Abstract
Background
. Th1 cells are implicated in numerous pulmonary inflammatory disorders, and adoptive transfer of alloreactive Th1 cells mediates lung injury and inflammation in mice. In response to Th1-mediated immune injury, CXCR3 ligands IP10 and MIG are markedly induced. Because Th1 cells express high levels of CXCR3, their recruitment and activity may be influenced by CXCR3 ligands.
Methods
. To examine the role of CXCR3 ligands, we inhibited CXCR3-ligand interaction by two approaches: 1) antibody ablation of CXCR3 ligands IP10 and MIG, and 2) use of cxcr3−/− mice.
Results
. Antibody neutralization of IP10 and MIG reduced Th1-cell mediated lung inflammation but did not alter Th1 cell influx in the lung. In contrast, a lack of CXCR3 on host cells had no effect on Th1 cells influx or acute inflammation. In vitro, ablation of endogenous IP10 and MIG inhibited antigen-mediated Th1 cell proliferation.
Conclusion
. These results suggest that the influx of alloreactive Th1 cells into the lung does not require CXCR3 ligands, but that these chemokines do affect Th1 cell proliferation and activity within the affected tissue. Other CXCR3+ leukocytes do not contribute to acute alloimmune injury.
Key Works: IP10, MIG, Macrophage
Background
Th1 lymphocytes have been implicated in a number of pulmonary diseases, including sarcoidosis (1, 2), hypersensitivity pneumonitis (3), idiopathic pneumonia syndrome (4), and allograft rejection (5, 6). To investigate how these cells initiate and amplify lung inflammation, we used a established murine model of lung injury induced by adoptive transfer of cloned allogeneic Th1 cells that recognize either Ly5a or Ly5b (7–13). These cells accumulate in the lung and induce a lung injury characterized by a mononuclear cell vasculitis and alveolitis, resembling graft versus host disease (7). Most of the inflammatory cells are host-derived, and the mononuclear inflammatory response is independent of host lymphocytes, suggesting that monocyte recruitment contributes to lung injury in this model (8). We previously reported that CXCR3 ligands, MIG (CXCL9/monokine induced by interferon-γ) and IP10 (CXCL10/interferon-γ-inducible 10-kD protein), are markedly induced in the lung 24 h after transfer of Th1 cells (11). These CXCR3 ligands localize to the inflammatory lesions and are expressed by macrophages, endothelium, and bronchial and alveolar epithelia. We used this model to assess if CXCR3 receptor-ligand interactions contribute to the pathogenesis of Th1 cell induced inflammation.
Th1 cells are the predominant cells that express CXCR3, and not surprisingly, increased CXCR3 ligands are associated with Th1-mediated inflammatory conditions (1, 2, 5, 6, 14, 15). A hallmark of allograft rejection is mononuclear cell infiltration, and CXCR3 and its ligands play an important and non-redundant role in this process. Mice lacking CXCR3 have prolonged allograft survival with reduced numbers of infiltrating CD4+, CD8+ T cells and macrophages (16). Allografts from IP10-deficient mice also have diminished alloreactivity (17). Clinically, these pathways also appear important in lung transplant rejection, as investigators have demonstrated an association between both acute and chronic rejection and elevated levels of IP10 and MIG in bronchoalveolar lavage fluid (BALF) (5, 18). In an experimental model of Idiopathic Pneumonia Syndrome after bone marrow transplantation, IP10 and MIG are increased in BALF (4). Hence, these CXC chemokines may be potential therapeutic targets to mitigate the injury induced by alloimmune responses.
Although Th1 cells express high levels of CXCR3, other leukocytes, such as eosinophils (19, 20), dendritic cells (21), and monocytes isolated from lymph nodes (22), also express CXCR3 to some degree. The differential expression of CXCR3 among leukocytes may be an important component of the mechanisms that direct their recruitment to specific organs. For example, CXCR3 ligands function in the recruitment of a subpopulation of monocytes to lymph nodes (22). More recently, other non-chemotactic functions have been ascribed to chemokines. In the case of CXCR3 ligands, they have been associated with angiogenesis (23), eosinophil degranulation (19), and augmented T lymphocyte proliferation in vitro (4, 24). Thus, CXCR3 and its two principal ligands, IP10 and MIG, may not only affect directed chemotaxis, but also influence activation of effector cells within an inflammatory setting.
In this study, we found that ablation of CXCR3 ligands markedly diminished alloimmune-mediated lung inflammation but did not affect the ability of the transferred Th1 cells to home to the lung nor the associated acute injury. Furthermore, CXCR3 on host cells was not required for Th1 cell-induced lung inflammation. These results indicate that IP10 and MIG function more to affect Th1 cell activity in the lung than to direct chemotaxis into this organ. In support of this conclusion, we also found that neutralization of IP10 and MIG inhibited Th1 cell proliferation in vitro, suggesting a potential mechanism whereby disruption of the CXCR3:CXCL axis may mitigate Th1 cell-induced lung inflammation.
Methods and Methods
Mice
C57BL/6(Ly5b), C57BL/6(Ly5a), and C57BL/6 RAG1-null mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 cxcr3−/− mice were generated and genotyped as described (16). All mice were housed in microisolator cages under specific pathogen-free conditions. All animal experiments were approved by the IACUC at the Fred Hutchinson Cancer Research Center.
Cell Culture
T cell clones specific for the Ly5a (clone 8F5) and Ly5b (clone 1A4) alleles were developed and maintained as described (7). In brief, Ly5b mice (C57BL/6) and congenic Ly5a mice were immunized with 13-mer Ly5a and Ly5b peptides, respectively. These peptides span the 3-amino acid motif coded by the polymorphism that define these alleles. CD4+ helper T cells specific for antigenic polymorphisms were elicited and cloned by limiting dilution. These T cell clones have a Th1 phenotype and produce interferon-γ and TNF-α. They are noncytolytic in vitro. The cells were maintained by periodic stimulation with Ly5 peptide or with allogeneic irradiated splenocytes in the presence of congenic irradiated splenocytes and grown in the presence of IL-2 (10 U/ml). Activated cells were harvested 1 day after stimulation, and resting cells were harvested 14–21 days after stimulation.
