Abstract
The interaction of renal basolateral organic anion transporter 3 (Oat3) with commonly used pharmacotherapeutics (e.g., NSAIDs, β-lactams, and methotrexate) has been studied extensively in vitro. However, the in vivo role of Oat3 in drug disposition, in the context of other transporters, glomerular filtration, and metabolism, has not been established. Moreover, recent investigations have identified inactive human OAT3 polymorphisms. Therefore, this investigation was designed to elucidate the in vivo role of Oat3 in the disposition of penicillin G and prototypical substrates using an Oat3 knockout mouse model. Oat3 deletion resulted in a doubling of penicillin’s half-life (P < 0.05) and a reduced volume of distribution (P < 0.01), together yielding a plasma clearance that was one-half (P < 0.05, males) to one-third (P < 0.001, females) of that in wild-type mice. Inhibition of Oat3 abolished the differences in penicillin G elimination between genotypes. Hepatic accumulation of penicillin was 2.3 times higher in male knockouts (P < 0.05) and 3.7 times higher in female knockouts (P < 0.001). Female knockouts also exhibited impaired estrone-3-sulfate clearance. Oat3 deletion did not impact p-aminohippurate elimination, providing correlative evidence to studies in Oat1 knockout mice that suggest Oat1 governs tubular uptake of p-aminohippurate. Collectively, these findings are the first to indicate that functional Oat3 is necessary for proper elimination of xenobiotic and endogenous compounds in vivo. Thus Oat3 plays a distinct role in determining the efficacy and toxicity of drugs. Dysfunctional human OAT3 polymorphisms or instances of polypharmacy involving OAT3 substrates may result in altered systemic accumulation of β-lactams and other clinically relevant compounds.
Keywords: kidney, liver, transport, Oat1, drug disposition
Pharmacotherapeutic efficacy and toxicity are governed by a multitude of pharmacodynamic and pharmacokinetic factors. The ability of a drug to reach its site of action for a desired duration is dependent on absorption, distribution, metabolism, and excretion (ADME), all of which are intimately related to transport mechanisms in barrier epithelia. The vast majority of drugs and their metabolites bear a negative or positive charge in the body, rendering them somewhat polar and hydrophilic. This polarity limits passive permeation across lipophilic barrier membranes throughout the body. Thus the disposition of most drugs is highly dependent on specialized carriers, or transporters, embedded in these barrier epithelia. Transporters mediate selective permeation of charged molecules through barrier membranes and are thought to play a pivotal role in detoxification and drug disposition (20, 27, 29). As their family names indicate, organic anion transporters selectively handle anions, while organic cation transporters handle cations. Organic anion transporters and organic cation transporters share some substrates, and their interaction with these substrates depends on the pH of the biophase (i.e., site of interaction with the transporter). Two organic anion transporters, Oat1 (Slc22a6) and Oat3 (Slc22a8), are expressed in the basolateral membrane of renal proximal tubule cells and have been identified as contributors to xenobiotic and endogenous organic anion secretion (13, 30–33). This membrane localization and function position them for an active role in determining drug exposure and efficacy.
In vitro studies have led to the identification of a number of substrates/inhibitors for Oat1 and Oat3, including anticancer drugs (e.g., methotrexate), antiviral compounds (e.g., adefovir), and β-lactam antibiotics (e.g., penicillin G) (6, 29). Additionally, some anionic compounds, typically with steroid backbones (e.g., estrone-3-sulfate), interact with Oat3, but not Oat1. The substrate profiles of Oat1 and Oat3 indicate that these transporters may have partially redundant roles in the kidney. However, in most cases, substrates shared by Oat1 and Oat3 have different affinities for the transporters, resulting in a concentration-dependent interaction in vivo.
To establish the in vivo role of Oat3 in the elimination of substrates both shared and unshared with Oat1, we assessed the plasma elimination of p-aminohippurate (Oat1 ≈ Oat3), estrone-3-sulfate (Oat3), and penicillin G (Oat3 > Oat1) in Oat3 knockout [Oat3(−/−)] and wild-type mice. Female Oat3(−/−) mice demonstrated decreased clearance of estrone-3-sulfate, and both male and female Oat3(−/−) mice exhibited a markedly reduced clearance of penicillin G. Additionally, indications of possible liver dysfunction were observed in the Oat3(−/−) mice. These findings suggest that Oat3 is actively engaged in organic anion elimination and distribution in the intact organism and that its perturbation may lead to impaired clearance of, and an elevated risk of hepatic exposure to, endogenous organic anions, pharmacotherapeutics, and toxicants. This finding thus raises the possibility of enhanced susceptibility of humans to low level toxicants, when possessing a nonfunctional Oat3 polymorphism (14, 34, 35) or in the situation of an Oat3 drug substrate overload. This may be the case in polypharmacy involving drugs such as NSAIDs, diuretics, and antibiotics.
METHODS
Chemicals and materials
[3H]p-aminohippurate, [3H]estrone-3-sulfate, and [14C]inulin were purchased from American Radiolabeled Chemicals (St. Louis, MO), and [3H]benzylpenicillin (penicillin G) was purchased from GE Healthcare Bio-Sciences (Piscataway, NJ). Probenecid and HPLC-grade reagents were purchased from Fisher Scientific (Hampton, NH). The HPLC and guard columns were purchased from Alltech Associates (Columbia, MD). Dispo-Equilibrium Dialyzers were acquired from Harvard Apparatus (Holliston, MA).
Animals
Male and female Oat3(−/−) (backcrossed onto C57BL/6 >6 times) and wild-type age-matched C57BL/6 mice (12–16 wk old for pharmacokinetic experiments and 6 mo old for quantitative PCR and organ function markers) were used in the present study. Mice were allowed food and water ad libitum and were housed in animal facilities maintained by the Medical University of South Carolina (MUSC) Division of Laboratory Animal Resources. The MUSC program for laboratory animal care has an assurance statement on file with the National Institutes of Health Office for the Protection from Research Risks/Department of Health and Human Services and has maintained full accreditation with the Association for Assessment and Accreditation of Laboratory Animal Care since 1987. All animal procedures were approved by the MUSC Institutional Animal Care and Use Committee (AR 2082, reapproved 9/1/2006).
Substrate elimination and distribution
Radiolabeled substrates 100 μCi/kg of p-aminohippurate (1.43 μg/kg), estrone-3-sulfate (0.53 μg/kg), or penicillin G (1.87 μg/kg), and 10 μCi/kg inulin (5.83 mg/kg) were administered in 5 μl of normal saline for each gram of weight, by bolus tail vein injection, to unanesthetized animals. In inhibition experiments, probenecid (100 mg/kg) was dissolved in dimethylsulfoxide (2 μl/g body wt) and injected intraperitoneally 15 min before intravenous substrate injection. This route of administration was determined in preliminary experiments to result in far greater bioavailability of probenecid compared with gavage, with a time of maximum concentration (Tmax) of 15 min and a maximum concentration achieved (Cmax) in plasma of ~1.0 mM. In inhibition experiments employing estrone-3-sulfate, the radiolabeled compounds penicillin G and inulin were mixed in the same saline injection solution with 30 mM estrone-3-sulfate and taurocholate (10 mg/ml). Taurocholate was considered ideal for solubilizing the lipophilic estrone-3-sulfate, as it is a physiological surfactant as well as an Oat3 substrate. For all experiments, serial blood samples (~35 μl) were obtained at the times indicated, and 100–200 mg of liver was harvested at the end of the experiment. Liver samples were blotted dry, weighed, and homogenized in 0.4 ml PBS using an electric tissue blender before the addition of 5 ml of scintillation fluid. Blood samples were centrifuged at 21,000 g for 5 min, and 10 μl of plasma was used for scintillation counting. Samples were counted in a Packard Tri-Carb 2900 TR liquid scintillation counter with external quench correction. Microcuries of radioactivity were converted to moles of substrate using the specific activity of the substrate. Substrate mass in the vascular space of liver tissue was calculated and subtracted from the total tissue level using established volumes (58 μl of plasma/g of liver) (24) and the measured plasma concentration at 30 min. The liver plasma volume using this literature method was nearly identical to the value obtained using measured inulin levels in the liver at 30 min (i.e., plasma volume in the liver was also calculated using the total mass of inulin in the liver piece and the known concentration in the plasma measured at 30 min).
