Abstract
Objective. Evaluation of the efficacy of green tea extract (GTE) in regulating chemokine production and chemokine receptor expression in human RA synovial fibroblasts and rat adjuvant-induced arthritis (AIA).
Methods. Fibroblasts isolated from human RA synovium were used in the study. Regulated upon activation normal T cell expressed and secreted (RANTES)/CCL5, monocyte chemoattractant protein (MCP)-1/CCL2, growth-regulated oncogene (GRO)α/CXCL1 and IL-8/CXCL8 production was measured by ELISA. Western blotting was used to study the phosphorylation of protein kinase C (PKC)δ and c-Jun N-terminal kinases (JNK). Chemokine and chemokine receptor expression was determined by quantitative RT–PCR. The benefit of GTE administration in rat AIA was determined.
Results. GTE (2.5–40 μg/ml) inhibited IL-1β-induced MCP-1/CCL2 (10 ng/ml), RANTES/CCL5, GROα/CXCL1 and IL-8/CXCL8 production in human RA synovial fibroblasts (P < 0.05). However, GTE inhibited MCP-1/CCL2 and GROα/CXCL1 mRNA synthesis in RA synovial fibroblasts. Furthermore, GTE also inhibited IL-1β-induced phosphorylation of PKCδ, the signalling pathway mediating IL-1β-induced chemokine production. Interestingly, GTE preincubation enhanced constitutive and IL-1β-induced CCR1, CCR2b, CCR5, CXCR1 and CXCR2 receptor expression. GTE administration (200 mg/kg/day p.o.) modestly ameliorated rat AIA, which was accompanied by a decrease in MCP-1/CCL2 and GROα/CXCL1 levels and enhanced CCR-1, -2, -5 and CXCR1 receptor expression in the joints of GTE administered rats.
Conclusions. Chemokine receptor overexpression with reduced chemokine production by GTE may be one potential mechanism to limit the overall inflammation and joint destruction in RA.
Keywords: Green tea, Chemokines, Chemokine receptors, Rheumatoid arthritis, Synovial fibroblasts, Complementary and alternative medicine, Adjuvant-induced arthritis
Introduction
RA is a chronic inflammatory disease leading to joint destruction mediated in part by the migration of inflammatory cells into the synovial tissue [1]. In response to the pro-inflammatory cytokines produced by macrophages, such as IL-1β and TNF-α, RA synovial fibroblasts produce chemokines that promote inflammation and neovascularization in the arthritic joint [1]. Although cytokine-specific biological agents have improved the treatment of RA, still in clinical practice, patients only respond partially to such therapies [2–4]. Thus, in order to achieve additional improvements in RA therapy, newer treatment combinations need to be tested.
Chemokines are a specialized family of small (8–10-kDa), structurally related proteins classified into four supergene families, C, CC, CXC and CX3C, based on the position of N-terminal cysteine residues [5]. CC and CXC chemokines are well-established regulators of gene transcription, cell proliferation and leucocyte trafficking to normal and inflamed tissues [6, 7]. Chemokines such as monocyte chemoattractant protein 1 (MCP-1)/CCL2, regulated upon activation normal T cell expressed and secreted (RANTES)/CCL5, growth-regulated oncogene α (GROα)/CXCL1 and IL-8/CXCL8 are potent chemotactic agents that are constitutively produced by RA synovial fibroblasts and further up-regulated upon cytokine stimulation [8]. The inhibition of chemokine–chemokine receptor interaction has shown promise in animal models of arthritis [9, 10]. Primarily, chemokines have been associated with the regulation of leucocyte trafficking to normal and inflamed tissues [11, 12]. However, in addition to leucocytes, the other non-haematopoietic cell types, including endothelial cells, fibroblasts and several tumour lineage cells, also express chemokine receptors [10–12].
Green tea (Camellia sinensis) is one of the most commonly consumed beverages in the world, with no significant adverse side effects [13]. Several epidemiological and experimental studies using animal models in the past two decades have verified the antioxidant, anti-inflammatory and anti-oncogenic properties of the various polyphenols named catechins found in green tea [14, 15]. Polyphenol-rich green tea extract (GTE) has shown inhibitory potential in selectively regulating cell growth, cell cycle and inducing apoptosis in cancer cells [16, 17]. Its anti-inflammatory properties were highlighted when the administration of GTE in drinking water to type II collagen immunized mice prevented the onset and severity of arthritis [18]. We recently showed that epigallocatechin-3-gallate (EGCG), a major constituent of GTE, inhibits IL-1β-stimulation CC/CXC chemokine production and MMP-2 activation in RA synovial fibroblasts [7]. We also showed recently that EGCG inhibits IL-6 synthesis and transsignalling in human RA synovial fibroblasts and rat adjuvant-induced arthritis (AIA) model [19]. The aim of the present study was to decipher the mechanism of CC/CXC chemokine regulation and CC/CXC chemokine receptor expression caused by GTE treatment in human RA synovial fibroblasts and rat AIA model.
Materials and methods
Antibodies and reagents
GTE was purchased from Sigma-Aldrich (Polyphenon 60 from green tea, Catalogue number P1204; St Louis, MO, USA). Recombinant IL-1β was purchased from R&D Systems (Minneapolis, MN, USA). Rabbit polyclonal antibodies against phosphoprotein kinase C (PKC)δ, total-PKCδ, phospho-c-Jun N-terminal kinases (JNK), total-JNK (Cell Signaling Technology, Beverly, MA, USA) and β-actin (Sigma-Aldrich, St Louis, MO, USA) were used in the study. All signalling inhibitors were purchased from Calbiochem (San Diego, CA, USA) and EMD Chemicals (Gibbstown, NJ, USA). ELISA kits for RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 were purchased from R&D Systems (Minneappolis, MN, USA). ELISA kits for MCP-1/CCL2 were purchased from Invitrogen (Carlsbad, CA, USA). Rat RANTES/CCL5, GROα/CXCL2 and MCP-1/CCL2 fluorescence-based Luminex assay kits were purchased from Millipore (Billerica, MA, USA).
Culture of human RA synovial fibroblasts
Fibroblasts were isolated from synovium obtained from RA patients [20] who had undergone total joint replacement surgery or synovectomy. The study was approved by the University of Michigan Institutional Review Board. Fresh synovial tissues were minced and digested in a solution of dispase, collagenase and DNase [7]. Cells were used at passage five or older, at which time they were a homogeneous population. RA synovial fibroblasts were grown in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere with 5% CO2. Upon confluence, cells were passaged by brief trypsinization, as previously described [7]. All treatments were performed in serum-free medium.
