Abstract
Introduction
Hyponatremia frequently complicates acute brain injury and may precipitate neurological worsening by promoting cerebral edema. An increase in brain water may be better managed through water excretion than with fluid restriction or hypertonic fluids. Vasopressin-receptor antagonists such as conivaptan, which promote free water excretion, may be ideal agents to treat this common and potentially serious disorder.
Methods
The efficacy of intermittent bolus doses of conivaptan to correct hyponatremia was examined in a consecutive series of patients treated in our neurointensive care unit. Patients were excluded if baseline sodium was over 135 mEq/l or if another conivaptan dose was given within 12 h. We assessed the proportion responding with a 4 or 6 mEq/l rise in sodium by 12 h, the change in sodium from baseline, and, in those not receiving another dose for at least 72 h, the long-term ability of a single dose to maintain sodium at least 4 mEq/l above baseline. We also recorded the effects of conivaptan on urine output and specific gravity, and noted any adverse events.
Results
A total of 25 doses given to 19 patients were included (out of 44 total doses administered in the study period). Sodium rose by 5.8 ± 3.2 mEq/l within 12 h, with 71% rising by at least 4 mEq/l and 52% manifesting at least a 6 mEq/l increase. In those receiving only a single dose, 69% maintained at least a 4 mEq/l rise up to 72 h. Conivaptan also consistently led to increased urine output and a significant drop in urine specific gravity (i.e., aquaresis). No cases of phlebitis were observed despite administration of conivaptan through peripheral IVs.
Conclusion
Intermittent dosing of conivaptan was effective in increasing free water excretion and correcting hyponatremia in neurologically ill patients. This supports its further evaluation for managing hyponatremia in this population.
Keywords: Hyponatremia, Inappropriate ADH syndrome, Sodium, Conivaptan, SIADH, Vasopressin receptors
Introduction
Hyponatremia is a frequent complication of acute brain injury, usually related to the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) [1–4]. In SIADH, the release of ADH (vasopressin) is not inhibited when plasma osmolality falls, resulting in excessive water retention in the renal tubules [5]. This abnormal water handling reduces extracellular tonicity, creating an osmotic gradient that promotes the shift of water into brain cells [6, 7]. This increase in brain water may worsen cerebral edema and precipitate neurological deterioration, especially in those with acute brain injury [8]. Unless it is corrected promptly and effectively, hyponatremia may increase morbidity for patients with acute neurological disorders by increasing the risk of seizures, elevations in intracranial pressure, and herniation [9].
Current options for the management of hyponatremia include fluid restriction, hypertonic saline, mineralocorticoids, and osmotic diuretics [10]. Fluid restriction alone may be insufficient to rapidly normalize serum sodium in symptomatic patients and, by promoting hypovolemia, can increase the risk of cerebral ischemia in patients with subarachnoid hemorrhage (SAH) [11, 12]. Administration of hypertonic saline may require central venous access and does not address the fundamental water imbalance that underlies euvolemic hyponatremia; rapid overcorrection and risk of osmotic demyelination is also a major concern [13, 14]. Fludrocortisone acetate enhances sodium retention through its mineralocorticoid activity. However, this agent has shown only limited efficacy in correcting hyponatremia and may precipitate volume overload through concurrent water retention [15, 16]. Osmotic diuretics such as mannitol lead to not only water but also significant urinary electrolyte losses, and can worsen volume depletion [17]. None of these agents promote renal-free water excretion and oppose the underlying pathophysiology of dilutional hyponatremia in SIADH.
Conivaptan (Vaprisol, Astellas Pharma) is a V1A- and V2 (vasopressin)-receptor antagonist [18]. In SIADH, vasopressin binding to the V2 receptors in the renal collecting ducts promotes free water reabsorption and, by antagonizing these receptors, conivaptan promotes water excretion while sparing electrolytes including sodium. This process, known as aquaresis, results in an increased serum sodium and, most importantly, does so through reversing the underlying pathophysiology of SIADH [19–21]. Conivaptan has been shown to increase water clearance and serum sodium when administered to patients with chronic SIADH [22, 23].