Penicillin G serum protein binding
Blood was acquired from anesthetized mice, and serum was obtained by centrifugation. Then, 2.77 μl (2.77 μCi) of [3H]benzylpenicillin was mixed with 450 μl of serum. Two 210-μl aliquots were taken from this solution, and 32 μl of PBS (pH 7.4) was mixed with one aliquot (i.e., control serum), while 32 μl of PBS containing estrone-3-sulfate (53 mM) and sodium taurocholate (18 mg/ml) was mixed with the other aliquot (i.e., competition serum). Then, 75 μl of either the control or competition [3H]benzylpenicillin serum was added to one chamber of a Dispo-Equilibrium Dialyzer (molecular weight cutoff of 5,000), and 75 μl of PBS was added to the opposite chamber. The apparatus was agitated at 200 rpm in an orbital shaker maintained at 37°C. At 1, 2, 4, and 6 h, 5-μl samples were taken from each side of the apparatus and placed in individual vials prefilled with scintillation cocktail. The buffer-to-serum [3H]benzylpenicillin concentration ratio was then calculated and plotted. The experiment was performed separately using serum from male and female mice. Each condition, control and competition, was assessed in triplicate.
Quantitative PCR
Total RNA was isolated from kidney and liver tissue using a Qiagen RNeasy Midi Kit according to the manufacturer’s protocols (Qiagen, Valencia, CA). One hundred micrograms of total RNA was subjected to digestion with DNase I and purified using a Qiagen RNeasy Mini Kit according to the instructions. Ten micrograms of DNase-treated RNA served as a template for first-strand cDNA synthesis (50-μl reaction volume). One microliter of the reaction served as a template for quantitative PCR (QPCR) using SybrGreen QPCR Master Mix (Stratagene, La Jolla, CA) on an Mx3000P QPCR instrument (Stratagene). All determinations were made in triplicate and repeated. No template control reactions and ROX reference dye were included in every run. Cycle parameters were as follows: 1 cycle for 10 min at 95°C, 40 cycles of denaturation at 95°C (30 s), annealing at 55°C (30 s), and elongation at 75°C (30 s), followed by a dissociation curve. Primer pairs were designed to cross intron-exon boundaries, and sequences are given (Table 1).
Table 1.
Nucleotide sequences of QPCR primers
| Transporter | Product Size, bp | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| Oat1 | 117 | 5′-CCATCGTGACTGAGTGGAAC-3′ | 5′-TGTCCGCCAGGTAGCCAAAC-3′ |
| Oat2 | 159 | 5′-GGCTGGGAGTACGACCGCTC-3′ | 5′-GCCAAACCTGTCAGACAAGT-3′ |
| Oat3 | 125 | 5′-CTTCAGAAATGCAGCTCTTG-3′ | 5′-ACCTGTTTGCCTGAGGACTG-3′ |
| Oat5 | 110 | 5′-ACCTTGGAGGTTGTGAAAAC-3′ | 5′-AGACAGATCCGCTTGCGAAG-3′ |
| Oatp1 | 101 | 5′-TGGGTCTGTGAATACAGATG-3′ | 5′-GGATATTCACTCCTGCACAG-3′ |
| Mdr1a/b | 146 | 5′-AGTGGGGGACAGAAACAGAG-3′ | 5′-CGGCCTTCTCTAGCCTTATC-3′ |
| Mrp2 | 151 | 5′-TGAATCTCGACCCTTTCAAC-3′ | 5′-TGCCTCTGCCCTATGCTCAG-3′ |
QPCR, quantitative PCR; Oat, organic anion transporter; Oatp, organic anion transporting polypeptide; Mdr, multidrug resistance protein; Mrp, multidrug resistance-associated protein.
Organ function markers
Blood was obtained by cardiac puncture in anesthetized mice, and serum was isolated by centrifugation. Serum analysis was performed by AniLytics (Gaithersburg, MD).
HPLC analysis
Probenecid was quantified in 25-μl samples of plasma deproteinized with 5 volumes of acetonitrile and injected (25-μl full-loop) for HPLC without evaporation. Samples were analyzed on a Finnigan LCQ Advantage Max LC-MS (Thermo Electron, Waltham, MA) equipped with a C18 column (5-μm particle size; 4.6-mm ID/150-mm length) maintained at 40°C. Mobile phase A was 50 mM formic acid (pH 3.0 via NH3) in deionized and distilled water, and mobile phase B was HPLC-grade methanol. The gradient was from 30 to 100% B over 10 min, and the column was flushed with 100% B before equilibration to initial conditions. Quantitation was by UV absorbance at 280 nm, and interpolation of the standard curve [peak area ratio of probenecid to internal standard (y-axis) vs. concentration of probenecid (x-axis)]. 4-Fluorobenzoic acid was used as the internal standard at 400 ng/μl and was added before deproteinization. Identity was confirmed using spiking and electrospray ionization mass spectrometry in the negative polarity setting. Probenecid was monitored at m/z = 284.1. Mass spectrometry conditions were selected using automatic instrument tuning to the ion of interest.
Pharmacokinetic and statistical analyses
Pharmacokinetic determinations were performed using WinNonlin software (Pharsight). Two-compartment intravenous bolus modeling was used with micro-constants and resulted in the lowest coefficients of variation for kinetic parameters compared with other models. Statistical significance of elimination curves was determined using 2-way ANOVA. Student’s t-test was used for all other comparisons. The α for significance was 0.05.
RESULTS
Pharmacokinetic analysis
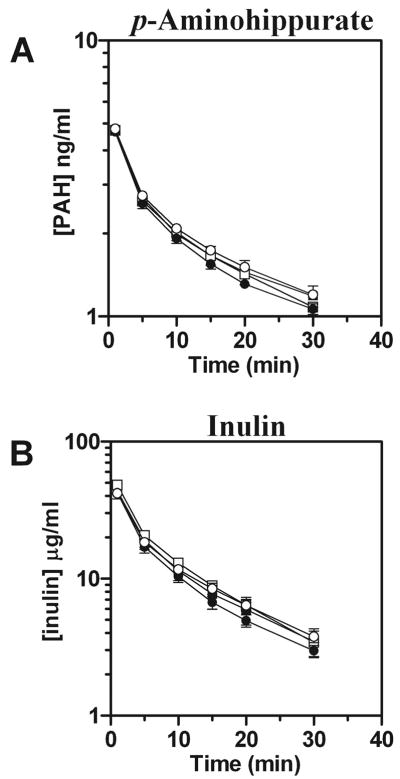
The plasma elimination profiles for p-aminohippurate were virtually identical between wild-type and Oat3(−/−) male and female mice (Fig. 1A). Furthermore, no significant differences in kinetic parameters were observed for p-aminohippurate between genotypes. The same was true for inulin (Fig. 1B).
