Abstract
OBJECTIVE
In rheumatoid arthritis (RA), telomeres of lymphoid and myeloid cells are age-inappropriately shortened, suggesting excessive turnover of hematopoietic precursor cells (HPC). We have examined functional competence (proliferative capacity, maintenance of telomeric reserve) of CD34+ HPC in RA.
METHODS
Frequencies of peripheral blood CD34+CD45+ HPC of 63 rheumatoid factor positive RA patients and 48 matched controls were measured by flow cytometry. Proliferative burst, cell cycle dynamics, and induction of lineage-restricted receptors were tested in purified CD34+ HPC after stimulating with early hematopoietins. Telomeric sequences were quantified by real-time PCR. HPC functions were correlated with disease duration, activity, severity, and treatment.
RESULTS
In healthy donors, CD34+ HPC accounted for 0.05% of nucleated cells; their numbers were strictly age-dependent and declined at a rate of 1.3%/year. In RA patients, CD34+ HPC frequencies were age-independently reduced to 0.03%. Upon growth factor stimulation, control HPC passed through 5 replication cycles over 4 days. In contrast, RA-derived HPC completed only 3 generations. Telomeres of RA CD34+ HPC were age-inappropriately shortened by 1,600 bp. All HPC defects were independent from disease duration, disease activity, and smoking status; and were present to the same degree in untreated patients.
CONCLUSIONS
In RA, circulating bone marrow-derived progenitor cells are diminished, with concentrations stagnated at levels typical for old control individuals. HPC from RA patients display growth factor non-responsiveness and sluggish cell cycle progression; marked telomere shortening indicates proliferative stress-induced senescence. Defective HPC function independent from disease activity markers suggests bone marrow failure as a potential pathogenic factor in RA.
Rheumatoid arthritis (RA) is a quintessential autoimmune syndrome characterized by tissue-destructive chronic inflammation. A reappraisal of RA disease manifestations over the last decade has emphasized the expanding spectrum of the rheumatoid syndrome, now including accelerated cardiovascular disease as well as increased susceptibility to lymphoma and infection (1–3). Although the broad use of immunosuppressants makes it difficult to distinguish between iatrogenic and disease-intrinsic pathogenic factors, it is clear that the overall immunocompetence of RA patients is compromised (4). Immune cells originate in the bone marrow (BM), raising the question of whether the BM itself has a role in the impaired immunocompetence and in the disease process defining RA (5). Hematological manifestations of RA are common, affecting all three major cell lineages of the BM (6, 7). Most striking are two neutropenic syndromes, Felty’s syndrome (FS) and T-cell large granular lymphocyte leukemia (TLGL) that are specifically associated with RA (8, 9).
While long known as the home of hematopoietic stem cells (HSC), the BM is also recognized as the origin of an increasing spectrum of tissue-specific stem and progenitor cells (10, 11). Stem cells perpetuate through self-renewal (12, 13) but also have to produce enormous numbers of offspring that differentiate into mature effector cells. These properties of self-renewal, multipotentiality, and differentiation are the bases for BM transplantation (14). Circulating BM-derived stem cells represent a small population of nucleated blood cells (15). Within the CD34+ population of circulating HSC, a subpopulation expresses the VEGF-receptor 2 and likely represents endothelial precursor cells (EPC) that give rise to new blood vessels (16, 17). VEGFR-2+ CD34+ cells have been localized in synovial tissue from RA as well as osteoarthritis patients (18), and a decreased number of EPC was found in RA patients (19), suggesting that circulating progenitors accumulate in and feed the inflammatory lesions. To the contrary, RA CD34+ BM cells generate more endothelial cells with a positive correlation between endothelial cell generation and microvessel density in synovitic tissue (20).
Whereas these studies propose pro-inflammatory and pro-immune functions for RA BM, other data indicate fundamentally impaired BM function in RA, with insufficient supply of progenitors leading to tissue failure and immune deficiency (6, 21). Specifically, in peripheral blood stem cell harvests, CD34+ cells were reduced to 50% in RA patients compared to healthy controls (22). Significantly lower percentages of CD34+ BM cells have been reported in RA patients candidated for autologous HSC transplantation (23).
In humans, CD34+ cells isolated from BM or from circulating blood represent a heterogeneous population and only a subset may represent true HSC, while other cells have already differentiated into multipotent hematopoietic progenitor cells (HPC). Such HPC produce different types of mature effector cells that have been implicated in autoimmunity and tissue inflammation in RA. Mature hematopoietic cells have a very short lifespan. The requirements for continuous cell production are immense; adult humans need to produce 1.5 million blood cells every second (24). Studies examining granulocyte telomeres have shown that this enormous proliferative stress is even more pronounced in RA (25). Neutrophils of RA patients have telomeric sequences shortened by more than 1,000 bp compared to age-matched controls (25). A similar telomeric loss is also seen in mature lymphocytes and has been associated with premature immunosenescence in RA. The current study has investigated HPC frequencies, function, and telomeric maintenance in RA patients. The studies reveal that the circulating HPC pool in RA is contracted to almost 50% and that surviving HPC fail to proliferate when driven with hematopoietins. Telomeres in RA-derived HPC are markedly shortened. These defects are independent from disease duration, severity, activity and treatment, raising the possibility that RA is associated with intrinsic abnormalities in HPC function.
Materials and Methods
Patients
This study was approved by the Emory University Institutional Review Board, and written informed consent was obtained from all participants. Samples were coded and those performing the experiments were not aware of the disease/control status of the samples.
Sixty-three consecutive adult patients attending the Rheumatology Clinic with diagnosis of seropositive RA according to the American College of Rheumatology 1987 revised criteria were included (26). Forty-eight age-, sex- and ethnically- frequency matched healthy subjects without history of chronic inflammatory diseases or immunosuppressive/chemotherapeutic therapy served as controls (Table 1). The cohorts were balanced for age, sex, and ethnicity. Pregnancy, history of cancer, sarcoidosis, active infection, and crystal arthropathy were exclusion criteria. All participants underwent clinical evaluation, and hospital records were reviewed. The following data were collected: disease duration, extra-articular manifestations, orthopedic surgeries, treatment, ESR, and CRP. In addition, disease activity was assessed using the FDA criteria (27). Accordingly, disease was considered active if at least three of the four following criteria were present: ≥ six painful joints, ≥ three joints with synovitis, morning stiffness duration ≥ 45 min, and erythrocyte sedimentation rate ≥ 28mm/h (Table 1).
Table 1.
