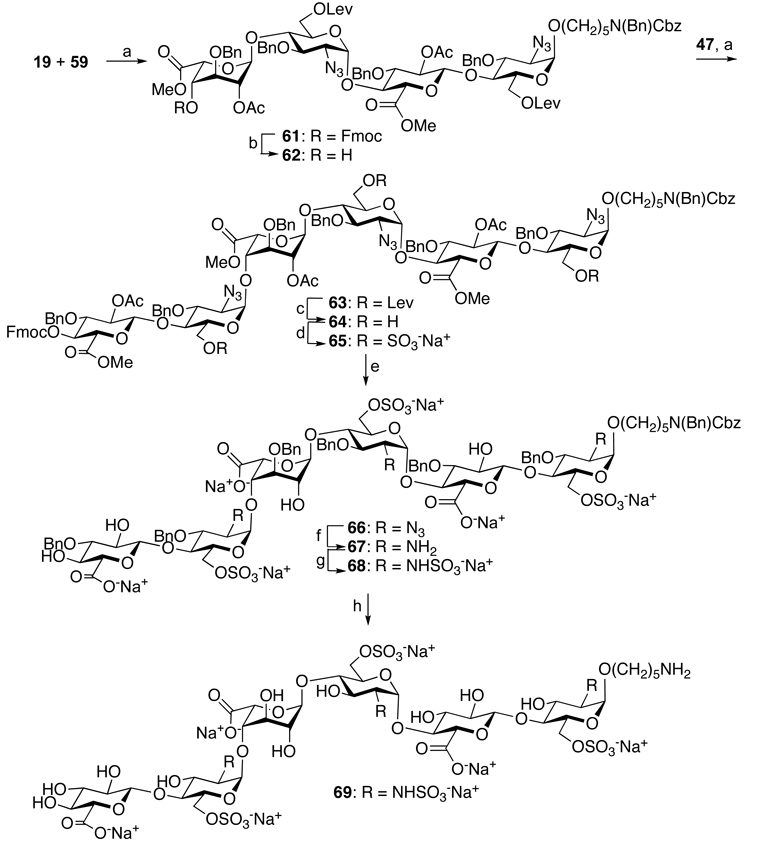
To demonstrate that the disaccharide acceptors and donors can be employed for modular HS oligosaccharide synthesis, hexasaccharide 69 was prepared from disaccharide modules 19, 47 and 59 (Scheme 4). Thus, a TMSOTf mediated glycosylation of trichloroacetimidate 59 with spacer-containing glycosyl acceptor 19 gave tetrasaccharide 61 in a yield of approximately 60% as only the alpha-anomer. The Fmoc protecting group of 61 was removed by standard conditions using Et3N in DCM and the resulting glycosyl acceptor 62 was coupled with glycosyl donor 47 to give the fully protected hexasaccharide 63 in a good yield and in this case, also only the α-anomer was observed. Next, sulfate esters were installed by removal of the Lev esters of 63 by treatment with hydrazine acetate in a mixture of toluene and ethanol followed by sulfation of the resulting hydroxyls of 64 with pyridinium sulfur trioxide to give compound 65. Next, the acetyl and methyl esters of 65 were saponified by a two-step procedure employing first LiOH in a mixture of hydrogen peroxide and THF34 and then sodium hydroxide in methanol to give partially deprotected 66. The azido moiety of 66 was reduced with trimethyl phosphine in THF in the presence of NaOH18,35 to give amine 67, which was immediately sulfated with pyridinium sulfur trioxide in the presence of triethylamine in methanol to give N-sulfate 68. Finally, the benzyl ethers and benzyloxycarbamate of 68 were removed by a two-step procedure36 involving hydrogenation over Pd/C in a mixture of MeOH/H2O which led to the removal of the spacer protecting groups followed by hydrogenation over Pd(OH)2 which led to the removal of the benzyl ethers to give HS oligosaccharides 69. Interestingly, a one step hydrogenation procedure proceeded very sluggishly and did not provide the target compound.
Scheme 4.
(a) Synthesis of hexasaccharide 69: (a) TMSOTf, −20°C to +5°C, 4°A sieves, 61: 64%; 63: 65%; (b) Et3N, DCM, 82%; (c) NH2NH2.HOAc, toluene/EtOH, 90%; (d) Py.SO3, DMF, 80%; (e) i) Et3N, DMF; ii) LiOH, H2O2, THF; ii) 4M NaOH, MeOH (58%, 3 steps), 80%; (f) PMe3, THF, NaOH, 65%; (g) N-sulfation: Py.SO3, MeOH, Et3N, 0.1 M NaOH, 50%; (i) i) Pd/C, H2, MeOH:H2O; ii) Pd(OH)2/C, H2, H2O, 67%
The 1H NMR spectra of the oligosaccharides were fully assigned by 1D and 2D NMR spectroscopy. The α-anomeric configuration of 2-azido-glucosides was confirmed by J1,2 coupling constants and by 13C chemical shifts of C-1 (~97 ppm). Furthermore, a downfield shifts of 0.5 ppm of H-6 was observed for O-sulfation of C-6 hydroxyls and 0.4 ppm of H-2 for N-sulfation.
Synthesis of a library of HS-oligosaccharides to probe inhibition of BACE-1
Alzheimer's disease is a progressive neurodegenerative disorder of the central nervous system that is characterized by the formation of β-amyloid peptides, which accumulate as perivascular or parenchymal deposits in brains. Cleavage of amyloid precursor protein (APP) by the aspartyl protease beta-site APP-cleaving enzyme 1 (BACE-1) generates a membrane-bound protein, which is further processed by the γ-secretase enzyme complex to generate the neurotoxic amyloid β-peptide. Selective inhibition of β-amyloid peptide formation could potentially slow or even reverse the devastating consequences of the disease.37 Indeed, experimental data from transgenic mouse models of Alzheimer's disease, BACE-1 knockout mice and pharmacological studies corroborate the potential usefulness of drugs that interfere with BACE-1 expression and/or enzymatic activity for the treatment of Alzheimer's disease.38
Heparan sulfate, which is a constituent of amyloid plaques, can interact with amyloid proteins, peptides, and fibrils, promote aggregation, and enhance the stability of fibrils. Soluble heparin and heparin analogues have been shown to inhibit these processes both in vitro and in vivo. Recently, however, it was shown that HS can inhibit the proteolytic activity of BACE-1; the putative mechanism is by blocking access to the enzyme active site, without interfering with APP processing by α- or γ-secretases.39 Systematic modification of porcine intestinal mucosal heparin was used to demonstrate a critical importance of 6-O-sulfates for inhibition of BACE-1.22 Furthermore, replacement of N-sulfate groups by acetamido moieties slightly impairs activity22,23 and derivatives containing N-acetyl and 2-O- and 6-O-sulfates had the highest anti- BACE-1 to anti-Xa activity ratio 22, demonstrating opportunities for optimizing therapeutic activities.
To probe the requirement of HS oligosaccharides for inhibition of BACE-1, a library of twelve tetrasaccharides (76–85, Figure 3) was prepared employing the disaccharide modules. Thus, tetrasaccharides 76–83, which contain C-6 sulfate esters but differ in the modification of the C-2 amino groups and the presence of glucuronic acid or idouronic acid moieties, were prepared. These compounds should reveal the importance of nature of the uronic acid moiety and the presence of an acetamido or N-sulfate group, for inhibitory activity. Furthermore, compounds 84 and 85 are derived from 80 and 78, respectively but have an additional sulfate ester at C-2 of an iduronic acid moiety.
Figure 3.
Putative synthetic HS ligands for BACE-1
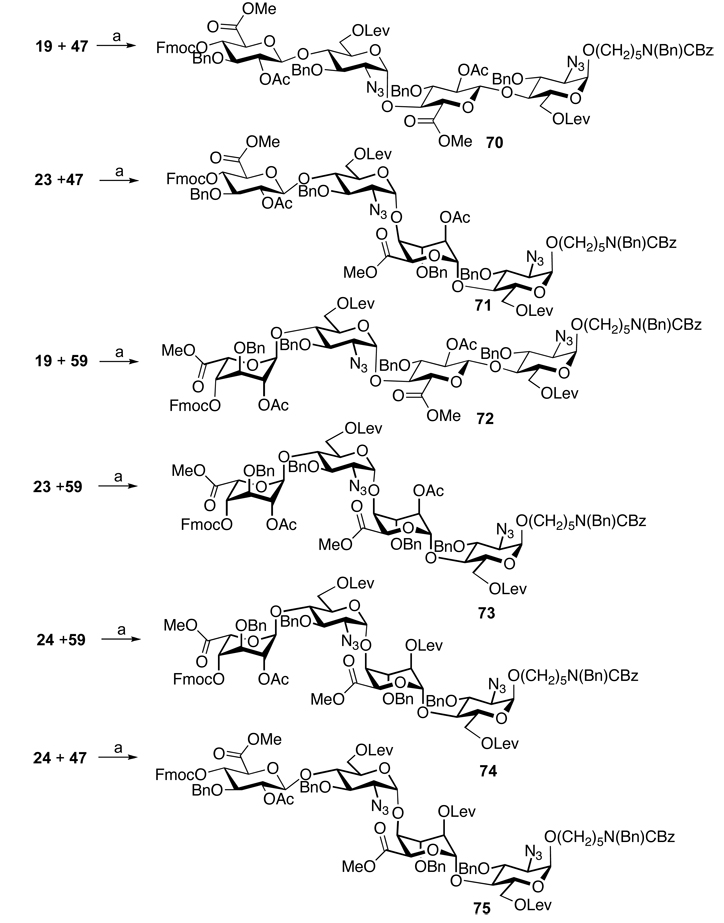
The target HS fragments could easily be prepared by employing the disaccharide modules 19, 23, 24, 47 and 59 combined with the sulfation and deprotection protocols described for the preparation of hexasaccharide 69. Thus, standard TMSOTf mediated glycosylation of glycosyl donors 19, 23 and 24 with glycosyl acceptor 47 and 59 in DCM gave the tetrasaccharides 70–75 in yields of approximately 60%, and fortunately in each case only the α-anomer was obtained (Scheme 5). Our preliminary studies had indicated that 2-deoxy-2-azido-glucopyranosyl trichloroacetimidates give excellent α-anomeric selectivities when modified with an acyl-protecting group at C-6 and employed in glycosylations with glycosyl acceptors of relatively low reactivity. This observation may be due to remote neighboring group participation of the C-6 ester.40 O-sulfation, deprotection and N-acetylation or N-sulfation was performed by standard procedures to give the target tetrasaccharides 76–86.