Preparation of GTE solution
A fresh stock solution of GTE (10 mg/ml) was prepared in water before each study, sterile filtered with 0.2-μm syringe filters and used in each experiment by adding directly to the culture medium.
HPLC analysis of GTE
A two-point standard for each catechin was prepared for calibration using 95% ethanol as diluting solution. Briefly, 100 mg of GTE sample, prepared in duplicate, were extracted into a 95% ethanol solution using sonication for 10 min and filtered into HPLC vials. HPLC conditions were as follows: Octadecyl Silane, 5 μm; column, 250 × 4.6 mm; flow rate, 1 ml/min; UV-Vis, 274 nm; run time, 45 min; injecting volume, 5 μl; mobile phase gradient between acetonitrile and 1% citric acid buffer. HPLC analysis was performed by Microbac Laboratories (Boulder, CO, USA).
Cell viability assay
The effect of GTE (10–100 μg/ml) on the viability of RA synovial fibroblasts (2 × 104/well in 96-well plate) was studied, as previously described [7], using the MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide]-based cell viability assay according to the instructions of the manufacturer (Molecular Probes, Invitrogen, Carlsbad, CA, USA).
Treatment of RA synovial fibroblasts with IL-1β and GTE
To study the effect of GTE on chemokine production, RA synovial fibroblasts were incubated with or without GTE (2.5–40 μg/ml) in serum-free medium for ∼12 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. After 24 h, culture supernatants were collected and centrifuged at 10 000 g for 5 min at 4°C to remove particulate matter, and stored at −80°C in fresh tubes. Using ELISA kits, culture supernatants were used to determine the quantities of MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8. To study the signalling mechanism of chemokine production by IL-1β, RA synovial fibroblasts were incubated with MAPK inhibitors (ERK½, PD98059; p38, SB203580; and JNK, SP600125; 10 μM), PKC inhibitors (general, Ro-318425; PKCα, Gö6976; and PKCδ, Rottlerin; 10 μM) or the NFκB inhibitor (PDTC; 200 μM) for 2 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h, and processed for determination of chemokine production. All inhibitors were purchased from Calbiochem (San Diego, CA, USA) and the concentrations used in this study were based on previous studies [7, 19].
Induction of arthritis by adjuvant
Female Lewis rats, ∼100 g (Harlan Laboratories, Indianapolis, IN, USA), were injected subcutaneously at the base of the tail with 300 μl (5 mg/ml) of lyophilized Mycobacterium butyricum (Difco Laboratories, Detroit, MI, USA) in sterile mineral oil. The day of adjuvant injection was considered 0 for all time points. Clinical parameters measured included articular index and ankle circumference. Articular index scores were recorded for each hind joint by a consistent observer blinded to the treatment regimen and then averaged for each animal. Scoring was performed on a 0–4 scale where 0 = no swelling or erythema, 1 = slight swelling and/or erythema, 2 = low to moderate oedema, 3 = pronounced oedema with limited joint usage and 4 = excess oedema with joint rigidity. Ankle circumferences were measured by the same blinded observer as described previously [21]. The increase in ankle circumference was presented as delta (Δ) ankle circumference. The Δ ankle circumferences of both the hind ankles from each animal were averaged and ‘n’ is represented as the number of animals used in each of the experimental groups. All animal studies were approved by the ethics committee of the University of Michigan.
Treatment of animals with GTE
GTE was brought into suspension in phosphate buffered saline (PBS). GTE (200 mg/kg) or PBS were administered daily by oral gavage, with treatment initiated on Day 7 after arthritis induction when the first signs of joint inflammation and pain are usually noted and continued until Day 16 [9, 22]. On Day 17, animals were anaesthetized for blood collection by cardiac puncture and then sacrificed for biochemical and cytokine analysis. For all comparisons, the PBS group served as a control. A daily dose of GTE (200 mg/kg) in rats corresponds to one-tenth of the ‘no observed-adverse-effect level’ dose used earlier for systemic toxicity study in rats following oral administration for 28 days [23].
Assay for rat MCP-1/CCL2, RANTES/CCL5 and GROα/CXCL2
Ankles used for ELISA were removed, snap frozen and homogenized on ice using a motorized homogenizer, followed by 30 s of sonication in 3 ml of PBS containing complete Mini protease inhibitor cocktail tablet (Roche, Indianapolis, IN, USA). Homogenates were centrifuged at 2500 g for 10 min, filtered through a 0.45-μm pore size filter (Millipore, Billerica, MA, USA) and stored at −80°C until use. Protein concentrations were measured using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Joint homogenates were analysed for rat MCP-1/CCL2, RANTES/CCL5 and GROα/CXCL2 using fluorescence-based Luminex assay kits (Millipore) according to the manufacturer’s protocol. The values obtained from the joint homogenates were normalized to protein content.
Western immunoblotting and analysis
To study the effect of GTE on signalling events, RA synovial fibroblasts were incubated with or without GTE (2.5–20 μg/ml) in serum-free RPMI 1640 for ∼12 h, followed by stimulation with IL-1β (10 ng/ml) for 20 min. Cells were lysed in cell lysis buffer containing 100 mM Tris (pH 7.4), 100 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1 mM NaF, 20 mM NaP2O4, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mM phenylmethylsulphonyl fluoride (PMSF) and protease inhibitors (one tablet per 10 ml; Roche, Indianapolis, IN, USA). Protein was measured using a BCA protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein (15 μg) were loaded and separated by SDS–PAGE and transferred onto nitrocellulose membranes (Bio-Rad, Richmond, CA, USA). Blots were probed using rabbit polyclonal antibodies specific for phospho-PKCδ isoforms, rabbit monoclonal phopho-JNK and rabbit polyclonal anti-β-actin. The immunoreactive protein bands were visualized by enhanced chemiluminescence. Densitometric analysis of the bands was performed using UN-SCAN-IT software, version 5.1 (Silk Scientific, Orem, UT, USA), and the data were analysed using Prism software (GraphPad Software, San Diego, CA, USA).
RNA extraction and quantitative RT–PCR
Total RNA was isolated from human RA synovial fibroblasts and rat ankles using RNAeasy mini RNA isolation kits in conjunction with QIAshredders (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol. Following isolation, RNA was quantified and checked for purity using a spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). cDNA was then prepared using a Reverse-IT MAX first-strand synthesis kit (Abgene, Rochester, NY, USA) as per the manufacturer’s protocol. The primer pairs used were based on published sequences [24–33] and are summarized in Table 1.
Table 1.