Induction of Lung Injury
Activated and resting cloned T cells (2, 5, or 10 × 106 cells in 0.5 ml PBS) were injected into the lateral tail vein of recipient mice. In some experiments, mice were pretreated with neutralizing rabbit antisera to murine IP10 or MIG via intraperitoneal injection. The antisera, which were generously provided by Dr. Thomas A. Hamilton, Cleveland Clinic Foundation, are specific for IP10 and MIG and do not have cross-reactivity to other known chemokines (15, 25). Ly5a mice (n = 4–6/group) were injected with neutralizing antiserum to murine IP10 (250 μl) and MIG (250 μl) or control rabbit serum (500 μl) 6 h before Th1 cell transfer. Following pretreatment, the mice received 5 × 106 anti-Ly5a Th1 cells via tail-vein injection.
Three days after Th1 cell transfer, mice were anesthetized with Avertin and exsanguinated by renal artery transection. The thorax was opened by midline sternotomy, and the trachea exposed and cannulated with an 18 g polypropylene catheter. The lungs were lavaged three times with 1 ml aliquots of sterile Ca/Mg-free PBS containing 0.6 mM EDTA. Bronchoalveolar lavage fluid (BALF) was centrifuged at 500×g to remove cells, and total protein in the supernatants was quantified by BCA assay (Sigma). The cell pellet was resuspended in 500 ml PBS/0.6 mM EDTA and counted using trypan blue exclusion as a measure of viability. Differential cell counts were performed on Wright-stained cytospun preparations.
Histology
At days 3, 7 and 14 after T-cell transfer, mice were anesthetized with Avertin (Aldrich) and exsanguinated by renal artery transection. Lungs were excised, inflated at 25 cm H20 pressure with 4% paraformaldehyde, fixed overnight, and embedded in paraffin. Lung sections (5 μm) were stained with hematoxylin and eosin. The histopathology was scored according to the extent and severity of vasculitis and inflammatory cell infiltration as follows: 0 = no abnormality; 1 = minimal inflammation involving <10% of venules; 2 = mild vasculitis involving 10–25% of venules; 3 = moderate vasculitis and perivascular inflammation involving >25% of venules; and 4 = severe vasculitis and perivasculitis with alveolar and interstitial inflammation. All slides were read and coded by 2 investigators in a blinded fashion.
Immunostaining
Sections were deparaffinized in HistoClear (National Diagnostics, Atlanta GA) and rehydrated through graded ethanol. Antigen retrieval was performed using antigen unmasking solution (Vector Labs, Burlingame CA) heated to 90°C for 30 min. Endogenous peroxidase was blocked using 3% hydrogen peroxide for 10 min. Avidin and Biotin block (Vector Labs, Burlingame, CA) was applied to each section for 10 min. Sections were incubated with primary antibody for 1 h at 37°C, and secondary antibody for 1 h at ambient temperature. After each incubation, the sections were washed three times in PBS. Secondary antibodies were labeled with the Vectastain ABC kit and colorimetric detection was done with diaminobenzidine staining per the manufacturer’s protocol (Vector Labs). Primary antibody was rat anti-mouse MAC-2 (a gift of Dr. Elaine Raines, University of Washington), and the secondary antibody was biotin-conjugated donkey anti-rat (R&D Systems, Minneapolis MN).
Flow Cytometry
Mouse lungs were minced into 1-mm pieces with scissors and then treated with bacterial collagenase (2 mg/ml; Sigma) and DNase (60 U/ml) for 1 h at 37°C on a rocking platform. Digested lungs were filtered through 70-μm nylon cell strainers (Becton Dickinson) and passed through 25 g needles. Released cells were collected by centrifugation and were washed in 0.5% BSA/PBS and incubated with rat IgG2b anti-mouse CD16/CD32 mAb (BD Biosciences) and biotin-labeled anti-mouse CD3e (BD Biosciences) at a concentration of 1 μg per 106 cells. The CD3+ cells were removed from the cellular suspension by column selection with streptavidin paramagnetic beads (Miltenyi Biotec). The remaining (negative) fraction was incubated with CD11b-binding paramagnetic beads, and CD11b+ cells were separated using a magnetic column. The CD3−/CD11b+ cells were incubated with rat IgG2b anti-mouse F4/80-APC monoclonal antibody (Caltag), rat IgG2b anti-mouse CD11b-PE monoclonal antibody (BD Biosciences), goat anti-mouse CXCR3 polyclonal antibody (Y-19; Santa Cruz), or isotype control antibodies (R&D, BD Biosciences). The secondary antibody for CXCR3 was FITC-conjugated mouse anti-goat IgG (Jackson Immunochemicals). Cells were analyzed using the Beckman Flow Cytometer, and analysis was performed using CellQuest software.
In Vivo Migration
8F5 T-cells were suspended in RPMI, 20% FBS at 107 cells/ml and incubated with 51Cr (20 μCi/ml; Amersham; t1/2 = 27.8 d) at 37°C for 1 h. Cells were resuspended in PBS after a Nycodenz wash. A total of 107 51Cr-labeled T-cells were injected into the tail vein of 24 Ly5a mice, of which 12 were pretreated with antisera to IP10 (250 μl) and MIG (250 μl) the day before Th1 cell transfer. Mice were sacrificed at 6 and 24 h after T-cell injection, and radioactivity in the blood, spleen, liver and lung was analyzed by gamma counting.
In Vitro T-Cell Proliferation Assay
A total of 5 × 104 T cells (8F5 clone) and 2.5 × 105 irradiated congenic splenocytes from C57BL/6 mice were plated in 96-well plates. The T cells were stimulated with Ly5a peptide (0.1 mg/ml), incubated at 37°C for 56 h, and pulsed for 16 h with 1.0 μCi of [3H]thymidine (Amersham). In separate experiments, neutralization experiments were done by adding antisera to MIG or IP10 or control rabbit serum at a dilution of 1:50 and 1:100 (5–8 wells/experiment). For stimulation experiments with MIG and IP10 (R&D Biosystems), the feeder cell and antigen concentrations were lowered to 1.25 × 105 cells/ml and 0.05 mg/ml, respectively, to avoid over-stimulating the Th1 cells. MIG (0.25, 0.5, 1.0 μg/ml), IP10 (0.5, 1.0 μg/ml), or carrier control (PBS/0.1% BSA) was added to stimulated Th1-cells (5–8 wells/experiment), incubated at 37°C for 56 h, and pulsed for 16 h with 1.0 μCi [3H]thymidine. All samples were harvested using an automated cell harvester and analyzed for radioactivity with an automated plate reader.
