Abstract
Objective. RA is associated with localized bone loss in the hands, as well as generalized osteoporosis. We evaluated the relationship between hand digital X-ray radiogrammetry BMD (DXR-BMD) and total hip and lumbar spine BMD.
Methods. We conducted a cross-sectional study of 138 post-menopausal women with RA. The DXR-BMD was calculated based on digitized hand radiographs. Measurements of the total hip and lumbar spine BMD were performed by a DXA-BMD (BMDa) scan. Patient and physician questionnaires and laboratory samples supplied information on relevant covariates. Separate multivariate linear regression models were constructed to determine the cross-sectional relationship between hand DXR-BMD (independent variable) and total hip or lumbar spine BMD (dependent variables).
Results. The cohort comprised women with a median age of 61 years and RA disease duration of 13 years. Seventy-six per cent were either RF and/or anti-cyclic citrullinated peptide (anti-CCP) positive and most had moderate disease activity [median disease activity score-28 joint count (DAS28) 3.7]. Hand DXR-BMD was significantly associated with total hip BMD (β = 0.61; P < 0.0001) and lumbar spine BMD (β = 0.62; P < 0.0008) in adjusted models.
Conclusions. This study suggests that hand DXR-BMD is associated with both the total hip and lumbar spine BMD among post-menopausal women with RA. The relationship between bone loss in the hands and generalized osteoporosis should be further explored in longitudinal studies of patients with RA.
Keywords: Rheumatoid arthritis, Osteoporosis, Bone mineral density, Digital X-ray radiogrammetry, Hand bone loss
Introduction
RA is characterized by generalized bone loss in the hands, as well as periarticular demineralization and focal bone erosion. Osteoporosis is a well-recognized feature of RA patients, present at a 2-fold higher rate and associated with significant morbidity [1]. The relationship between hand bone loss in RA and generalized osteoporosis is not clear. By studying this relationship, clues to shared mechanisms may be discovered, perhaps leading to an improvement in the management and treatment of both types of bone loss associated with RA.
Digital X-ray radiogrammetry (DXR) is a technique to measure the hand BMD using digitized hand radiographs [2, 3]. Its utility was initially limited due to imprecision, but adding digitized images to the method with automated regions of interest for BMD calculation improved reproducibility [4, 5]. A recently developed imaging algorithm has improved precision with a coefficient of variation of 0.42% [4].
The DXR-BMD technology has been used to evaluate hand bone loss in RA and measures cortical BMD in the shaft of the second, third and fourth metacarpal bones, a site distinct from but adjacent to the periarticular region. An automated analysis of the cortical thickness of these regions of interest results in a metacarpal index calculation. These regions of interest have been measured in prior work [6] and found to be reliable and precisely defined. An estimate of the hand DXR-BMD is then obtained by calculating the volume of bone per projected area and adjusting for porosity of the bone, using geometric values to obtain an areal measurement [2]. The hand DXR-BMD measuring cortical metacarpal bone has been shown to strongly correlate with hand DXA-BMD (BMDa) measuring whole-hand cortical and trabecular bone (r = 0.88; P < 0.001) [7, 8]. The hand DXR-BMD is associated with RA disease severity as measured by radiographic progression [9, 10]. There is emerging evidence for the role of the hand DXR-BMD as a marker of disease activity and potential outcome measure in RA [7, 11]. If hand DXR-BMD is truly a measurement of ongoing bone loss and can predict radiographic progression and/or generalized osteoporosis, it may be a useful tool to help clinicians in the prognostication of RA patients along with currently available data.
Prior work [6] has demonstrated a correlation between hand DXR- and DXA-BMD measurement of the total femur BMD (r = 0.59; P < 0.01) and lumbar spine BMD (r = 0.45; P < 0.01), but this study was limited by lack of information on glucocorticoid usage and serological status. The hand DXR-BMD has been associated with osteoporotic fracture risk both in non-RA and RA populations [10, 12]. However, detailed analysis of the association between hand DXR-BMD and generalized osteoporosis as measured by both total hip and lumbar spine BMD is lacking.