Fig. 1.
Plasma elimination of p-aminohippurate (PAH) in male and female Oat3(−/−) and wild-type mice. [3H]PAH and [14C]inulin were concomitantly administered by intravenous injection, and plasma levels were determined by scintillation counting at 1, 5, 10, 15, 20, and 30 min. A: plasma elimination profile of PAH. B: plasma elimination profile of inulin. Values are means ± SE; n = 3–5. Significance was assessed between genotypes of the same gender by ANOVA. Squares, males; circles, females; open symbols, Oat3(−/−); closed symbols, wild-type.
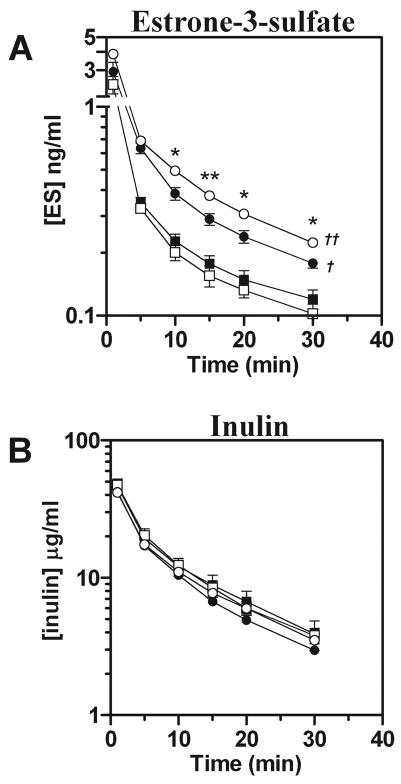
In contrast, plasma levels of estrone-3-sulfate were significantly elevated in females compared with males while inulin levels remained comparable (Fig. 2, A and B). Additionally, plasma estrone-3-sulfate levels were increased in female Oat3(−/−) animals compared with wild-type (Fig. 2A). In contrast to p-aminohippurate, for estrone-3-sulfate female Oat3(−/−) mice exhibited a significantly elevated area under the concentration vs. time curve (AUC) and lower total clearance compared with wild-type (Table 2).
Fig. 2.
Plasma elimination of estrone-3-sulfate (ES) in male and female Oat3(−/−) and wild-type mice. [3H]ES and [14C]inulin were concomitantly administered by intravenous injection, and plasma levels were determined by scintillation counting at 1, 5, 10, 15, 20, and 30 min. A: plasma elimination profile of ES. B: plasma elimination profile of inulin. Values are means ± SE; n = 4. Symbols are defined as in Fig. 1. Significance was assessed between genotypes of the same gender by t-test (*P < 0.05, **P < 0.01) and between genders of the same genotype by ANOVA (†P < 0.05, ††P < 0.01).
Table 2.
Kinetic analysis of iv bolus estrone-3-sulfate, penicillin G, and inulin in wild-type and Oat3(−/−) mice
| Kinetic Parameter |
||||||
|---|---|---|---|---|---|---|
| Sex | Compound | Genotype | Vdss, ml/kg | AUC | Total Clearance, ml · min−1 · kg−1 | Elimination Half-Life, min |
| Male | ES | Wild-type (n = 4) | 786±78 | 14±1.8 | 39±4.8 | 2.59±0.09 |
| Oat3(−/−) (n = 4) | 682±104 | 14±1.7 | 39±4.6 | 2.01±0.19 | ||
| PCN G | Wild-type (n = 4) | 406±20 | 65±8 | 29±3.6 | 5.21±0.46 | |
| Oat3(−/−) (n = 4) | 270±31† | 118±11† | 16±1.6* | 9.01±1.71 | ||
| Inulin | Wild-type (n = 11) | 184±11 | 447±35 | 14±1.0 | 4.92±0.25 | |
| Oat3(−/−) (n = 11) | 186±15 | 457±25 | 13±0.8 | 5.27±0.49 | ||
| Female | ES | Wild-type (n = 4) | 469±85 | 21±2.0 | 25±2.0 | 3.07±0.41 |
| Oat3(−/−) (n = 4) | 324±44 | 27±2.0* | 20±1.0* | 2.13±0.48 | ||
| PCN G | Wild-type (n = 4) | 349±25 | 55±2.8 | 34±1.7 | 3.74±0.34 | |
| Oat3(−/−) (n = 4) | 209±13† | 167±23‡ | 11±1.4‡ | 8.07±2.64 | ||
| Inulin | Wild-type (n = 12) | 215±16 | 325±26 | 19±1.4 | 3.92±0.25 | |
| Oat3(−/−) (n = 13) | 214±22 | 389±30 | 16±1.2 | 4.45±0.52 | ||
Values are means ± SE. Area under the concentration vs. time curve to infinity (AUC) expressed as min · ng−1 · ml−1 for estrone-3-sulfate (ES) and penicillin G (PCN G) and min · μg−1 · ml−1 for inulin. n, No. of animals; Vdss, volume of distribution at steady state. Plasma was sampled periodically for 30 min (see Figs 2 and 3), and 2-compartment iv bolus modeling was used. Significant differences (*P < 0.05, †P < 0.01, ‡P < 0.001) are for Oat3(−/−) compared with wild-type for adjacent rows.
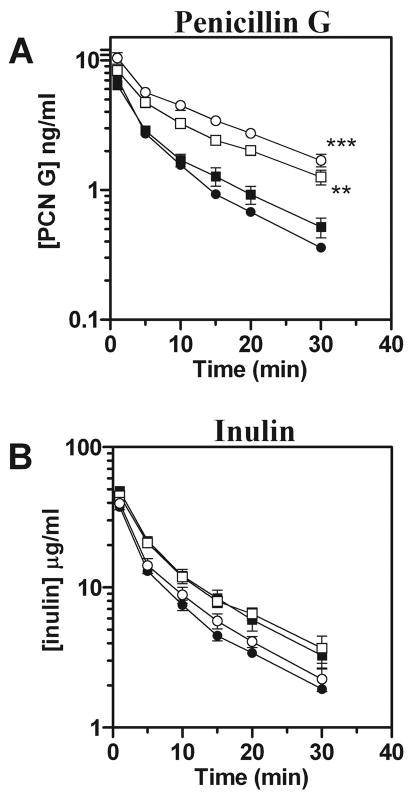
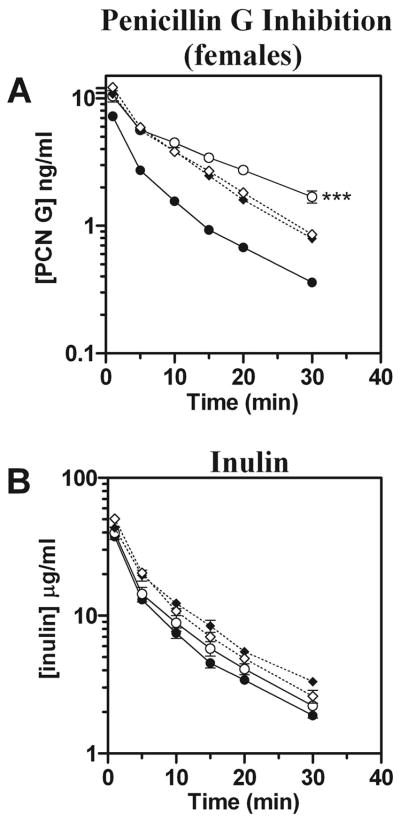
Plasma clearance of penicillin G exhibited the most striking differences between genotypes. Levels of penicillin G were markedly higher in male and female Oat3(−/−) mice compared with wild-type, whereas inulin levels remained comparable between genotypes (Fig. 3, A and B). The differences between males and females observed for estrone-3-sulfate were not evident with penicillin G. Both genders of Oat3(−/−) mice exhibited significantly elevated hepatic levels of penicillin G, 30 min after administration, compared with wild-type [2.3 times higher in male knockouts (P < 0.05) and 3.7 times higher in female knockouts (P < 0.001)]. All observed pharmacokinetic parameters for penicillin G were substantially different in Oat3(−/−) mice and indicated that they have a reduced volume of distribution and total clearance of penicillin G, with an increased AUC (Table 2). Furthermore, Oat3(−/−) mice demonstrated a nearly twofold greater half-life for penicillin G than wild-type mice (Table 2, P < 0.05 for males and females pooled).
