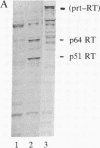
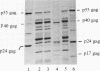
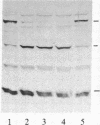
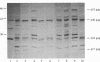
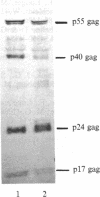
Abstract
The protease encoded by the human immunodeficiency virus (HIV) processes the viral gag and gag-pol protein precursor by posttranslational cleavage. In this study we have demonstrated by site-specific mutagenesis (Asp----Thr) and by pepstatin A inhibition that the recombinant HIV protease is an aspartic-type protease. Furthermore, incubation of HIV-infected H9 cells with pepstatin A inhibited part of the intracellular processing of the HIV gag protein yet had no apparent toxicity on HIV-infected cells during 48 hr of incubation.
Full text
PDF

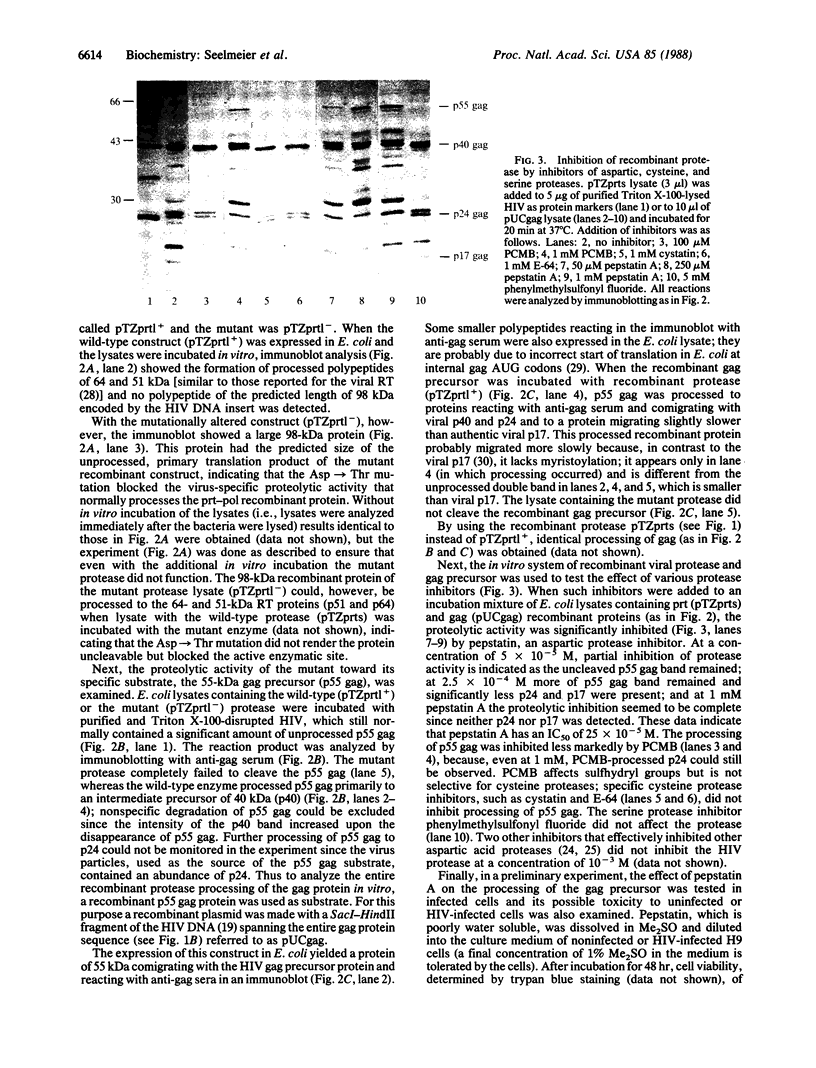
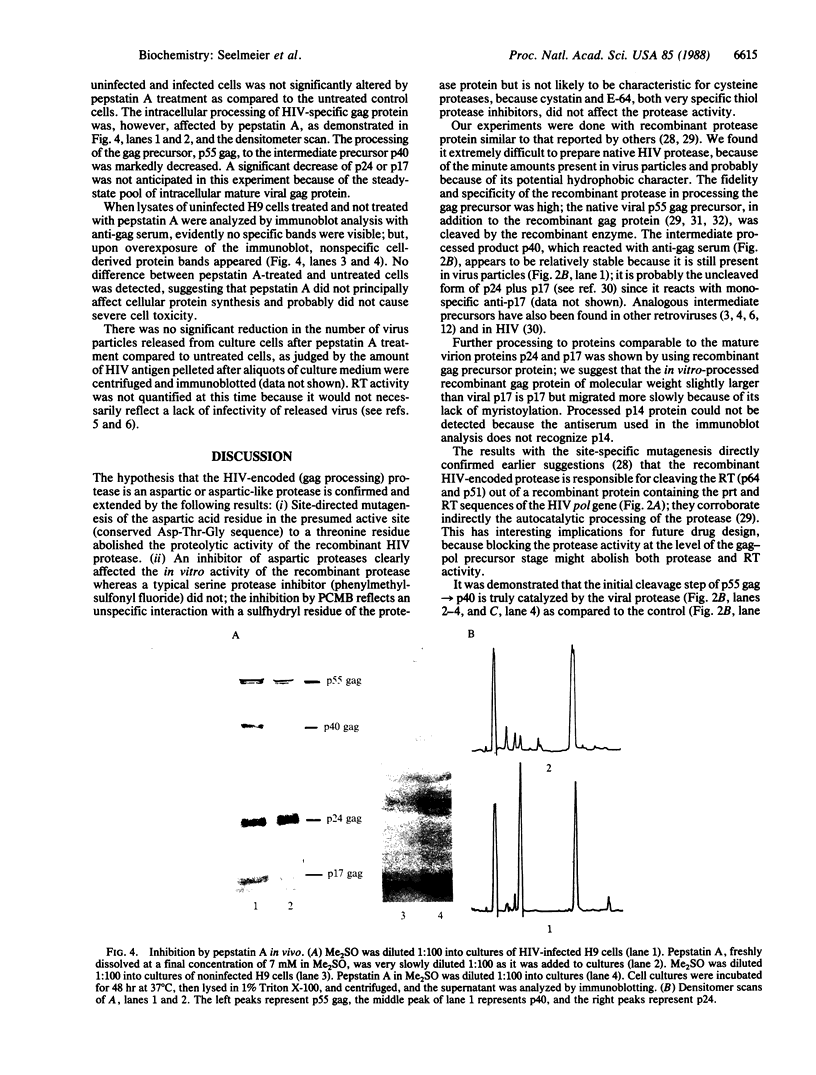

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Sonigo P., Barré-Sinoussi F., Chermann J. C., Tiollais P., Montagnier L., Wain-Hobson S. Molecular cloning of lymphadenopathy-associated virus. Nature. 1984 Dec 20;312(5996):757–760. doi: 10.1038/312757a0. [DOI] [PubMed] [Google Scholar]
- Bonnevie O., Svendsen L. B., Holst-Christensen J., Johansen T. S., Søltoft J., Christiansen P. M. Double-blind randomised clinical trial of a pepsin-inhibitory pentapeptide (pepstatin) in the treatment of duodenal ulcer. Gut. 1979 Jul;20(7):624–628. doi: 10.1136/gut.20.7.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. A deletion mutation in the 5' part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol. 1985 Mar;53(3):899–907. doi: 10.1128/jvi.53.3.899-907.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck C., Gorniak J. G., Strickler J. E., Meek T. D., Metcalf B. W., Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmerie W. G., Loeb D. D., Casavant N. C., Hutchison C. A., 3rd, Edgell M. H., Swanstrom R. Expression and processing of the AIDS virus reverse transcriptase in Escherichia coli. Science. 1987 Apr 17;236(4799):305–308. doi: 10.1126/science.2436298. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Greenbaum L. M., Grebow P., Johnston M., Prakash A., Semente G. Pepstatin, an inhibitor of leukokinin formation and ascitic fluid accumulation. Cancer Res. 1975 Mar;35(3):706–710. [PubMed] [Google Scholar]