Sequences of the primer pairs used for chemokines and their receptors
| Genes | Species | Forward | Reverse |
|---|---|---|---|
| MCP-1/CCL2 | Human | 5′-TCCAGCATGAAAGTCTCTGC-3′ | 5′-TGGAATCCTGAACCCACTTC-3′ |
| RANTES/CCL5 | Human | 5′-AGCTACTCGGGAGGCTAAGG-3′ | 5′-GAGGCATGCTGACTTCCTTC-3′ |
| GROα/CXCL2 | Human | 5′-AGGGAATTCACCCCAAGAAC-3′ | 5′-CACCAGTGAGCTTCCTCCTC-3′ |
| IL-8/CXCL8 | Human | 5′-TTGGCAGCCTTCCTGATT-3′ | 5′-AACTTCTCCACAACCCTCTG-3′ |
| CCR1 | Human | 5′-ACGGAGGTGATCGCCTACAC-3′ | 5′-CGGAACCTGTCACCAACGAA-3′ |
| CCR2b | Human | 5′-GCTGGTCCTGCCGCTG-3′ | 5′-CACCAAGCAGGGTTTTCA-3′ |
| CCR5 | Human | 5′-GGTTGGGGTGGGATAGGG-3′ | 5′-TTTAAATGTAGAGGGGGATCCT-3′ |
| CXCR1 | Human | 5′-TCCTGGGAAATGACACAGCA-3′ | 5′-AAGCCAAAGGTGTGAGGCAG-3 ′ |
| CXCR2 | Human | 5′-GGGCAACAATACAGCAAACT-3′ | 5′-GCACTTAGGCAGGAGGTCTT-3′ |
| CCR1 | Rat | 5′-GGAGTTCACTCACCATACCTGTAG-3′ | 5′-GGTCCAGAGGAGGAAGAA-3′ |
| CCR2 | Rat | 5′-CGCAGAGTTGACAAGTTGTG-3′ | 5′-GCCATGGATGAACTGAGGTA-3′ |
| CCR5 | Rat | 5′-CACCCTGTTTCGCTGTAGGAATG-3′ | 5′-GCAGTGTGTCATCCCAAGAGTCTC-3′ |
| CXCR1 | Rat | 5′-CATCTTCCGCCAGGCATATAAA-3′ | 5′-GGGACAGACCACGCAATGTT-3′ |
| CXCR2 | Rat | 5′-CAGCAGTGTTCTGTTGCTAGCCT-3′ | 5′-CCAAGTGTCTCTTCTGGATCAGTGT-3′ |
Chemokine and chemokine receptor expression was measured by real-time RT–PCR in RA synovial fibroblasts and joints. β-actin was used as a housekeeping gene. RNA was isolated from RA synovial fibroblasts and rat joints using RNAeasy mini RNA isolation kits in conjunction with QIAshredders (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Following the isolation, RNA was quantified and checked for purity by spectrophotometry (Nanodrop Technologies, Wilmington, DE, USA). cDNA was then prepared using a Reverse-IT MAX first-strand synthesis kit, with anchored oligo-DT RNA primers (Abgene, Rochester, NY, USA) following the manufacturer’s protocol. Quantitative PCR (qPCR) was performed using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol, using specific primer sequences (Table 1). Diluted cDNA was mixed with Platinum SYBR Green qPCR SuperMix-UDG, forward and reverse primers specific for each gene (0.2 μM final concentrations), and incubated at the following cycles: 50°C for 2 min, 95°C for 2 min and 40 cycles of 95°C for 30 s, 55°C for 30 s and 68°C for 30 s using an Eppendorf Mastercycler ep realpex thermal cycler (Eppendorf, Hamburg, Germany). All samples were run in duplicate and analysed using Eppendorf software. Control reactions for product identification consisted of analysing the melting peaks (in degree celsius). A threshold to detect fluorescence above background is determined by the software. The level of expression of each mRNA and their estimated crossing points or cycle threshold (Ct) was determined relative to the standard preparation using the LightCycler computer software.
Relative gene expression was calculated using ΔCt method with β-actin as housekeeping gene 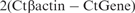 and the fold increase over mRNA levels of untreated cells was then determined.
and the fold increase over mRNA levels of untreated cells was then determined.
Statistical analysis
Statistical analysis to determine the effect of GTE treatment on RA synovial fibroblasts in vitro for chemokine production and chemokine receptor expression was performed using the Kruskall–Wallis one-way analysis of variance (ANOVA) test, followed by Dunnett’s multiple comparison post hoc test with P < 0.05 chosen as the level of significance. Data obtained from rat AIA clinical measurements, chemokine production and chemokine receptor expression were analysed using Student’s t-test with P < 0.05 between two groups was considered statistically significant.
Results
Composition of GTE
HPLC analysis showed that GTE contains ∼36% (w/w) of catechins, which included epigallocatechin (EGC, 11.5%), epicatechin (EC, 8%), epicatechin gallate (8%) and EGCG (5.9%).
Inhibition of IL-1β-induced RA synovial fibroblast chemokine production by GTE
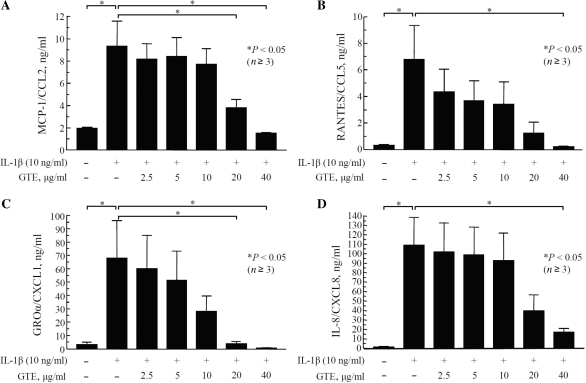
IL-1β is a potent inducer of chemokine production in RA synovial fibroblasts [18]. Excessive production of chemokines by RA synovial fibroblasts has been shown to induce proliferation of these cells and facilitate invasion into the adjacent tissues [7]. In the present study, stimulation of RA synovial fibroblasts with IL-1β (10 ng/ml) for 24 h resulted in a 5-, 21-, 21- and 96-fold induction of MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8, production, respectively, as compared with untreated controls (P < 0.05; n ⩾ 4). Pretreatment with GTE caused a significant, but differential, dose-dependent inhibition of IL-1β-induced MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 production. GTE at 40 μg/ml almost completely blocked IL-1β-induced MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 production in RA synovial fibroblasts (Fig. 1A–D). MTT assay to assess the viability of RA synovial fibroblasts showed that GTE (10–100 μg/ml) had no effect on the viability of cultured RA synovial fibroblasts at 24 h.
Fig. 1.