Results
Ablation of IP10 and MIG Reduces Immune-mediated Lung Inflammation
We used a established murine model of lung injury induced by adoptive transfer of cloned allogeneic Th1 cells that recognize either Ly5a or Ly5b (7–13). These cells accumulate in the lung and induce a lung injury characterized by a host-derived mononuclear cell vasculitis and alveolitis, resembling graft versus host disease. This injury is dependent on activation of the Th1 cells by their antigen, Ly5a or Ly5b. As shown in figure 1F and consistent with our previous work (8, 13), the infusion of non-alloreactive Th1 cells (anti-Ly5b cells into Ly5a mice) failed to elicit visible lung inflammation.
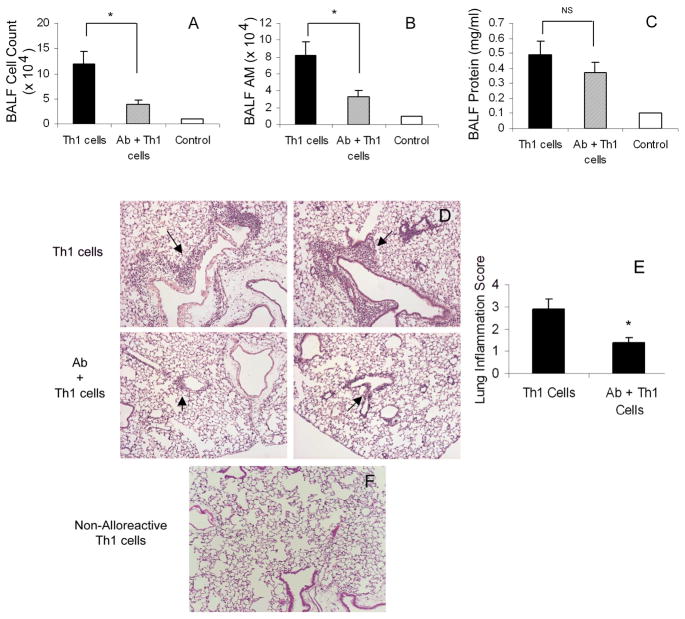
Figure 1. Th1-induced Lung Injury.
Ly5a mice were injected intravenously with 5 ×106 anti-Ly5a Th1 cells 6 h after IP administration of nonimmune rabbit serum (Th1 cells) or IP10/MIG antisera (Ab + Th1). Control mice received nonimmune rabbit serum but no Th1 cells (Control). Mice were sacrificed 3 days after Th1 cell transfer, and the lungs were lavaged and processed for histology. A) Total BALF cells. B) Total BALF alveolar macrophage (AM) cell numbers. C) BALF protein concentration. D) Representative images from mice pretreated with nonimmune rabbit serum or IP10/MIG neutralizing antisera showing reduced Th1-induced mononuclear cell vasculitis (arrows). E) Lung Inflammation score (see methods) of mice pretreated with either nonimmune rabbit serum or IP10/MIG antisera. Values are mean ± SD, *p <0.05. F) Infusion of non-alloreactive Th1 cells (anti-Ly5b) into Ly5a mice. Lungs were collected 3 days after transfer and processed for histology. The representative image demonstrates no obvious lung inflammation at day 3.
We previously demonstrated that levels are IP10 and MIG are markedly elevated in lungs 24 h following transfer of allogeneic Th1-cells (11). To assess if these CXCR3 ligands contribute to immune-mediated lung injury, Ly5a mice were pretreated 6 h before anti-Ly5a Th1-cell transfer with either control rabbit serum or with a combination of IP10 and MIG antisera. At 3 days post-immune injury, mice pretreated with neutralizing IP10/MIG antisera had reduced lung injury compared to control mice. Antisera-treated mice had significantly less lung inflammation as demonstrated by a 3-fold reduction inflammatory cells recovered from BALF (Fig. 1A,B) and overtly diminished airway inflammation in sections of whole lung (Fig. 1D,E). In contrast, although we saw a trend toward protection, total BALF protein did not differ significantly between immune-injured and antisera-treated mice (Fig. 1C). These data indicate that CXCR3 ligands promote the early inflammatory response to Th1 cells but that these chemokines are not critical for acute injury manifested by vascular/epithelial permeability.
Immune Injury Does Not Selectively Induce Influx of CXCR3+ Macrophages
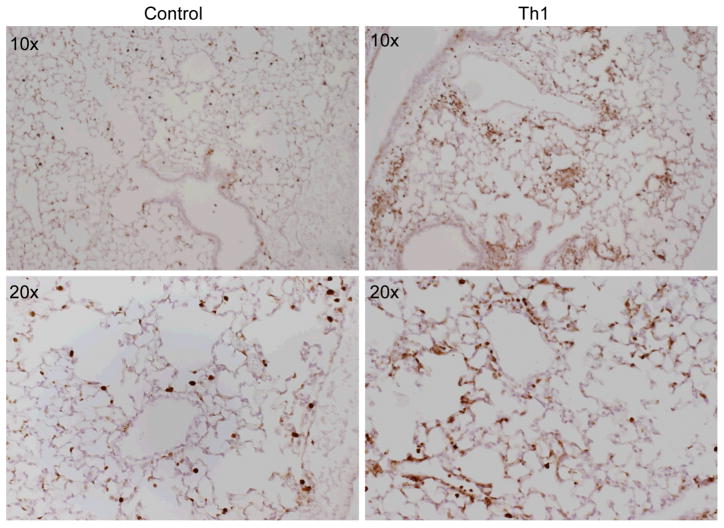
We previously showed that a majority of the inflammatory cells recruited to the lung after allogeneic Th1 infusion are host-derived (8). This mononuclear inflammatory response occurs in the absence of host lymphocytes (8), suggesting that macrophage recruitment contributes to lung inflammation. To confirm these observations, we performed immunohistochemistry using an anti-macrophage antibody, MAC-2 (26, 27) on lung tissue obtained from mice treated with allogeneic Th1 cells 3 days prior to harvest (anti-Ly5a cells into Ly5a mice) and control mice (untreated). The results of the immunostaining (Fig. 2) demonstrate that a majority of the cellular infiltrates observed at day 3 is comprised of macrophages.