- Katoh I., Yasunaga T., Ikawa Y., Yoshinaka Y. Inhibition of retroviral protease activity by an aspartyl proteinase inhibitor. Nature. 1987 Oct 15;329(6140):654–656. doi: 10.1038/329654a0. [DOI] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Rein A., Shibuya M., Odaka T., Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity. Virology. 1985 Sep;145(2):280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline sarcoma virus-coded polyprotein: enzymatic cleavage by a type C virus-coded structural protein. J Virol. 1979 Feb;29(2):649–656. doi: 10.1128/jvi.29.2.649-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. A., Schaber M. D., Skalka A. M., Ganguly K., Wong-Staal F., Reddy E. P. HTLV-III gag protein is processed in yeast cells by the virus pol-protease. Science. 1986 Mar 28;231(4745):1580–1584. doi: 10.1126/science.2420008. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Von der Helm K. Characterization of a virus-specific proteolytic activity processing the gag precursor of the simian sarcoma-associated virus. Virology. 1987 Feb;156(2):246–252. doi: 10.1016/0042-6822(87)90404-1. [DOI] [PubMed] [Google Scholar]
- Madisen L., Travis B., Hu S. L., Purchio A. F. Expression of the human immunodeficiency virus gag gene in insect cells. Virology. 1987 May;158(1):248–250. doi: 10.1016/0042-6822(87)90262-5. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigaki T., Sugawara K., Ito Y. Effect of protease inhibitors on the activation of Epstein-Barr virus repressed in cultured lymphoid cells. Intervirology. 1981;16(1):49–52. doi: 10.1159/000149247. [DOI] [PubMed] [Google Scholar]
- Mous J., Heimer E. P., Le Grice S. F. Processing protease and reverse transcriptase from human immunodeficiency virus type I polyprotein in Escherichia coli. J Virol. 1988 Apr;62(4):1433–1436. doi: 10.1128/jvi.62.4.1433-1436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl L. H., Taylor W. R. A structural model for the retroviral proteases. Nature. 1987 Sep 24;329(6137):351–354. doi: 10.1038/329351a0. [DOI] [PubMed] [Google Scholar]