While the approved dosing for conivaptan is a 20 mg bolus followed by 20 mg/day continuous infusion over 1–4 days [24], intermittent bolus dosing may permit a similar but rapid and more titratable effect, while avoiding the problems encountered with the infusion. Administration of a bolus to healthy adults resulted in a significant rise in plasma osmolality, an increase in urine output (UOP), and an associated fall in urine osmolality. Peak effect was measured by 2 h and persisted for 6 h [25]. However, no clinical studies have examined conivaptan administered as a bolus without concurrent use of an infusion, or used it in neurologically ill patients with acute hyponatremia, despite a strong theoretical basis for both novel interventions [21]. Therefore we sought to test whether a single dose of conivaptan would rapidly and durably correct hyponatremia in patients with acute neurological conditions.
Methods
All patients admitted to the neurointensive care unit (NICU) at our institution who received a dose of conivaptan between May 2006 and June 2007 were retrospectively identified from a prospectively collected ICU database. Conivaptan, administered as a 20- or 40-mg bolus given over 30 min with no subsequent infusion, was used as a novel treatment modality for acute euvolemic hyponatremia in this setting. A central line was not required for administration of conivaptan. It was prescribed at the discretion of the attending NICU physician, either as monotherapy or in combination with a continuous infusion of hypertonic fluids (ranging from 1.25% to 2% saline). It was generally considered in the setting of symptomatic hyponatremia, in patients at high risk for worsening cerebral edema, or in those with hyponatremia resistant to traditional modalities. Its use was avoided in patients with SAH if cerebral salt wasting (CSW) was suspected. No patients were concurrently treated with osmotic diuretics, boluses of hypertonic saline, fludrocortisone, or fluid restriction. As our intention was to analyze the ability of conivaptan to correct hyponatremia and examine its actions over at least a 12-h time period, doses were excluded if the pre-treatment sodium was over 135 mEq/l or if another dose was given within 12 h.
Data collected included patient demographics, primary neurological diagnosis, admission GCS, duration of hyponatremia prior to conivaptan dose, the dose given, and concurrent use of hypertonic saline. Serum sodium was measured before treatment, between 4 and 12 h after each dose (usually at 8 h), and then once or twice daily, for 72 h when possible. Urine output (UOP) and specific gravity were measured in each patient every 2 h and we calculated net fluid balance over a 12-h period after dosing. Venous phlebitis occurring during the bolus was recorded, as was any hypotension (defined as a drop in mean arterial pressure of ≥20% within 12 h of dosing).
Analysis and Endpoints
The doses of conivaptan were separated into three groups for analysis. Group 1 included only those doses where no further dose was given within 72 h, to allow evaluation of the sustained effect of a single bolus. Group 2 included those doses in group 1 as well as those where a subsequent dose was administered between 12 and 72 h after the first (i.e., all eligible doses); this group formed the basis of our primary analysis of the acute effects of conivaptan. Group 3 contained all excluded doses. Although excluded from our primary analysis, we present this data to allow an unbiased perspective on the full spectrum of doses given.
We defined thresholds for response to conivaptan as an increase in serum sodium of at least 4 or 6 mEq/l within 12 h of a single dose. These cutoffs were selected to allow comparison with existing literature on the efficacy of conivaptan [22, 23]. We measured the change in sodium from pre-treatment to the peak value within the evaluated time frame, and analyzed this change using a paired t-test for each group. Hyponatremia was considered corrected when the sodium rose to >135 mEq/l. Furthermore, we assessed all the above endpoints up to 72 h for group 1, and evaluated how often a rise of 4 mEq/l was sustained at 72 h after a single dose. Excessive correction was defined as ≥12 mEq/l increase in serum sodium within 24 h of a dose. The mean sodium values closest to 4, 8, 24, 48, and 72 h after each dose (as available) were plotted for group 1. Mean hourly UOP and specific gravities were also calculated for 12 h after each eligible dose. Using ANOVA, we compared the peak sodium response at 12 h between 20 and 40 mg doses, in those on isotonic vs. hypertonic fluids, and between groups 2 and 3 (included and excluded patients). We also compared the response rate (proportion with sodium increasing by 6 mEq/l) between these two doses, fluid types, and groups, using the chi-squared test.