Scheme 5.
(a) Synthesis of library of tetrasaccharides. (a) TMSOTf, DCM, −20°C to 5°C, molecular sieves (70: 61%, 71: 62%, 72: 64%, 73: 62%, 74: 51%, 75: 59%)
The ability of the HS oligosaccharides to inhibit BACE-1 cleavage of APP was assessed using a fluorescent resonance energy transfer (FRET) peptide cleavage assay employing the Swedish amino acid variant FRET peptide 5-FAM-Glu-Val-Asn-Leu-Asp-Ala-Phe-Lys(QXL520)-OH. When intact, the amino terminal fluorophore is quenched, but upon enzymatic cleavage, it is released from quencher and fluoresces at 520 nm.
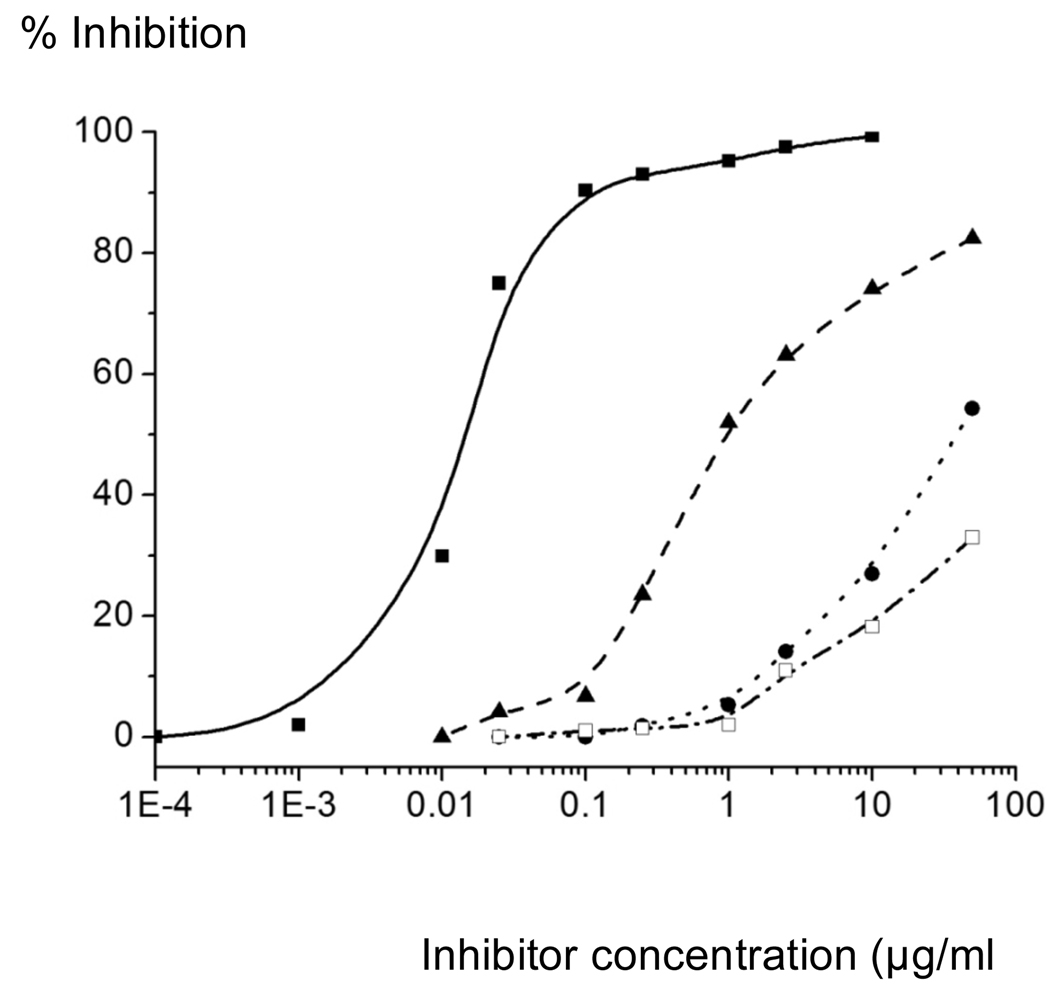
As can be seen in Table 1 and Figure 4, HS-oligosaccharide 83 was able to inhibit the cleavage of the peptide by BACE-1 with relatively high potency. Several derivatives such as 76, 77 and 82 displayed modest activity whereas compounds 78, 79, 81, 84, and 86 had low- or no inhibitory activity. Compound 83 is a tetrasaccharide composed of two iduronic acid moieties that are sulfated at the C-6 hydroxyls and two glucosamine moieties modified by N-sulfates. Interestingly, compound 82, which has acetamido moieties instead of N-sulfates, has 10-fold reduced potency highlighting the importance of the N-sulfates of 83 for optimal inhibitory activity. Replacement of one of the iduronic acid moieties by a glucuronic acid derivative, as in compounds 79 and 81, led to a large reduction in inhibitory activity. Surprisingly compounds 76 and 77, which contain two glucuronic acid moieties, displayed reasonable activity and in this case, HS oligosaccharide 76 having acetamido moieties was somewhat more active than compound 77 modified by N-sulfates. Previous studies have indicated that the binding cleft of BACE-1 can accommodate relatively large HS oligosaccharides,22 and thus it is conceivable that derivatives 76/77 and 82/83 bind in different regions of the cleft explaining the difference in structure-activity-relationship. Finally, a sulfate ester at C-2 of an uronic acid moiety, as in compound 84–86, had low- or no inhibitory activity.
Table 1.
Inhibitory activities of synthetic compounds and heparin for cleave of FRET peptide by BACE-1
| HS | IC50 (µg/ml) | IC50 (µmol) |
|---|---|---|
| heparin | 0.02 | - |
| 76 | 39 | 35 |
| 77 | 97 | 78 |
| 78 | >300 | - |
| 79 | 240 | 195 |
| 80 | 104 | 94 |
| 81 | 290 | 236 |
| 82 | 35 | 32 |
| 83 | 4.6 | 3.7 |
| 84 | >300 | - |
| 85 | 167 | 138 |
| 86 | >300 | - |
| 69 | 64 | 36 |
Figure 4.
Dose response inhibition curves for selected compounds compared to porcine mucosal heparin in the FRET peptide cleavage assay (solid squares, heparin; triangles, compound 83; circles, compound 77 and open squares, compound 81).
The synthetic HS-oligosaccharides are less active than full-length HS polysaccharide, which was expected because previous observations with natural oligosaccharides suggest that the binding site of BACE-1 can accommodate relatively large HS-oligosaccharides.22 However, the fact that a tetrasaccharide displayed considerable inhibitory activity indicates that a library of such compounds is appropriate for identifying lead compounds, which provide an attractive starting point for the synthesis of a focused library of larger oligosaccharides. The attraction of such an approach is that the preparation of a representative library of tetrasaccharides is an achievable task whereas preparation of a library of larger HS-oligosaccharides remains a considerable challenge.
Conclusions
The modular synthetic approach reported here utilizes a relatively small number of selectively protected disaccharide building blocks that can easily be converted into glycosyl donors and acceptors, which in turn can be employed for the convenient preparation of libraries of well-defined HS oligosaccharides. Such a collection of compounds can be employed for structure activity relationship studies for HS binding proteins. Key features of the approach include the use of Lev esters for those hydroxyls that need sulfation, an Fmoc carbonate as a temporary protecting group for the C-4’ hydroxyl for the preparation of glycosyl acceptors, an anomeric TDS group for glycosyl donor synthesis and acetyl esters and benzyl ethers as permanent protecting groups. Trichloroacetimidate methodology41 was employed for reliable oligosaccharide assembly and in each glycosylation only the required α-anomer was obtained. Furthermore, it was found that installation of the uronic acid moieties could best be performed at the disaccharide stage by selective TEMPO/BIAB mediated oxidation of the C-6 hydroxyl of a glucoside or idoside to the corresponding carboxylic acid. The utility of the modular building blocks has been illustrated by the preparation of a library of twelve oligosaccharides and importantly, a standard set of reaction conditions could be employed for the preparation of all target compounds. The HS oligosaccharides were employed to probe structural features of HS for inhibition of the protease, BACE-1. The significant and complex variations in activity with structural changes observed in this study support the view that important functional information is embedded in HS sequences.10,15 Furthermore, the most active derivative identified in this study provides an attractive lead compound for the preparation more potent compounds for BACE-1, which may find use as a therapeutic agent for Alzheimer's disease. The synthetic compounds are also equipped with an artificial aminopentyl spacer, which offers an opportunity for HS-oligosaccharide array development. Such an array is expected to provide a unique tool for rapid ligand identification for HS-binding proteins.
Experimental Section
General glycosylation procedure for synthesis of disaccharides (37–40, 49–52)
Glycosyl thioethyl donor (1.2 eq based on acceptor) and 2-azido-2-deoxy-α-D-glucopyranoside acceptor (1.0 eq) were combined in a flask, co-evaporated with toluene (3× 3mL) and dissolved in DCM to maintain a concentration of 0.02 M (based on donor). Powdered freshly activated 4Å molecular sieves (weight of sieves equal to the combined weight of donor and acceptor) were added and the mixture was stirred for 30 min at ambient temperature and then cooled to 0°C. NIS (1.2 eq) and TMSOTf (0.1 eq) were added to the mixture and stirring was continued until TLC indicated disappearance of glycosyl donor (~15 min). The reaction mixture was allowed to warm to +5°C and then quenched by the addition of DTBMP. The mixture was filtered, the filtrate was concentrated in vacuo and the residue was purified by silica gel column chromatography using a stepwise gradient of toluene and EtOAc (90/10 → 65/35, v/v).