By comparing the measurement of hand BMD by DXR with the measurement of generalized osteoporosis by conventional DXA, important relationships between the two types of bone loss may be better elucidated. We examined the relationship of hand DXR-BMD with total hip and lumbar spine BMD in a cohort of post-menopausal women with established RA. We hypothesized a direct relationship between hand DXR-BMD and both total hip and lumbar spine DXA-BMD, and that this relationship would be stronger in specific subgroups.
Methods
Study design
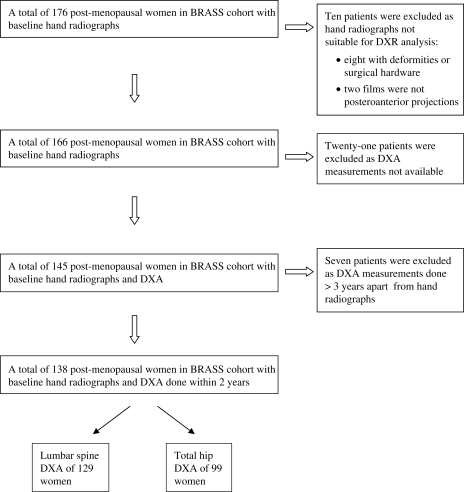
The study population consisted of women with RA who received their rheumatological care at an academic medical centre. All eligible patients were enrolled in the Brigham Rheumatoid Arthritis Sequential Study (BRASS): a longitudinal RA patient registry. Our study population represents a subset of the BRASS cohort, including only 138 post-menopausal women who had had bilateral hand radiographs and a total hip and/or lumbar spine DXA done within 2 years of each other (Fig. 1). The Institutional Review Board (Partners Human Research Committee) approval was obtained for this study. All patients in the BRASS cohort provided informed patient consent for participation in the BRASS, and our study population represents a subset of the BRASS cohort.
Fig. 1.
Cohort assembly. Patients were selected on the basis of having baseline hand radiographs and total hip and/or lumbar spine DXA measurements performed within 2 years of each other. Patients were excluded on the basis of either technical limitations of the hand DXR-BMD measurement or DXA measurements that were not available or >3 years from the date of the hand radiograph.
Hand DXR-BMD measurements
The hand DXR-BMD was measured at the metacarpal bones of the second, third and fourth digits, using a methodology developed by Sectra (Sweden) [6]. Regions of interest were defined on hand radiographs at fixed distances of 2.0 cm for the second, 1.8 cm for the third and 1.6 cm for the fourth metacarpals. Digitized hand radiograph images were collected every 2 years as part of the BRASS. The digitized image of the hand radiograph closest in time to the DXA was analysed using the DXR-online service (Sectra Imtec AB, Linkoping, Sweden).
Total hip and lumbar spine DXA measurements
All patients underwent a DXA scan on a Hologic QDR 4500 densitometer (Hologic, Bedford, MA, USA). Lumbar levels with artefact from sclerosis or compression fracture were removed from the reading. Short-term in vivo reproducibility for the posteroanterior spine is 0.026 gm/cm2 and for the lateral spine is 0.034 gm/cm2. Long-term in vivo reproducibility, determined by calculating the residual variation around a line connecting measurements made at 0, 3 and 6 months for each patient in before study, is between 1 and 2% for the total hip and lumbar spine DXA, and the reproducibility is independent of the patient's absolute BMD. Readers of the bone density measurement were blinded to the patient's clinical status.