Figure 2. Increased Macrophage Recruitment 3 days Post-Th1 Infusion.
These photomicrographs are representative images of whole lung stained with anti-macrophage antibody, MAC-2 (a gift of Elaine Raines, University of Washington). Control lung represents untreated mice, Th1 lung represents Ly5a mice that have received allogeneic Th1-cells (anti-Ly5a) intravenously 3 days before harvest. Lungs have been fixed in 4% paraformaldehyde and paraffin-embedded. The primary antibody is rat anti-mouse MAC-2, the secondary antibody is biotin-labeled donkey anti-rat ab. Colorimetric detection was done with diaminobenzidine staining (Vector Lab) and the tissue counterstained with hematoxylin.
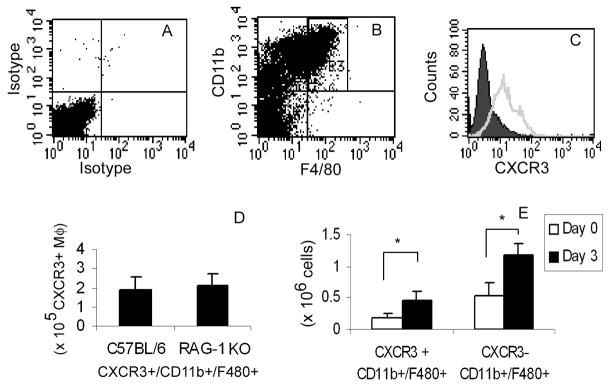
To assess if Th1-cell injury and the resultant burst in IP10 and MIG levels mediate a selective influx of CXCR3+ macrophages, we quantified the levels of these cells in the lung by FACS. Macrophages were isolated from whole lung digests of control (untreated) mice using magnetic affinity selection to remove CD3+ lymphocytes and enrich for CD11b+ leukocytes. The collected cells were analyzed by FACS with antibodies specific for CD11b, F4/80, and CXCR3 (Fig. 3B,C), as well as isotope control antibodies (Fig. 3A). We identified a population of CXCR3-expressing macrophages representing approximately 2 × 105 cells per uninjured lung (or 30.8 ± 1.7% of CD11b+/F480+ cells; Fig. 3C). To ensure no contamination with CXCR3-expressing lymphocytes, we used the same affinity isolation procedure to isolate myeloid cells from lymphocyte-deficient RAG1-null mice. These mice have the same number of CXCR3-expressing macrophages as do Ly5a mice (Fig. 3D).
Figure 3. Isolation of Pulmonary CXCR3+ Macrophages.
CD3-depleted/CD11b+ selected cells from whole lung digests were stained with anti-CD11b-PE, anti-F4-80-APC, goat anti-mCXCR3, or control antibody. Dead cells were gated out by 7-AAD staining. Panels A-D represent cells isolated from naïve mice. A) Cells stained with isotype control antibodies. B) Identification of macrophages co-expressing CD11b and F4/80 representing 23.4 ± 0.4% of live cells (R3). C) CD11b+/F4-80+ cells stained with control IgG (solid) or CXCR3 antibody (open). D) Quantification of CXCR3+/CD11b+/F480+ cells (CXCR3+ MΦ) from C57BL/6 mice and RAG-1 knock-out mice. E) Quantification of CXCR3- and CXCR3+ Macrophages at Day 0 and Day 3 post-Th1 cell transfer. Values are mean ± SD (*p value <0.05).
We then assessed if CXCR3 expression or the numbers of CXCR3+ macrophages in the lung change after Th1-injury. The mean fluorescent intensity of CXCR3 on isolated macrophages did not differ between cells from lungs of control or immune-injured mice (125 ± 25 vs. 103 ±17 control vs. Th1-treated mice respectively, p=0.49), indicating that the expression of CXCR3 per macrophage is not affected by alloreactive Th1 cells. In contrast, the number of CXCR3-expressing macrophages increased about 2-fold in response to immune-mediated injury. The increase in macrophage numbers was similar to the increase of other (i.e., CXCR3−) myeloid cell populations following Th1 cell-induced injury (Fig. 3E). These findings indicate that host CXCR3+ cells are not selectively recruited to the lung following Th1 cell-induced injury.
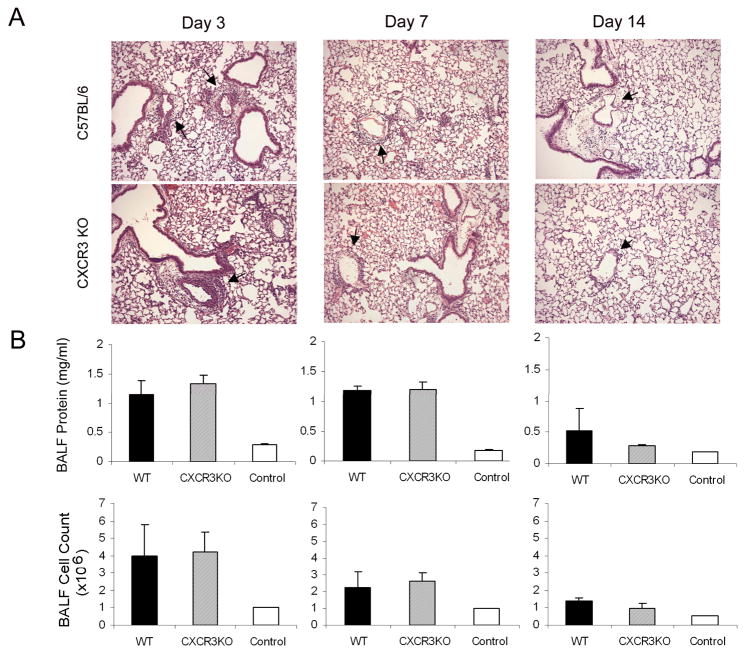
Th1 Cell-induced Inflammation and Injury are Not Reduced in cxcr3−/− Mice
Antibody-neutralization of CXCR3 ligands may reduce lung inflammation by blocking the recruitment of host CXCR3+ myeloid cells or by affecting the alloreactive activity of the transferred Th1 cells. To discern between these possibilities, we assessed Th1 cell-mediated inflammation in recipient cxcr3−/− mice. Th1 cells (anti-Ly5b) were injected into wildtype (Ly5b) C57BL/6 and age and sex-matched cxcr3−/− mice. Lungs and BALF were harvested 3, 7, and 14 days later. We saw no difference between genotypes in total lung inflammation, BALF protein, or histopathology at any time post-transfer (Fig. 4). These data indicate that although CXCR3 is expressed on myeloid cells, this chemokine receptor is not essential for leukocyte influx into the lung following alloimmune injury. Although non-immune serum was used as a control, we cannot exclude non-specific effects of the anti-serum, including T cell priming or loss of cell subsets. However, others using these antisera have not reported any non-specific effects, including T cell priming (15).