General procedure for benzylidene acetal cleavage of disaccharides
Method A
To a solution of disaccharide (37, 49) in DCM was added ethanethiol (6 eq) and p-TsOH (0.2 eq) and the resulting solution was stirred at ambient temperature for 1 h. The reaction mixture was quenched by the addition of Et3N and concentrated in vacuo and the residue was purified by silica gel column chromatography to give pure product.
Method B
A solution of a disaccharide (38–40, 50–52) in a mixture of DCM:TFA:H2O (0.06 M, 10/1/0.1, v/v/v) was stirred at ambient temperature for 30 min. The reaction mixture was concentrated in vacuo and the residue co-evaporated with toluene. The residue was purified by silica gel column chromatography using a mixture of toluene and EtOAc to give pure product.
General procedure for TEMPO/BAIB mediated oxidation and esterification by diazomethane (41–44, 53–56)
To a vigorously stirred solution of the diol (0.3 M solution) in a mixture of DCM: H2O (2/1, v/v) was added TEMPO (0.2 eq) and BAIB (2.5 eq). Stirring was continued until TLC indicated complete conversion of the starting material to a spot of lower Rf (~45 min). The reaction mixture was quenched by the addition of aqueous Na2S2O3 (10%, 10 mL). The mixture was extracted with EtOAc (2× 10 mL) and the combined aqueous layers were back-extracted with EtOAc (10 mL). The combined organic layers were dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The oily residue was dissolved in THF (0.1 M) and treated with an excess of freshly prepared ethereal solution of diazomethane until the reaction mixture stayed yellow. The excess diazomethane was quenched by the addition of AcOH until the reaction mixture became colorless. The mixture was concentrated in vacuo, co-evaporated with toluene and the residue was purified by silica gel column chromatography to yield a methyl ester.
General Procedure for synthesis of Fmoc protected disaccharides
To a 0.03 M solution of disaccharide in DCM at 0°C was added FmocCl (10 eq) and DMAP (0.01 eq). The reaction mixture was brought to room temperature and stirring was continued until TLC indicated complete consumption of the starting material (~2 h). After quenching the reaction with MeOH (50 µL), the mixture was diluted with DCM (50 mL), washed with saturated aqueous sodium bicarbonate (2× 50 mL), brine (50 mL). The organic phase was dried (MgSO4) filtered and the filtrate concentrated in vacuo. The residue was chromatographed over silica gel using a gradient of hexanes and EtOAc to give Fmoc carbonate protected disaccharide.
General procedure for silyl ether cleavage
A disaccharide was dissolved in THF (0.05 M) followed by the addition of HF.pyridine (100 eq). After stirring for 18 h, the reaction mixture was diluted with DCM (50 mL), and washed with water (50 mL), saturated aqueous sodium bicarbonate (50 mL), and brine (50 mL). The organic phase was dried (MgSO4), filtered and the filtrate concentrated in vacuo. The residue was chromatographed over silica gel using a gradient of hexanes and EtOAc to give pure lactol.
General procedure for preparation of trichloroacetimidates (45–48, 57–60)
To a solution of the lactol in DCM (2 mL for 0.08 mmol) was added finely powdered anhydrous K2CO3 (2 eq.). After stirring at room temperature for 1.5 h, the reaction mixture was filtered and the filtrate concentrated in vacuo. The residue was chromatographed over silica gel using a mixture of hexanes and EtOAc containing 0.01% pyridine to yield a trichloroacetimidate donor.
General procedure for preparation of tetrasaccharides (70–75)
Disaccharide trichloroacetimidate donor (1.2 eq based on acceptor) and disaccharide acceptor (1.0 eq) were combined in a flask, co-evaporated with toluene (3 × 3 mL) and dissolved in DCM to maintain a concentration of (0.04–0.05 M). Powdered freshly activated 4Å molecular sieves (weight of sieves equal to the combined weight of donor and acceptor) were added and the mixture was stirred for 30 min at ambient temperature and then cooled to −20°C. TMSOTf (0.1 eq) was added and stirring was continued until TLC indicated the disappearance of donor (~15 min). The reaction was allowed to warm to +5°C and then quenched by the addition of pyridine (5 µL) after 1 hour. The mixture was filtered, the filtrate was concentrated in vacuo and the residue was purified by silica gel column chromatography using a gradient of toluene and EtOAc to give pure tetrasaccharide.
General procedure for Fmoc cleavage of tetrasaccharide
A tetrasaccharide was dissolved in a mixture of DCM (2.4 mL for 0.12 mmol) and triethylamine (0.6 mL). After stirring for 2 h, the reaction mixture was concentrated in vacuo and the residue was purified by silica gel column chromatography using a gradient of hexanes and EtOAc to afford a tetrasaccaharide acceptor.
General procedure for cleavage of Lev esters
Anhydrous hydrazine acetate (5 eq per Lev group) was added to a solution of the starting material in a mixture of ethanol and toluene (2/1, v/v, 5 mL for 150 mg). Stirring was continued until TLC analysis (toluene/EtOAc 1/1, v/v or hexanes/EtOAc 1/1, v/v) indicated disappearance of starting material (~2 h). The reaction mixture was diluted with DCM (30 mL), washed with water (3 × 25 mL), brine (25 mL), dried (MgSO4) and filtered. The filtrate was concentrated and the residue was purified by silica gel column chromatography using a gradient of hexanes or toluene and EtOAc to afford product.
General procedure for O-sulfation
Sulfur trioxide pyridine complex (10 eq. per OH) was added to a solution of the starting material in DMF (1.0 mL for 0.02 mmol). The mixture was stirred at ambient temperature for 2–4 h until TLC (CHCl3, CH3OH 90/10, v/v) indicated completion of the reaction. After the addition of pyridine (0.2 mL) and CH3OH (0.5 mL) stirring was continued for 30 min. The mixture was concentrated in vacuo (bath temperature 20°C) and the residue was applied to a column of Iatrobeads (1.5 g), which was eluted with a gradient of CH3OH in CHCl3 (96/4 → 88/12 v/v, containing 0.2% pyridine). The fractions containing product were concentrated in vacuo (bath temperature 20°C) and the residue was immediately passed through a column of Biorad 50X8 Na+ resin (0.6 × 5cm) using CH3OH as eluent providing the product as sodium salt.
General procedure for Fmoc cleavage
Et3N (0.1 mL) was added to a solution of the starting material in DMF (1.0 mL for 0.02 mmol). The reaction mixture was stirred for ~1.5 hrs until TLC (CHCl3/CH3OH, 85/15, v/v) indicated disappearance of starting material. The reaction mixture was concentrated in vacuo (bath temperature 20°C), and the residue was passed through a column of Biorad 50X8 Na+ resin (0.6 × 5cm) using CH3OH as eluent. Fractions containing product were concentrated in vacuo and the residue chromatographed over Iatrobeads (1.2 g) using a gradient of CH3OH in CHCl3 (94/5 → 88/12, v/v) as eluent. Appropriate fractions were concentrated in vacuo providing product, which was directly used in the next step.
General procedure for saponification of methyl esters and de-O-acetylation
A premixed solution of 30% solution of H2O2 in water (100 eq per CO2Me) and 1M LiOH (50 eq per CO2Me) were added to a solution of the starting material in THF (0.02 M). The reaction mixture was stirred at room temperature for 8 h. A 4N solution of NaOH (1.0 mL) was added until pH 14. The reaction mixture was left stirring for 18 h at room temperature. In the case that the reaction had not gone to completion, stirring was continued at 35°C for an additional 12 h. The mixture was then brought to pH 8–8.5 by addition of AcOH and the mixture was concentrated in vacuo (bath temperature 20°C). The residue was vortexed with water and applied to a RP-18 column (10 times the weight of starting material), which was eluted with a stepwise gradient of H2O and CH3OH (from 90/10 → 70/30, v/v). The appropriate fractions were concentrated in vacuo (bath temperature 20°C) and the residue was passed through a column of Biorad 50X8 Na+ resin (0.6 × 5cm) using CH3OH as eluent providing product.
General procedure for reduction of azide group
A 1M solution of PMe3 in THF (8 eq per azide group)) was added to the solution of the starting material in THF (1.0 mL for 0.013 mmol). 0.1 M NaOH (10 eq per azido group) was added and the mixture was stirred at room temperature for 5 h. The progress of the reaction was monitored by TLC (CHCl3/CH3OH/H2O 70/30/5, v/v/v and RP-18 plates with H2O/CH3OH 40/60, v/v). The presence of amino groups was confirmed using ninhydrin as visualizing agent (In some cases, an additional amount of PMe3 solution was added to achieve completion of the reaction). The pH was then adjusted to 8.5 by careful addition of AcOH and the mixture concentrated in vacuo (bath temperature 20°C). The residue was vortexed with water and applied to a small RP-18 silica gel column (10 times the weight of starting material), which was eluted with a stepwise gradient of H2O and CH3OH (from 90/10 → 40/60, v/v). The appropriate fractions were concentrated in vacuo and the residue was passed through a column Biorad 50X8 Na+ resin (0.6 × 5cm) using CH3OH as eluent providing product.