Covariates
Participants completed a questionnaire with the assistance of a trained interviewer at baseline and then annually. The questionnaire included information about past and present arthritis medications, non-arthritis medications, comorbidities, smoking history and surgeries. In an additional semi-annual self-administered questionnaire, patients provided demographical information, new medical conditions, medication use, new symptoms, health-care resource use and assessed functional status with the multidimensional HAQ (MDHAQ) [13]. Rheumatologists completed a questionnaire documenting the ACR criteria for RA diagnosis, the 28-joint count, comorbid conditions, overall disease activity on a numerical rating scale of 0–100 and medication changes made at the visit [14].
Laboratory tests performed at baseline include the RF with anti-cyclic citrullinated peptide (anti-CCP) antibody performed annually. Hand radiographs are performed at baseline and every 2 years. Glucocorticoid use was measured as the self-reported cumulative lifetime dose with high glucocorticoid usage defined as >5 g over a lifetime (the 75th quartile of cumulative lifetime glucocorticoid usage) [15].
Since the baseline hand radiograph and the total hip and/or lumbar spine DXA measurements were not performed at the same time point, a time difference variable was defined to account for the time lag between the two measurements. The time difference was the time in months between the date of the hand radiograph and the date of the total hip and/or lumbar spine DXA.
Statistical analysis
We examined the cross-sectional relationship between the baseline hand DXR-BMD and both total hip and lumbar spine DXA-BMD. These relationships were analysed in two separate linear regression models, with the dependent variables being total hip and lumbar spine DXA-BMD. Variables tested as possible confounders or effect modifiers included age, disease duration, BMI, glucocorticoid use, smoking (in pack-years), MDHAQ score, disease activity score-28 joint count (DAS28), RF and CCP status and current osteoporosis medications [16]. Variables that changed the hand DXR-BMD parameter estimate in bivariate models by >10% were placed in the multivariate models as these were deemed to be clinically relevant. Differences in R2 were used to quantify the effect of adding and subtracting variables from the models.
To elucidate potential relationships between hand DXR-BMD and both total hip and lumbar spine BMD, we stratified by age, seropositivity, smoking status, cumulative glucocorticoid usage and disease duration. Our goal was to assess whether these factors modified the relationship between hand DXR-BMD and total hip and lumbar spine DXA-BMD. All statistical analysis was performed using SAS (version 9.1, Cary, NC, USA).
Results
Patient characteristics
Our study cohort consisted of 138 post-menopausal women with RA with a mean age of 61 years. The median duration of RA was 13 years with 19% having ⩽5-year duration (Table 1). The majority of patients were positive for RF and/or anti-CCP (76%). Eighty-six per cent were on DMARD therapy at baseline. The median DAS28 score was 3.7, reflecting moderate disease activity, and the median MDHAQ score was 0.6 (range 0–3). The sample had a median BMI of 26 kg/m2.
Table 1.
Patient characteristics
| Variables | |
|---|---|
| Age, mean (s.d.), years | 61 (10) |
| Aged >61 years, n (%) | 63 (46) |
| Race (Caucasian), n (%) | 127 (93) |
| BMI, median (IQR), kg/m2 | 26 (23–30) |
| DMARDs (yes), n (%) | 118 (86) |
| Smoking, median (IQR), pack-years | 1 (0–17) |
| High glucocorticoid usea, n (%) | 41 (30) |
| No history of glucocorticoid use, n (%) | 41 (30) |
| Current osteoporosis medication use, n (%) | 19 (14) |
| Disease duration, median (IQR), years | 13 (7–23) |
| Early RAb, n (%) | 26 (19) |
| Seropositive (RF and/or anti-CCP), n (%) | 105 (76) |
| RF status positive, n (%) | 88 (64) |
| Anti-CCP positive, n (%) | 87 (63) |
| MDHAQ score, median (IQR) | 0.6 (0.2–1.0) |
| DAS28, median (IQR) | 3.7 (2.5–4.9) |
| Time difference, median (IQR), monthsc | 7 (1–14) |
| Hand DXR-BMD, mean (s.d.), g/cm2 | 0.52 (0.09) |
| Total hip DXA, mean (s.d.), g/cm2 | 0.85 (0.11) |
| Lumbar spine DXA, mean (s.d.), g/cm2 | 0.97 (0.16) |
Lumbar spine BMD analysis consisted of 129 patients; total hip BMD analysis consisted of 99 patients. IQR is given between 25th and 75th percentiles. aHigh glucocorticoid use is defined as a cumulative lifetime exposure of >5 g. bEarly RA defined as disease duration ⩽5 years. cVariable created to account for time difference (in months) between date of hand X-ray for hand DXR measurement and date of total hip or lumbar spine DXA for BMD measurement.