Demographic characteristics of study populations and clinical characteristics of the RA patients.
| Controls | RA | p | |
|---|---|---|---|
| No. of subjects | 48 | 63 | |
| Sex, %female/%male | 77/23 | 82/18 | 0.47 |
| Age, mean ± SD years | 52 ± 14 | 55 ± 14 | 0.26 |
| Ethnicity, % | 0.14 | ||
| African American | 67 | 65 | |
| White | 27 | 17.5 | |
| Hispanic | 6 | 17.5 | |
| Disease duration, mean ± SD years | 9.93 ± 9.85 | ||
| Active disease*, % | 56 | ||
| Extra-articular manifestations, % | 41 | ||
| Nodules | 34 | ||
| Cytopenia | 6 | ||
| Sicca | 13 | ||
| Joint replacements, % | 30 | ||
| HLA-DR4 pos / neg, % | 30 / 70 | ||
| Tobacco yes / no, % | 16 /84 | ||
| DMARDs naïve, % | 11 | ||
| Medications, % | |||
| Corticosteroids | 75 | ||
| Methotrexate | 54 | ||
| Hydroxychloroquine | 31 | ||
| Sulfasalazine | 23 | ||
| TNF inhibitors | 10 | ||
Active disease defined by FDA criteria (presence of ≥ 3 of the following: morning stiffness (>45 min), swollen joints (>3), tender joints (>6), sed rate (>28mm)
Flow Cytometric enumeration of CD34+ cells
Circulating CD34+ cells were quantified with ProCount™ (BD Bioscience; San Jose, Calif) according to the manufacturer’s protocol (15).
CD34 subset isolation
Peripheral blood mononuclear cells (PBMC) from 40 ml of blood were separated by gradient centrifugation (Mediatech, Inc, Herndon, VA), and CD34+ cells were purified using the Direct CD34 Progenitor Isolation kit and AutoMACS magnetic separation device (Miltenyi Biotec Inc, Auburn, CA). Cells were counted and assessed for viability by trypan blue dye exclusion.
CD34+ cells expressing CD133, CD71, CD45RA, CD38, CD33, CD7, and CD10 surface markers were assessed by flow cytometry using appropriate monoclonal antibodies (BD Bioscience; San Jose, Calif).
CFSE fluorescent dye labeling and liquid culture system
Freshly isolated CD34+ cells were stained with the vital stain carboxyfluoroscein succinimidyl ester (CFSE), and 50,000–80,000 CFSE-labeled cells were expanded in defined medium for hematopoietic cells (StemSpam™ H3000, StemCell Technologies) supplemented with 100ng/ml rh Flt-3 ligand, 100ng/ml rh stem cell factor (SCF), 20ng/ml rh IL-3, and 20ng/ml rh IL-6 (StemSpam™ CC100 Cytokine cocktail, StemCell Technologies). Cultures were seeded in flat bottom 96-well plates at 5,000 to 7,500 cells per well, and incubated for 18 days. On days 4, 8, 11 and 18, aliquots were stained with antibodies specific for cell surface markers, including CD34-APC, CD117-PE, CD11c-PE and CD14-PerCp (all from BD Biosciences, San Jose, CA) and analyzed using a FACSort flow cytometer (BD Biosciences). Cell proliferation was assessed by FACS CFSE dilution at day 4 of culture. The CFSE intensity of gated CD34 was determined using a histogram analysis. The peak fluorescence intensity of unstimulated cells was used as a reference point for all cytokine stimulated cells. The number of cell divisions was calculated by counting the number of peaks relative to the reference point.
Telomere length measurement
Telomere length of freshly isolated CD34+ cells was measured by real-time PCR modified from the Cawthon's method (28, 29). Genomic DNA was purified using the Wizard Genomic DNA purification kit (Promega) and quantified through the copy number of a single copy gene (36B4). PCR was conducted with 5 µl of 0.1 ng/µl genomic DNA and 15 µl of PCR solutions containing sense and anti-sense primers (2000 nM for telomere and 300 nM for 36B4), 3mM MgCl2, 0.2X SYBR Green, 0.75 U Platinum Taq (Invitrogen). Primer sequences are as follows: telomere sense, 5’-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3'; telomere anti-sense, 5’-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3'; 36B4 sense, 5'-CAGCAAGTGGGAAGGTGTAATCC-3'; 36B4 anti-sense, 5'-CCCATTCTATCATCAACGGGTACAA-3'. Mixtures were incubated for one cycle of 15 min at 95 degrees, amplified over 40 PCR cycles with 5 seconds at 95 degrees, 10 seconds at 56 degrees, and 60 seconds at 72 degrees. For 36B4 PCR, mixtures were incubated for one cycle of 15 min at 95 degrees, amplified over 40 PCR cycles for 10 seconds at 95 degrees, 30 seconds at 58 degrees, and 30 seconds at 72 degrees. Reference samples of genomic DNA with known telomere length were used to standardize the assay.
HLA-DRB1*04 typing
Genomic DNA was extracted and amplified by nested PCR with HLA-DRB1*04 specific primers as previously described (25).
Statistical analysis
Summary statistics included mean, median, and range for quantitative measurements and the frequency and percentage for categorical variables. Demographic characteristics, frequencies of HPCs, and CD34 telomere lengths were compared between groups and within the RA patients among different subgroups by Wilcoxon rank sum test or Kruskal-Wallis test and the chi-square test for categorical items. Correlations between clinical data and cell counts were examined by multivariate analysis using linear regression.