General procedure for selective N-acetylation
Acetic anhydride (10 eq per NH2) was added to a solution of the starting material in a mixture of anhydrous CH3OH (500 µL for 0.011 mmol) and Et3N (20 eq per NH2) at 0°C. The progress of the reaction was monitored by TLC (silica gel: CHCl3/CH3OH/H2O, 60/30/3, v/v/v, and RP18 silica gel: H2O/CH3OH, 40/60, v/v). After 5 h, another portion of Et3N and Ac2O was added at 0°C. After stirring for 1h at room temperature, the mixture was co-evaporated with toluene in vacuo (bath temperature 20°C), and the residue passed through a short column of Biorad 50X8 Na+ resin (0.6 × 5 cm) using a mixture of CH3OH and H2O (90/10, v/v) as eluent and appropriate fractions were concentrated in vacuo. The residue was vortexed with water and applied to small RP-18 column (20 times the weight of starting material), which was eluted with a stepwise gradient of H2O and CH3OH (from 90/10 → 40/60, v/v). The appropriate fractions were concentrated in vacuo to obtain N-acetylated product.
General procedure for selective N-sulfation
SO3.Py (5 eq per NH2) was added to the starting material in CH3OH (1mL for 0.006 mmol) in a mixture of triethylamine (0.3 mL) and 0.1M NaOH (2 eq. per NH2) at 0°C. The progress of the reaction was monitored by TLC (silica gel TLC: EtOAc/pyridine/water/CH3CO2H, 8/5/3/1, v/v/v/v and RP-18 TLC: H2O/CH3OH, 60/40, v/v). Two additional portions of SO3.Py were added at 0°C after 1 h and 2 h. After stirring for an additional 8 h, the reaction mixture was co-evaporated with water (bath temperature 20°C) and the residue passed through a short column of Biorad 50X8 Na+ resin (0.6 × 5cm) using CH3OH and H2O (90/10, v/v) as eluent. Appropriate fractions were concentrated in vacuo and the residue was vortexed with water and applied to small RP-18 silica gel column (20 times the weight of starting material), which was then eluted with a stepwise gradient of H2O and CH3OH (90/10 → 40/60, v/v). The appropriate fractions were concentrated in vacuo to provide N-sulfated product.
General procedure for global debenzylation
Pd/C (10%, 1.5 time the weight of starting material) was added to a solution of the starting material in CH3OH and H2O (1/1, v/v, 1 mL for 5 mg). The mixture was placed under an atmosphere of hydrogen and the progress of the reaction monitored by TLC (silica gel, CHCl3/CH3OH/H2O 60/40/10, v/v/v and EtOAc/pyridine/water/CH3CO2H, 3/5/3/1, v/v/v). The hydrogenation was stopped when TLC indicated the disappearance of the starting material and the presence of a ninhydrin positive-main spot (2 h). The mixture was filtered through a PTFE syringe filter (0.2 mm, 13 mm) and washed with mixture of CH3OH and H2O (1/1, v/v, 2 mL) and the solvents were concentrated in vacuo. The residue was dissolved in distilled water (1.5 mL) and palladium hydroxide on carbon (Degussa type, 20%, 1.5 times the weight of starting material) added. The resulting mixture was placed under an atmosphere of hydrogen and after 12 h, TLC (EtOAc/pyridine/water/CH3CO2H 4/5/3/1, v/v/v/v) indicated the completion of the reaction. The mixture was filtered through a PTFE syringe filter and the residue was washed with H2O (2 mL). The filtrate was freeze-dried and the residue was passed through a short column of Biorad 50X8 Na+ resin (0.6 × 2.5 cm) using H2O as the eluent and the appropriate fractions were freeze dried to provide the final product.
5-aminopentyl [(β-D-glucopyranosyluronate)-(1→4)-(2-deoxy-2-sulfoamino-6-O-sulfonato-α-D-glucopyranoside)-(1→4)-O-(α-L-idopyranosyluronate)-(1→4)-O-(2-deoxy-2-sulfoamino-6-O-sulfanate-α-D-glucopyranoside)-(1→4)-(β-D-glucopyranosyluronate)]-(1→4)-O-2-deoxy-N-sulfoamino-6-O-sulfonate-α-D-glucopyranoside nonasodium salt (69)
Hexasaccharide 63 (73.5 mg, 0.028 mmol) was subjected to the sequence of deprotection steps including delevulinoylation, O-sulfation, Fmoc cleavage, saponification, deacetylation, azide reduction, N-sulfation and global debenzylation according to the general procedures to provide hexasaccharide 69 (4.8 mg). [α]D25: +126.4 (c = 0.33, H2O): 1H NMR (800 MHz, D2O): δ 5.58 (d, 1H, J = 3.7 Hz, H1c), 5.33 (d, 1H, J = 3.6 Hz, H1E), 5.13 (d, 1H, J = 3.6 Hz, H1A), 4.99 (bs, 1H, H1E), 4.80 (d, 1H, J = 2.17 Hz, H5D), 4.59 (s, 1H, J = 8.0 Hz, H1F), 4.58 (s, 1H, J = 8.0 Hz, H1B), 4.46 (bd, 1H, J = 9.7 Hz, H6aE), 4.41 (bd, 1H, J = 9.6 Hz, H6aA), 4.31( bd, 1H, J = 10.4 Hz, H6aC), 4.25 (dd, 1H, J = 6.0 Hz, J = 11.1 Hz, H6bA), 4.17 ( m, 2H, H6bE, H6bC), 4.10 ( t, 1H, J = 3.6 Hz, H3D), 4.04-4.01 (m, 3H, H4D, H5A, H5E), 3.97 (bd, 1H, J = 10.04 Hz, H5C), 3.84 (t, 1H, J = 9.1 Hz, H3B), 3.80-3.65 (m, 10H, H5B, H5F, H4E, H2D, H4B, H3A, OCHH Linker, H4C, H3E, H4A), 3.62 (t, 1H, J = 9.7 Hz, H3C), 3.56-3.56-3.47 (m, 3H, OCHH Linker, H3F, H4F), 3.36 (t, 1H, J = 8.6 Hz, H2B), 3.32 (t, 1H, J = 8.5 Hz, H2F), 3.27 (dd, 1H, J = 3.6 Hz, J = 10.2 Hz, H2A), 3.26 (dd, 1H, J = 3.6 Hz, J = 10.0 Hz, H2C), 3.24 (dd, 1H, J = 3.6 Hz, J = 10.5 Hz, H2E) 3.0 (m, 2H, CH2N Linker), 1.72-1.62 (m, 4H, 2 × CH2 Linker), 1.52-1.45 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C41H68N4O49S6: 796.0644, found: 796.0634 [M-2H]2−; calcd. for C41H67N4O49S6: 530.3738, found: 530.3721 [M-3H]3−.
5-aminopentyl [(β-D-glucopyranosyluronate)-(1→4)-(2-acetamido-2-deoxy-6-O-sulfanate-α-D-glucopyranoside)-(1→4)-O-(β-D-glucopyranosyluronate-(1→4)-O-2-acetamido-2-deoxy-6-O-sulfanate-α-D-glucopyranoside tetrasodium salt (76)
Tetrasaccharide 70 (101 mg, 0.052 mmol) was subjected to the sequence of deprotection steps including delevulinoylation, O-sulfation, Fmoc cleavage, saponification, deacetylation, azide reduction to give a diamino tetrasaccharide. Part of this material was subjected to N-acetylation and global debenzylation according to the general procedures to give the tetrasaccharide 76 (3.0 mg). [α]D25: +70 (c= 1, H2O); 1H NMR (800 MHz, D2O): δ 5.40 (d, 1H, J = 3.8 Hz, H1C), 4.85 (d, 1H, J = 3.2 Hz, H1A), 4.55-4.54 (m, 2H, H1B, H1D), 4.42 (bd, 2H, H6aC, H6aA), 4.23 (dd, 1H, J = 6.2 Hz, J = 11.4 Hz, H6bA), 4.16 (dd, 1H, J = 1.9 Hz, J = 11.1 Hz, H6bC), 4.04-4.00 (m, 2H, H5C, H5A), 3.96 (m, 3H, H2C, H2A, H3A), 3.82-3.78 (m, 2H, H3C, H5B), 3.74-3.67 (m, 6H, H4B, H4C, H5D, OCHH Linker, H3B, H4A), 3.51 (t, 1H, J = 9.3 Hz, H3D), 3.50-3.46 (m, 2H, OCHH Linker, H4D), 3.32 (dd, 2H, J = 7.9 Hz, J = 9.3 Hz, H2B, H2D), 2.98 (t, 2H, J = 7.7 Hz, CH2N Linker), 2.02, 2.00 (2s, 3H each, 2 × CH3, NAc), 1.69-1.59 (m, 4H, 2 × CH2 Linker), 1.46-1.43 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C33H54N3O29S2: 1020.2290, found: 1020.2312 [M-H]1−; calcd. for C33H53N3O29S2: 509.6109, found: 509.6118 [M-2H]2−.