Association of hand DXR-BMD with total hip and lumbar spine DXA-BMD
We found that hand DXR-BMD was associated with both total hip and lumbar spine DXA-BMD. The parameter estimate (β-coefficient) for hand DXR-BMD in the unadjusted total hip model was 0.57 (P < 0.0001). The parameter estimate (β-coefficient) for hand DXR-BMD and in the unadjusted lumbar spine model was 0.45 (P = 0.004). After adjusting for relevant confounders, the multivariate linear regression models found that hand DXR-BMD was still significantly associated with DXA-BMD, having a β-coefficient of 0.61 in the total hip model (P < 0.0001) and 0.62 in the lumbar spine model (P = 0.0008) (Tables 2 and 3). The R2 of the total hip model was 0.34 and lumbar spine model was 0.17.
Table 2.
Linear regression analyses of hand DXR-BMD, covariates and total hip BMD
| Variable | Univariate parameter estimate (β-coefficient) | P-value | Multivariate parameter estimate (β-coefficient) | P-value |
|---|---|---|---|---|
| Hand DXR-BMD | 0.57 | <0.0001 | 0.61 | <0.0001 |
| Age at hand radiograph, years | −0.003 | 0.007 | –a | –a |
| Disease duration, years | −0.001 | 0.37 | 0.002 | 0.04 |
| BMI, kg/m2 | 0.007 | 0.0003 | 0.006 | <0.0002 |
| DMARDs (yes) | −0.047 | 0.16 | –a | –a |
| High glucocorticoid useb | −0.027 | 0.26 | –a | –a |
| MDHAQ score | −0.012 | 0.43 | –a | –a |
| DAS28 | 0.006 | 0.42 | –a | –a |
| Seropositive (RF and/or anti-CCP) | −0.05 | 0.06 | –a | –a |
| Current osteoporosis medications | −0.047 | 0.22 | –a | –a |
| Smoking, pack-years | −0.001 | 0.61 | –a | –a |
| Aged >61 years | −0.016 | 0.46 | –a | –a |
| Time difference measurementc | 0.003 | 0.025 | –a | –a |
Total hip BMD model consisted of 99 patients. In the multivariate model, all variables listed in Table 1 were tested for entry into the model and remained if there was a >10% change in the hand DXR-BMD parameter estimate. R2 is found to be 0.34. aVariables not included in the multivariate model. bHigh glucocorticoid use defined as a cumulative lifetime exposure of >5 g. cVariable created to account for time difference (in months) between date of hand radiograph for hand DXR measurement and date of total hip or lumbar spine DXA for BMD measurement.
Table 3.