Fig. 3.
Plasma elimination of penicillin G (PCN G) in male and female Oat3(−/−) and wild-type mice. [3H]PCN G and [14C]inulin were concomitantly administered by intravenous injection, and plasma levels were determined by scintillation counting at 1, 5, 10, 15, 20, and 30 min. A: plasma elimination profile of PCN G. B: plasma elimination profile of inulin. Values are means ± SE; n = 4. Symbols are defined as in Fig. 1. Significance was assessed between genotypes of the same gender by ANOVA (**P < 0.01, ***P < 0.001).
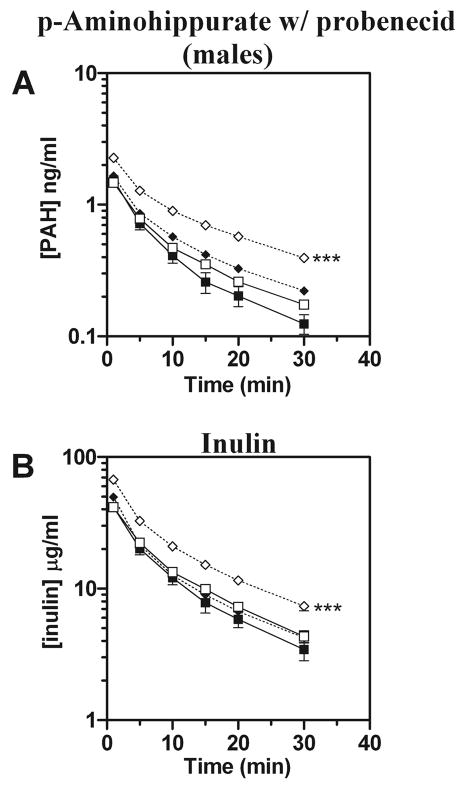
The elimination of p-aminohippurate in the presence of the potent organic anion transporter inhibitor probenecid was also investigated. In the presence of probenecid (100 mg/kg ip 15 min before p-aminohippurate and inulin administration), plasma p-aminohippurate levels were elevated in male wild-type mice while inulin levels were unaffected (Fig. 4, A and B). However, both p-aminohippurate and inulin levels were significantly elevated in male Oat3(−/−) mice in response to probenecid (Fig. 4, A and B). To rule out a difference in bioavailability of intraperitoneally administered probenecid in Oat3(−/−) and wild-type mice as a cause of the observed difference in response, probenecid plasma levels were quantified 2 min before p-aminohippurate and inulin administration using HPLC. Probenecid levels were not significantly different between Oat3(−/−) and wild-type mice: (1.14 ± 0.17 vs. 1.04 ± 0.06 mM, n = 5).
Fig. 4.
Plasma elimination of p-aminohippurate (PAH) in male Oat3(−/−) and wild-type mice in the presence or absence of probenecid. [3H]PAH and [14C]inulin were concomitantly administered by intravenous injection in the presence or absence of probenecid (~1 mM in plasma via HPLC) that was administered intraperitoneally 15 min before injection. Plasma levels were determined by scintillation counting at 1, 5, 10, 15, 20, and 30 min. A: plasma elimination profile of PAH. B: plasma elimination profile of inulin. Values are means ± SE; n = 3–7. Squares, no inhibition; diamonds, inhibition; open symbols, Oat3(−/−); closed symbols, wild-type. Significance was assessed between genotypes given the same treatment by ANOVA (***P < 0.001).
Therefore, to examine the effect of organic anion transporter inhibition without the complicating effect of probenecid on inulin levels in knockout animals, elimination of radiolabeled penicillin G was measured in the presence of a transporter-saturating dose of unlabeled estrone-3-sulfate, a high-affinity Oat3 substrate that does not interact with Oat1 [Figs. 5A (males) and 6A (females)]. In both males and females, inhibition with estrone-3-sulfate resulted in plasma levels of penicillin G that were no different between genotypes. In stark contrast to penicillin G’s pharmacokinetics in the absence of inhibition, in the presence of an Oat3-saturating level of estrone-3-sulfate all measured penicillin G pharmacokinetic parameters were nearly identical between wild-type and Oat3(−/−) mice (Table 3). Furthermore, penicillin’s volume of distribution and plasma clearance resembled that of inulin under inhibition conditions.
Fig. 5.
Plasma elimination of PCN G in male Oat3(−/−) and wild-type mice in the presence or absence of an Oat3-saturating dose of ES. [3H]PCN G and [14C]inulin were concomitantly administered by intravenous injection with or without unlabeled ES. Plasma levels were determined by scintillation counting at 1, 5, 10, 15, 20, and 30 min. A: plasma elimination profile of PCN G. B: plasma elimination profile of inulin. Values are means ± SE; n = 4. Symbols are as defined in Fig. 4. Data without inhibition were reproduced from Fig. 3 and do not represent a separate experiment. Significance was assessed between genotypes given the same treatment by ANOVA (**P < 0.01).
Table 3.
Effect of inhibition with estrone-3-sulfate on penicillin G and inulin pharmacokinetics in wild-type and Oat3(−/−) mice
| Kinetic Parameter |
||||||
|---|---|---|---|---|---|---|
| Sex | Compound | Genotype | Vdss, ml/kg | AUC | Total Clearance, ml · min−1 · kg−1 | Elimination Half-Life, min |
| Male | PCN G | Wild-type (n = 4) | 198±9 | 135±9 | 13±1 | 3.84±0.18 |
| Oat3(−/−) (n = 4) | 196±13 | 125±7 | 14±1 | 4.61±0.52 | ||
| Inulin | Wild-type (n = 4) | 161±6 | 555±51 | 11±1 | 3.77±0.38 | |
| Oat3(−/−) (n = 4) | 164±11 | 536±24 | 12±1 | 5.31±0.36 | ||
| Female | PCN G | Wild-type (n = 4) | 203±18 | 113±4 | 17±1 | 4.31±0.26 |
| Oat3(−/−) (n = 4) | 191±5 | 124±6 | 16±1 | 4.68±0.75 | ||
| Inulin | Wild-type (n = 4) | 201±17 | 412±33 | 16±1 | 4.72±0.39 | |
| Oat3(−/−) (n = 4) | 184±15 | 407±33 | 16±1 | 4.15±0.21 | ||
Values are means ± SE. AUC expressed as min · ng−1 · ml−1 for PCN G and min · μg−1 · ml−1 for inulin. n, No. of animals. PCN G and inulin were injected concomitantly with excess ES (dissolution facilitated with taurocholate), and plasma was sampled periodically for 30 min (see Figs. 5 and 6). Two-compartment iv-bolus modeling was used. See Table 2 for PCN G data without inhibition.