Results
Circulating HPC are diminished in RA
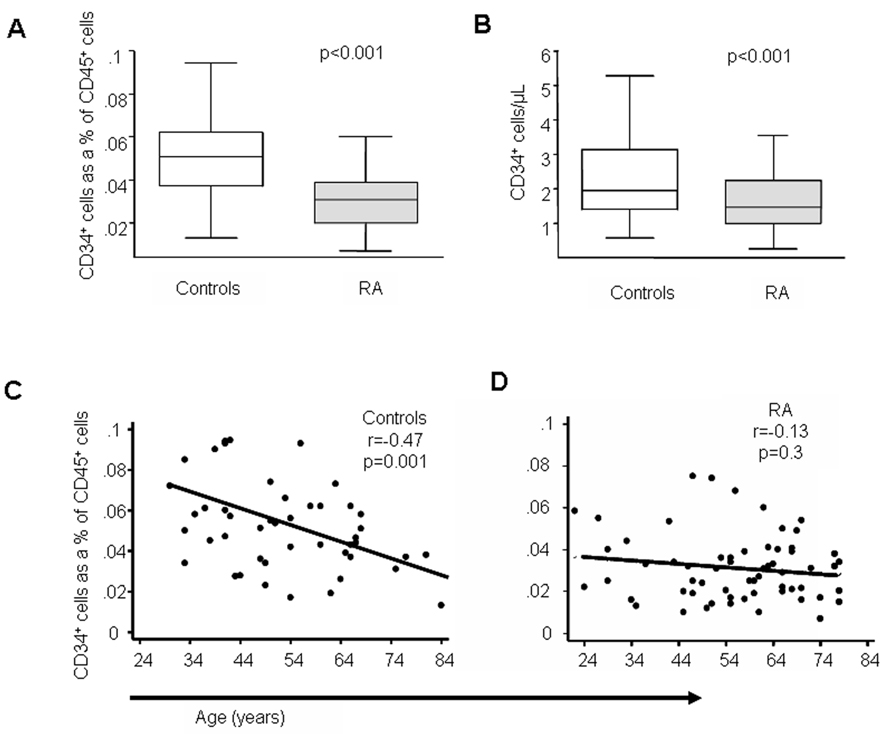
Within the peripheral blood, circulating BM-derived progenitor cells represent a small population of nucleated CD45+ cells. When transplanted in sufficient cell numbers, circulating CD34+ cells permanently reconstitute myeloablated recipients in all blood cell lineages. Besides true stem cells, the CD34+ cell population also contains multipotent progenitor cells, the parent cells of oligopotent progenitors (24). To study the biology of HPCs in RA, we accessed CD34+ cells in whole blood samples and determined their frequencies in a cohort of 63 RA patients (Table 1) and 48 controls matched for age, sex, and ethnicity. In control individuals, CD34+ cells accounted for 0.05 % of CD45+ nucleated cells. Frequencies were significantly lower in RA patients where only 0.03% of the CD45+ population expressed the CD34 marker (Figure 1). The almost 50% reduction in frequencies for RA patients was statistically significant at the p<0.001 level. Depletion of CD34+ cells in RA blood was confirmed by measuring absolute cell numbers per µl of blood (Figure 1).
Figure 1. Depletion of circulating HPC in RA.
Freshly isolated blood samples from 63 patients with RA and 48 age and sex matched controls were analyzed for CD34+CD45+ cells by flow cytometry. Number of CD34+ cells expressed as percentage of all nucleated cells (A). Absolute numbers of CD34+ cells per µl of blood (B). Age-related decline in the number of CD34+ cells in controls (C). Frequencies of CD34+ cells in RA patients are no longer age dependent (D). Box plots show medians (bold black lines), interquartile ranges (boxes), and values within 1.5 interquartile ranges from the upper or lower edge of the box (whiskers).
To identify factors influencing the size of the CD34+ HPC pool, donor age was correlated with cell numbers (Figure 1C). In healthy controls, age was a strong predictor of the proportion of CD34+ nucleated cells (r=−0.47, p 0.001) with an almost 50% loss in HPC frequencies between the ages of 25 and 75 years. The lower threshold of HPC frequencies appeared to be at 0.02%. In contrast, many of the RA patients had frequencies close to or even below 0.02 % of CD34+ HPC independent of their age (Figure1D) (r= −0.13, p=0.3).
To rule out the possibility that the population of CD34+ HPC cells circulating in controls and RA patients were in a different stage of differentiation, we profiled CD34+ cells for the following markers: CD133, CD71, CD45RA, CD38, CD33, CD7, CD10, and HLA-DR. The markers were detected on variable subsets of CD34+ cells with no statistical difference between controls and patients (data not shown).
In summary, the studies suggested that the pool of circulating CD34+ HPC is contracted in RA patients with frequencies close to the lower threshold of this biologic system, normally reached during the 8th decade of life.
Loss of circulating HPC is independent from disease duration and activity
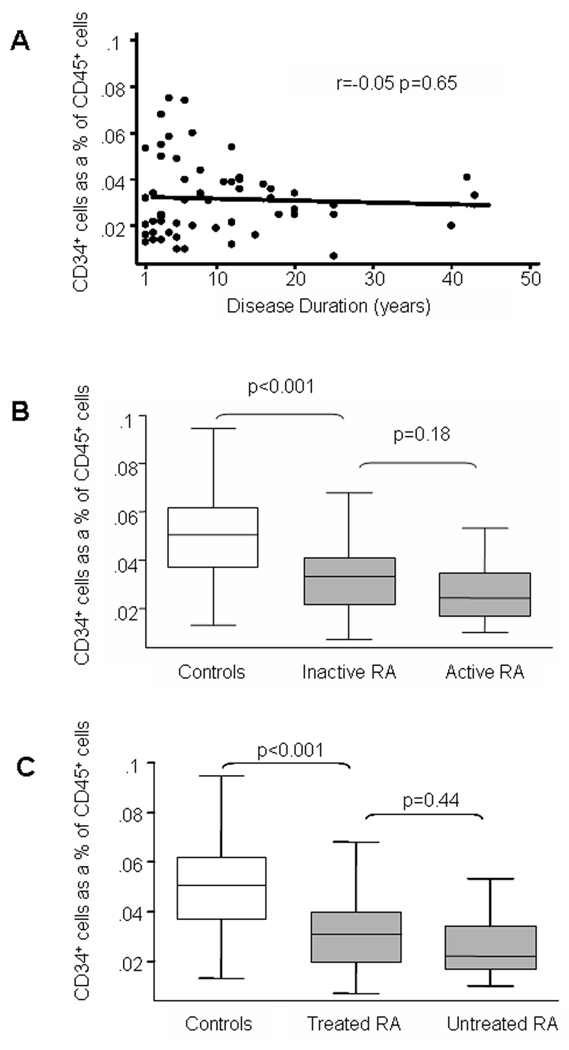
Depletion of BM-derived cells in the blood of RA patients could be a consequence of reduced reserve, enhanced attrition, or altered distribution. Each of these parameters could be affected by the disease process itself. Therefore, we examined the impact of disease duration, disease activity, and treatment on the representation of blood CD34+ cells. HPC frequencies did not decline with increasing disease duration (Figure 2A), and no correlation existed between the percentage of CD34+ cells and the duration of RA (r=−0.05, p=0.65). Remarkably, even patients with disease of less than 5 years had low counts for CD34+ cells, often lower than 0.02%. Factors associated with disease severity (tobacco use, presence of nodules, radiographic bone erosions, and inheritance of the HLA-DR4 haplotype), disease duration, and disease activity score were included in a multivariate model. None of these variables were predictors of a decreased HPC number in RA patients. Due to the ethnic composition of the patient cohort consisting mostly of African American individuals, the frequency of HLA-DR4 reached only 30%. Fifty-six percent of the 63 RA patients had active disease at the time of analysis. Patients with active disease and well-managed disease were indistinguishable in terms of depletion of circulating CD34+ cells (p=0.5; Figure 2B). Also, the study cohort included 7 patients who were untreated when CD34+ cells were harvested (Figure 2C). HPC frequencies were reduced to the same degree in treated and untreated patients (p< 0.001 controls versus treated patients, p=0.4 treated versus untreated patients).