Inhibition of IL-1β-induced production of MCP-1/CCL2 (A), RANTES/CCL5 (B), GROα/CXCL2 (C) and IL-8/CXCL8 (D) by GTE in RA synovial fibroblasts.RA synovial fibroblasts (2 × 104/well) were incubated with GTE (0–40 μg/ml) for 12 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. The production of MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 in culture supernatants was measured using a commercially available ELISA kit. The values were mean and SEM. n: number of RA synovial fibroblast donors used.
GTE (2.5–40 μg/ml) inhibited IL-1β-induced MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8, production ranging from 9 to 84%, 36 to 97%, 12 to 99% and 6 to 84%, respectively. Interestingly, the inhibitory concentration 50% (IC50) values for MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 production by GTE were 23.0 (4.2), 6.6 (0.8), 9.1 (1.3) and 13.6 (3.5) μg/ml, respectively.
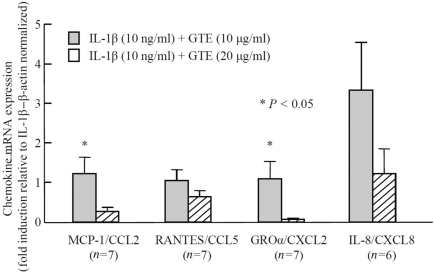
Transcriptional effect of GTE on IL-1β-induced chemokine production
To further strengthen our understanding of the chemokine inhibition, we studied the effect of the GTE on IL-1β-induced chemokine production at the mRNA level. In the present study, the stimulation of RA synovial fibroblasts with IL-1β (10 ng/ml) for 24 h resulted in a 290-, 2000-, 14 000- and 27 000-fold induction of MCP-1/CCL2, RANTES/CCL5,GROα/CXCL2 and IL-8/CXCL8 mRNA expression, respectively, as compared with untreated controls. Surprisingly, treatment with GTE differentially modulated the IL-1β-induced MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 mRNA expression (Fig. 2). GTE at 10 μg/ml had no effect on MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 transcription, when compared with IL-1β-treated values. GTE at 20 μg/ml inhibited MCP-1/CCL2 and GROα/CXCL2 mRNA synthesis by 73% (P < 0.05) and 94% (P < 0.01), respectively. A trend was observed for RANTES/CCL5 expression, which was inhibited by 36%. However, GTE at 10 μg/ml induced IL-8/CXCL8 mRNA synthesis (not significant, NS), whereas GTE at 20 μg/ml had no inhibitory effect on IL-8/CXCL8 mRNA synthesis. Overall, the results suggest that GTE possesses an ability to suppress chemokine production at the transcriptional level.
Fig. 2.
Modulation of IL-1β-induced MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 expression by GTE in RA synovial fibroblasts.RA synovial fibroblasts (2 × 105/well) were incubated with GTE (10–20 μg/ml) for 12 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. The mRNA expression of MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 was analysed by qRT–PCR. The values were normalized to their respective β-actin mRNA levels and expressed as mean and SEM. *P < 0.05 vs treatment with IL-1β alone. n: number of RA synovial fibroblast donors used.
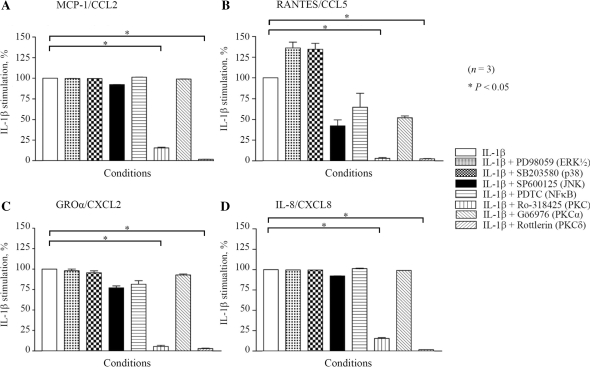
Role of PKCδ in IL-1β-induced chemokine production
To study the signalling pathways involved in IL-1β-induced production of chemokines, RA synovial fibroblasts were pretreated with different signalling inhibitors as described in detail in the ‘Materials and methods’ section. The conditioned medium was utilized to estimate the levels of MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8. The general PKC and the specific PKCδ inhibitors almost completely blocked IL-1β-induced MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 production in RA synovial fibroblasts (P < 0.001; Fig. 3A–D). In addition, we observed a trend in the inhibition of RANTES production by SP600125, a JNK inhibitor (Fig. 3B).
Fig. 3.
Involvement of PKCδ in the regulation of IL-1β-induced chemokine production.RA synovial fibroblasts (2 × 105/well) were pretreated with MAPK inhibitors (ERK½, PD98059; p38, SB203580; and JNK, SP600125; 10 μM), PKC inhibitors (general, Ro-318425; PKCα, Gö6976; and PKCδ, Rottlerin; 10 μM) and NF-κB inhibitor (PDTC; 200 μM) for 2 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h and processed for estimation of MCP-1/CCL2 (A), RANTES/CCL5 (B), GROα/CXCL2 (C) and IL-8/CXCL8 (D) production. Values are mean and SEM. *P < 0.05 vs treatment with IL-1β alone. n: number of RA synovial fibroblast donors used.
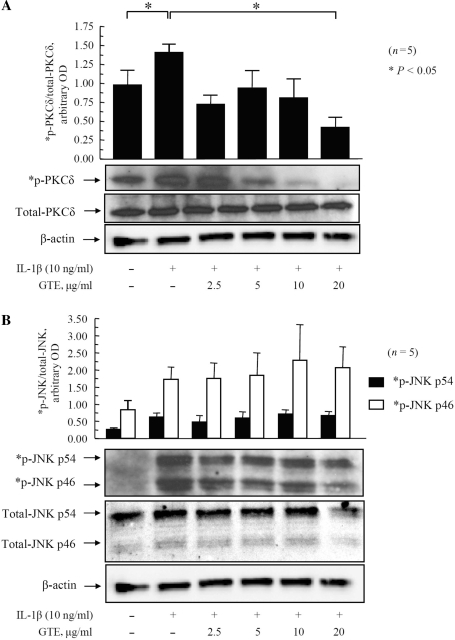
Modulation of PKCδ signalling pathways by GTE to inhibit IL-1β-induced chemokine production
In light of these observations, we evaluated the effect of GTE treatment on IL-1β-induced phosphorylation of PKCδ and JNK (Fig. 4A and B). Densitometric analysis of protein bands showed that IL-1β (10 ng/ml) increased phospho-PKCδ to almost 2-fold as compared with the untreated samples (P < 0.05; Fig. 4A). Treatment of RA synovial fibroblasts with GTE (2.5–20 μg/ml) caused a dose-dependent inhibition in the expression of phospho-PKCδ ranging from 43 to 73% (P < 0.05 for GTE 5, 10 and 20 μg/ml; Fig. 4A). Similarly, IL-1β (10 ng/ml) increased the expression of phospho-JNKp46 and -p54 to almost ∼3-fold as compared with the untreated samples (P < 0.05 for phospho-JNKp54; Fig. 4B). Normalization of the phosphorylated p46 and p54 isoforms of JNK with their respective total p46 and p54 protein levels showed that GTE had no significant inhibitory effect on any of the JNK isoforms as compared with IL-1β-treated levels (Fig. 4B). These results provide evidence that GTE may regulate chemokine production predominantly via the PKCδ pathway in RA synovial fibroblasts.