Penicillin G serum protein binding
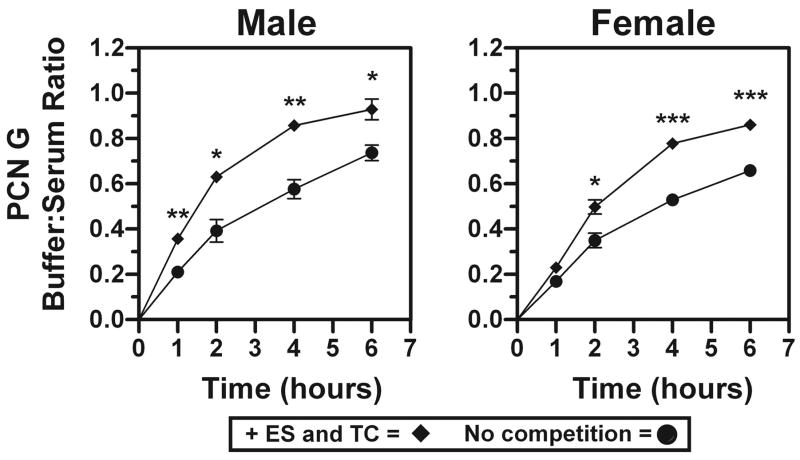
Since combined estrone-3-sulfate and taurocholate accelerated the elimination rate of penicillin G in Oat3(−/−) mice (Figs. 5A and 6A; note slope of terminal elimination and Table 2 compared with Table 3), the ability of these compounds to displace penicillin G from its serum protein binding site(s) was determined (Fig. 7). In both male and female Oat3(−/−) mouse serum, combined estrone-3-sulfate and taurocholate resulted in significantly enhanced partitioning of penicillin G into the buffer chamber of the dialyzer, indicating that displacement of penicillin G from serum protein had occurred.
Fig. 6.
Plasma elimination of PCN G in female Oat3(−/−) and wild-type mice in the presence or absence of an Oat3-saturating dose of ES. [3H]PCN G and [14C]inulin were concomitantly administered by intravenous injection with or without unlabeled ES. Plasma levels were determined by scintillation counting at 1, 5, 10, 15, 20, and 30 min. A: plasma elimination profile of PCN G. B: plasma elimination profile of inulin. Values are means ± SE; n = 4. Symbols are as defined in Fig. 4. Data without inhibition were reproduced from Fig. 3 and do not represent a separate experiment. Significance was assessed between genotypes given the same treatment by ANOVA (***P < 0.001).
Fig. 7.
PCN G serum protein binding and displacement with combined ES and taurocholate (TC). Dialysis was performed using male or female Oat3(−/−) mouse serum spiked with [3H]PCN G. To determine the partitioning of [3H]PCN G into the buffer chamber in the absence and presence of excess ES combined with TC, 5-μl buffer and serum chamber samples were acquired at 1, 2, 4, and 6 h. Values are means of buffer:serum ratio ± SE; n = 3 replicates using serum pooled from 3 male or female mice. Asterisks represent significant differences between control (no competition) and competition (+ES and TC) by t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
QPCR
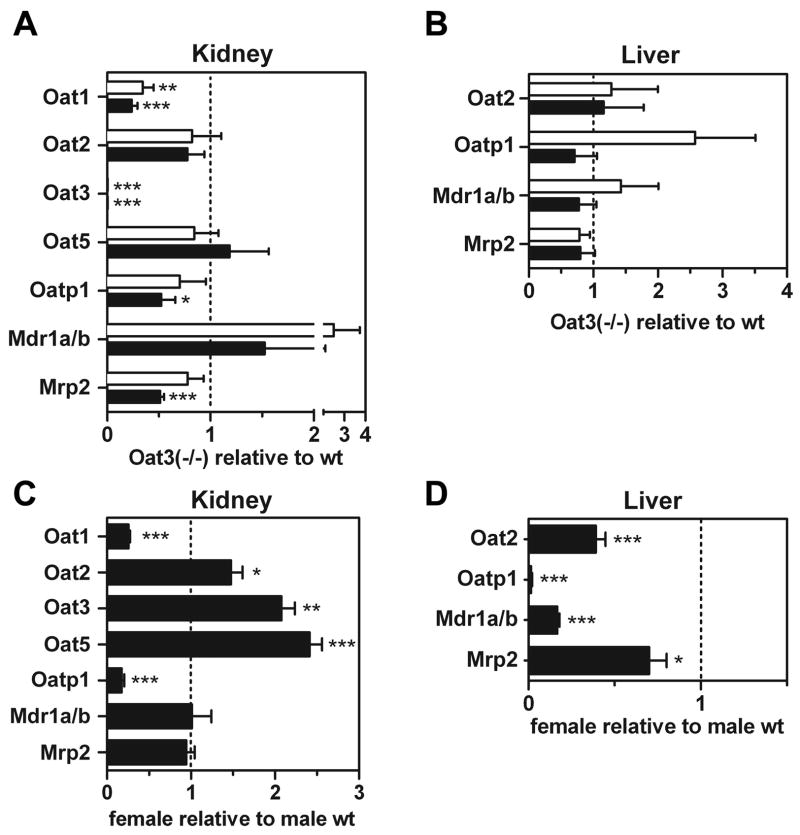
To determine whether loss of Oat3 affected the level of gene expression of other transporters and to elucidate gender differences in transporter expression, renal and hepatic mRNA expression levels were examined by QPCR in male and female Oat3(−/−) and wild-type mice (Fig. 8, A–D). As expected, Oat3 message was undetectable in Oat3(−/−) mice. Renal expression of Oat2 and Oat5 was unaffected by loss of Oat3 (Fig. 8A). However, Oat1 renal message levels in both genders of Oat3(−/−) animals were less than half that observed in wild-type animals (Fig. 8A). Male Oat3(−/−) mice also exhibited reduced expression of organic anion transporting polypeptide 1 (Oatp1) and multidrug-resistance-associated protein 2 (Mrp2) in the kidney (Fig. 8A). No differences in hepatic mRNA levels were observed between Oat3(−/−) and wild-type mice (Fig. 8B).
Fig. 8.
Quantitative PCR (QPCR) determination of transporter mRNA levels in Oat3(−/−) and wild-type male and female mice. Transporter expression levels were determined in kidney and liver from 6-mo-old mice by QPCR. A: renal transporter levels in knockout relative to wild-type animals. Open bars, females; closed bars, males. B: hepatic transporter levels in knockout relative to wild-type animals (open bars, females; closed bars, males). C: renal expression levels in female wild-type relative to male wild-type animals. D: hepatic expression levels in female wild-type relative to male wild-type animals. Values are mean mRNA levels relative to comparator ± SE; n = 6 for each bar. Asterisks represent significant differences from unity (dotted line) by t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
Differences in mRNA levels between genders of wild-type mice were also investigated. In the kidney, a female predominance existed for Oat2, Oat3, and Oat5, while a male predominance existed for Oat1 and Oatp1 (Fig. 8C). In the liver, male mice exhibited greater expression of Oat2, Oatp1, multidrug resistance protein 1 (Mdr1), and Mrp2 compared with female mice (Fig. 8D).