Results
A total of 174 patients admitted to the NICU over the study period developed hyponatremia, of which 24 patients ultimately received 44 doses of conivaptan. Nineteen doses were excluded (15 given ≤12 h apart and four doses with pre-treatment sodium >135 mEq/l). This left 25 doses given to 19 patients eligible for primary analysis, of which 20 doses could be analyzed up to 72 h, forming group 1. Characteristics of our patient population are summarized in Table 1. Duration of hyponatremia was generally <24–48 h, and most patients had disorders putting them at risk for cerebral edema (e.g., intracranial hemorrhage, traumatic brain injury, brain tumors). Seven of 25 doses (28%) were given concomitantly with infusions of hypertonic saline (1.25–2%).
Table 1.
Patient characteristics
| Variable | Group 1 (single dose) | Group 2 (all included) | Group 3 (all excluded) | All doses |
|---|---|---|---|---|
| Diagnosis: all (patients/doses) | 18/20 | 19/25 | 9/19 | 24/44 |
| Intracerebral hemorrhage | 4/4 | 4/4 | 1/2 | 5/6 |
| Subarachnoid hemorrhage | 4/5 | 4/6 | 1/2 | 5/8 |
| Brain tumor | 3/3 | 4/4 | 5/11 | 5/15 |
| Guillain–Barré syndrome | 2/3 | 2/3 | 0 | 2/3 |
| Traumatic brain injury | 2/2 | 2/3 | 0 | 2/3 |
| Other* | 3/3 | 3/5 | 2/4 | 5/9 |
| Gender (male/female), patients | 13/5 | 14/6 | 7/2 | 13/11 |
| Conivaptan doses (20/40 mg) | 17/3 | 20/5 | 11/8 | 31/13 |
| Fluid type (NS/HS) | 14/6 | 18/7 | 9/10 | 27/17 |
| Admission GCS (median, IQR) | 14 (12.75–15) | 14 (9.5–15) | 13 (3–14) | 13.5 (8.5–15) |
| Admission GCS ≤8 (%) | 7% | 18% | 43% | 25% |
| Duration of hyponatremia prior to dose (median, IQR) | 22 h (10–48) | 20 h (10–48) | 9 h (0–24) | 12.5 h (6–24) |
Other diagnoses included subdural hematoma, ischemic stroke, and normal-pressure hydrocephalus
Note: All doses forming group 1 are also included in group 2
NS normal saline, HS hypertonic saline
Natremic Response
Examining 12-h sodium response (Table 2) among all 25 included doses, we found that over half had a 6 mEq/l rise, and 71% corrected to normal even at this early time point. The mean sodium change from baseline to 12-h peak was 5.8 ±3.2 mEq/l (P < 0.0001). This response was clearly greater than that seen in excluded patients, where only 11% had a 6 mEq/l rise in sodium, and less than half corrected after their dose.
Table 2.