5-aminopentyl [(β-D-glucopyranosyluronate)-(1→4)-(2-deoxy-2-N-sulfoamino-6-Osulfonate-α-D-glucopyranoside)-(1→4)-O-(β-D-glucopyranosyluronate-(1→4)-O-2-deoxy-2-N-sulfoamino-2-deoxy-6-O-sulfonate-α-D-glucopyranoside hexasodium salt (77)
A diamino tetrasaccharide obtained by partial deprotection of 70 was subjected to N-sulfation and global debenzylation according to the general procedures to give tetrasaccharide 77 (6.8 mg). [α]D25: +33 (c= 1, H2O); 1H NMR (800 MHz, D2O): δ 5.62 (d, 1H, J = 3.7 Hz, H1C), 5.12 (d, 1H, J = 3.7 Hz, H1A), 4.58 (d, 1H, J = 7.9 Hz, H1B), 4.56 (d, 1H, J = 7.9 Hz, H1D), 4.43 (dd, 1H, J = 1.9 Hz, J = 11.0 Hz, H6aC), 4.41 (dd, 1H, J = 2.0 Hz, J = 11.2 Hz, H6aA), 4.23 (dd, 1H, J = 6.0 Hz, J = 11.2 Hz, H6bA), 4.15 (dd, 1H, J = 1.9 Hz, J = 11.0 Hz, H6bC), 4.01 (ddd, 1H, J = 2.0 Hz, J = 6.0 Hz, J = 10.0 Hz, H5A), 3.99 (bd, 1H, J = 10.0 Hz, H5C), 3.84(t, 1H, J = 8.8 Hz, H3B), 3.80-3.71(m, 6H, H5B, H4B, H4C, H5D, OCHH Linker, H3A), 3.66-3.64 (m, 2H, H3C, H4A), 3.56-3.54 (m, 1H, OCHH Linker), 3.53-3.46 (m, 2H, H3D, H4D), 3.35 (dd, 1H, J = 7.9 Hz, J = 9.4 Hz, H2B), 3.32 (dd, 1H, J = 8.0 Hz, J = 9.2 Hz, H2D), 3.26 (dd, 1H, J = 3.7 Hz, J = 8.2 Hz, H2A), 3.23(dd, 1H, J = 3.7 Hz, J = 10.8 Hz, H2C), 2.99 (t, 2H, J = 7.4 Hz, CH2N Linker), 1.70-1.61 (m, 4H, 2 × CH2 Linker), 1.50-1.44 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C29H50N3O33S4: 1096.1215, found: 1096.1237 [M-H]1−; calcd. for C29H49N3O33S4: 547.5571, found: 547.5580 [M-2H]2−
5-Aminopentyl [β-D-glucopyranosyluronate-(1→4)-(2-acetamido-2-deoxy-6-O-sulfonate-α-D-glucopyranoside)-(1→4)-α-L-idopyranosyluronate)]-( 1→4)-2-acetamido-2-deoxy-6-O-sulfonate-α-D-glucopyranoside tetrasodium salt (78)
Tetrasaccharide 71 (53 mg, 0.027 mmol) was subjected to the sequence of deprotection steps including delevulinoylation, O-sulfation, Fmoc cleavage, saponification, deacetylation, azide reduction to obtain a diamino tetrasaccharide. Part of this material was subjected to N-acetylation and global debenzylation according to the general procedure to give tetrasaccharide 78 (2.5 mg). [α]D25: +22 (c = 0.5, H2O); 1H NMR (800 MHz, D2O): δ 5.16 (d, 1H, J = 3.8 Hz, H1C), 4.96 (d, 1H, J = 3.3 Hz, H1B), 4.86 (d, 1H,. J = 3.6 Hz, H1A), 4.70 (d, 1H, J = 2.9 Hz, H5B), 4.56 (d, 1H, J = 7.9 Hz, H1D), 4.43 (dd, 1H, J = 2.6 Hz, J = 11.2 Hz, H6aC), 4.33 (dd, 1H, J = 2.0 Hz, J = 11.2 Hz, H6aA), 4.25 (dd, 1H, J = 5.7 Hz, H6bA), 4.01 (dd, 1H, J = 1.9 Hz, H6bC), 4.08 (m, 1H, H5C), 4.06 (t, 1H, J = 3.4 Hz, H4B), 4.03 (m, 1H, H5A), 3.96 (dd, 1H, J = 10.4 Hz, H2C), 3.94 (dd, 1H, J = 3.6 Hz, J = 10.7 Hz, H2A), 3.88 (dd, J = 3.4 Hz, J = 5.9 Hz, H3B), 3.82 (dd, J = 8.7 Hz, H3A, J = 10.7 Hz), 3.78-3.73 (m, 3H, H3C, H4C, H5D), 3.72-3.65 (m, 3H, H2B, H4A, OCHH Linker), 3.53-3.47 (m, 3H, H3D, H4D, OCHH Linker), 3.33 (dd, 1H, J = 7.9 Hz, J = 9.4 Hz, H2D), 2.98 (t, 2H, J = 7.6 Hz, CH2NH2 Linker), 2.01, 1.98 (2s, 3H each, 2 × CH3, NHAc), 1.70-1.58 (m, 4H, 2 × CH2 Linker) and 1.48-1.40 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C33H54N3O29S2: 1020.2290, found: 1020.2273 [M-H]1−; calcd. for C33H53N3O29S2: 509.6109, found: 509.6119 [M-2H]2−; calcd. for C33H52N3O29S2: 339.4048, found: 339.4051 [M-3H]3−.
5-Aminopentyl [β-D-glucopyranosyluronate-(1→4)-(2-deoxy-2-sulfoamino-6-O-sulfonate-α-D-glucopyranoside)-(1→4)-α-L-idopyranosyluronate)]-( 1→4)-2-deoxy-2-sulfoamino-6-O-sulfonate-α-D-glucopyranoside hexasodium salt (79)
The second portion of the diamino tetrasaccharide obtained above was subjected to N-sulfation and global debenzylation according to the general procedure described above to obtain the tetrasaccharide 79 (4.1 mg). [α]D25: +40 (c = 0.5, H2O); 1H NMR (800 MHz, D2O): δ 5.36 (d, 1H, J = 3.6 Hz, H1C, 5.13 (d, 1H, J = 3.7 Hz, H1A), 4.99 (d, 1H, J = 2.4 Hz, H1B), 4.72 (d, 1H, J = 2.3 Hz, H5B), 4.57 (d, 1H, J = 8.0 Hz, H1D), 4.46 (dd, 1H, J = 2.0 Hz, J = 11.0 Hz, H6aC), 4.32 (dd, 1H, J = 1.7 Hz, J = 11.0 Hz, H6aA), 4.23 (dd, 1H, J = 5.5 Hz, J = 11.0 Hz, H6bA), 4.18 (dd, 1H, J = 1.7 Hz, J = 11.0 Hz, H6bC), 4.10 (dd, 1H, J = 3.5 Hz, J = 4.4Hz, H3B), 4.05 (bt, 1H, J = 4.0 Hz, H4B), 4.04-4.00 (m, 2H, H5A, H5C), 3.77-3.64 (m, 7H, H2B, H3A, H3C, H4A, H4C, H5D, OCHH Linker), 3.57-3.51 (m, 2H, H3D, OCHH Linker), 3.48 (t, 1H, J = 9.3 Hz, H4D), 3.33 (dd, 1H, J = 8.0 Hz, J = 9.1 Hz, H2D), 3.27 (dd, 1H, J = 3.7 Hz, J = 9.9 Hz, H2A), 3.24 (dd, 1H, J = 10.5 Hz, H2C), 3.02 (t, 2H, J = 7.4 Hz, CH2NH2), 1.70-1.60 (m, 4H, 2 × CH2 Linker), 1.52-1.42 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C29H50N3O33S4: 1096.1215, found: 1096.1247 [M-H]1−; calcd. for C29H49N3O33S4: 547.5571, found: 547.5591 [M-2H]2−; C29H48N3O33S4: 364.7023, found: 364.7034 [M-3H]3−.
5-aminopentyl [(α-L-idopyranosyluronate)-(1→4)-O-(2-acetamido-2-deoxy-6-O-sulfonate-α-D-glucopyranoside)-(1→4)-O-(β-D-glucopyranosyluronate)]-(1→4)-(2-acetamido-2-deoxy-6-O-sulfonate-α-D-glucopyranoside tetrasodium salt (80)
Tetrasaccharide 72 (89 mg, 0.045 mmol) was subjected to the sequence of deprotection steps including delevulinoylation, O-sulfation, Fmoc cleavage, saponification, deacetylation, azide reduction to obtain the diamino tetrasaccharide. One portion of the diamino tetrasaccharide was subjected to N-acetylation and global debenzylation according to the general procedure described above to give tetrasaccharide 80 (5.8 mg). [α]D25: +140 (c = 0.5, H2O); 1H NMR (800 MHz, D2O): δ 5.38 (d, 1H, J = 3.8 Hz, H1C), 4.87-4.85 (m, 2H, H1A, H1D), 4.57 (d, 1H, J = 3.8 Hz, H5D), 4.55 (d, 1H, J = 7.9 Hz, H1B), 4.42 (dd, 1H, J = 1.9 Hz, J = 11.2 Hz, H6aA), 4.35 (dd, 1H, J = 2.0 Hz, J = 11.0 Hz, H6aC), 4.25 (dd, 1H, J = 6.0 Hz, J = 11.2 Hz, H6bA), 4.17 (dd, 1H, J = 1.8 Hz, J = 11.0 Hz, H6bC), 4.05-4.02 (m, 1H, H5A), 4.01- 3.98 (m, 1H, H5C), 3.94-3.88 (m, 3H, H2A, H2C, H3A), 3.87 (dd, 1H, J = 4.2 Hz, J = 5.6Hz (H4D), 3.79 (d, 1H, J = 9.5 Hz, H5B), 3.77- 3.66 (m, 6H, H3B, H3C, H3D, H4B, H4C, OCHH Linker), 3.64 (dd, 1H, J = 8.0 Hz, J = 10.0 Hz, H4A), 3.56 ( dd, 1H, J = 2.6 Hz, J = 7.1 Hz, H2D), 3.50 (m, 1H, OCHH Linker), 3.33 (dd, 1H, J = 9.6 Hz, J = 9.6 Hz, H2B), 2.98 (t, 2H, J = 7.7 Hz, CH2NH2), 2.05 and 2.03 (2s, 3H each, 2 × CH3, NHAc), 1.74-1.60 (m, 4H, 2 × CH2 Linker), 1.50-1.42 (m, 2H, CH2 Linker). C33H54N3O29S2: 1020.2290, found: 1020.2319 [M-H]1−; calcd. for C33H53N3O29S2: 509.6109, found: 509.6129 [M-2H]2−; calcd. for C33H52N3O29S2: 339.4048, found: 339.4056 [M-3H]3−.