Organ function markers
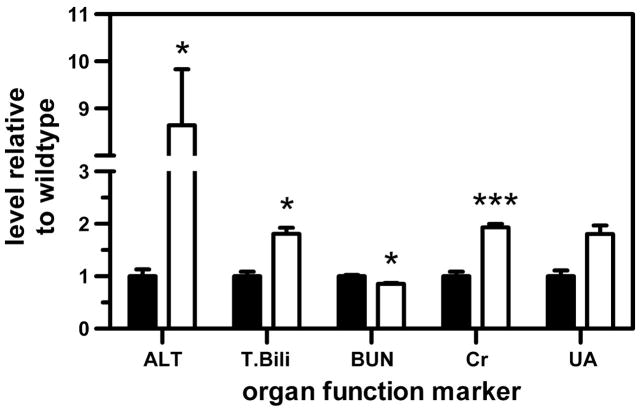
Liver and kidney tissue morphology was found to be normal in a previous investigation of Oat3(−/−) mice (31). Here we investigated biochemical markers of organ function in 6-mo-old male mice to reveal less overt indications of dysfunction. Liver and kidney function marker levels were significantly different between Oat3(−/−) and wild-type mice but were within the normal range with the exception of alanine aminotransferase in knockout mice (Fig. 9). Absolute measurements were [wild-type vs. Oat3(−/−)]: alanine aminotransferase, 23.0 ± 7.5 vs. 198.8 ± 67.0 U/l; total bilirubin, 0.08 ± 0.02 vs. 0.15 ± 0.02 mg/dl; blood urea nitrogen, 26.7 ± 1.6 vs. 22.8 ± 1.0 mg/dl; creatinine, 0.25 ± 0.05 vs. 0.48 ± 0.04 mg/dl; and uric acid, 3.5 ± 0.94 vs. 6.32 ± 1.39 mg/dl.
Fig. 9.
Organ function marker levels in serum of 6-mo-old Oat3(−/−) (open bars) and wild-type (closed bars) male mice. ALT, alanine aminotransferase; T. Bili, total bilirubin; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid. Significant differences for Oat3(−/−) vs. wild-type are indicated (*P < 0.05, ***P < 0.001).
DISCUSSION
The continual identification of novel substrates for renal transporters using heterologous in vitro expression systems provides valuable information for predicting xenobiotic-xenobiotic and xenobiotic-endobiotic interactions, as well as consequences of nonfunctional transporter polymorphisms. However, the well-recognized limitation of these preliminary determinations is that they do not indicate the true relevance of a transporter in handling a substrate in the context of whole-body metabolism, extrarenal transport, and glomerular filtration. To attribute an abnormality in blood or organ chemical composition to a transporter perturbation, the relevance of the transporter to the disposition of the chemical must first be determined in vivo. The goal of this investigation was to determine the in vivo role of Oat3 in the elimination and distribution of substrates demonstrated to have diverse transporter selectivity in vitro, ranging from relatively promiscuous substrates to Oat3-selective substrates. The substrates chosen represent those interacting with partially redundant renal transporters (i.e., p-aminohippurate with Oat1 and Oat3), those interacting with partially redundant renal and hepatic transporters (i.e., estrone-3-sulfate with Oat3 and the renal and hepatic organic anion transporting polypeptides), and those interacting predominantly with Oat3 (i.e., penicillin G). The expectation is that the elimination of substrates that interact more specifically with Oat3 and rely primarily on the kidney for removal will be reduced to the greatest extent by Oat3 deletion. For every animal, the nontransported compound, [14C]inulin, was injected concomitantly with the tritiated substrate of interest. This measure served to rule out any differences in glomerular filtration and hemodynamics between mice and also indicated dosing consistency. It was determined that none of these factors were confounding, as no differences in inulin handling were identified between mice of different genotypes (Figs. 1B, 2B, 3B, 5B, 6B, and Tables 2 and 3).
p-Aminohippurate is the prototypical organic anion transporter substrate and is also used as a diagnostic drug for measuring renal blood flow and transport capacity (4, 12). When examined in vitro, the renal basolateral organic anion transporters Oat1 and Oat3 have displayed species differences in their affinities for p-aminohippurate. Human OAT1 exhibits an ~10-fold stronger affinity for p-aminohippurate than human OAT3, and rabbit Oat3 does not transport p-aminohippurate (7, 9, 36). However, rat Oat1 and Oat3 interact with p-aminohippurate with similar affinities in vitro (22, 32). While a Km for p-aminohippurate on mouse Oat3 has not been reported, mouse Oat1 exhibits a similar affinity for p-aminohippurate as rat Oat1 and Oat3 (23). A recent investigation demonstrated that Oat1 knockout mice exhibit impaired p-aminohippurate elimination (13), substantiating the in vivo role of Oat1 in handling this compound and suggesting that if compensatory upregulation of Oat3 occurs, the degree of compensation is not sufficient to completely counteract the effect of Oat1 deletion. In the current investigation, we have determined that Oat3 deletion does not result in impaired p-aminohippurate elimination (Fig. 1A). Despite renal Oat1 mRNA levels exhibiting a substantial reduction in the Oat3(−/−) mice (Fig. 8A), p- aminohippurate elimination was not altered, suggesting that Oat1 function may not be compromised in the Oat3(−/−) mice. Additionally, despite the current and previous observation (5) of lower renal Oat1 mRNA levels in wild-type female mice relative to males, p-aminohippurate elimination was similar between genders. Collectively, current evidence suggests that Oat1 plays a greater role in p-aminohippurate elimination than Oat3 in vivo. It is important to note that since the current study assessed low-dose p-aminohippurate, and maximum transport capacity was not evaluated, it remains uncertain whether differences in Oat1 protein expression are present between the two genotypes. Clearly, comparison of Oat1 protein expression levels in wild-type and Oat3(−/−) animals is central to determining the compensatory potential of this partially redundant transporter. However, custom-generated and commercially available anti-rat and anti-mouse Oat1 antisera failed to recognize mouse Oat1 via Western blotting or immunohistochemistry.
In a separate experiment, p-aminohippurate was administered with probenecid to determine the impact of organic anion transporter inhibition on p-aminohippurate handling (Fig. 4). Interestingly, Oat3(−/−) males exhibited a response to probenecid that was more pronounced than that in wild-type males; however, this heightened response occurred with inulin as well. Thus it appears that Oat3(−/−) mice demonstrate a response to probenecid that involves an effect on hemodynamics or the glomeruli, whereas wild-type mice only demonstrate an effect on transport (i.e., inulin was not affected by probenecid in wild-type mice). To rule out a difference in bioavailability of intraperitoneally administered probenecid between knockout and wild-type mice, probenecid levels were measured by HPLC 2 min before injection of p-aminohippurate and inulin. The probenecid levels were determined to be ~1 mM in all mice (probenecid levels in humans reach ~0.5 mM for prevention of cidofovir nephrotoxicity) (26). Further investigation is needed to elucidate the mechanism for heightened responsiveness of Oat3(−/−) mice to probenecid. For the current study, because of this unexplained effect of probenecid in knockout mice, the Oat3 substrate estrone-3-sulfate, which bears the added benefit of avoiding inhibition of Oat1, was used to inhibit penicillin G elimination.