Figure 4. Th1-induced Injury in cxcr3−/− Mice.
C57BL/6 (WT) and cxcr3−/− (CXCR3 KO) mice received 2 × 106 anti-Ly5b Th1 cells intravenously. Lungs were harvested for lavage and histopathology 3, 7, and 14 days later. A) Representative images from C57BL/6 (top) and cxcr3−/− mice (bottom) show similar patterns of inflammation at all time points. Pulmonary vasculitis (arrows) seen at days 3 and 7, nearly resolved by day 14. B) BALF protein and cell counts from C57BL/6, CXCR3 KO, and control (no Th1 cells) mice. Values are mean ± SD (n = 5).
Neutralization of IP10 and MIG Does Not Alter Th1 Cell Influx into Lung
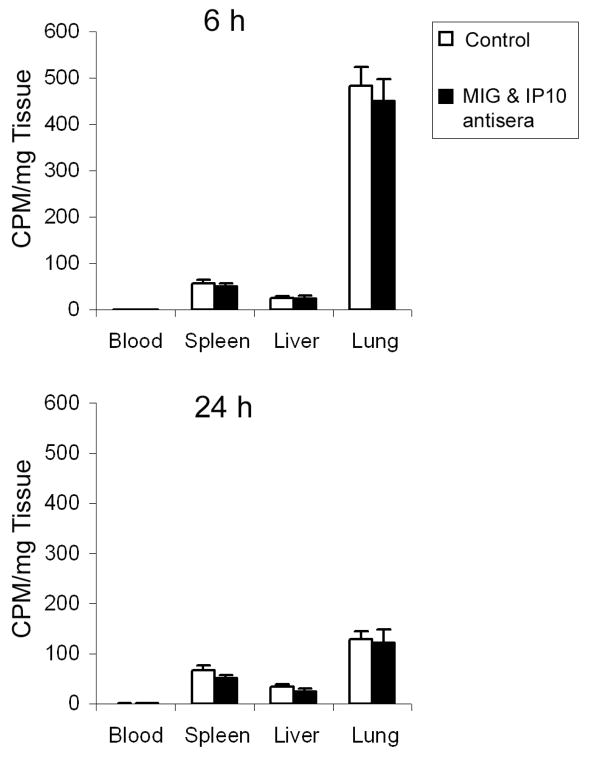
The observations that cxcr3−/− mice were not protected against alloimmune-mediated injury (Fig. 4), whereas antibody neutralization of IP10 and MIG did block host inflammation (Fig. 1) but did not affect host injury (Fig. 1C) suggest that the CXCR3 ligands function within the lung environment to modulate Th1 cell activity. To assess this idea, we quantified Th1 cell recruitment to the lung. For this, we tracked the distribution of 51Cr-labeled Th1 cells after adoptive transfer. At 6 h post-transfer, most Th1 cells had moved into the lung, with fewer cells detected in the spleen and liver (Fig. 5). At 24 h post-transfer, radioactivity in the lung decreased about 4-fold, indicating clearance of Th1 cells, with little change in the low levels in spleen and liver. Antibody neutralization of IP10 and MIG did not affect the influx of Th1 cells into lung or the other organs nor did it affect Th1 cell retention (Fig. 5). These findings indicate that CXCR3 ligands are not critical for Th1 cells to home into the lung.
Figure 5. Inhibition of IP10 and MIG Does Not Alter Th1 Homing.
Mice were injected with 51Cr-labeled Th1 cells and 6 h later were treated with rabbit serum (open bars) or IP10 and MIG antisera (black bars). Mice were sacrificed 6 h (upper panel) or 24 h (lower panel) after Th1 cell transfer. The blood, spleen, liver and lung were harvested from each mouse and weighed, and radioactivity was counted. Values are mean ± SD (n = 6).