Linear regression analyses of hand DXR-BMD, covariates and lumbar spine DXA
| Variable | Univariate Parameter estimate (β-coefficient) | P-value | Multivariate Parameter estimate (β-coefficient) | P-value |
|---|---|---|---|---|
| Hand DXR-BMD | 0.45 | 0.004 | 0.62 | 0.0008 |
| Age at hand radiograph, years | −0.003 | 0.10 | –a | –a |
| Disease duration, years | 0.001 | 0.28 | 0.003 | 0.03 |
| BMI, kg/m2 | 0.003 | 0.04 | 0.006 | 0.02 |
| DMARDs (yes) | −0.024 | 0.56 | –a | –a |
| High glucocorticoid useb | −0.01 | 0.76 | –a | –a |
| MDHAQ score | −0.01 | 0.47 | −0.014 | 0.47 |
| DAS28 | −0.006 | 0.54 | –a | –a |
| Seropositive (RF and/or anti-CCP) | −0.03 | 0.38 | –a | –a |
| Current osteoporosis medications | −0.027 | 0.51 | –a | –a |
| Smoking, pack-years | 0.001 | 0.93 | 0.001 | 0.47 |
| Aged >61 years | −0.014 | 0.62 | –a | –a |
| Time difference measurementc | −0.001 | 0.39 | −0.002 | 0.10 |
Total hip BMD model consisted of 99 patients. In the multivariate model, all variables listed in Table 1 were tested for entry into the model and remained if there was a >10% change in the hand DXR-BMD parameter estimate. R2 was found to be 0.17. aVariables not included in the multivariate model. bHigh glucocorticoid use defined as a cumulative lifetime exposure of >5 g. cVariable created to account for time difference (in months) between date of hand radiograph for hand DXR measurement and date of total hip or lumbar spine DXA for BMD measurement.
Modification of effect by seropositivity, disease duration and age >61 years
To further delineate the relationships between hand DXR-BMD and both total hip and lumbar spine DXA-BMD, we stratified the models by serological status (positive or negative), disease duration (⩽5 and >5 years) and age (⩽61 or >61 years). We did not find evidence of effect modification by seropositivity, disease duration or age in the hip model, nor did we find evidence of effect modification by seropositivity or disease duration in the spine model. There was effect modification of the relationship between hand DXR-BMD and spine DXA-BMD by age (P < 0.05). There was no effect modification with smoking (pack-years) or cumulative glucocorticoid use.
Discussion
Bone disease in RA has at least two components: first, local inflammation leads to both erosions and periarticular osteopenia; secondly, generalized osteoporosis results from multiple factors [17, 18]. Osteoporosis carries the risk of fracture, and localized joint destruction leads to pain, disability and a decrease in functional status. Systemic bone loss in RA is related to RA-specific factors such as disease duration, disease severity and medication use (such as glucocorticoids), as well as other determinants of osteoporosis such as post-menopausal status and race [19]. Similarly, hand bone loss in RA may be from RA-specific factors and generalized osteoporosis disease determinants, although these complex relationships are yet to be fully determined. Low BMI is an established risk factor for osteoporosis [20]. In our population of post-menopausal women with established RA, we found that hand DXR-BMD was a significant predictor of both total hip and lumbar spine BMD, even after adjusting for multiple confounders. Disease duration and BMI were also important predictors of the total hip and lumbar spine BMD.
The relationship between hand DXR-BMD and lumbar spine DXA-BMD was modified by age. Our study included only post-menopausal women ranging in age from 51 to 71 years. Our models stratified by age revealed that among women who were aged <61 years, the hand DXR-BMD was more highly associated with the lumbar spine BMD. This finding of an increased association of hand BMD with spine BMD in younger women also implies that the effect of increasing age plays a more prominent role in determining generalized bone loss at the spine as other data have suggested [21]. Future studies may enroll pre-menopausal women with RA to examine this relationship in further detail and to ascertain the extent that RA disease-specific factors may influence generalized osteoporosis.
Hand bone loss in RA occurs early in the course of disease, whereas generalized osteoporosis may take longer to develop [22–24]. The hand DXR-BMD has been associated with radiographic progression, which manifests as the disease duration increases [25]. Thus, it is possible that the hand DXR-BMD has more utility in predicting generalized osteoporosis among post-menopausal women who have longer disease duration, compared with those with early RA; however, our analysis was limited by the smaller number in the early RA group with disease duration ⩽5 years (n = 26). To investigate this hypothesis more thoroughly, a prospective longitudinal study of the baseline hand DXR-BMD and serial measurements of the total hip and lumbar spine BMD would be necessary.