Estrone-3-sulfate is an excreted conjugated metabolite of estrogen that has been used in vitro for tracking Oat3 function. Estrone-3-sulfate interacts with Oat3 (31), but it also interacts with most liver and renal Oatps (17). However, since estrone-3-sulfate does not interact with Oat1 (31), it is possible that Oat3 loss may have a greater impact on estrone-3-sulfate elimination than on p-aminohippurate in vivo. In this investigation, female Oat3(−/−), but not male Oat3(−/−) mice exhibited reduced estrone-3-sulfate clearance (Fig. 2A and Table 2). Additionally, male mice of either genotype demonstrated a greater plasma clearance of estrone-3-sulfate compared with females, which appeared to be caused by a greater volume of distribution in males (Fig. 2A and Table 2). These two findings suggest that male mice possess a mechanism for estrone-3-sulfate clearance that is more active than that in females. Perhaps the most likely explanation is that males have greater Oatp function in the liver and/or kidneys than females, and thus the impact of Oat3 knockout is less pronounced in males and their estrone-3-sulfate clearance is greater. This hypothesis is supported by the present finding that female mice have substantially lower Oatp1 mRNA levels in kidney and liver than male mice (Fig. 8, C and D). Similar determinations have been made in both mice and rats (8, 25). Furthermore, biliary elimination of a related compound, estradiol-17-β-D-glucuronide, has been shown to be more active in male rats than in females (15).
Penicillin G (benzylpenicillin) is a pharmacotherapeutic substrate that is transported by Oat3 in vitro and interacts to a lesser degree with Oat1 (18, 19, 21). This antibiotic is eliminated primarily by the kidney (~80%) (11), is 65% protein bound (28), demonstrates some affinity for human hepatic OATPs (16, 17), and is 20–30% metabolized hepatically (10). Thus these properties place the elimination burden on carrier-mediated mechanisms in the kidney. This transporter dependency was historically exploited by practitioners using probenecid (a pan-inhibitor of organic anion transporters) to extend the residence time of penicillin and other β-lactams in the systemic circulation. The current investigation provides the first definitive in vivo confirmation that Oat3 is a major transporter responsible for penicillin G elimination, despite the presence of possible redundant transporters. Both male and female Oat3(−/−) mice exhibited markedly impaired penicillin G elimination (Fig. 3A and Table 2) in addition to substantially heightened hepatic levels 30 min after administration (~2- to 4-fold higher). These findings suggest that the human OAT3 polymorphisms which were demonstrated to result in dysfunctional or nonfunctional transport in vitro may be of true relevance in patients (14). Similar findings have also been reported with human OAT1 polymorphisms in vitro (1) and Oat1 knockout mice (13). The difference in penicillin G elimination between Oat3(−/−) and wild-type mice is completely abolished in the presence of an Oat3-saturating dose of estrone-3-sulfate (Figs. 5A and 6A and Table 3). This finding supports the contention that penicillin G elimination is highly dependent on Oat3 function. Since estrone-3-sulfate is substantially protein bound in the blood (2, 3), the high dose given to inhibit penicillin G elimination was theorized to displace penicillin G from its protein binding site. Dialysis was employed to confirm that such displacement can occur (Fig. 7). The buffer-to-serum ratio of penicillin G approached unity only in the presence of estrone-3-sulfate, suggesting that the displacement of penicillin G from serum protein was nearly complete. Thus, while estrone-3-sulfate will inhibit Oat3-mediated transport in wild-type mice and Oatp-mediated transport in both wild-type and Oat3(−/−) mice, resulting in less Oat3-mediated tubular uptake of penicillin G in wild-type mice and less Oatp-mediated hepatic uptake of penicillin G in both wild-type and Oat3(−/−) mice, it will also facilitate glomerular filtration of penicillin G by raising the free fraction in the blood. The expectation is therefore that with an excess level of estrone-3-sulfate, all Oat3- and Oatp-mediated penicillin G transport should be abolished, and penicillin G in the blood should be free from protein. If these hypotheses hold true, penicillin G clearance should approach that of inulin, which is not protein bound and is not transported, but is merely removed by glomerular filtration. Table 3 provides evidence that this is the case for both male and female Oat3(−/−) and wild-type mice. Interestingly, Oat3(−/−) mice demonstrate a greater penicillin G elimination rate (i.e, shorter half-life) when estrone-3-sulfate is present (Tables 2 and 3). This finding suggests that when Oat3 is absent, and therefore cannot be inhibited, estrone-3-sulfate’s enhancement of penicillin G’s glomerular filtration contributes to a net increase in penicillin elimination. On the other hand, in wild-type mice, since enhanced glomerular filtration is opposed by inhibition of Oat3, the net elimination rate of penicillin G does not change substantially.
The present findings indicate that Oat3 plays a major role in elimination and distribution of certain pharmacotherapeutics such as β-lactam antibiotics. Furthermore, these results indicate that impaired renal secretion resulting from perturbation of Oat3 is not entirely compensated for by other transport mechanisms. This is evidenced in the elevated systemic levels of estrone-3-sulfate and penicillin G (Figs. 2A and 3A). The elevated liver accumulation of penicillin G in Oat3(−/−) mice bears important implications for hepatotoxicity resulting from more toxic endogenous and xenobiotic Oat3 substrates. Elevated alanine aminotransferase and total bilirubin, and reduced blood urea nitrogen, in the Oat3(−/−) mice supports the hypothesis that hepatotoxicity may have already ensued as a result of such accumulation of endogenous organic anions (Fig. 9).
This study highlights the importance of Oat3 as a major mediator of penicillin G elimination and distribution. This information serves as a further contribution to the continuing efforts to identify the specific molecular entities responsible for mediating the disposition of antibiotics and other xenobiotic and endogenous compounds of clinical relevance. Future studies are necessary to elucidate the contribution of Oat3 to the elimination of other β-lactam antibiotics in vivo. A recent investigation regarding the role of renal basolateral Oat1 in β-lactam transport provided an indication that different β-lactam antibiotics have markedly different transport properties on the same transporter, and penicillin G exhibits a relatively weak interaction with Oat1 compared with other β-lactams (19). The major impact of Oat3 deletion on penicillin G elimination at such a low concentration in the blood suggests that other low-level substrates can potentially accumulate to abnormal levels in humans with compromised Oat3 function. Therefore, drug competition on Oat3 or the presence of nonfunctional polymorphisms of Oat3 should be considered as potential risk factors for accumulation of its substrates in the systemic circulation and in the liver. Oat3 substrates that are also actively transported into the liver by other transporters are especially likely candidates for causing hepatotoxicity as a result of Oat3 inhibition/dysfunction.
Acknowledgments
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01-DK-067216 (D. H. Sweet), by fellowship provisions from the American Foundation for Pharmaceutical Education (A. L. VanWert), and by the NIH C06-RR-015455 from the Extramural Research Facilities Program of the National Center for Research Resources. A portion of this work was presented at the annual meeting of the American Association of Pharmaceutical Scientists, San Antonio, TX, October 29–November 2, 2006 (poster W5308).