5-aminopentyl [(L-α-idopyranosyluronate)-(1→4)-O-(2-deoxy-2-sulfoamino-6-O-sulfonate-α-D-glucopyranoside)-(1→4)-O-(β-D-glucopyranosyluronate)]-(1→4)-2-deoxy-2-sulfoamino-6-O-sulfonate-α-D-glucopyranoside hexasodium salt (81)
The second portion of the diamino tetrasaccharide obtained above was subjected to N-sulfation and global debenzylation according to the general procedure described above to give tetrasaccharide 81 (5.7 mg). [α]D25: -5.4 (c = 0.5, H2O); 1H NMR (800 MHz, D2O): δ 5.61 (d, 1H, J = 3.8 Hz, H1C), 5.13 (d, 1H, J = 3.6 Hz, H1A), 4.86 (d, 1H, J = 4.7 Hz, H1D), 4.58-4.54 (m, 2H, H1B, H5D), 4.43 (dd, 1H, J = 1.8 Hz, J = 11.1 Hz, H6aA), 4.35 (dd, 1H, J = 2.0 Hz, J = 11.1 Hz, H6aC), 4.24 (dd, 1H, J = 6.1 Hz, J = 11.1 Hz, H6bA), 4.17 (dd, 1H, J = 2.0 Hz, J = 11.1 Hz, H6bC), 4.04-4.01 (m, 1H, H5A), 3.97-3.92 (m, 1H, H5C), 3.88 (dd, 1H, J = 4.0 Hz, J = 6.0 Hz, H4D), 3.84 (dd, 1H, J = 8.7 Hz, J = 9,3 Hz, H3B), 3.80 (d, 1H, J = 9.6 Hz, H5B), 3.76 (dd, 1H, J = 8.6 Hz, J = 9.3 Hz, H4B), 3.75-3.70 (m, 4H, H3A, H3D, H4C, OCHH Linker), 3.65 (dd, 1H, J = 9.0 Hz, J = 10.0 Hz, H4A), 3.62 (dd, 1H, J = 9.2 Hz, J = 10.3 Hz, H3C), 3.57-3.53 (m, 2H, H2D, OCHH Linker), 3.36 (dd, 1H, J = 8.0 Hz, H2B), 3.29-3.26 (m, 2H, H2A, H2C), 3.00 (t, J = 7.6 Hz, CH2NH2), 1.72-1.60 (m, 4H, 2 × CH2 Linker), 1.52-1.42 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C29H50N3O33S4: 1096.1215, found: 1096.1251 [M-H]1−; calcd. for C29H49N3O33S4: 547.5571, found: 547.5590 [M-2H]2−; C29H48N3O33S4: 364.7023, found: 364.7030 [M-3H]3−.
5-aminopentyl [(α-L-idopyranosyluronate)-(1→4)-(2-acetamido-2-deoxy-6-O-sulfonate-α-D-glucopyranoside)-(1→4)-O-(α-L-idopyranosyluronate-(1→4)-O-2-acetamido-2-deoxy-6-O-sulfonate-α-D-glucopyranoside tetrasodium salt (82)
Tetrasaccharide 73 (148 mg, 0.076 mmol) was subjected to the sequence of deprotection steps including delevulinoylation, O-sulfation, Fmoc cleavage, saponification, deacetylation, azide reduction to obtain the diamino tetrasaccharide. One portion of the diamino tetrasaccharide was subjected to N-acetylation and global debenzylation according to the general procedure described above to give tetrasaccharide 82 (4.32 mg). [α]D23: +70 (c = 0.35, H2O); 1H NMR (800 MHz, D2O): δ 5.14 (d, 1H, J = 3.7 Hz, H1C), 4.94 (d, 1H, J = 3.2 Hz, H1B), 4.85 (d, 1H, J = 3.7 Hz, H1A), 4.83 (d, 1H, J = 4.6 Hz, H1D), 4.68 (d, 1H, J = 2.7 Hz, H5B), 4.55 (d, 1H, J = 3.9 Hz, H5D), 4.34-4.31 (m, 2H, H6aC, H6aA),4.23 (dd, 1H, J = 6.6 Hz, J = 11.2 Hz, H6bA), 4.2 (dd, 1H, J = 1.7 Hz, J = 11 Hz, H6bC), 4.05 (t, 1H, J = 3.2 Hz, H4B), 4.04-4.01 (m, 2H, H5C, H5A), 3.96 (dd, 1H, J = 3.7 Hz, J = 10.2 Hz, H2C), 3.91 (dd, 1H, J = 3.7 Hz, J = 10.7 Hz, H2A), 3.87(dd, 1H, J = 3.7 Hz, J = 5.9 Hz, H3B), 3.85 (dd, 1H, J = 4.1 Hz, J = 6.1 Hz, H4D), 3.80 (t, 1H, J = 9.8 Hz, H3A), 3.74-3.64 (m, 7H, H4C, H3C, H3D, H2B, H4A, H2B, OCHH Linker), 3.53 (dd, 1H, J = 4.6 Hz, J = 7.3 Hz), 3.50-3.47 (m, 1H, OCHH Linker), 2.97 (t, 2H, J = 7.6 Hz, CH2N Linker), 2.00, 1.98 (2s, 3H each, 2× CH3, NHAc), 1.69-1.56 (m, 4H, 2× CH2 Linker), 1.45-1.41 (m, 2H, CH2 Linker). C33H54N3O29S2: 1020.2290, found: 1020.2279 [M-H]1−; calcd. for C33H53N3O29S2: 509.6109, found: 509.6113 [M-2H]2−; calcd. for C33H52N3O29S2: 339.4048, found: 339.4046 [M-3H]3−.
5-aminopentyl [(α-L-idopyranosyluronate)-(1→4)-(2-deoxy-2-N-sulfoamino-6-O-sulfonate-α-D-glucopyranoside)-(1→4)-O-(α-L-idopyranosyluronate-(1→4)-O-2-deoxy-2-N-sulfoamino-6-O-sulfonate-α-D-glucopyranoside hexasodium salt (83)
The second portion of the diamino tetrasaccharide obtained above was subjected to N-sulfation and global debenzylation according to the general procedure described above to give tetrasaccharide 83 (6.5 mg). [α]D23: +24.4 (c = 0.3, H2O); 1H NMR (800 MHz, D2O): δ 5.33 (d, 1H, J = 3.7 Hz, H1C), 5.11 (d, 1H, J = 3.7 Hz, H1A), 4.97 (d, 1H, J = 2.0 Hz, H1B), 4.85 (d, 1H, J = 4.9 Hz, H1D), 4.71 (d, 1H, J = 2.2 Hz, H5B), 4.57 (d, 1H, J = 3.9 Hz, H5D), 4.35 (dd, 1H, J = 2.2 Hz, J = 11.2 Hz, H6aC), 4.29 (dd, 1H, J = 1.7 Hz and 11 Hz, H6aA), 4.23 (dd, 1H, J = 5.4 Hz, J = 11.2 Hz, H6bA), 4.17 (dd, 1H, J = 1.7 Hz, J = 11 Hz, H6bC), 4.08 (t, 1H, J = 4.0 Hz, H3B), 4.04 (t, 1H, J = 2.6 Hz, H4B), 4.00-3.98 (bm, 2H, H5A, H5C), 3.85 (dd, 1H, J = 3.9 Hz, J = 5.9 Hz , H4D), 3.74-3.61 (m, 7H, OCHH Linker, H2B, H4C, H3D, H4A, H3A, H3C), 3.55-3.52 (m, 2H, OCHH Linker, H2D), 3.25 (dd,1H, J = 3.9 Hz, J = 10.3 Hz, H2A), 3.23 (dd, 1H, J = 3.7 Hz, J = 10.8 Hz, H2C), 2.98 (t, 2H, J = 7.5 Hz, CH2N Linker), 1.69-1.62 (m, 4H, 2× CH2 Linker), 1.49-1.44 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C29H50N3O33S4: 1096.1215, found: 1096.1251 [M-H]1−; calcd. for C29H49N3O33S4: 547.5571, found: 547.5563 [M-2H]2−; C29H48N3O33S4: 364.7023, found: 364.7012 [M-3H]3−.
5-Aminopentyl [(α-L-idopyranosyluronate)-(1→4)-O-(2-acetamido-2-deoxy-6-O-sulfonate-α-D-glucopyranoside)-(1→4)-O-(2-O-sulfonato-α-L-idopyranosyl-uronate)]-(1→4)-2-deoxy-2-acetamido-6-O-sulfonate-α-D-glucopyranoside penta sodium salt (84)
Tetrasaccharide 74 (17 mg, 0.0085 mmol) was subjected to the sequence of deprotection steps including delevulinoylation, O-sulfation, Fmoc cleavage, saponification, deacetylation, azide, N-acetylation and global debenzylation according to the general procedure described above to give tetrasaccharide 84 (1.6 mg). [α]D25: 26 (c = 0.5, H2O); 1H NMR (500 MHz, D2O): δ 5.22 (bs, 1H, H1B), 5.14 (d, 1H, J = 3.6 Hz, H1C), 4.87 (d, 1H, J = 3.6 Hz, H1A), 4.85 (d, 1H, J = 5.2 Hz, H1D), 4.80 (d, 1H, J = 2.3 Hz, H5B), 4.57 (d, 1H, J = 4.1 Hz, H5D), 4.40 (dd, 1H, J = 1.5 Hz, J = 11.5 Hz, H6aA or C), 4.36 (dd, 1H, J = 3.0 Hz, J = 11.0Hz, H6aA or C), 4.29 (bs, 1H, H2B), 4.28-4.20 (m, 3H, H6bA, H6bC, H3B), 4.09- 4.01 (m, 4H, H2C, H4B, H5A, H5C), 3.94 (dd, 1H, J = 3.6 Hz, J = 10.5 Hz, H2A), 3.89-3.83 (m, 2H, H3A, H4D), 4.78-4.67 (m, 5H, H3C, H3D, H4A, H4C, OCHH Linker), 3.55 (dd, 1H, J = 7.3Hz, H2D), 3.53-3.48 (m, 1H, OCHH Linker), 3.10 (t, 2H, J = 7.4Hz, CH2NH2) 2.05 and 2.02 (2s, 3H each, 2× CH3, NHAc) 1.74-1.5 (m, 2H, 2 × CH2 Linker), 1.59-1.48 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C33H53N3O32S3: 549.5893, found: 549.5890 [M-H]2−; calcd. for C33H52N3O32S3: 366.0571, found: 366.0582 [M-3H]3−.