DXA is traditionally measured at sites that include the trabecular bone, particularly those involved in clinically significant morbidity from fractures such as the spine and femur. Inaccuracies in measurements at the lumbar spine may be due to the degenerative joint disease that is unrelated to RA disease activity. In addition, sclerotic changes from the degenerative joint and disc disease in the lumbar spine may create artefact that falsely increases the overall lumbar spine BMD. The DXA measurement in our study attempted to account for such inaccuracies by removing any sclerotic artefact from the reading. About 27% of the patients had four or less vertebrae in the analysis. RA does not typically involve the lumbar spine, but can involve the total hip, particularly with secondary OA and the cervical spine. In our analysis, we found that there was a similar relationship between hand DXR-BMD and both total hip and lumbar spine DXA-BMD, as indicated by the parameter estimates (β-coefficients) of 0.61 in the total hip and 0.62 in the lumbar spine models. Prior study [26] has found that radiographic joint damage in RA as measured by the Larsen score correlates with the BMD of the total hip but not the lumbar spine [26]. The R2 of the total hip model was 0.34 compared with 0.17 in the lumbar spine, indicating that the explanatory power of the model to account for the variability was higher in the total hip vs the lumbar spine. This increased explanatory power of the total hip model as compared to the lumbar spine model is consistent with some prior studies noting that RA radiographic damage is associated more with the total hip compared with lumbar spine BMD.
Several important limitations of our study need to be discussed. This study was an analysis of cross-sectional data. Hand radiographs performed to measure the hand DXR-BMD and the DXA scans were not done on the same day. Although the time between the studies was taken into account in our analysis through the time difference variable, this may have affected our results. Despite this drawback, we still found a strong association between hand DXR-BMD and total hip and lumbar spine DXA-BMD. We did not have data on current physical activity level or vitamin D at the time of the hand DXR-BMD measurement and it is known that low activity level is a putative risk factor for the development of osteoporosis [27]. Vitamin D serum levels were also not available but may play a role in both RA disease activity and osteoporosis [28]. Lastly, measurement of the hand DXR-BMD and the total hip and lumbar spine DXA-BMD in a population of healthy post-menopausal women would serve as an important comparison group and may be the subject of future research.
The regions of interest used in our study were areas of the metacarpal cortical bone anatomically close to the areas of active joint inflammation, but not contained within the joint itself. Our analysis demonstrates that in this cohort of post-menopausal women with established RA, hand DXR-BMD is significantly associated with both total hip and lumbar spine BMD, after adjustment for multiple confounders. Other studies have shown that hand DXR-BMD may be a better predictor of radiographic progression in patients with early RA as compared with patients with longer disease duration, and there may be a role for serial measurements of hand DXR-BMD over time to evaluate the change in DXR-BMD as a predictor of the DXA-BMD and radiographic progression [29]. Future prospective studies might focus on RA patients with both early and established disease and women who are pre- and post-menopausal, as these results may have implications for osteoporosis-prevention strategies [30, 31]. Since hand radiographs are routinely used in the management of patients with RA, DXR-BMD may have a role in assessing both the RA-specific bone effects and generalized osteoporosis, among patients with RA.
Acknowledgements
We gratefully acknowledge the technical support in obtaining the hand digital X-ray radiogrammetry (hand DXR-BMD) measurements, specifically Jakob Algulin Sectra (Sweden), Johan Kalvesten, Sectra (Sweden) and the Brigham Rheumatoid Arthritis Sequential Study (BRASS) cohort.
Funding: This study was supported by BiogenIdec and Crescendo Biosciences. This work was partially supported by the NIH (R21 AG027066). S.P.D.'s effort is supported by the NIH (T32 AR007530, Institutional Training Grant) and the American College of Rheumatology Research and Education Fund Physician Scientist Development Award. D.H.S. also receives salary support from other NIH grants (AR 055989 and AR 047782).