Figure 2. Correlation of HPC frequencies with disease duration, disease activity, and treatment of RA.
Frequencies of CD34+ HPC were determined in the blood of RA patients as described in Figure 1 and correlated with clinical parameters. HPC numbers were equally reduced in patients with early and long-standing disease (A). HPC were reduced to a similar degree in patients with active and well-controlled RA (B). Depletion of HPC did not correlate with therapy; untreated patients were indistinguishable from those on immunosuppressive treatment (C). Box plots were used for data presentation as described in Figure 1.
In summary, the loss of circulating CD34+ HPC appeared to be a disease-intrinsic defect in RA, not a reflection of persistent inflammatory activity or anti-inflammatory treatment.
CD34+ HPC from RA patients are impaired in their proliferative burst
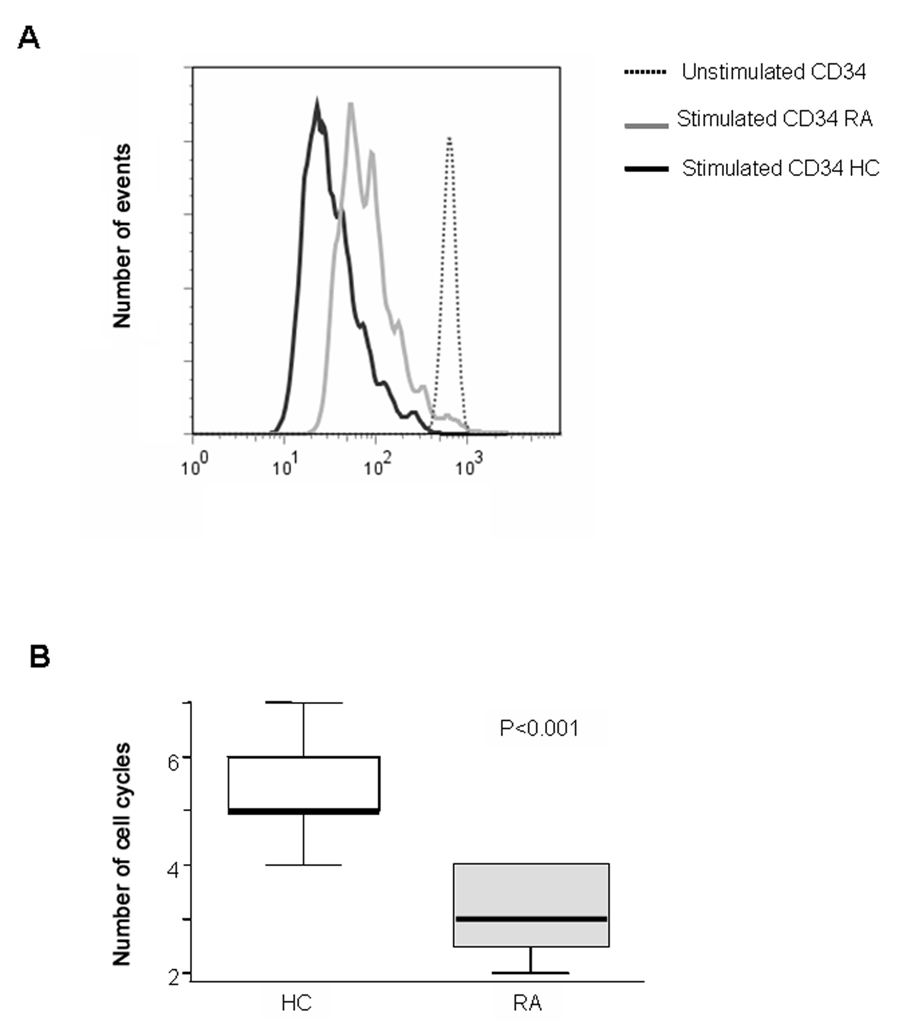
A principal functional capacity of stem cells and multipotent progenitor cells is the production of massive numbers of offspring (12, 13). When signaled by growth and differentiation factors, parent cells enter the cell cycle and expand. To test whether HPC of RA patients have intact proliferative capacity, purified circulating CD34+ cells were driven into maximal proliferation by a cocktail of four early hematopoietins: IL-3, IL-6, SCF, and Flt3L. Pilot experiments established optimal concentrations of each growth factor. To examine cell cycle entry and passage, CD34+ cells were labeled with CFSE, and FACS analysis was used to objectively measure the cell replication at day 4. CD34+ cells responded vigorously to the growth factor cocktail (Figure 3A). Cells from control individuals essentially all entered the cell cycle and rapidly progressed through 4–6 generations. On average, CD34+ HPC divided once every 20–24 hours. A different picture emerged for CD34+ HPC from RA patients; these cells passed through only 3 doublings by day 4, and 10–15% never entered the cell cycle. Figure 3B gives a direct comparison between the numbers of cell cycles completed by control and RA-derived CD34+ cells and shows the impaired proliferative capacity of the patients’ HPC (p<0.001).
Figure 3. Impaired proliferative potential of HPC in RA.
Freshly isolated CD34+ cells from RA (n=16) and controls (n=12) were stained with CFSE and cultured with a growth factor cocktail (IL-3, IL-6, Flt3L, SCF). At day 4, progressive generations of expanding HPC were distinguished by CFSE dilution, and the number of cell cycles was determined by comparing CFSE fluorescence in stimulated and unstimulated /non-proliferated cells. A representative flow cytometry is shown in (A). Numbers of cell cycles completed by control and RA-derived HPC after 4 days of growth factor stimulation are shown as box plots as described for Figure 1 (B).