Fig. 4.
Inhibition of IL-1β-induced PKCδ phosphorylation by GTE in RA synovial fibroblasts.RA synovial fibroblasts (2 × 105/well) were treated with GTE (2.5–20 μg/ml) for 12 h, followed by stimulation with IL-1β (10 ng/ml) for 20 min. Cells were lysed in extraction buffer containing protease inhibitors, and the total and phosphorylated (p) PKCδ (A) and JNK (B) were determined by western blotting. A representative blot for each protein is shown. Equal loading of protein was verified by re-probing the blots for β-actin. Values are mean and SEM of RA synovial fibroblasts obtained from six different donors. *P < 0.05 vs treatment with IL-1β. OD: optical density; n: number of RA synovial fibroblast donors used.
GTE-induced chemokine receptor expression in vitro
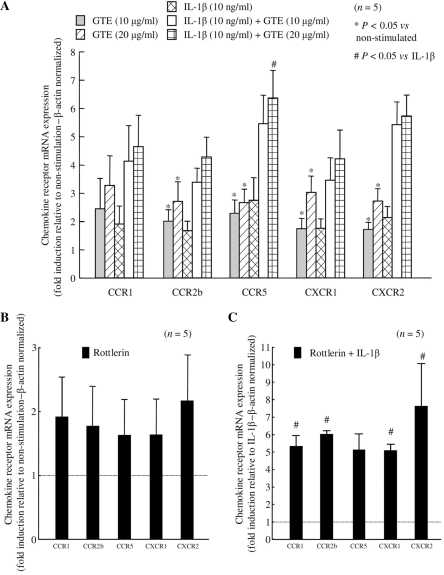
Since chemokines execute their detrimental effects via their respective chemokine receptors, we extended our study and tested the effect of GTE on constitutive and induced CC/CXC receptor expression by qRT–PCR. RA synovial fibroblasts were pre-incubated with GTE (10 and 20 μg/ml) for ∼12 h, followed by stimulation with or without IL-1β (10 ng/ml) for 24 h. The qRT–PCR analysis showed that GTE was able to induce CCR1, CCR2b, CCR5, CXCR1 and CXCR2 dose-dependent expression (n ⩾ 5; P < 0.05 for CCR2b, -5, CXCR1 and -2 at 20 μg/ml). IL-1β had no significant effect on any of the RA synovial fibroblast CCR or CXCR expression, whereas GTE pre-incubation further enhanced the IL-1β-induced expression of these chemokine receptors. These results suggest that GTE, by enhancing receptor expression, may enhance the binding of CC/CXC chemokines to their receptors, thereby reducing the amounts of these chemokines available to induce chemotaxis (Fig. 5A).
Fig. 5.
Enhancement of IL-1β-induced CCR1, CCR2b, CCR5, CXCR1 and CXCR2 mRNA expression by GTE in RA synovial fibroblasts.(A) RA synovial fibroblasts (2 × 105/well) were incubated with or without GTE (10–20 μg/ml) for 12 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. The mRNA expression of CCR1, CCR2b, CCR5, CXCR1 and CXCR2 was analysed by qRT–PCR. (B) RA synovial fibroblasts (2 × 105/well) were incubated with or without Rottlerin (10 μM) for 25 h. (C) RA synovial fibroblasts (2 × 105/well) were incubated with or without Rottlerin (10 μM) for 1 h, followed by stimulation with IL-1β (10 ng/ml) for 24 h. The mRNA expression of CCR1, CCR2b, CCR5, CXCR1 and CXCR2 was analysed by qRT–PCR. The values were normalized to the respective β-actin mRNA levels and expressed as mean and SEM. *P < 0.05 vs treatment with IL-1β alone. n: number of RA synovial fibroblast donors used.
PKCδ inhibitor mimics GTE in increasing chemokine receptor expression
To further validate the utility of GTE-mediated increase in CC/CXC receptor expression, we tested the effect of the PKCδ inhibitor on constitutive and IL-1β-induced CC/CXC chemokine receptor mRNA expression. The qRT–PCR analysis showed that PKCδ blockade enhanced CC/CXC receptor expression by ∼2-fold as compared with untreated levels (NS; Fig. 5B). We also observed 5-fold further synergistic induction in CC/CXC receptor expression by the PKCδ inhibitor when cells were treated with IL-1β (P < 0.05 for CCR1, CCR2b, CXCR1 and CXCR2; Fig. 5C). These results suggest that GTE might be mediating its effect on CC/CXC receptors via modulation of these signalling pathways.
GTE administration ameliorates rat AIA
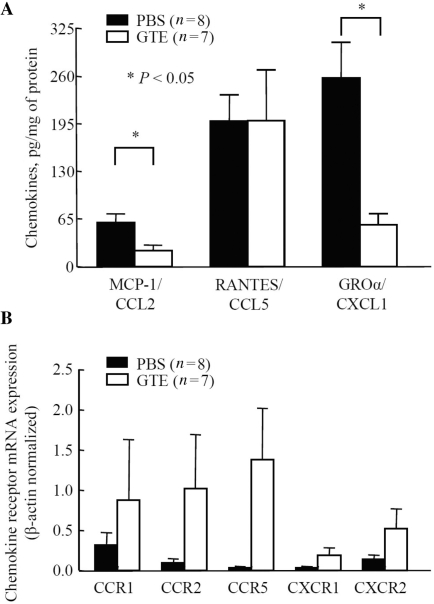
We utilized a rat AIA model in which chemokine production and chemokine receptor expression peaks in the arthritic joints around Day 18, which coincides with maximum inflammation and joint destruction [19, 34]. To study in vivo modulation of CC/CXC chemokines by GTE in rats, a daily dose (200 mg/kg/oral gavage) was administered starting from Day 7 to 16. Interestingly, the evaluation of RANTES/CCL5, MCP-1/CCL2 and GROα/CXCL2 levels in the joint homogenates of animal sacrificed at Day 17 showed that GTE treatment significantly inhibited MCP-1/CCL2 and GROα/CXCL2 levels by almost ∼64 and ∼78%, respectively (P < 0.05 for both), with no change in the level of RANTES/CCL5, when compared with the PBS group (Fig. 6A). However, the qRT–PCR analysis showed that GTE administration was able to induce CCR1, CCR2b, CCR5 and CXCR1 expression in the ankles of the GTE administered group compared with the PBS group (Fig. 6B).