IP10 and MIG Enhance Th1 Cell Proliferation
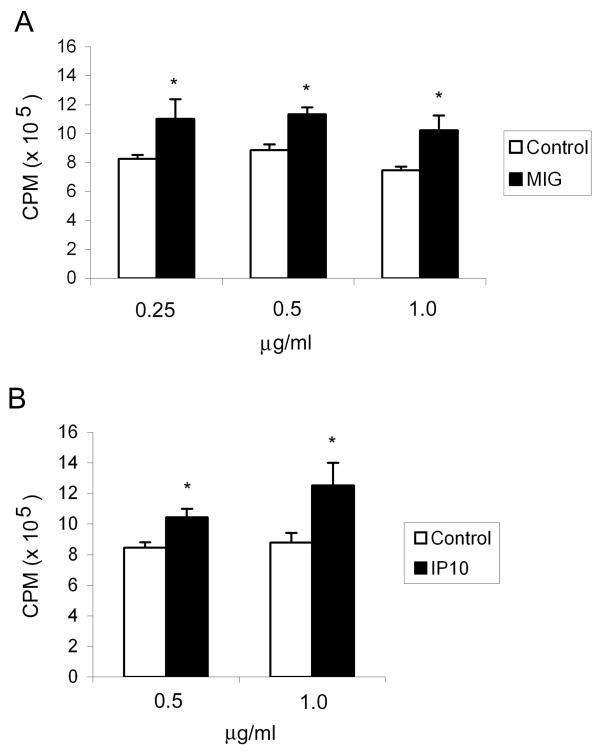
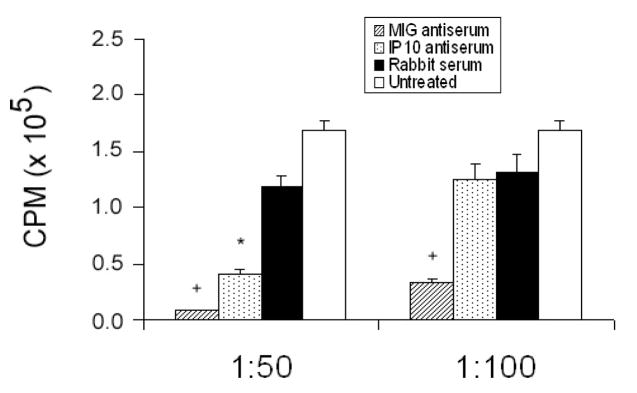
To assess if IP10 and MIG affect Th1 cell activity, we measured antigen-specific proliferation in the presence of recombinant IP10 or MIG or antisera to IP10 and MIG. Anti-Ly5a Th1 cells were stimulated with Ly5a peptide in the presence of irradiated congenic splenocytes. In the absence of Ly5a antigen, Th1-cells do not proliferate. At the time of antigen stimulation, MIG (0.25–1.0 μg/ml), IP10 (0.5–1.0 μg/ml), or carrier control were added to the cultures. Both MIG and IP10 augmented Th1-cell proliferation about 33% (Fig. 6A) and 40% (Fig. 6B), respectively. Although this enhancement by exogenous MIG and IP10 may seem modest, endogenous CXCR3 ligands could potentiate antigen-induced Th1 proliferation. Indeed, both MIG and IP10 antisera significantly inhibited Th1 cell proliferation, with virtually no Th1 cell proliferation detected at a dilution of 1:50 of either antibody (Fig. 7).
Figure 6. IP10 and MIG Stimulate Th1 Cell Proliferation.
Anti-Ly5a Th1-cells were stimulated with Ly5a peptide in the presence of IP10, MIG or carrier control. The cells were incubated at 37°C for 56 h and pulsed for 16 h with 1.0 μCi [3H]thymidine. The results are the mean ± SD of 3 experiments, with 5–8 replicates per experiment. *p <0.05
Figure 7. IP10 and MIG Ablation Inhibits Th1-cell Proliferation.
Anti-Ly5a Th1-cells were stimulated with Ly5a peptide in the presence of irradiated congenic splenocytes. At the time of stimulation, antisera to IP10 and MIG or control rabbit serum was added at a dilution of 1:50 or 1:100. Untreated samples received Ly5a peptide only. The cells were incubated at 37°C for 56 h and pulsed for 116 h with 1.0 μCi [3H]thymidine. The results are the mean ± SD of 3 experiments, with 5–8 replicates per experiment. p <0.05: anti-IP10 (*) or anti-MIG (+) vs. control rabbit serum.
Discussion
This study was designed to investigate the role of CXCR3 and its two principal ligands, IP10 and MIG, in a murine model of lung injury induced by adoptive transfer of alloreactive Th1 cells. This model replicates the early inflammatory response to Th1 cells, which is relevant to numerous Th1-mediated inflammatory disorders, including allograft rejection and Idiopathic Pneumonia Syndrome. Our earlier work showed that IP10 and MIG are highly induced in the lung 24 h following Th1 cell transfer and localize to the inflammatory foci within the lung (11). Because the induction of these chemokines precedes the host leukocyte influx, we hypothesized that CXCR3 ligands control the Th1 cells’ ability to induce lung inflammation. Indeed, if we neutralized the IP10 and MIG with functional blocking antisera, we markedly attenuated the host inflammatory response without altering donor Th1 cell influx or retention in the lung. Furthermore, inflammation and injury were unaffected in cxcr3−/− mice, and we did not observe selective recruitment of CXCR3+ macrophages or other leukocytes following Th1-induced lung injury. Together, these findings indicate that CXCR3-expression by host leukocytes is not required for the inflammatory response to Th1 cells and indicate that CXCR3 ligands affect donor Th1 cell function.
Although IP10 and MIG neutralization did not affect Th1 cell recruitment, these ligands have been shown by others to function in T lymphocyte recruitment (5, 15, 28, 29). In our model, other pathways may be more important for the initial homing of Th1 cells to the lung. As shown in other tissues (30, 31), Th1 cell recruitment to the lung involves P- and E- selectin expression by the vascular endothelium and selectin ligands on Th1 lymphocytes (10). Other adhesion proteins, such as lymphocyte function-associated antigen (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) are also important in Th1 lymphocyte adhesion and pulmonary localization (12, 32). In addition, in our model, Th1 cell localization to the lung occurs early (within 1 h of adoptive transfer) and precedes IP10 and MIG induction. Thus, it is not surprising that initial homing of the Th1 cells was unaffected by inhibition of IP10 and MIG. In other alloimmune injury models (such as Idiopathic Pneumonia Syndrome), in which there is a source of circulating donor Th1 cells, CXCR3 and its ligands are involved in sustained donor lymphocyte recruitment (4).