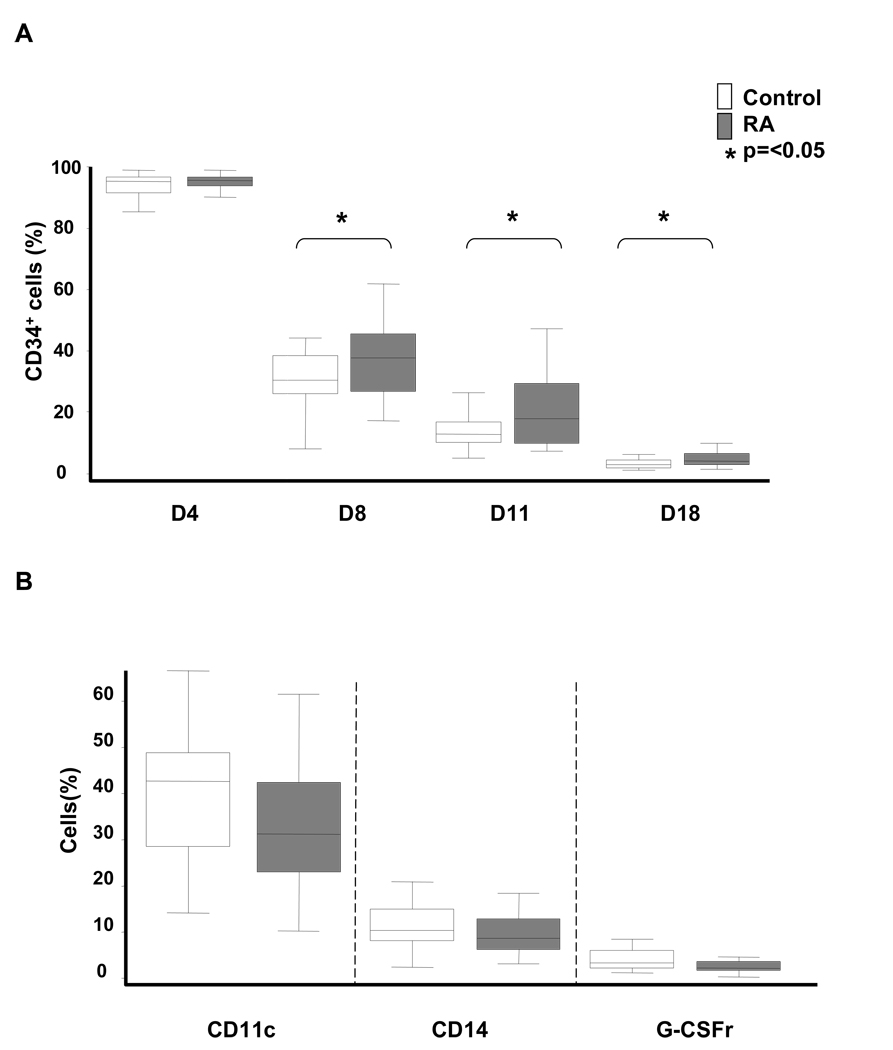
Only true stem cells are capable of self renewal without entering the differentiation program. Signals and microenvironmental conditions that preserve characteristics of immortal stem cells while such cells can reproduce are still not understood (30). When exposed to growth and differentiation factors, most CD34+ cells will begin to differentiate, acquire lineage markers, and lose the CD34 marker (24). To study whether delayed proliferation of RA CD34+ cells was connected with a shift in cell differentiation, cultured CD34+ cells were monitored for the expression of CD34 and the acquisition of the differentiation markers CD11c, CD14, and G-CSF receptor. CD34 expression was maintained for the first 4 days of growth factor-driven expansion followed by a rapid loss of this surface molecule (Figure 4). By day 18, almost all cells had converted to the CD34− phenotype. This process of CD34 loss was delayed amongst RA-derived CD34+ HPC (p=<0.05 for days 8, 11, and 18 of the culture system). Even by day 18, RA-derived HPC still contained a subset of CD34+ cells. These data confirmed that HPC from RA patients failed to pass through the cell cycle appropriately and were sluggish in differentiating into lineage-committed precursor cells.
Figure 4. Delayed loss of CD34 on expanding HPC from RA patients.
Freshly isolated CD34+ cells from 17 patients and 17 controls were driven with early hematopoietins as described in Figure 3. On days 4, 8, 11 and 18, cells were harvested and analyzed for the surface expression of CD34, CD11c, CD14, and G-CSF receptor by flow cytometry. Frequencies of cells retaining CD34 (A) or acquiring CD11c (B, left), CD14 (B, middle), or G-CSFR (B, right) are shown as box plots in RA (gray boxes) and controls (white boxes) as described in Figure 1.
While the in vitro expansion cultures were not intended to evaluate progenitor cell differentiation, the conditions induced lineage-specific markers on a fraction of the proliferating cells. FACS analysis of control and RA cells derived from CD34+ HPC did not reveal statistically significant differences. Thirty to forty percent of cells acquired CD11c, indicating a bias towards myeloid lineage differentiation. By day 18, about 10% of the cells were positive for the monocyte marker CD14, and 2–4% of the cells expressed the G-CSF receptor.