Fig. 6.
GTE administration suppresses chemokine production, but up-regulates chemokine receptor mRNA expression, in rat AIA joints.(A) MCP-1/CCL2, RANTES/CCL5 and GROα/CXCL2 levels in joint homogenates were determined by ELISA. Values represent the mean ± SEM of the chemokine concentration in the joints from eight or seven rats after normalization to their respective protein values. (B) The mRNA expression of CCR1, CCR2b, CCR5 and CXCR1 was analysed in rat joints from GTE- or PBS-administered groups. The values were normalized to the respective β-actin mRNA levels. Values shown are mean ± SEM of the joints from eight rats (PBS group) or seven rats (GTE group).
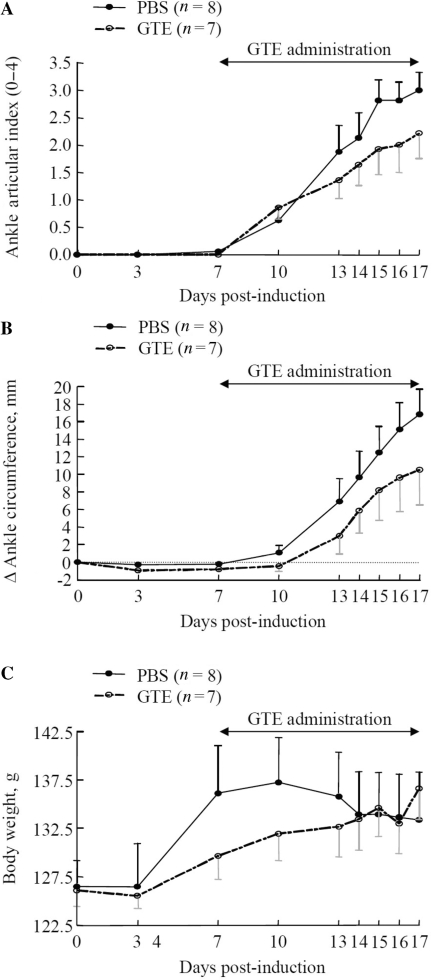
In GTE-fed animals, we also observed a reduction in the clinical measurements of joint inflammation and arthritis (Fig. 7A–C). The articular index and the Δ circumference of the ankles in GTE administered rats was ∼28% (P = 0.212) and ∼38% (P = 0.187) lower, respectively, than the PBS group at Day 17 (Fig. 7A and B) by Day 17. The level of significance (P < 0.05) could not be achieved in this experiment, which may be attributed to several contributing factors including the late start of the dosing regimen, the number of animals (n) in the study and the day of termination of the study for evaluation. Nonetheless, the present study clearly suggests that consumption of GTE during the development of AIA in rats was able to retard the course of the disease in GTE-administered rats when compared with the PBS group. Importantly, the trend of CCR and CXCR receptor expression in human RA synovial fibroblasts was similar to that observed in rat AIA, suggesting a potential mechanism by which GTE may suppress inflammation and joint destruction in RA. In addition, GTE (200 mg/kg) administration showed no adverse effect on the body weight gain of the treated animals (Fig. 7C). Overall, these results suggest that GTE ameliorates rat AIA by preferentially down-regulating the concentrations of chemokines and enhancing the expression of chemokine receptors in the joints.
Fig. 7.
GTE ameliorates AIA in rats.(A and B) GTE (200 mg/kg/day, p.o.) was administered daily from Day 7 to 16. Articular index scores were averaged for each animal and Δ ankle circumferences (minus Day 0) values were averaged for each animal before group comparisons and final values represented as the mean of ‘n’ number of animals per group. Mean ± SEM is shown for ankle articular index scores (range 0–4) and ankle Δ circumferences (in millimetres) determined on indicated days after adjuvant injection. (C) The mean ± SEM increases in body weight measured on the indicated days after adjuvant injection. n: number of animals used for the clinical assessment. Details are provided in the ‘Materials and methods’ section.
Discussion
In the present study, we provide evidence of the ability of GTE to differentially regulate chemokine production and chemokine receptor expression in human RA synovial fibroblasts and rat AIA. These results suggest that GTE may play an important role in modifying the responses of RA synovial fibroblasts towards stimuli generated by cytokines and chemokines.
CC/CXC chemokines are elevated in RA SFs when compared with the other joint disorders [35, 36]. MCP-1/CCL2 and RANTES/CCL5 are the members of the CC subfamily and have been shown to chemoattract lymphocytes and monocytes [37], whereas GROα/CXCL2 and IL-8/CXCL8 are the members of the CXC chemokine sub-group that possess both potent chemoattractant and neutrophil activation functions [8, 38]. These chemokines are produced by RA synovial fibroblasts and are up-regulated upon stimulation with IL-1β [7, 39–41]. In animal models of arthritis, the expression of these chemokines generally coincides with the onset of clinical symptoms [42–45], and we targeted this by GTE administration. In the present study, GTE significantly inhibited the expression of MCP-1/CCL2 and GROα/CXCL2 in rat AIA. Additionally, GTE blocked IL-1β-induced MCP-1/CCL2, RANTES/CCL5, GROα/CXCL2 and IL-8/CXCL8 production by RA synovial fibroblasts in a concentration-dependent manner. When we explored the effect of GTE at mRNA level in RA synovial fibroblasts, we observed a paradoxical effect of GTE on IL-8/CXCL8 expression. Previously, GTE was described to specifically inhibit IL-8/CXCL8 secretion while inducing de novo synthesis of IL-8/CXCL8 in a human gastrointestinal cell line [46], suggesting differences in its regulation between transcription and translation events. A similar mechanism could explain the fact that in RA synovial fibroblasts, GTE decreased IL-8/CXCL8 production in culture supernatants, but not at the mRNA level.
In the present study, we confirmed at the mRNA level that CC/CXC receptors were not restricted to T cells, but were also expressed by RA synovial fibroblasts as previously described by PCR and flow cytometry [47, 48]. However, previous data supported the notion that chemokine receptors expressed by RA synovial fibroblasts are very stable despite stimulation with TNF-α, IL-1β and IFN-γ over 24 h [47]. In addition, our data show that IL-1β (10 ng/ml) is also capable of modestly inducing CCR5 and CXCR2. In the light of the present findings, there are two potential mechanisms that could be suggested for these unique differential effects induced by GTE. First, by inhibiting chemokine synthesis, GTE directly reduces the chemokine pool available for CC/CXC receptor binding. Secondly, by increasing chemokine receptor expression, GTE maximizes chemokine ligand–receptor binding that consequently reduces the amount of free chemokine available for binding to leucocytes or monocytes/macrophages, thus acting as a chemokine trap and reducing the chemotactic environment [1]. However, this explanation warrants further testing of the effect of GTE on other cell types that are present in the joint that may contribute to chemotactic activity and angiogenesis [1, 28].