Because antibody ablation of IP10 and MIG reduced Th1 cell-induced lung injury without affecting the recruitment of donor Th1 cells, we speculated that these ligands control Th1 cell function. Indeed, we demonstrated that Th1 cell proliferation was enhanced by the addition of IP10 or MIG proteins. Although this response was arguably marginal and required high doses of chemokines, the required addition of Ly5a antigen potently stimulated the cells, which could not proliferate much faster. In contrast, Th1 cell proliferation was substantially inhibited by IP10 and MIG antisera, underscoring an important role of these chemokines in mediating alloimmune lung injury. Due to low recovery of Th1 cells from the lung at 3 days post transfer, we were not able to assess proliferation rate in vivo (using CSFE-labeled T cells or thymidine incorporation, data not shown). Similarly, other investigators have been unable to demonstrate CXCR3-dependent lymphocyte proliferation in vivo, despite clear effects in vitro (4, 24). Alternatively, CXCR3 ligands may also mediate survival of effector cells as suggested in CXCR3-expressing myeloma cells (33) and shown for other chemokine ligands, such as CXCL12 (34, 35). Future studies will need to address the role of enhance Th1-cell survival in our model.
Our data are consistent with other published reports in which T lymphocyte activation and proliferation are enhanced by stimulating the CXCR3 pathway or repressed by inhibiting this system (4, 24). In contrast to our findings, these studies and others (15, 36) also find CXCR3-dependent recruitment of lymphocytes. However, these models differ from ours in that they involve transplant of cardiac or marrow allografts, rather than a simplified single infusion of allogeneic Th1 cells. Unlike our model in which host lymphocytes are not required for lung inflammation (8) and donor lymphocytes traffic to the lung independent of CXCR3 ligands, allograft rejection is characterized by intense CD8+ and modest CD4+ T cell infiltration. Thus, it is not surprising that inhibition of CXCR3 or its ligands reduces recruitment of these CXCR3-expressing lymphocytes in other more complex allograft models (15).
Furthermore, in these allograft models, any in vivo anti-proliferative effect of IP10 or MIG antisera may be compensated by other proinflammatory pathways. Our simplified model of Th1 cell induced lung inflammation allows us to dissect the effect of donor and host-specific expression of CXCR3, and provides further support for a role of the CXCR3/ligand axis in lung inflammation that is independent of chemotaxis.
In our model, adoptive transfer of allogeneic Th1 cells results in a macrophage-predominant vasculitis and alveolitis by 3 days. Although the absolute number of donor Th1 cells in these lesions may be small relative to the number of cells in the infiltrate, these Th1 cells are capable of amplifying inflammatory signals and leukocyte recruitment through their effector functions. Thus, early inhibition of their activation or proliferation can have significant down-stream effects on host inflammatory cell recruitment and lung inflammation.
In summary, we found that host expression of CXCR3 is dispensable for Th1 cell induced lung inflammation. However, we found that neutralizing antisera to IP10 and MIG decreased Th1 cell induced lung inflammation without altering donor Th1 cell recruitment or retention within the lung. These ligands augment Th1 cell proliferation in vitro, a process that can be abrogated by neutralizing antisera, indicating another mechanism by which CXCR3 ligands may contribute to Th1 cell induced lung injury. These findings suggest that neutralization of CXCR3 ligands may be an effective target for inhibiting Th1 cell mediated lung inflammation, and it may do so by multiple mechanisms.
Acknowledgments
This work was supported by R01-HL069442, T32-HL07287, and F32-HL078110.
We thank Dr. Thomas Hamilton (Cleveland Clinic Foundation) for providing IP10 and MIG antisera, Elaine Raines (University of Washington) for providing the Mac-2 antibody, and Drs. William Parks and Lynn Schnapp (University of Washington) for their careful review of the manuscript.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- IP10
Interferon-γ-inducible 10-kD protein
- I-TAC
interferon-γ-inducible T cell α chemoattractant
- MIG
monokine induced by interferon-γ
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Authors Contributions
AM and JC designed the study and wrote the manuscript. AM and KB carried out the experiments. BL generated and provided CXCR3-null mice. All the authors have read and approved the final manuscript.
References
- 1.Agostini C, Cassatella M, Zambello R, Trentin L, Gasperini S, Perin A, Piazza F, Siviero M, Facco M, Dziejman M, Chilosi M, Qin S, Luster AD, Semenzato G. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161(11):6413–20. [PubMed] [Google Scholar]
- 2.Prasse A, Georges CG, Biller H, Hamm H, Matthys H, Luttmann W, Virchow JC., Jr Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin Exp Immunol. 2000;122(2):241–8. doi: 10.1046/j.1365-2249.2000.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamasaki H, Ando M, Brazer W, Center DM, Cruikshank WW. Polarized type 1 cytokine profile in bronchoalveolar lavage T cells of patients with hypersensitivity pneumonitis. J Immunol. 1999;163(6):3516–23. [PubMed] [Google Scholar]
- 4.Hildebrandt GC, Corrion LA, Olkiewicz KM, Lu B, Lowler K, Duffner UA, Moore BB, Kuziel WA, Liu C, Cooke KR. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J Immunol. 2004;173(3):2050–9. doi: 10.4049/jimmunol.173.3.2050. [DOI] [PubMed] [Google Scholar]
- 5.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158(5):1703–11. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stinn JL, Taylor MK, Becker G, Nagano H, Hasegawa S, Furakawa Y, Shimizu K, Libby P, Mitchell RN. Interferon-gamma-secreting T-cell populations in rejecting murine cardiac allografts: assessment by flow cytometry. Am J Pathol. 1998;153(5):1383–92. doi: 10.1016/s0002-9440(10)65725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Chatta GS, Rubin WD, Clark JG, Hackman RC, Madtes DK, Ligitt DH, Kusunoki Y, Martin PJ, Cheever MA. T cells specific for a polymorphic segment of CD45 induce graft-versus-host disease with predominant pulmonary vasculitis. J Immunol. 1998;161(2):909–18. [PubMed] [Google Scholar]