Telomeric ends of CD34+ HPC are prematurely shortened in RA
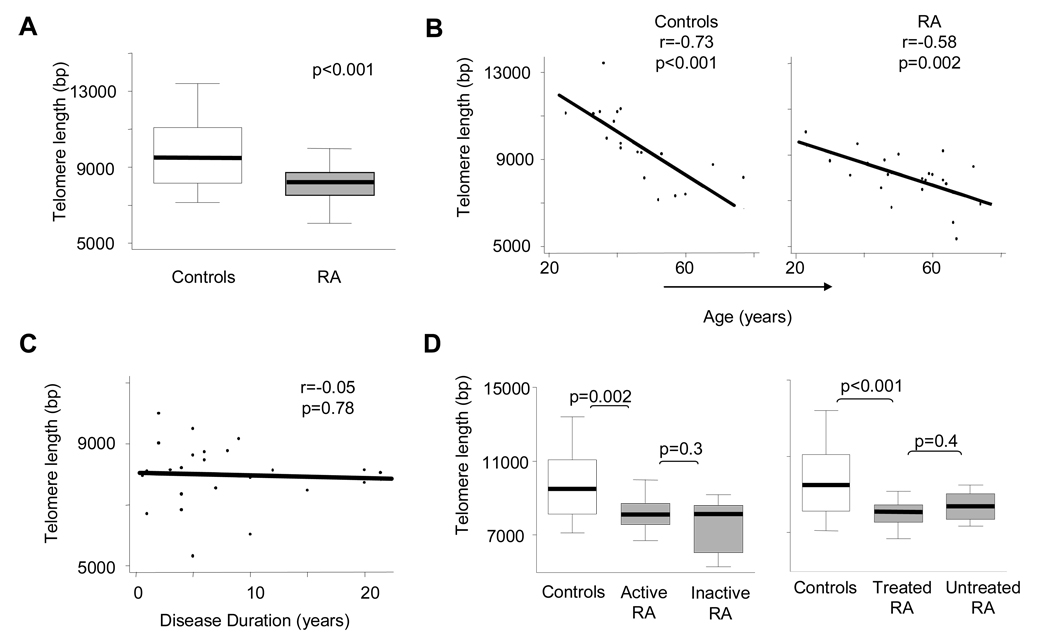
The sluggishness of cell cycle progression for RA-derived CD34+ HPC raised the question of whether these cells had features of cellular senescence. Whereas the senescence program is cell-type specific, a common mechanism for all cells involves telomere shortening (31). To compare the proliferative history of HPC in controls and patients, we isolated CD34+ HPC from 25 patients and 21 controls and measured the lengths of telomeric sequences (Figure 5). As expected, HPC telomeric ends were longer than in differentiated hematopoietic cells, such as lymphocytes and granulocytes. In CD34+ HPC from controls, telomeres were 9,597±1,615 bp (mean ± SD). Length of telomeric sequences was clearly dependent upon the donor’s age (r=−0.73, p<0.001). Between the ages of 25 and 75 years, control individuals lost almost 5,000 bp from the telomeric ends, attesting to the enormous proliferative pressure imposed on CD34+ cells. On average, CD34+ HPC shorten their telomeres by 94 bp per year. Up to the age of 75 years, the relationship between telomere length and age appeared linear. In the RA cohort, telomeres measured 7990±1031 bp (mean ± SD) and thus were 1,600 bp shorter than in the controls. Telomeric length of CD34+ HPC was still age-dependent in RA patients (r=−0.58, p=0.002); however, the slope of the curve was less steep. The discrepancy of HPC telomeres between patients and controls was particularly relevant in younger individuals. Patients aged 20 to 30 years old already had HPC telomeres shortened to less than 9,000 bp, a benchmark reached by 50- to 60-year-old controls. On average, RA-derived HPC lost 45 bp of their telomeric ends per year of life, emphasizing abnormal proliferative turnover of surviving progenitor cells.
Figure 5. Accelerated telomeric erosion in CD34+ HPC in RA.
Circulating CD34+ HPC were isolated from fresh blood of 21 controls and 25 patients. DNA was extracted, and the lengths of telomeric sequences were measured by real-time PCR. Telomeric ends were 1,600 bp shorter in the RA cohort compared to age-matched controls (A). The relationship between donor age and telomeric lengths in HPC for controls and patients is shown (B). Patients were categorized as active or inactive and treated or untreated as described in Figure 2. Telomeric lengths in CD34+ HPC did not correlate to disease duration (C). Telomeres were shortened to the same degree in patients with active and inactive RA (D). Patients on treatment displayed a similar loss of telomeres as untreated patients (D).
Accelerated telomeric erosion in RA is independent from disease activity and treatment
The chronic inflammation of RA itself may affect HPC function (23). If so, HPC telomeres should be the shortest in patients with high disease activity, and long-standing RA should progressively erode telomeric ends. Similarly, RA patients are routinely immunosuppressed, and that treatment may impair BM function. To address these possibilities, the impact of disease duration, activity, and therapy was examined in the RA cohort. No correlation could be found between disease duration and telomeric shortening; telomeres remained essentially stable over a disease duration of 20 years (Figure 5C). Patients with active and inactive disease both had shortened HPC telomeric ends with no statistical difference between the inactive and active disease categories (Figure 5D, p=0.3). The RA cohort included 4 patients that were untreated at the time of analysis, and their HPC telomeres were eroded to the same degree as in patients that had received immunosuppressive treatments (Figure 5D, p= 0.4).
In summary, premature telomeric loss was independent from disease activity markers raising the question of whether defective telomere maintenance is a disease-intrinsic defect and not a consequence of inflammation and drug therapy.
Discussion
Almost all hematopoietic cells have been implicated in disease mechanisms typical for RA (32). Neutrophils and mast cells are involved at the level of inflammatory target tissue damage. T cells, B cells, and dendritic cells contribute to the breakdown of self-tolerance and autoimmunity. All of these cells derive from common progenitors that survive in the BM and serve as a reserve to generate the different hematopoietic lineages. Data presented here provide evidence that such progenitor cells are functionally defective, impairing their proliferative expansion to provide more committed oligopotent and lineage-constrained progenitors. Age-inappropriate restriction of telomeric sequences identifies RA HPC as cells that have been exposed to excessive proliferative stress, in line with the concept that HPC turnover, renewal, and survival are fundamentally dysfunctional in RA. Since BM stem cell populations can also acquire nonhematopoietic fates (12, 13) and transdifferentiate into hepatocytes, skeletal muscle, cardiomyocytes, neuronal cells, endothelial cells, and various types of epithelial cells (33), such functional defects may reach far beyond the hematopoietic hierarchy and affect repair mechanisms in a variety of organ systems. While BM suppression, somehow mediated by inflammation, has traditionally been considered to explain the impairments of hematopoiesis in RA (6, 21), our data could not delineate a correlation of the duration of RA, disease activity, disease severity (tobacco use, presence of nodules, radiographic bone erosions and presence of HLA-DR4 haplotype), or treatment with reduced frequencies, impaired cell cycle progression, or telomeric shortening of HPC. Thus, results from the current study raise the possibility that BM-derived HPC are intrinsically dysfunctional in RA and should be considered a disease-relevant cell type in the RA syndrome.
The success of BM transplantation has provided unequivocal evidence that CD34+ cells isolated from BM or circulating blood have stem cell activity (16). BM, CD34+ cells live in specialized niches critically determining their fate and cell cycle behavior. They can be mobilized to circulate into the blood where they continuously replenish organ-residing progenitor cells. In the present study, the population of CD34+ cells in RA patients was reduced to 60% of control levels. CD34+ frequencies were contracted irrespective of whether measured as a proportion of nucleated cells or as cells per µl of blood. In healthy individuals, numbers of circulating CD34+ cells were strongly correlated with donor age, suggesting that the BM reserve is slowly expended during adult life. In RA, donor age no longer predicted frequencies. Instead, in many patients levels fell below 0.02% of CD45+ nucleated cells. In controls, such low levels were only reached in donors older than 75 years, suggesting that the CD34+ HPC pool in RA patients is severely depleted.