Natremic response by 12 h for all doses (divided by groups)
| Group 1 n = 20 | Group 2 n = 25 | Group 3 n = 19 | |
|---|---|---|---|
| Baseline Na+ | 131.4 ± 3.0 | 131.4 ± 3.0 | 132.8 ± 5.1 |
| Peak Na+ (within 12 h) | 137.1 ± 2.8† | 136.7 ± 2.7† | 135.5 ± 4.3† |
| Peak change in Na+ | 6.31 ± 3.0 | 5.76 ± 3.2 | 2.33 ± 2.8¶ |
| Increase by ≥4 mEq/l | 12/16 (75%) | 15/21 (71%) | 8/18 (44%) |
| Increase by ≥6 mEq/l | 9/16 (56%) | 11/21 (52%) | 2/18 (11%)‡ |
| Na+ correct >135 | 12/16 (75%) | 15/21 (71%) | 8/18 (44%) |
Na+ = serum sodium (mEq/l)
P < 0.0001 compared to baseline Na+ (paired t-test);
P = 0.001 compared to group 2 (ANOVA);
P < 0.01 compared to group 2 (chi-squared)
Group 1 showed an increase from baseline to peak sodium of 8.0 mEq/l over 72 h (P < 0.0001). While 40% achieved their maximal natremic response within 8 h of the dose, others continued to rise beyond this time point, with 25% having peak sodium 72 h after a single dose. Mean sodium values for these 20 doses are plotted in Fig. 1. Of the 16 with data at 72 h, all had a response (rise by 4 mEq/l) after conivaptan, and 11 of these (69%) sustained this change up to 72 h without another dose being given. Eighteen of 20 doses given resulted in sodium correcting (Table 3), and the two that did not normalize in this group had a significantly lower baseline sodium than those which did correct (126 vs. 132, P = 0.005). However, both doses resulted in a sodium rise of at least 4 mEq/l. Of all 44 doses given, only one resulted in excessive correction (in combination with 1.25% saline infusion), with a 13 mEq/l rise in sodium at 24 h; however, no clinical sequelae were noted.
Fig. 1.
Mean serum sodium after conivaptan (group 1)
Table 3.
Natremic response for doses in group 1 out to 72 h
| Endpoint | Results |
|---|---|
| Peak Na+ | 139.40 ± 4.82† |
| Peak change in Na+ | 8.0 ± 4.1 |
| Change in Na+ at 72 h | 5.12 ± 4.0† |
| Increase by ≥4 mEq/l | 19/20 (95%) |
| Increase by ≥6 mEq/l | 14/20 (70%) |
| Na+ correct >135 | 18/20 (90%) |
| Sustained rise ≥4 at 72 h | 11/16 (69%) |
Na+ = serum sodium (mEq/l)
P < 0.0001 compared to baseline Na+ (paired t-test)
The rate of response for patients receiving normal saline (7 of 16) was equivalent to that seen in those receiving hypertonic saline (four of five), with the change in sodium within 12 h 5.3 ± 3.1 mEq/l with isotonic fluids compared to 7.2 ± 3.4 on hypertonic saline (P = 0.26). The dose of conivaptan also revealed no effect on efficacy, with 8 of 16 in the 20 mg group responding compared to three of five in the 40 mg group. The mean 12-h sodium change was 5.6 ± 3.4 mEq/l in 20 mg group compared to 6.2 ± 2.4 mEq/l after a 40 mg dose (P = 0.73).
Effects on Urine Output and Specific Gravity
Hourly UOP tripled as early as 2 h after the conivaptan dose (Fig. 2), and remained elevated for 6 h, with return to baseline by 12 h. A concomitant decrease in urine specific gravity was also observed. Mean specific gravity rapidly decreased from a baseline of 1.010 ± 0.005 to a nadir of 1.005 ± 0.005 at 2–4 h, with a return to baseline by 12 h indicating the end of the aquaretic effect. This increased UOP generally resulted in a slightly negative fluid balance, but only four of 25 patients were one liter or more negative 12 h after the dose.
Fig. 2.
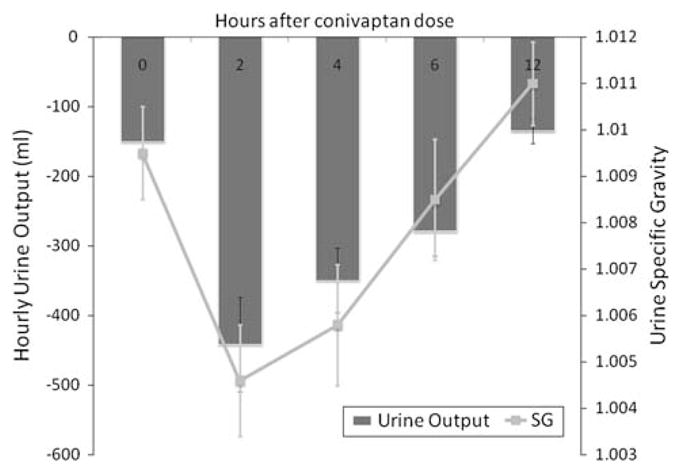
Urine output and specific gravity after conivaptan (group 2)
Adverse Drug Reactions
Conivaptan was safely administered through a peripheral IV, with no venous irritation or cases of phlebitis observed. No significant hypotension was seen after any administered doses.