- Pohl J., Zaoral M., Jindra A., Jr, Kostka V. Purification of pepsins and cathepsin D by affinity chromatography on Sepharose 4B with an immobilized synthetic inhibitor. Anal Biochem. 1984 Jun;139(2):265–271. doi: 10.1016/0003-2697(84)90001-0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Szelke M., Leckie B., Hallett A., Jones D. M., Sueiras J., Atrash B., Lever A. F. Potent new inhibitors of human renin. Nature. 1982 Oct 7;299(5883):555–557. doi: 10.1038/299555a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk V., Brzin J., Longer M., Ritonja A., Eropkin M., Borchart U., Machleidt W. Protein inhibitors of cysteine proteinases. III. Amino-acid sequence of cystatin from chicken egg white. Hoppe Seylers Z Physiol Chem. 1983 Nov;364(11):1487–1496. doi: 10.1515/bchm2.1983.364.2.1487. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Morishima H., Matsuzaki M., Hamada M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot (Tokyo) 1970 May;23(5):259–262. doi: 10.7164/antibiotics.23.259. [DOI] [PubMed] [Google Scholar]
- Veronese F. D., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Biochemical and immunological analysis of human immunodeficiency virus gag gene products p17 and p24. J Virol. 1988 Mar;62(3):795–801. doi: 10.1128/jvi.62.3.795-801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Translational readthrough of an amber termination codon during synthesis of feline leukemia virus protease. J Virol. 1985 Sep;55(3):870–873. doi: 10.1128/jvi.55.3.870-873.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. A comparison of avian and murine retrovirus polyprotein cleavage activities. Virology. 1981 May;111(1):239–250. doi: 10.1016/0042-6822(81)90668-1. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- Yuasa Y., Shimojo H., Aoyagi T., Umezawa H. Effect of protease inhibitors on focus formation by murine sarcoma virus. J Natl Cancer Inst. 1975 May;54(5):1255–1256. doi: 10.1093/jnci/54.5.1255. [DOI] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]