Furthermore, we observed that GTE reduced IL-1β-induced chemokine production at 24 h, which is largely attributed to the decreased expression of chemokines relevant to RA [1]. In the present study, GTE also blocked IL-1β-induced phosphorylation of PKCδ. We extended our previous observation demonstrating that the regulation of PKCδ plays an important role in controlling IL-1β-induced chemokine production and chemokine receptor expression in RA synovial fibroblasts [7], and that GTE possesses the ability to control their expression by targeting the PKCδ pathway in RA synovial fibroblasts. However, RANTES production was also reduced by a JNK inhibitor, which may suggest complexity of the involvement of multiple pathways in chemokine regulation. Data from animal models suggest that blockade of chemokine receptors may suppress arthritis by inhibiting neutrophil migration to inflamed joints [49]. However, the acceleration of arthritis in chemokine receptor knockout animals suggests their regulatory role in the development of arthritis, which warrants further investigation [50].
The concentration of GTE used to inhibit chemokine production and chemokine receptor expression may be achieved by consuming GTE as a dietary supplement and may possibly have a preventative effect in regulating the course and clinical outcomes of RA in humans. On this line of investigation, it will be interesting to suggest future studies to evaluate the influence of dietary consumption of GTE on first-line conventional treatment options for RA such as MTX or other DMARDs for the possible better clinical outcome in animal models of arthritis. Further studies may be designed to improve the clinical outcome in animal models of RA through modification of the dose, and frequency of GTE administration may provide a better outcome and benefits of GTE in RA. In conclusion, the novel findings of this study are that GTE inhibits CC/CXC chemokine production, but up-regulates CC/CXCR chemokine receptor expression, in both RA synovial fibroblasts and rat AIA. Hence, it is suggested that the prophylactic use of GTE alone or as an adjunct treatment for human RA may be a beneficial therapeutic approach.

Acknowledgements
The authors thank the National Disease Research Interchange for providing RA synovial tissues. S.A. designed and participated in this study. H.M. and S.A. performed experiments and wrote the manuscript. H.M., J.H.R., P.L.C. and S.A. participated in animal studies. A.E.K. participated in writing the manuscript and provided her support to the study. All the authors were involved in drafting/revising the manuscript and have read and approved the version of the manuscript for publication.
Funding: This study was supported by the NIH grants AT-003633, AR-055741 and AR-48267, the Frederick G. L. Huetwell and William D. Robinson, M.D. Professorship in Rheumatology, the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, the French Society of Rheumatology, the French Rheumatologist Network, Lavoisier and the Philippe Foundation.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Koch AE. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis Rheum. 2005;52:710–21. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- 2.van Vollenhoven RF, Klareskog L. Clinical responses to tumor necrosis factor alpha antagonists do not show a bimodal distribution: data from the Stockholm tumor necrosis factor alpha followup registry. Arthritis Rheum. 2003;48:1500–3. doi: 10.1002/art.11027. [DOI] [PubMed] [Google Scholar]
- 3.Feltelius N, Fored CM, Blomqvist P, et al. Results from a nationwide postmarketing cohort study of patients in Sweden treated with etanercept. Ann Rheum Dis. 2005;64:246–52. doi: 10.1136/ard.2004.023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marotte H, Pallot-Prades B, Grange L, et al. The shared epitope is a marker of severity associated with selection for, but not with response to, infliximab in a large rheumatoid arthritis population. Ann Rheum Dis. 2006;65:342–7. doi: 10.1136/ard.2005.037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacon K, Baggiolini M, Broxmeyer H, et al. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067–8. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- 6.Szekanecz Z, Koch AE. Therapeutic inhibition of leukocyte recruitment in inflammatory diseases. Curr Opin Pharmacol. 2004;4:423–8. doi: 10.1016/j.coph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed S, Pakozdi A, Koch AE. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2006;54:2393–401. doi: 10.1002/art.22023. [DOI] [PubMed] [Google Scholar]
- 8.Unemori EN, Amento EP, Bauer EA, Horuk R. Melanoma growth-stimulatory activity/GRO decreases collagen expression by human fibroblasts. Regulation by C-X-C but not C-C cytokines. J Biol Chem. 1993;268:1338–42. [PubMed] [Google Scholar]
- 9.Shahrara S, Proudfoot AE, Woods JM, et al. Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 2005;52:1907–19. doi: 10.1002/art.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson Z, Power CA, Weiss C, et al. Chemokine inhibition—why, when, where, which and how? Biochem Soc Trans. 2004;32:366–77. doi: 10.1042/bst0320366. [DOI] [PubMed] [Google Scholar]
- 11.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 13.Cooper R, Morre DJ, Morre DM. Medicinal benefits of green tea: Part I. Review of noncancer health benefits. J Altern Complement Med. 2005;11:521–8. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- 14.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–84S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 15.Akai Y, Nakajima N, Ito Y, Matsui T, Iwasaki A, Arakawa Y. Green tea polyphenols reduce gastric epithelial cell proliferation and apoptosis stimulated by Helicobacter pylori infection. J Clin Biochem Nutr. 2007;40:108–15. doi: 10.3164/jcbn.40.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129:173–9. doi: 10.1016/s0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 17.Oak MH, El Bedoui J, Schini-Kerth VB. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem. 2005;16:1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Haqqi TM, Anthony DD, Gupta S, et al. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci USA. 1999;96:4524–9. doi: 10.1073/pnas.96.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed S, Marotte H, Kwan K, et al. Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production. Proc Natl Acad Sci USA. 2008;105:14692–7. doi: 10.1073/pnas.0802675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Woods JM, Katschke KJ, Volin MV, et al. IL-4 adenoviral gene therapy reduces inflammation, proinflammatory cytokines, vascularization, and bony destruction in rat adjuvant-induced arthritis. J Immunol. 2001;166:1214–22. doi: 10.4049/jimmunol.166.2.1214. [DOI] [PubMed] [Google Scholar]