Discussion
Conivaptan, a novel aquaretic, appears to be an effective treatment option for the correction of acute hyponatremia in neurologically injured patients. The effect of a bolus without use of an infusion proved to be rapid, with most responding with a significant rise in sodium by 12 h. The degree of response was also clinically significant, with sodium increasing almost 6 mEq/l above baseline within 12 h. In the select group of patients who received only a single dose within 72 h, most corrected to within the normal range, with a maximal rise of 8 mEq/l over this time. These results compare favorably to those reported using a conivaptan bolus combined with a continuous infusion in patients with chronic hyponatremia, where mean time to rise ≥4 mEq/l was 24 h, and a similar 70% achieved ≥6 mEq/l rise within 72 h [24].
Our population had acute hyponatremia, with median duration of low sodium prior to conivaptan being under 24 h. While the risk of osmotic complications from rapid correction of hyponatremia is thought to be low in such an acute setting, we also rarely observed overcorrection after a single dose of conivaptan. Indeed, prompt normalization of hyponatremia is desirable in this population of mostly brain-injured patients to avoid worsening cerebral edema and precipitating neurological deterioration from increased brain water. Greater caution should be taken in those with more prolonged duration of hyponatremia, where risks of rapid correction are higher.
We confirmed a robust aquaretic response to this vasopressin receptor antagonist, with a significant increase in the production of dilute urine over the 12 h period following each dose. Although this counteracts the water retention that underlies SIADH, caution should be taken when using conivaptan in any patient at risk of volume depletion, especially patients with possible CSW [12]. We continued intravenous fluids in all these patients, not allowing them to become excessively negative in terms of fluid balance, and did not observe any ischemic complications, even when administering conivaptan to patients with SAH. However, we recommend caution in this group, especially when risk of cerebral ischemia and CSW is high.
A continuous infusion of hypertonic saline was utilized in 28% of the conivaptan doses. While we cannot fully separate out the relative contributions of these agents, we believe that the conivaptan-induced aquaresis was largely responsible for the rise in sodium we observed after each dose. A slow infusion of 1.25–2% saline would not be expected to result in such an acute and rapid response, and most patients had already been receiving such solutions with a stable or falling sodium prior to conivaptan administration. We also did not find an incremental efficacy when both agents were used together, suggesting that conivaptan has its effects largely independent of the fluid type used concurrently, and may be a suitable agent as monotherapy for acute hyponatremia.
While the current dosing recommendation for conivaptan is a 20 mg bolus followed by a continuous infusion over 1–4 days, there are safety concerns with the use of this regimen. Infusion-site reactions and venous phlebitis occurred in up to 73% of patients receiving a 20 mg/day infusion in company-sponsored trials [24]. This high incidence of vascular irritation necessitates administration of conivaptan through large veins, in addition to changing the infusion site every 24 h. Conivaptan also requires a dedicated intravenous line due to limited compatibility data. These management issues are minimized when it is dosed as an intermittent bolus as in our novel study; no cases of venous irritation were reported among any doses given using this protocol despite some being administered through peripheral IVs. Not only is this an advantage over the traditional infusion regimen, but being able to rapidly correct hyponatremia without central access may be a significant advantage over the use of hypertonic saline. Additional conivaptan bolus doses may be given as required if sodium correction is not adequately sustained and can be titrated to desired effect.