5-Aminopentyl [(β-D-glucopyranosyluronate)-(1→4)-O-(2-acetamido-2-deoxy-6-O-sulfonato- α-D-glucopyranoside)-(1→4)-O-(2-O-sulfonato-α-L-idopyranosyluronate)]-(1→4)-2-deoxy-2-acetamido-6-O-sulfonate-α-D-glucopyranoside penta sodium salt (85)
Tetrasaccharide 75 (159 mg, 0.079 mmol) was subjected to the sequence of deprotection steps including delevulinoylation, O-sulfation, Fmoc cleavage, saponification, deacetylation, azide reduction to obtain the diamino tetrasaccharide. One portion of the diamino tetrasaccharide was subjected to N-acetylation and global debenzylation according to the general procedure described above to give tetrasaccharide 85 (9.3 mg). [α]D25: +26 (c = 0.5, H2O); 1H NMR (800 MHz, D2O): δ 5.14 (bs, 1H, H1B), 5.10 (d, 1H, J = 3.7 Hz, H1C), 4.83 (d, 1H, J = 3.7 Hz, H1A), 4.78 (d, 1H, J = 2.3 Hz, H5B), 4.54 (d, 1H, J = 7.9 Hz, H1D), 4.41 (dd, 1H, J = 2.7 Hz, J = 11.0 Hz, H6aC), 4.36 (d, 1H, J = 1.8 Hz, J = 11.2 Hz, H6aA), 4.26 (bd, J = 3.2 Hz, H2B), 4.25-4.21 (m, 2H, H6bA, H6bC), 4.18 (bt, 1H, H3B), 4.09-4.05 (m, 1H, H5C), 4.05-4.01 (m, 2H, H4B, H5A), 3.99 (dd, 1H, J = 3.7 Hz, J = 10.2 Hz, H2C), 3.92 (dd, 1H, J = 3.7 Hz, J = 10.6 Hz, H2A), 3.82 (dd, 1H, J = 8.8 Hz, J = 10.4 Hz, H3A), 3.76-3.72 (m, 3H, H3C, H4C, H5D), 3.71-3.68 (m, 2H, H4A, OCHH Linker), 3.53-3.46 (m, 3H, H3D, H4D, OCHH Linker), 3.32 (dd, 1H, J = 9.2 Hz, H2D), 2.98 (t, 2H, J = 7.6 Hz, CH2NH2), 2.03 and 2.00 (2s, 3H each, 2× CH3, NHAc), 1.70-1.58 (m, 4H, 2 × CH2 Linker), 1.48-1.42 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C33H53N3O32S3: 549.5893, found: 549.5879 [M-H]2−; calcd. for C33H52N3O32S3: 366.0571, found: 366.0559 [M-3H]3−.
5-Aminopentyl [(β-D-glucopyranosyluronate)-(1→4)-O-(2-deoxy-2-sulfamino-6-O-sulfonate-α-D-glucopyranoside)-(1→4)-O-(2-O-sulfonate-α-L-idopyranosyluronate)-(1→4)-2-deoxy-2-sulfoamino-6-O-sulfonate-α-D-glucopyranoside heptasodium salt (86)
A second portion of the diamino tetrasaccharide obtained above was subjected to N-sulfation and global debenzylation according to the general procedure described above to give tetrasaccharide 86 (5.0 mg). [α]D25: +79 (c = 1.3, H2O); 1H-NMR (800 MHz, D2O): δ 5.40 (d, 1H, J = 3.3 Hz, H1C), 5.23 (d, 1H, J = 2.7 Hz, H1B), 5.10 (d, 1H, J = 3.3 Hz, H1A), 4.70 (d, 1H, J = 2.7 Hz, H1B), 4.57 (d, 1H, J = 7.9 Hz, H1D), 4.45 (bd, 1H, J = 11.0 Hz, H6aC), 4.35 (bd, 1H, J = 11.0 Hz, H6aA), 4.30- 4.25 (m, 2H, H2B, H6bA), 4.20 (m, 1H, H6bC), 4.16 (dd, 1H, J = 4.0 Hz, J = 5.7 Hz, H3B), 4.09-4.06 (m, 2H, H4B and H5C), 4.00 (m, 1H, H5A), 3.77-3.68 (m, 5H, H3A, H4A, H4C, H5D, OCHH Linker), 3.66 (dd, 1H, J = 9.1 Hz, J = 9.5 Hz, H3C), 3.56-3.53 (m, 1H, OCHH Linker), 3.51 (t, 1H, J = 9.2 Hz, H3D), 3.47 (t, 1H, J = 9.2 Hz, H4D), 3.32 (dd, 1H, J = 8.4 Hz, J = 9.2 Hz, H2D), 3.27-3.23 (m, 2H, H2A, H2C), 3.00 (t, 2H, J = 7.4Hz, CH2NH2), 1.72-1.55 (m, 4H, 2 × CH2 Linker), 1.50-1.40 (m, 2H, CH2 Linker). ESI-MS: m/z: calcd. for C29H49N3O36S5: 587.5355, found: 587.5347 [M-H]2−; calcd. for C29H48N3O36S5: 391.3546, found: 391.3535 [M-3H]3−.
BACE inhibition assays
The ability of the compounds to inhibit BACE-1 cleavage of APP was assessed using a fluorescent resonance energy transfer (FRET) peptide cleavage assay employing the FRET peptide HiLyteFluor™488-Glu-Val-Asn-Leu-Asp-Ala-Glu-Phe-Lys(QXL520)-OH (Anaspec Inc, San Jose, CA), containing the Swedish amino acid variant. Assays were performed in triplicate in 96-well black plates (Greiner Bio-One Ltd) in a total volume of 100 µl of 20 mM sodium acetate, 0.1% Triton-X-100, pH 4.5 using 114 pmoles FRET peptide/well and 0.1 µg of recombinant human BACE-1 (R & D Systems; specific activity >3.5 pmol/min/µg). Inhibitors were added at 0.01 to 50 µg/mL and mixed with enzyme prior to addition of substrate. Appropriate controls for enzyme activity and background fluorescence were employed and plates were incubated (2 h, 25 °C) with reaction stopped by addition of 100 µl 2.5 M sodium acetate. Fluorescence 480ex/520em was measured on a Polarstar plate reader (BMG LabTechnologies, U.K.) and data were analyzed by plotting log concentration of inhibitor against percent inhibition and using a logistic sigmoidal curve fitting function using OriginPro 8 software (OriginLab Corporation, MA).
Supplementary Material
Acknowledgement
This research was supported by the Center for Research Resource of the National Institutes of Health (Grant No. 2 P41 RR005351 (G.-J.B.), Human Frontier Science Program (Grant RGP0062, G.-J.B. and J.E.T.), Medical Research Council UK (Senior Research Fellowship to J.E.T.) and the Biotechnology and Biological Sciences Research Council (grant to J.E.T). We thank Elizabeth Edwards and Alexandra Holme for technical assistance with the BACE inhibition assays.
Footnotes
Supporting Information Available: Copies of NMR spectra of synthetic intermediates and final products, and synthetic procedures and NMR assignments for intermediates.