- 8.Clark JG, Madtes DK, Hackman RC, Chen W, Cheever MA, Martin PJ. Lung injury induced by alloreactive Th1 cells is characterized by host-derived mononuclear cell inflammation and activation of alveolar macrophages. J Immunol. 1998;161(4):1913–20. [PubMed] [Google Scholar]
- 9.Clark JG, Mandac JB, Dixon AE, Martin PJ, Hackman RC, Madtes DK. Neutralization of tumor necrosis factor-alpha action delays but does not prevent lung injury induced by alloreactive T helper 1 cells. Transplantation. 2000;70(1):39–43. [PubMed] [Google Scholar]
- 10.Clark JG, Mandac-Dy JB, Dixon AE, Madtes DK, Burkhart KM, Harlan JM, Bullard DC. Trafficking of Th1 cells to lung: a role for selectins and a P-selectin glycoprotein-1-independent ligand. Am J Respir Cell Mol Biol. 2004;30(2):220–7. doi: 10.1165/rcmb.2003-0208OC. [DOI] [PubMed] [Google Scholar]
- 11.Dixon AE, Mandac JB, Madtes DK, Martin PJ, Clark JG. Chemokine expression in Th1 cell-induced lung injury: prominence of IFN-gamma-inducible chemokines. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L592–9. doi: 10.1152/ajplung.2000.279.3.L592. [DOI] [PubMed] [Google Scholar]
- 12.Dixon AE, Mandac JB, Martin PJ, Hackman RC, Madtes DK, Clark JG. Adherence of adoptively transferred alloreactive Th1 cells in lung: partial dependence on LFA-1 and ICAM-1. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L583–91. doi: 10.1152/ajplung.2000.279.3.L583. [DOI] [PubMed] [Google Scholar]
- 13.Dixon AE, Mandac JB, Martin PJ, Madtes DK, Hackman RC, Clark JG. Alloreactive Th1 cells localize in lung and induce acute lung injury. Chest. 1999;116(1 Suppl):36S–37S. doi: 10.1378/chest.116.suppl_1.36s. [DOI] [PubMed] [Google Scholar]
- 14.Ross DJ, Moudgil A, Bagga A, Toyoda M, Marchevsky AM, Kass RM, Jordan SC. Lung allograft dysfunction correlates with gamma-interferon gene expression in bronchoalveolar lavage. J Heart Lung Transplant. 1999;18(7):627–36. doi: 10.1016/s1053-2498(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 15.Miura M, Morita K, Kobayashi H, Hamilton TA, Burdick MD, Strieter RM, Fairchild RL. Monokine induced by IFN-gamma is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J Immunol. 2001;167(6):3494–504. doi: 10.4049/jimmunol.167.6.3494. [DOI] [PubMed] [Google Scholar]
- 16.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192(10):1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193(8):975–80. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169(2):1037–49. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 19.Jinquan T, Jing C, Jacobi HH, Reimert CM, Millner A, Quan S, Hansen JB, Dissing S, Malling HJ, Skov PS, Poulsen LK. CXCR3 expression and activation of eosinophils: role of IFN-gamma-inducible protein-10 and monokine induced by IFN-gamma. J Immunol. 2000;165(3):1548–56. doi: 10.4049/jimmunol.165.3.1548. [DOI] [PubMed] [Google Scholar]
- 20.Katoh S, Fukushima K, Matsumoto N, Ehara N, Matsumoto K, Yamauchi A, Hirashima M. Accumulation of CXCR3-expressing eosinophils and increased concentration of its ligands (IP10 and Mig) in bronchoalveolar lavage fluid of patients with chronic eosinophilic pneumonia. Int Arch Allergy Immunol. 2005;137(3):229–35. doi: 10.1159/000086335. [DOI] [PubMed] [Google Scholar]
- 21.Kohrgruber N, Groger M, Meraner P, Kriehuber E, Petzelbauer P, Brandt S, Stingl G, Rot A, Maurer D. Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands. J Immunol. 2004;173(11):6592–602. doi: 10.4049/jimmunol.173.11.6592. [DOI] [PubMed] [Google Scholar]
- 22.Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J Exp Med. 2001;194(9):1375–84. doi: 10.1084/jem.194.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005 doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Whiting D, Hsieh G, Yun JJ, Banerji A, Yao W, Fishbein MC, Belperio J, Strieter RM, Bonavida B, Ardehali A. Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J Immunol. 2004;172(12):7417–24. doi: 10.4049/jimmunol.172.12.7417. [DOI] [PubMed] [Google Scholar]
- 25.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161(2):927–32. [PubMed] [Google Scholar]
- 26.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147(4):1016–28. [PMC free article] [PubMed] [Google Scholar]
- 27.Dong S, Hughes RC. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen) Glycoconj J. 1997;14(2):267–74. doi: 10.1023/a:1018554124545. [DOI] [PubMed] [Google Scholar]
- 28.Mohan K, Cordeiro E, Vaci M, McMaster C, Issekutz TB. CXCR3 is required for migration to dermal inflammation by normal and in vivo activated T cells: differential requirements by CD4 and CD8 memory subsets. Eur J Immunol. 2005;35(6):1702–11. doi: 10.1002/eji.200425885. [DOI] [PubMed] [Google Scholar]
- 29.Koga S, Auerbach MB, Engeman TM, Novick AC, Toma H, Fairchild RL. T cell infiltration into class II MHC-disparate allografts and acute rejection is dependent on the IFN-gamma-induced chemokine Mig. J Immunol. 1999;163(9):4878–85. [PubMed] [Google Scholar]
- 30.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385(6611):81–3. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 31.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272(5258):60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann JC, Jablonski-Westrich D, Haubold U, Gutierrez-Ramos JC, Springer T, Hamann A. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J Immunol. 2003;171(5):2588–93. doi: 10.4049/jimmunol.171.5.2588. [DOI] [PubMed] [Google Scholar]
- 33.Giuliani N, Bonomini S, Romagnani P, Lazzaretti M, Morandi F, Colla S, Tagliaferri S, Lasagni L, Annunziato F, Crugnola M, Rizzoli V. CXCR3 and its binding chemokines in myeloma cells: expression of isoforms and potential relationships with myeloma cell proliferation and survival. Haematologica. 2006;91(11):1489–97. [PubMed] [Google Scholar]
- 34.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23(9):1324–32. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 36.Yun JJ, Fischbein MP, Whiting D, Irie Y, Fishbein MC, Burdick MD, Belperio J, Strieter RM, Laks H, Berliner JA, Ardehali A. The role of MIG/CXCL9 in cardiac allograft vasculopathy. Am J Pathol. 2002;161(4):1307–13. doi: 10.1016/S0002-9440(10)64407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]