A limitation of a retrospective analysis of this type lies in the lack of predetermined or standardized selection criteria for the use of conivaptan. Conivaptan was used only in a small subset (approximately 15%) of the hyponatremic patients seen in our NICU, and was prescribed usually when other therapies had failed, or when we felt patients were at risk for acute neurological deterioration.
Another important limitation lies in the fact that many patients received a second dose within 72 h of the first, making it difficult to properly analyze these doses for the sustained effects of conivaptan. Patients in group 1 appeared to be a select subset who had a better response and therefore did not require repeat dosing. Caution should therefore be taken in interpreting the encouraging (70%) response rate with these 20 doses. The early response rate was also the highest in group 1 (56%), lower in group 2 (52%), and the lowest in those patients excluded, where it was only 11%. Overall, excluded patients received more than one dose likely related to a limited response, with sodium rising on average 2.3 mEq/l compared to 5.8 mEq/l in those not requiring a repeat dose within 12 h. This means that patients included in our primary analysis comprise a subset of better responders. However, even when analyzing all doses (including those excluded) to get a sense of overall response rate, we still found that 59% had a rise by at least 4 mEq/l within 12 h. This may provide an unbiased perspective of the efficacy of conivaptan in this broad population.
Conclusions
Conivaptan, a vasopressin-receptor antagonist, offers a promising new approach to the treatment of hyponatremia in the neurologically injured patient. We have shown that a single bolus of 20 or 40 mg is effective for the correction of acute hyponatremia in this population. The safety and rapid efficacy of intermittent bolus dosing appears to be advantageous with an effect that is maintained for up to 72 h despite elimination of the problematic continuous infusion. It may also have a number of advantages over the use of hypertonic saline, including the correction of hyponatremia through reversal of the underlying free water excess present in many of these patients. Further controlled studies are needed to confirm our findings.
Contributor Information
Theresa Murphy, Email: txm5120@bjc.org, Department of Pharmacy, Neurology/Neurosurgery Intensive Care Unit, Barnes-Jewish Hospital, Washington University School of Medicine, 216 S. Kingshighway Blvd, Saint Louis, MO 63110, USA.
Rajat Dhar, Department of Neurology and Neurological Surgery, Washington University School of Medicine, Saint Louis, MO, USA.
Michael Diringer, Department of Neurology and Neurological Surgery, Washington University School of Medicine, Saint Louis, MO, USA.
References
- 1.Lester MC, Nelson PB. Neurological aspects of vasopressin release and the syndrome of inappropriate secretion of antidiuretic hormone. Neurosurgery. 1981;8(6):735–40. doi: 10.1097/00 006123-198106000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Doczi T, Tarjanyi J, Huszka E, Kiss J. Syndrome of inappropriate secretion of antidiuretic hormone after head injury. Neurosurgery. 1982;10(6 Pt 1):685–8. doi: 10.1227/00006123-198206010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Suri MF, Sung GY, et al. Prognostic significance of hypernatremia and hyponatremia among patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;50(4):749–55. doi: 10.1097/00006123-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Rabinstein AA, Wijdicks EF. Hyponatremia in critically ill neurological patients. Neurologist. 2003;9(6):290–300. doi: 10.1097/01.nrl.0000095258.07720.89. [DOI] [PubMed] [Google Scholar]
- 5.Multz AS. Vasopressin dysregulation and hyponatremia in hospitalized patients. J Intensive Care Med. 2007;22(4):216–23. doi: 10.1177/0885066607301360. [DOI] [PubMed] [Google Scholar]