Several mechanisms could be implicated in minimizing the blood CD34+ pool. Besides a genuine reduction in the BM, CD34+ cell mobilization could be impaired. Alternatively, these cells may rapidly leave the blood, being recruited into inflamed tissues where they are needed for repair functions. CD34+ cells are present in inflamed synovium (18). However, osteoarthritic tissues contain similar numbers of progenitors, excluding inflammatory cytokines and chemokines as the only mechanisms orchestrating recruitment. Progenitor cells have also been isolated out of synovial fluid (34). Support for a reduction of the HPC pool in RA comes from studies describing reduced numbers of BM CD34+ cells and reduced recovery of CD34+ populations in RA patients undergoing BM transplantation (22, 23). Finally, EPC are markedly reduced in RA patients, suggesting an age-inappropriate demise affecting several CD34+ subpopulations (19).
Even more remarkable than the depletion of circulating CD34+ cells in RA was the impairment of cell cycle progression. Culture conditions were chosen to give optimal proliferative expansion and used a cytokine cocktail including all early hematopoietins. By day 4, control HPC had passed through 5 cell cycles, thus generating 32 cells from one precursor. In contrast, RA-derived CD34+ cells only completed 3 cell cycles, translating into an 8-fold expansion. Whereas true stem cells are mostly resting cells, their immediate progeny has to proliferate massively in order to service the enormous demand for differentiated hematopoietic cells (31). Restriction of this capacity must have implications for the fundamental principles of hematopoiesis. As primitive progenitors proliferate, they lose the CD34 marker (24). By day 8, most CD34+ HPC had differentiated into CD34− offspring. The process of downregulating CD34 and replicating are linked; CFSE-low cells (highly proliferative) consistently had lower CD34 density than CFSE-high cells (data not shown). Delayed CD34 loss on RA HPC was thus in line with deficient proliferation. Similar findings have been reported for a murine model of Down syndrome where BM CD34+ cells have drastically reduced growth capacity (35). Like RA, Down syndrome is associated with premature immune aging and T-cell telomere shortening (36), suggesting possible links between CD34 pool dynamics and shaping of the peripheral immune system.
In our culture system, we did not detect differences in the frequencies of CD11c, CD14, or G-CSFR-positive cells as indicators of myeloid differentiation. The cultures were not designed to probe lymphoid differentiation pathways. Children almost always develop lymphocytic leukemia whereas myeloid leukemia is a disease of the elderly, supporting the concept that lymphoid differentiation deteriorates with progressive age while less functional HPC can sustain myeloid differentiation (37). Gene expression studies and functional analysis of aged murine stem cells have suggested that HSC aging is accompanied by loss of stem cell quiescence, differential capacity to generate committed myeloid and lymphoid precursors, and systemic downregulation of genes mediating lymphoid specification (37). We would therefore expect that the molecular abnormalities seen in RA HPC would be most relevant for the generation of lymphocytes and less pertinent for myeloid repopulation.
Telomeres are useful tools for assessing the history of cellular stress exposure. Telomeric length in PBMC is associated with longevity (38). Inherited defects in telomere maintenance, e.g. in dyskeratosis congenita, lead to BM failure (39, 40) and progressive combined immunodeficiency (41). In RA patients, telomeres of mature CD4+ T cells are shortened by 1,000–1,500 bp, a defect already present in naïve cells untouched by immune responses. Premature telomere erosion in RA also involves granulocytes, pointing towards abnormalities in HPC biology (25). Although stem cells should be able to repair their telomeres to secure their immortality, these cells are not spared from the aging process. Telomere studies in patients after BM transplantation demonstrate accelerated loss, resulting from a small stem cell reserve and high demand for cell production (42). In RA, thirty-year-old patients have telomeric sequences normal for 60-year-old controls, suggesting profound differences in the HPC lifecycle and replication.
Through yet unknown mechanisms, chronic inflammation is considered to suppress BM function, raising the question of how the disease process impacts HPC dynamics. If the chronicity of RA causes HPC depletion, slowing of the cell cycle and age-inappropriate loss of telomeres, all of these defects should become more significant with long-standing disease. Also, patients with active disease should be distinguishable from those with well-controlled disease. However, for none of these clinical parameters could we demonstrate a correlation with HPC abnormalities. Our study therefore raises the question of whether abnormal generation, turnover, and survival of HPC may even precede the rheumatoid disease process, representing risk factors leading to autoimmunity.
How would diminution of CD34+ HPC and their failure to respond appropriately to growth factors influence immune function and possibly enhance the risk for chronic inflammatory, tissue-destructive disease? A series of studies has established that the immune system of RA patients is prematurely aged (43). The remodeled T-cell pool is filled with end-differentiated senescent memory T cells (44). Such T cells have altered tissue trafficking (45), are biased to pro-inflammatory action, and preferentially interact with mesenchymal cells, such as synovial fibroblasts, in peripheral tissues (46). Senescent CD4 T cells accumulate in unstable atherosclerotic plaque and have been implicated in plaque destabilization, providing a link to the increased cardiovascular risk of RA patients (47–49). Premature senescence of the RA immune system is not limited to the memory T-cell pool but also involves naïve T cells (43), the reserve pool of T cells protecting from infection and malignancy, and enabling tissue repair. Defects in naïve T cells may well explain why RA patients are at increased risk for lymphoma and infection (4). The current study redirects the search for premature immunosenescence away from the peripheral immune system toward the origins of all hematopoietic cells. Viewing RA as a disease of abnormal HPC function would provide unprecedented opportunities to re-address underlying etiologies and design novel therapeutic interventions.
Acknowledgments
The authors thank Dr. Mourad Tighiouart for statistical advice and Tamela Yeargin for editing the manuscript.
Grant numbers and sources of support: This work was funded in part by grants from the National Institutes of Health (RO1 AR 42527, RO1 AI 44142, and RO1 AR 41974).