References
- 1.Bleasby K, Hall LA, Perry JL, Mohrenweiser HW, Pritchard JB. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6) J Pharmacol Exp Ther. 2005;314:923–931. doi: 10.1124/jpet.105.084301. [DOI] [PubMed] [Google Scholar]
- 2.Bouhamidi R, Gaillard JL, Silberzahn P, Martin B. Binding of estrogen-3-sulfates to stallion plasma and equine serum albumin. J Steroid Biochem Mol Biol. 1992;42:345–349. doi: 10.1016/0960-0760(92)90138-9. [DOI] [PubMed] [Google Scholar]
- 3.Bow DA, Perry JL, Simon JD, Pritchard JB. The impact of plasma protein binding on the renal transport of organic anions. J Pharmacol Exp Ther. 2006;316:349–355. doi: 10.1124/jpet.105.093070. [DOI] [PubMed] [Google Scholar]
- 4.Brenner BM, Rector FC. Brenner & Rector’s The Kidney. 7. Philadelphia, PA: Saunders; 2004. Total renal blood flow. [Google Scholar]
- 5.Buist SC, Klaassen CD. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1–3; Slc22a6–8) mRNA levels. Drug Metab Dispos. 2004;32:620–625. doi: 10.1124/dmd.32.6.620. [DOI] [PubMed] [Google Scholar]
- 6.Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol. 2003;146:95–158. doi: 10.1007/s10254-002-0003-8. [DOI] [PubMed] [Google Scholar]
- 7.Cha SH, Sekine T, Fukushima Ji Kanai Y, Kobayashi Y, Goya T, Endou H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- 8.Cheng X, Maher J, Lu H, Klaassen CD. Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol. 2006;70:1291–1297. doi: 10.1124/mol.106.025122. [DOI] [PubMed] [Google Scholar]
- 9.Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56:570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- 10.Cole M, Kenig MD, Hewitt VA. Metabolism of penicillins to penicilloic acids and 6-aminopenicillanic acid in man and its significance in assessing penicillin absorption. Antimicrob Agents Chemother. 1973;3:463–468. doi: 10.1128/aac.3.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittert LW, Griffen WO, Jr, LaPiana JC, Shainfeld FJ, Doluisio JT. Pharmacokinetic interpretation of penicillin levels in serum and urine after intravenous administration. Antimicrobial Agents Chemother (Bethesda) 1969;9:42–48. doi: 10.1128/AAC.9.1.42. [DOI] [PubMed] [Google Scholar]
- 12.Dowling TC, Frye RF, Fraley DS, Matzke GR. Characterization of tubular functional capacity in humans using para-aminohippurate and famotidine. Kidney Int. 2001;59:295–303. doi: 10.1046/j.1523-1755.2001.00491.x. [DOI] [PubMed] [Google Scholar]
- 13.Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, Barshop BA, Kaler G, Nigam SK. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in Oat1 knock-out mice. J Biol Chem. 2006;281:5072–5083. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- 14.Erdman AR, Mangravite LM, Urban TJ, Lagpacan LL, Castro RA, de la Cruz M, Chan W, Huang CC, Johns SJ, Kawamoto M, Stryke D, Taylor TR, Carlson EJ, Ferrin TE, Brett CM, Burchard EG, Giacomini KM. The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am J Physiol Renal Physiol. 2006;290:F905–F912. doi: 10.1152/ajprenal.00272.2005. [DOI] [PubMed] [Google Scholar]
- 15.Gotoh Y, Kato Y, Stieger B, Meier PJ, Sugiyama Y. Gender difference in the Oatp1-mediated tubular reabsorption of estradiol 17β-D-glucuronide in rats. Am J Physiol Endocrinol Metab. 2002;282:E1245–E1254. doi: 10.1152/ajpendo.00363.2001. [DOI] [PubMed] [Google Scholar]
- 16.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 17.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Kusuhara H, Sugiyama D, Ito K, Ueda S, Endou H, Sugiyama Y. Functional involvement of rat organic anion transporter 3 (rOat3; Slc22a8) in the renal uptake of organic anions. J Pharmacol Exp Ther. 2002;300:746–753. doi: 10.1124/jpet.300.3.746. [DOI] [PubMed] [Google Scholar]
- 19.Jariyawat S, Sekine T, Takeda M, Apiwattanakul N, Kanai Y, Sophasan S, Endou H. The interaction and transport of beta-lactam antibiotics with the cloned rat renal organic anion transporter 1. J Pharmacol Exp Ther. 1999;290:672–677. [PubMed] [Google Scholar]
- 20.Kerb R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006;234:4–33. doi: 10.1016/j.canlet.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda M, Kusuhara H, Endou H, Sugiyama Y. Rapid elimination of cefaclor from the cerebrospinal fluid is mediated by a benzylpenicillin-sensitive mechanism distinct from organic anion transporter 3. J Pharmacol Exp Ther. 2005;314:855–861. doi: 10.1124/jpet.105.085027. [DOI] [PubMed] [Google Scholar]
- 22.Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem. 1999;274:13675–13680. doi: 10.1074/jbc.274.19.13675. [DOI] [PubMed] [Google Scholar]
- 23.Kuze K, Graves P, Leahy A, Wilson P, Stuhlmann H, You G. Heterologous expression and functional characterization of a mouse renal organic anion transporter in mammalian cells. J Biol Chem. 1999;274:1519–1524. doi: 10.1074/jbc.274.3.1519. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther. 2000;292:1048–1052. [PubMed] [Google Scholar]
- 25.Lu R, Kanai N, Bao Y, Wolkoff AW, Schuster VL. Regulation of renal Oatp mRNA expression by testosterone. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F332–F337. doi: 10.1152/ajprenal.1996.270.2.F332. [DOI] [PubMed] [Google Scholar]
- 26.Selen A, Amidon GL, Welling PG. Pharmacokinetics of probenecid following oral doses to human volunteers. J Pharm Sci. 1982;71:1238–1242. doi: 10.1002/jps.2600711114. [DOI] [PubMed] [Google Scholar]
- 27.Shitara Y, Horie T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci. 2006;27:425–446. doi: 10.1016/j.ejps.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Standiford HC, Jordan MC, Kirby WM. Clinical pharmacology of carbenicillin compared with other penicillins. J Infect Dis. 1970;122(Suppl):S9–13. doi: 10.1093/infdis/122.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 29.Sweet DH. Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicol Appl Pharmacol. 2005;204:198–215. doi: 10.1016/j.taap.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol Renal Physiol. 2003;284:F763–F769. doi: 10.1152/ajprenal.00405.2002. [DOI] [PubMed] [Google Scholar]
- 31.Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 [Oat3 (Slc22a8)] knockout mice. J Biol Chem. 2002;277:26934–26943. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- 32.Sweet DH, Wolff NA, Pritchard JB. Expression cloning and characterization of rOat1. The basolateral organic anion transporter in rat kidney. J Biol Chem. 1997;272:30088–30095. doi: 10.1074/jbc.272.48.30088. [DOI] [PubMed] [Google Scholar]
- 33.Tahara H, Kusuhara H, Maeda K, Koepsell H, Fuse E, Sugiyama Y. Inhibition of Oat3-mediated renal uptake as a mechanism for drug-drug interaction between fexofenadine and probenecid. Drug Metab Dispos. 2006;34:743–747. doi: 10.1124/dmd.105.008375. [DOI] [PubMed] [Google Scholar]
- 34.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 35.Xu G, Bhatnagar V, Wen G, Hamilton BA, Eraly SA, Nigam SK. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)] Kidney Int. 2005;68:1491–1499. doi: 10.1111/j.1523-1755.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Groves CE, Bahn A, Barendt WM, Prado MD, Rodiger M, Chatsudthipong V, Burckhardt G, Wright SH. Relative contribution of OAT and OCT transporters to organic electrolyte transport in rabbit proximal tubule. Am J Physiol Renal Physiol. 2004;287:F999–F1010. doi: 10.1152/ajprenal.00156.2004. [DOI] [PubMed] [Google Scholar]