References
- 1.Esko JD, Selleck SB. Annu. Rev. Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi NS, Mancera RL. Chem. Biol. Drug Des. 2008;72:455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 3.Capila I, Linhardt RJ. Angew. Chem. Int. Ed. Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; Hacker U, Nybakken K, Perrimon N. Nat. Rev. Mol. Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]; Whitelock JM, Iozzo RV. Chem. Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]; Van Vactor D, Wall DP, Johnson KG. Curr. Opin. Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]; Bishop JR, Schuksz M, Esko JD. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 4.Ori A, Wilkinson MC, Fernig DG. Front. Biosci. 2008;13:4309–4338. doi: 10.2741/3007. [DOI] [PubMed] [Google Scholar]
- 5.Johnson Z, Proudfoot AE, Handel TM. Cytokine Growth Factor Rev. 2005;16:625–636. doi: 10.1016/j.cytogfr.2005.04.006. [DOI] [PubMed] [Google Scholar]; Parish CR. Nat. Rev. Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]; Taylor KR, Gallo RL. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]; Chen Y, Gotte M, Liu J, Park PW. Mol. Cells. 2008;26:415–426. [PubMed] [Google Scholar]; Zacharski LR, Lee AY. Expert Opin. Investig. Drugs. 2008;17:1029–1037. doi: 10.1517/13543784.17.7.1029. [DOI] [PubMed] [Google Scholar]
- 6.Lindahl U, Backstrom G, Thunberg L, Leder IG. Proc. Natl. Acad. Sci. U S A. 1980;77:6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]; Petitou M, van Boeckel CA. Angew. Chem. Int. Ed. Engl. 2004;43:3118–3133. doi: 10.1002/anie.200300640. [DOI] [PubMed] [Google Scholar]; de Kort M, Buijsman RC, van Boeckel CAA. Drug Discov. Today. 2005;10:769–779. doi: 10.1016/S1359-6446(05)03457-4. [DOI] [PubMed] [Google Scholar]
- 7.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 8.Grootenhuis PD, Westerduin P, Meuleman D, Petitou M, van Boeckel CA. Nat. Struct. Biol. 1995;2:736–739. doi: 10.1038/nsb0995-736. [DOI] [PubMed] [Google Scholar]
- 9.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, 3rd, Nishi A, Hsieh-Wilson LC. Nat. Chem. Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 10.Kreuger J, Spillmann D, Li JP, Lindahl U. J. Cell Biol. 2006;174:323–327. doi: 10.1083/jcb.200604035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karst NA, Linhardt RJ. Curr. Med. Chem. 2003;10:1993–2031. doi: 10.2174/0929867033456891. [DOI] [PubMed] [Google Scholar]; Poletti L, Lay L. Eur. J. Org. Chem. 2003:2999–3024. [Google Scholar]; Noti C, Seeberger PH. Chem. Biol. 2005;12:731–756. doi: 10.1016/j.chembiol.2005.05.013. [DOI] [PubMed] [Google Scholar]; Linhardt RJ, Dordick JS, Deangelis PL, Liu J. Semin. Thromb. Hemost. 2007;33:453–465. doi: 10.1055/s-2007-982076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Bos LJ, Codee JDC, Litjens REJN, Dinkelaar J, Overkleeft HS, van der Marel GA. Eur. J. Org. Chem. 2007:3963–3976. doi: 10.1021/jo070704s. [DOI] [PubMed] [Google Scholar]; Sun ZC, Wei Z, Wei KM. Prog. Chem. 2008;20:1136–1142. [Google Scholar]
- 13.Yates EA, Guimond SE, Turnbull JE. J. Med. Chem. 2004;47:277–280. doi: 10.1021/jm0309755. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl U, Li JP, Kusche-Gullberg M, Salmivirta M, Alaranta S, Veromaa T, Emeis J, Roberts I, Taylor C, Oreste P, Zoppetti G, Naggi A, Torri G, Casu B. J. Med. Chem. 2005;48:349–352. doi: 10.1021/jm049812m. [DOI] [PubMed] [Google Scholar]; Chen J, Jones CL, Liu J. Chem. Biol. 2007;14:986–993. doi: 10.1016/j.chembiol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guimond S, Turnbull JE. Curr. Biol. 1999;9:1343–1346. doi: 10.1016/s0960-9822(00)80060-3. [DOI] [PubMed] [Google Scholar]
- 16.de Paz JL, Angulo J, Lassaletta JM, Nieto PM, Redondo-Horcajo M, Lozano RM, Gimenez-Gallego G, Martin-Lomas M. Chembiochem. 2001;2:673–685. doi: 10.1002/1439-7633(20010903)2:9<673::AID-CBIC673>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]; Petitou M, Imberty A, Duchaussoy P, Driguez PA, Ceccato ML, Gourvenec F, Sizun P, Herault JP, Perez S, Herbert JM. Chem. Eur. J. 2001;7:858–873. doi: 10.1002/1521-3765(20010216)7:4<858::aid-chem858>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; Codee JDC, Overkeelft HS, van der Marel GA, van Boeckel CAA. Drug Discovery Today: Technologies. 2004;1:317–326. doi: 10.1016/j.ddtec.2004.11.017. [DOI] [PubMed] [Google Scholar]; Lubineau A, Lortat-Jacob H, Gavard O, Sarrazin S, Bonnaffe D. Chem. Eur. J. 2004;10:4265–4282. doi: 10.1002/chem.200306063. [DOI] [PubMed] [Google Scholar]; de Paz JL, Martin-Lomas M. Eur. J. Org. Chem. 2005:1849–1858. [Google Scholar]; Zhou Y, Lin F, Chen JF, Yu B. Carbohydr. Res. 2006;341:1619–1629. doi: 10.1016/j.carres.2006.02.020. [DOI] [PubMed] [Google Scholar]; Chen JF, Zhou Y, Chen C, Xu WC, Yu B. Carbohydr. Res. 2008;343:2853–2862. doi: 10.1016/j.carres.2008.06.011. [DOI] [PubMed] [Google Scholar]; Chen C, Yu B. Bioorg. Med. Chem. Lett. 2009;19:3875–3879. doi: 10.1016/j.bmcl.2009.03.155. [DOI] [PubMed] [Google Scholar]
- 17.Lee JC, Lu XA, Kulkarni SS, Wen YS, Hung SC. J. Am. Chem. Soc. 2004;126:476–477. doi: 10.1021/ja038244h. [DOI] [PubMed] [Google Scholar]
- 18.Noti C, de Paz JL, Polito L, Seeberger PH. Chem. Eur. J. 2006;12:8664–8686. doi: 10.1002/chem.200601103. [DOI] [PubMed] [Google Scholar]
- 19.Haller M, Boons GJ. J. Chem. Soc., Perkin Trans. 1. 2001:814–822. [Google Scholar]; Haller MF, Boons GJ. Eur. J. Org. Chem. 2002;2002:2033–2038. [Google Scholar]
- 20.Prabhu A, Venot A, Boons GJ. Org. Lett. 2003;5:4975–4978. doi: 10.1021/ol0359261. [DOI] [PubMed] [Google Scholar]
- 21.Gavard O, Hersant Y, Alais J, Duverger V, Dilhas A, Bascou A, Bonnaffe D. Eur. J. Org. Chem. 2003:3603–3620. [Google Scholar]; Orgueira HA, Bartolozzi A, Schell P, Litjens R, Palmacci ER, Seeberger PH. Chem. Eur. J. 2003;9:140–169. doi: 10.1002/chem.200390009. [DOI] [PubMed] [Google Scholar]; Lu LD, Shie CR, Kulkarni SS, Pan GR, Lu XA, Hung SC. Org. Lett. 2006;8:5995–5998. doi: 10.1021/ol062464t. [DOI] [PubMed] [Google Scholar]; Polat T, Wong CH. J. Am. Chem. Soc. 2007;129:12795–12800. doi: 10.1021/ja073098r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patey SJ, Edwards EA, Yates EA, Turnbull JE. J. Med. Chem. 2006;49:6129–6132. doi: 10.1021/jm051221o. [DOI] [PubMed] [Google Scholar]
- 23.Patey SJ, Edwards EA, Yates EA, Turnbull JE. Neurodegener. Dis. 2008;5:197–199. doi: 10.1159/000113701. [DOI] [PubMed] [Google Scholar]
- 24.Zhu T, Boons GJ. Tetrahedron: Asymmetry. 2000;11:199–205. [Google Scholar]
- 25.Boons GJ. Contemp. Org. Synth. 1996;3:173–200. [Google Scholar]; Demchenko AV. Synlett. 2003:1225–1240. [Google Scholar]
- 26.Bongat AFG, Demchenko AV. Carbohydr. Res. 2007;342:374–406. doi: 10.1016/j.carres.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Vasella A, Witzig C, Chiara JL, Martinlomas M. Helv. Chim. Acta. 1991;74:2073–2077. [Google Scholar]; Alper PB, Hung SC, Wong CH. Tetrahedron Lett. 1996;37:6029–6032. [Google Scholar]
- 28.van Boeckel CAA, Beetz T, Vos JN, de Jong AJM, van Aelst SF, van den Bosch RH, Mertens JMR, van der Vlugt FA. J. Carbohydr. Chem. 1985;4:293–321. [Google Scholar]; Tabeur C, Machetto F, Mallet JM, Duchaussoy P, Petitou M, Sinay P. Carbohydr. Res. 1996;281:253–276. doi: 10.1016/0008-6215(95)00346-0. [DOI] [PubMed] [Google Scholar]; Tatai J, Osztrovszky G, Kajtar-Peredy M, Fugedi P. Carbohydr, Res. 2008;343:596–606. doi: 10.1016/j.carres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Basten JEM, Dreef-Tromp CM, de Wijs B, van Boeckel CAA. Bioorg. Med. Chem. Lett. 1998;8:1201–1206. doi: 10.1016/s0960-894x(98)00196-6. [DOI] [PubMed] [Google Scholar]
- 30.Veeneman GH, van Leeuwen SH, van Boom JH. Tetrahedron Lett. 1990;31:1331–1334. [Google Scholar]
- 31.Davis NJ, Flitsch SL. Tetrahedron Lett. 1993;34:1181. [Google Scholar]
- 32.DeMico A, Margarita R, Parlanti L, Vescovi A, Piancatelli G. J. Org. Chem. 1997;62:6974–6977. [Google Scholar]; van den Bos LJ, Codee JD, van der Toorn JC, Boltje TJ, van Boom JH, Overkleeft HS, van der Marel GA. Org. Lett. 2004;6:2165–2168. doi: 10.1021/ol049380+. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Gaekwad J, Wolfert MA, Boons GJ. Chem. Eur. J. 2008;14:558–569. doi: 10.1002/chem.200701165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas H, Basten JEM, van Dinther TG, Meuleman DG, van Aelst SF, van Boeckel CAA. Tetrahedron. 1990;46:8207–8228. [Google Scholar]; Rochepeau-Jobron L, Jacquinet JC. Carbohydrate Research. 1998;305:181–191. doi: 10.1016/s0008-6215(98)00298-5. [DOI] [PubMed] [Google Scholar]
- 35.Brewer M, Rich DH. Org. Lett. 2001;3:945–948. doi: 10.1021/ol015612i. [DOI] [PubMed] [Google Scholar]
- 36.Venot A, Swayze EE, Griffey RH, Boons GJ. Chembiochem. 2004;5:1228–1236. doi: 10.1002/cbic.200400105. [DOI] [PubMed] [Google Scholar]
- 37.Golde TE. J. Clin. Invest. 2003;111:11–18. doi: 10.1172/JCI17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang WP, Koelsch G, Wong S, Downs D, Da H, Weerasena V, Gordon B, Devasamudram T, Bilcer G, Ghosh AK, Tang J. J. Neurochem. 2004;89:1409–1416. doi: 10.1111/j.1471-4159.2004.02452.x. [DOI] [PubMed] [Google Scholar]; Asai M, Hattori C, Iwata N, Saido TC, Sasagawa N, Szabo B, Hashimoto Y, Maruyama K, Tanuma S, Kiso Y, Ishiura S. J. Neurochem. 2006;96:533–540. doi: 10.1111/j.1471-4159.2005.03576.x. [DOI] [PubMed] [Google Scholar]
- 39.Scholefield Z, Yates EA, Wayne G, Amour A, McDowell W, Turnbull JE. J. Cell Biol. 2003;163:97–107. doi: 10.1083/jcb.200303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demchenko AV, Rousson E, Boons GJ. Tetrahedron Lett. 1999;40:6523–6526. [Google Scholar]; Crich D, Hu TS, Cai F. J. Org. Chem. 2008;73:8942–8953. doi: 10.1021/jo801630m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt RR, Kinzy W. Adv. Carbohydr. Chem. Biochem. 1994;50:21–123. doi: 10.1016/s0065-2318(08)60150-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.