- 22.Tatsuo MA, Carvalho WM, Silva CV, Miranda AE, Ferreira SH, Francischi JN. Analgesic and antiinflammatory effects of dipyrone in rat adjuvant arthritis model. Inflammation. 1994;18:399–405. doi: 10.1007/BF01534437. [DOI] [PubMed] [Google Scholar]
- 23.Chengelis CP, Kirkpatrick JB, Regan KS, et al. 28-Day oral (gavage) toxicity studies of green tea catechins prepared for beverages in rats. Food Chem Toxicol. 2008;46:978–89. doi: 10.1016/j.fct.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Marin V, Farnarier C, Gres S, et al. The p38 mitogen-activated protein kinase pathway plays a critical role in thrombin-induced endothelial chemokine production and leukocyte recruitment. Blood. 2001;98:667–73. doi: 10.1182/blood.v98.3.667. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Wang D, Wang Y, Xu Q. Simvastatin down regulates mRNA expression of RANTES and CCR5 in posttransplant renal recipients with hyperlipidemia. Transplant Proc. 2006;38:2899–904. doi: 10.1016/j.transproceed.2006.08.136. [DOI] [PubMed] [Google Scholar]
- 26.Nomiyama H, Otsuka-Ono K, Miura R, et al. Identification of a novel CXCL1-like chemokine gene in macaques and its inactivation in hominids. J Interferon Cytokine Res. 2007;27:32–7. doi: 10.1089/jir.2007.0099. [DOI] [PubMed] [Google Scholar]
- 27.Patel L, Charlton SJ, Chambers JK, Macphee CH. Expression and functional analysis of chemokine receptors in human peripheral blood leukocyte populations. Cytokine. 2001;14:27–36. doi: 10.1006/cyto.2000.0851. [DOI] [PubMed] [Google Scholar]
- 28.Cheong JY, Cho SW, Choi JY, et al. RANTES, MCP-1, CCR2, CCR5, CXCR1 and CXCR4 gene polymorphisms are not associated with the outcome of hepatitis B virus infection: results from a large scale single ethnic population. J Korean Med Sci. 2007;22:529–35. doi: 10.3346/jkms.2007.22.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidemann J, Ogawa H, Dwinell MB, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 30.Cowell RM, Silverstein FS. Developmental changes in the expression of chemokine receptor CCR1 in the rat cerebellum. J Comp Neurol. 2003;457:7–23. doi: 10.1002/cne.10554. [DOI] [PubMed] [Google Scholar]
- 31.Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci Lett. 2004;355:236–40. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Cowell RM, Xu H, Parent JM, Silverstein FS. Microglial expression of chemokine receptor CCR5 during rat forebrain development and after perinatal hypoxia-ischemia. J Neuroimmunol. 2006;173:155–65. doi: 10.1016/j.jneuroim.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Valles A, Grijpink-Ongering L, de Bree FM, Tuinstra T, Ronken E. Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol Dis. 2006;22:312–22. doi: 10.1016/j.nbd.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Shahrara S, Amin MA, Woods JM, Haines GK, Koch AE. Chemokine receptor expression and in vivo signaling pathways in the joints of rats with adjuvant-induced arthritis. Arthritis Rheum. 2003;48:3568–83. doi: 10.1002/art.11344. [DOI] [PubMed] [Google Scholar]
- 35.Erdem H, Pay S, Serdar M, et al. Different ELR (+) angiogenic CXC chemokine profiles in synovial fluid of patients with Behcet’s disease, familial Mediterranean fever, rheumatoid arthritis, and osteoarthritis. Rheumatol Int. 2005;26:162–7. doi: 10.1007/s00296-004-0524-3. [DOI] [PubMed] [Google Scholar]
- 36.Hosaka S, Akahoshi T, Wada C, Kondo H. Expression of the chemokine superfamily in rheumatoid arthritis. Clin Exp Immunol. 1994;97:451–7. doi: 10.1111/j.1365-2249.1994.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 38.Koch AE, Kunkel SL, Harlow LA, et al. Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis. J Clin Invest. 1994;94:1012–8. doi: 10.1172/JCI117414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volin MV, Campbell PL, Connors MA, Woodruff DC, Koch AE. The effect of sulfasalazine on rheumatoid arthritic synovial tissue chemokine production. Exp Mol Pathol. 2002;73:84–92. doi: 10.1006/exmp.2002.2460. [DOI] [PubMed] [Google Scholar]
- 40.Koch AE, Kunkel SL, Shah MR, et al. Growth-related gene product alpha. A chemotactic cytokine for neutrophils in rheumatoid arthritis. J Immunol. 1995;155:3660–6. [PubMed] [Google Scholar]
- 41.Koch AE, Kunkel SL, Harlow LA, et al. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90:772–9. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szekanecz Z, Halloran MM, Volin MV, et al. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:1266–77. doi: 10.1002/1529-0131(200006)43:6<1266::AID-ANR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 43.Thornton S, Duwel LE, Boivin GP, Ma Y, Hirsch R. Association of the course of collagen-induced arthritis with distinct patterns of cytokine and chemokine messenger RNA expression. Arthritis Rheum. 1999;42:1109–18. doi: 10.1002/1529-0131(199906)42:6<1109::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Matsukawa A, Yoshimura T, Fujiwara K, Maeda T, Ohkawara S, Yoshinaga M. Involvement of growth-related protein in lipopolysaccharide-induced rabbit arthritis: cooperation between growth-related protein and IL-8, and interrelated regulation among TNFα, IL-1, IL-1 receptor antagonist, IL-8, and growth-related protein. Lab Invest. 1999;79:591–600. [PubMed] [Google Scholar]
- 45.Halloran MM, Woods JM, Strieter RM, et al. The role of an epithelial neutrophil-activating peptide-78-like protein in rat adjuvant-induced arthritis. J Immunol. 1999;162:7492–500. [PubMed] [Google Scholar]
- 46.Netsch MI, Gutmann H, Aydogan C, Drewe J. Green tea extract induces interleukin-8 (IL-8) mRNA and protein expression but specifically inhibits IL-8 secretion in caco-2 cells. Planta Med. 2006;72:697–702. doi: 10.1055/s-2006-931597. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Vicuna R, Gomez-Gaviro MV, Dominguez-Luis MJ, et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004;50:3866–77. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- 48.Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol. 2001;167:5381–5. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- 49.Coelho FM, Pinho V, Amaral FA, et al. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis Rheum. 2008;58:2329–37. doi: 10.1002/art.23622. [DOI] [PubMed] [Google Scholar]
- 50.Quinones MP, Estrada CA, Kalkonde Y, et al. The complex role of the chemokine receptor CCR2 in collagen-induced arthritis: implications for therapeutic targeting of CCR2 in rheumatoid arthritis. J Mol Med. 2005;83:672–81. doi: 10.1007/s00109-005-0637-5. [DOI] [PubMed] [Google Scholar]