References
- 1.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 2.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 3.Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how "high-grade" systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 4.Weyand CM, Goronzy JJ, Kurtin PJ. Lymphoma in rheumatoid arthritis: an immune system set up for failure. Arthritis Rheum. 2006;54:685–689. doi: 10.1002/art.21674. [DOI] [PubMed] [Google Scholar]
- 5.Weyand CM, Goronzy JJ. Stem cell aging and autoimmunity in rheumatoid arthritis. Trends Mol Med. 2004;10:426–433. doi: 10.1016/j.molmed.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Berthelot JM, Bataille R, Maugars Y, Prost A. Rheumatoid arthritis as a bone marrow disorder. Semin Arthritis Rheum. 1996;26:505–514. doi: 10.1016/s0049-0172(96)80039-4. [DOI] [PubMed] [Google Scholar]
- 7.Bowman SJ. Hematological manifestations of rheumatoid arthritis. Scand J Rheumatol. 2002;31:251–259. doi: 10.1080/030097402760375124. [DOI] [PubMed] [Google Scholar]
- 8.Burks EJ, Loughran TP., Jr Pathogenesis of neutropenia in large granular lymphocyte leukemia and Felty syndrome. Blood Rev. 2006;20:245–266. doi: 10.1016/j.blre.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Lamy T, Loughran TP., Jr Clinical features of large granular lymphocyte leukemia. Semin Hematol. 2003;40:185–195. doi: 10.1016/s0037-1963(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 10.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 11.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 13.McCulloch EA, Till JE. Perspectives on the properties of stem cells. Nat Med. 2005;11:1026–1028. doi: 10.1038/nm1005-1026. [DOI] [PubMed] [Google Scholar]
- 14.Jansen J, Hanks S, Thompson JM, Dugan MJ, Akard LP. Transplantation of hematopoietic stem cells from the peripheral blood. J Cell Mol Med. 2005;9:37–50. doi: 10.1111/j.1582-4934.2005.tb00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnett D, Janossy G, Lubenko A, Matutes E, Newland A, Reilly JT. Guideline for the flow cytometric enumeration of CD34+ haematopoietic stem cells. Prepared by the CD34+ haematopoietic stem cell working party. General Haematology Task Force of the British Committee for Standards in Haematology. Clin Lab Haematol. 1999;21:301–308. doi: 10.1046/j.1365-2257.1999.00253.x. [DOI] [PubMed] [Google Scholar]
- 16.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler BL, Valtieri M, Porada GA, De Maria R, Muller R, Masella B, et al. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999;285:1553–1558. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 18.Ruger B, Giurea A, Wanivenhaus AH, Zehetgruber H, Hollemann D, Yanagida G, et al. Endothelial precursor cells in the synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2004;50:2157–2166. doi: 10.1002/art.20506. [DOI] [PubMed] [Google Scholar]
- 19.Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D, et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111:204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 20.Hirohata S, Yanagida T, Nampei A, Kunugiza Y, Hashimoto H, Tomita T, et al. Enhanced generation of endothelial cells from CD34+ cells of the bone marrow in rheumatoid arthritis: possible role in synovial neovascularization. Arthritis Rheum. 2004;50:3888–3896. doi: 10.1002/art.20729. [DOI] [PubMed] [Google Scholar]
- 21.Papadaki HA, Marsh JC, Eliopoulos GD. Bone marrow stem cells and stromal cells in autoimmune cytopenias. Leuk Lymphoma. 2002;43:753–760. doi: 10.1080/10428190290016854. [DOI] [PubMed] [Google Scholar]
- 22.Snowden JA, Nink V, Cooley M, Zaunders J, Keir M, Wright L, et al. Composition and function of peripheral blood stem and progenitor cell harvests from patients with severe active rheumatoid arthritis. Br J Haematol. 1998;103:601–609. doi: 10.1046/j.1365-2141.1998.01073.x. [DOI] [PubMed] [Google Scholar]
- 23.Porta C, Caporali R, Epis O, Ramaioli I, Invernizzi R, Rovati B, et al. Impaired bone marrow hematopoietic progenitor cell function in rheumatoid arthritis patients candidated to autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:721–728. doi: 10.1038/sj.bmt.1704407. [DOI] [PubMed] [Google Scholar]
- 24.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schonland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100:13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 27.Soubrier M, Dougados M. Selecting criteria for monitoring patients with rheumatoid arthritis. Joint Bone Spine. 2005;72:129–134. doi: 10.1016/j.jbspin.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gil ME, Coetzer TL. Real-time quantitative PCR of telomere length. Mol Biotechnol. 2004;27:169–172. doi: 10.1385/MB:27:2:169. [DOI] [PubMed] [Google Scholar]
- 30.Ross J, Li L. Recent advances in understanding extrinsic control of hematopoietic stem cell fate. Curr Opin Hematol. 2006;13:237–242. doi: 10.1097/01.moh.0000231420.92782.8f. [DOI] [PubMed] [Google Scholar]
- 31.Greenwood MJ, Lansdorp PM. Telomeres, telomerase, and hematopoietic stem cell biology. Arch Med Res. 2003;34:489–495. doi: 10.1016/j.arcmed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 33.Udani VM. The continuum of stem cell transdifferentiation: possibility of hematopoietic stem cell plasticity with concurrent CD45 expression. Stem Cells Dev. 2006;15:1–3. doi: 10.1089/scd.2006.15.1. [DOI] [PubMed] [Google Scholar]
- 34.Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001;167:1758–1768. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- 35.Jablonska B, Ford D, Trisler D, Pessac B. The growth capacity of bone marrow CD34 positive cells in culture is drastically reduced in a murine model of Down syndrome. C R Biol. 2006;329:726–732. doi: 10.1016/j.crvi.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, et al. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 39.Lieberman L, Dror Y. Advances in understanding the genetic basis for bone-marrow failure. Curr Opin Pediatr. 2006;18:15–21. doi: 10.1097/01.mop.0000192520.48411.fa. [DOI] [PubMed] [Google Scholar]
- 40.Vulliamy T, Dokal I. Dyskeratosis congenita. Semin Hematol. 2006;43:157–166. doi: 10.1053/j.seminhematol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Sznajer Y, Baumann C, David A, Journel H, Lacombe D, Perel Y, et al. Further delineation of the congenital form of X-linked dyskeratosis congenita (Hoyeraal-Hreidarsson syndrome) Eur J Pediatr. 2003;162:863–867. doi: 10.1007/s00431-003-1317-5. [DOI] [PubMed] [Google Scholar]
- 42.Brummendorf TH, Balabanov S. Telomere length dynamics in normal hematopoiesis and in disease states characterized by increased stem cell turnover. Leukemia. 2006;20:1706–1716. doi: 10.1038/sj.leu.2404339. [DOI] [PubMed] [Google Scholar]
- 43.Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Nakajima T, Goronzy JJ, Weyand CM. Tissue trafficking patterns of effector memory CD4+ T cells in rheumatoid arthritis. Arthritis Rheum. 2005;52:3839–3849. doi: 10.1002/art.21482. [DOI] [PubMed] [Google Scholar]
- 46.Sawai H, Park YW, Roberson J, Imai T, Goronzy JJ, Weyand CM. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2005;52:1392–1401. doi: 10.1002/art.21140. [DOI] [PubMed] [Google Scholar]
- 47.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 48.Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, et al. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res. 2006;98:524–531. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]