- 6.Pasantes-Morales H, Lezama RA, Ramos-Mandujano G, Tuz KL. Mechanisms of cell volume regulation in hypoosmolality. Am J Med. 2006;119(Suppl 1 7):S4–11. doi: 10.1016/j.amjmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Gullans SR, Verbalis JG. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med. 1993;44:289–301. doi: 10.1146/annurev.me.44.020193.001445. [DOI] [PubMed] [Google Scholar]
- 8.Bhardwaj A. Neurological impact of vasopressin dysregulation and hyponatremia. Ann Neurol. 2006;59(2):229–36. doi: 10.1002/ana.20788. [DOI] [PubMed] [Google Scholar]
- 9.Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102(1):67–77. doi: 10.1016/S0002-9343(96)00274-4. [DOI] [PubMed] [Google Scholar]
- 10.Diringer MN, Zazulia AR. Hyponatremia in neurologic patients: consequences and approaches to treatment. Neurologist. 2006;12(3):117–26. doi: 10.1097/01.nrl.0000215741.01699.77. [DOI] [PubMed] [Google Scholar]
- 11.Singhi SC, Singhi PD, Srinivas B, et al. Fluid restriction does not improve the outcome of acute meningitis. Pediatr Infect Dis J. 1995;14(6):495–503. doi: 10.1097/00006454-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol. 1985;17(2):137–40. doi: 10.1002/ana.410170206. [DOI] [PubMed] [Google Scholar]
- 13.Gross P, Reimann D, Henschkowski J, Damian M. Treatment of severe hyponatremia: conventional and novel aspects. J Am Soc Nephrol. 2001;12(Suppl 17):S10–4. [PubMed] [Google Scholar]
- 14.Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol. 2007;2(6):1110–7. doi: 10.2215/CJN.00910207. [DOI] [PubMed] [Google Scholar]
- 15.Woo MH, Kale-Pradhan PB. Fludrocortisone in the treatment of subarachnoid hemorrhage-induced hyponatremia. Ann Pharmacother. 1997;31(5):637–9. [PubMed] [Google Scholar]
- 16.Wijdicks EF, Vermeulen M, van Brummelen P, van Gijn J. The effect of fludrocortisone acetate on plasma volume and natriuresis in patients with aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 1988;90(3):209–14. doi: 10.1016/0303-8467 (88)90023-6. [DOI] [PubMed] [Google Scholar]
- 17.Porzio P, Halberthal M, Bohn D, Halperin ML. Treatment of acute hyponatremia: ensuring the excretion of a predictable amount of electrolyte-free water. Crit Care Med. 2000;28(6):1905–10. doi: 10.1097/00003246-200006000-00037. [DOI] [PubMed] [Google Scholar]
- 18.Cawley MJ. Hyponatremia: current treatment strategies and the role of vasopressin antagonists. Ann Pharmacother. 2007;41(5):840–50. doi: 10.1345/aph.1H502. [DOI] [PubMed] [Google Scholar]
- 19.Hays RM. Vasopressin antagonists—progress and promise. N Engl J Med. 2006;355(20):2146–8. doi: 10.1056/NEJMe068236. [DOI] [PubMed] [Google Scholar]
- 20.Palm C, Pistrosch F, Herbrig K, Gross P. Vasopressin antagonists as aquaretic agents for the treatment of hyponatremia. Am J Med. 2006;119(Suppl 1 7):S87–92. doi: 10.1016/j.amjmed.2006.05. 014. [DOI] [PubMed] [Google Scholar]
- 21.Rabinstein AA. Vasopressin antagonism: potential impact on neurologic disease. Clin Neuropharmacol. 2006;29(2):87–93. doi: 10.1097/00002826-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447–57. doi: 10.1159/000106456. [DOI] [PubMed] [Google Scholar]
- 23.Ghali JK, Koren MJ, Taylor JR, et al. Efficacy and safety of oral conivaptan: a V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J Clin Endocrinol Metab. 2006;91(6):2145–52. doi: 10.1210/jc.2005-2287. [DOI] [PubMed] [Google Scholar]
- 24.Prescribing information. Deerfield Ill: Astellas Pharma US, Inc; 2007. Vaprisol (conivaptan hydrochloride injection) [Google Scholar]
- 25.Burnier M, Fricker AF, Hayoz D, Nussberger J, Brunner HR. Pharmacokinetic and pharmacodynamic effects of YM087, a combined V1/V2 vasopressin receptor antagonist in normal subjects. Eur J Clin Pharmacol. 1999;55(9):633–7. doi: 10.1007/s002280050685. [DOI] [PubMed] [Google Scholar]