Disclosure statement: M.E.W. has received research support and acts as a consultant to Biogen Idec, Crescendo Bioscience and Millennium Pharmaceuticals. N.A.S. receives grant support from Biogen Idec, Crescendo Biosciences, AMGEN and Bristol Myers Squibb Foundation. All other authors have declared no conflicts of interest.
References
- 1.Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Reduced bone mineral density in male rheumatoid arthritis patients: frequencies and associations with demographic and disease variables in ninety-four patients in the Oslo County Rheumatoid Arthritis Register. Arthritis Rheum. 2000;43:2776–84. doi: 10.1002/1529-0131(200012)43:12<2776::AID-ANR18>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen JT, Andersen PB, Rosholm A, Bjarnason NH. Digital X-ray radiogrammetry: a new appendicular bone densitometric method with high precision. Clin Phys. 2000;20:330–5. doi: 10.1046/j.1365-2281.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 3.Cootes TF, Taylor CJ. Anatomical statistical models and their role in feature extraction. Br J Radiol. 2004;77:S133–9. doi: 10.1259/bjr/20343922. [DOI] [PubMed] [Google Scholar]
- 4.Ward KA, Cotton J, Adams JE. A technical and clinical evaluation of digital X-ray radiogrammetry. Osteoporos Int. 2003;14:389–95. doi: 10.1007/s00198-003-1386-3. [DOI] [PubMed] [Google Scholar]
- 5.Bottcher J, Pfeil A, Rosholm A, et al. Influence of image-capturing parameters on digital X-ray radiogrammetry. J Clin Densitom. 2005;8:87–94. doi: 10.1385/jcd:8:1:087. [DOI] [PubMed] [Google Scholar]
- 6.Rosholm A, Hyldstrup L, Backsgaard L, Grunkin M, Thodberg HH. Estimation of bone mineral density by digital X-ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int. 2001;12:961–9. doi: 10.1007/s001980170026. [DOI] [PubMed] [Google Scholar]
- 7.Hoff M, Haugeberg G, Kvien TK. Hand bone loss as an outcome measure in established rheumatoid arthritis: 2-year observational study comparing cortical and total bone loss. Arthritis Res Ther. 2007;9:R81. doi: 10.1186/ar2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelke K, Gluer CC. Quality and performance measures in bone densitometry—part 1: errors and diagnosis. Osteoporos Int. 2006;17:1283–92. doi: 10.1007/s00198-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 9.Bottcher J, Malich A, Pfeil A, et al. Potential clinical relevance of digital radiogrammetry for quantification of periarticular bone demineralization in patients suffering from rheumatoid arthritis depending on severity and compared with DXA. Eur Radiol. 2004;14:631–7. doi: 10.1007/s00330-003-2087-1. [DOI] [PubMed] [Google Scholar]
- 10.Haugeberg G, Lodder MC, Lems WF, et al. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) Collaborative Study. Ann Rheum Dis. 2004;63:1331–4. doi: 10.1136/ard.2003.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottcher J, Pfeil A. Diagnosis of periarticular osteoporosis in rheumatoid arthritis using digital X-ray radiogrammetry. Arthritis Res Ther. 2008;10:103. doi: 10.1186/ar2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouxsein ML, Palermo L, Yeung C, Black DM. Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int. 2002;13:358–65. doi: 10.1007/s001980200040. [DOI] [PubMed] [Google Scholar]
- 13.Pincus T, Yazici Y, Bergman M. Development of a multi-dimensional health assessment questionnaire (MDHAQ) for the infrastructure of standard clinical care. Clin Exp Rheumatol. 2005;23(5 Suppl. 39):S19–28. [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Solomon DH, Stedman M, Licari A, Weinblatt ME, Maher N, Shadick N. Agreement between patient report and medical record review for medications used for rheumatoid arthritis: the accuracy of self-reported medication information in patient registries. Arthritis Rheum. 2007;57:234–9. doi: 10.1002/art.22549. [DOI] [PubMed] [Google Scholar]
- 16.Department of Rheumatology UMC, Nijmegen. [(20 May 2008, date last accessed)]. http://www.das-score.nl/www.das-score.nl/index.html. [Google Scholar]
- 17.Goldring SR, Gravallese EM. Mechanisms of bone loss in inflammatory arthritis: diagnosis and therapeutic implications. Arthritis Res. 2000;2:33–7. doi: 10.1186/ar67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laan RF, Buijs WC, Verbeek AL, et al. Bone mineral density in patients with recent onset rheumatoid arthritis: influence of disease activity and functional capacity. Ann Rheum Dis. 1993;52:21–6. doi: 10.1136/ard.52.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev. 2005;208:228–51. doi: 10.1111/j.0105-2896.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 20.De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 21.Oelzner P, Schwabe A, Lehmann G, et al. Significance of risk factors for osteoporosis is dependent on gender and menopause in rheumatoid arthritis. Rheumatol Int. 2008;28:1143–50. doi: 10.1007/s00296-008-0576-x. [DOI] [PubMed] [Google Scholar]
- 22.Devlin J, Lilley J, Gough A, et al. Clinical associations of dual-energy X-ray absorptiometry measurement of hand bone mass in rheumatoid arthritis. Br J Rheumatol. 1996;35:1256–62. doi: 10.1093/rheumatology/35.12.1256. [DOI] [PubMed] [Google Scholar]
- 23.Goldring SR. Periarticular bone changes in rheumatoid arthritis: pathophysiological implications and clinical utility. Ann Rheum Dis. 2009;68:297–9. doi: 10.1136/ard.2008.099408. [DOI] [PubMed] [Google Scholar]
- 24.Guler-Yuksel M, Bijsterbosch J, Goekoop-Ruiterman YP, et al. Changes in bone mineral density in patients with recent onset, active rheumatoid arthritis. Ann Rheum Dis. 2008;67:823–8. doi: 10.1136/ard.2007.073817. [DOI] [PubMed] [Google Scholar]
- 25.Forslind K, Keller C, Svensson B, Hafstrom I. Reduced bone mineral density in early rheumatoid arthritis is associated with radiological joint damage at baseline and after 2 years in women. J Rheumatol. 2003;30:2590–6. [PubMed] [Google Scholar]
- 26.Lodder MC, de Jong Z, Kostense PJ, et al. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann Rheum Dis. 2004;63:1576–80. doi: 10.1136/ard.2003.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregg EW, Cauley JA, Seeley DG, Ensrud KE, Bauer DC. Physical activity and osteoporotic fracture risk in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1998;129:81–8. doi: 10.7326/0003-4819-129-2-199807150-00002. [DOI] [PubMed] [Google Scholar]
- 28.Leventis P, Patel S. Clinical aspects of vitamin D in the management of rheumatoid arthritis. Rheumatology. 2008;47:1617–21. doi: 10.1093/rheumatology/ken296. [DOI] [PubMed] [Google Scholar]
- 29.Hoff M, Haugeberg G, Odegard S, et al. Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann Rheum Dis. 2009;68:324–9. doi: 10.1136/ard.2007.085985. [DOI] [PubMed] [Google Scholar]
- 30.Tourinho TF, Capp E, Brenol JC, Stein A. Physical activity prevents bone loss in premenopausal women with rheumatoid arthritis: a cohort study. Rheumatol Int. 2008;28:1001–7. doi: 10.1007/s00296-008-0554-3. [DOI] [PubMed] [Google Scholar]
- 31.Solomon DH, Finkelstein JS, Shadick N, et al. The relationship between focal erosions and generalized osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum. 2009;60:1624–31. doi: 10.1002/art.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]