Abstract
The protein kinase Akt is a critical regulator of cell function and its overexpression and activation have been functionally linked to numerous pathologies such as cancer. Previous reports regarding the mechanism regulating Akt's activation have revealed two phosphorylation events, at threonine 308 (T308) and serine 473 (S473), as necessary for the full activation of the kinase in response to insulin. For this reason and because of the availability of phospho-specific antibodies to both T308 and S473, many studies that focus on Akt's role in governing cell function rely on the measurement of these two sites to understand changes in kinase activity. Recent evidence, however, suggests the involvement of other phosphorylation sites; for example, in Src-transformed and epidermal growth factor (EGF)- treated cells, tyrosine phosphorylation has been found important for full kinase activation. In this study, we probed the quantitative reliability of using S473 and/or T308 phosphorylation as surrogates for Akt kinase activity acress diverse treatment conditions. We performed quantitative western blots and kinase activity assays on lysates generated during a two hour time course from two cell lines treated with either EGF or insulin. From the resulting ∼250 quantitative measurements of phosphorylation and activity, we found that both T308 and S473 phosphorylation accurately captured quantitative changes in EGF-stimulated cells, but not in insulin-stimulated cells. Morever, in all but one condition studied, we found a tight correlation between the onset of phosphorylation and dephosphorylation for both sites, despite the fact that they do not share common kinase- or phosphatase-mediated regulation. In sum, using a quantitative approach to study Akt activation identified ligand-dependent limits for the use of T308 or S473 as proxies for kinase activity and suggests the coregulation of Akt phosphorylation and dephosphorylation.
Keywords: Akt, Protein kinase B (PKB), Epidermal Growth Factor (EGF), Insulin (INS), Serine/Threonine phosphorylation
Introduction
The protein kinase Akt/PKB is a critical regulator of cellular functions such as apoptosis, proliferation, and migration [1, 2]. Its well-established role in the governance of cell survival implicates it as a critical signaling node in cancer, and its overexpression and increased activation has been found in a variety of cancers such as those occurring in the breast, neck, and lungs [3]. Prior studies into the mechanism of Akt's kinase activity have revealed that growth factor or insulin induced activation of phosphoinositide 3-kinase (PI3K) leads to the generation of 3,4,5 phosphatidylinositol (PIP3) and subsequent recruitment of Akt to the plasma membrane via its pleckstin homology (PH) domain. Once at the membrane, Akt is phosphorylated by phospoinositide-dependent kinase 1 (PDK1) on the threonine 308 residue (T308) that resides in its activation loop. In addition, phosphorylation of the serine 473 residue (S473) located in the carboxy-terminal hydrophobic domain occurs via a kinase whose identity has been debated [4-6], although recent evidence identifies a Rictor-mTOR complex as the responsible kinase [7]. The T308 and S473 phosphorylation sites have been the primary focus of a large number of biochemical studies into Akt's mechanism of activation. Salient results include the finding that phosphorylation on both the S473 and T308 sites is necessary for full kinase activation in response to insulin [with S473 phosphorylation alone inducing no activity and T308 phosphorylation alone inducing approximately one-third maximum activity [8]], that S473 phosphorylation precedes and promotes T308 phosphorylation [9], and that the dephosphorylation of the two sites often occurs differentially and through separate phosphatases [10, 11]. Facile measurement of these two sites using western blot or immunostaining techniques has been enabled by the availability of phospho-specific antibodies against T308 and S473.
Although the majority of work regarding Akt activation has focused on phosphorylation at T308 and S473, recent evidence suggests the existence of other phosphorylation sites that regulate kinase activity. In particular, tyrosine phosphorylation has been identified as a key regulatory mechanism in Src-transformed cells, cells stimulated with epidermal growth factor (EGF), and cells treated with the tyrosine phosphatase inhibitor pervanadate [12, 13]. Threonine 72 and serine 246 have also recently been identified as autophosphorylated sites that regulate kinase activity [14]. Furthermore, uncoupling of T308 phosphorylation and kinase activity after initial kinase activation has been reported in response to insulin [11].
Given evidence that phosphorylation at multiple sites other than T308 and S473 are important for kinase activity, especially in systems highly relevant to cancer such as Src-transformed cell lines or those exposed to elevated levels of EGF ligands, we raised the question of how reliably inferences can be made about Akt kinase activity and its role in signaling from interrogation of phosphorylation at S473 and/or T308 alone. To address this question, we quantified kinase activity in addition to T308 and S473 phosphorylation in two different cell lines, a Chinese hamster ovary cell line transfected with EGFR (CHO-EGFR) and a colon carcinoma cell line (HT-29), treated individually with EGF or insulin. We measured kinase activity over two hours to capture the initial kinase activation profile (with a time scale typically under 5 minutes) as well as deactivation or sustained activity at longer times, since both acute and longer term responses may be important for Akt's governance of cellular phenotype. Our results show that phosphorylation at T308 and S473 provides an accurate representation of kinase activity in the case of EGF stimulation in both cell lines. T308 and S473 phosphorylation, however, fail to capture elements of kinase activity in response to insulin treatment, most notably in the case of HT-29 cells where early phase oscillations in activity are not reflected by T308 or S473 phosphorylation levels. Additionally, our data suggest that both phosphorylation and dephosphorylation at T308 and S473 are tightly coupled under most conditions studied.
Materials and Methods
Cell Culture and Treatment
HT-29 cells (ATCC) were grown in McCoy's 5A medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). CHO K1 cells, transfected with EGFR-GFP as described previously [15], were grown in high-glucose Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate, 1mM non-essential amino acids, 100 units/ml penicillin and 100 μg/ml streptomycin. The growth medium was supplemented with 500 μg/ml of G418 for plasmid expression maintenance and selectivity.
For lysis, cells were seeded at 50,000 cells/cm2, grown for 48 hrs, and then stimulated with 100 ng/ml EGF (Peprotech) or 500 ng/ml insulin (Sigma) for the indicated times. Cells were lysed in 1% Triton X-100, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 50 mM β-glycerophosphate, 20 mM sodium pyrophosphate, 30 mM NaF, 1 mM benzamidine, 2 mM EGTA, 200μM NaVO4, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 1 μg/ml microcystin-LR. Protein concentrations were determined with a micro bicinchoninic acid assay (Pierce).
Western Blotting
To quantify phosphorylation levels, 80 μg of lysate were resuspended in 40 μl of sample buffer [100 mM DTT, 2% SDS, 10% glycerol, 0.01% bromophenol blue, 62.5 mM Tris-HCl (pH 6.8)]. Gel electrophoreses (10% polyacrylamide gel) was followed by transfer to polyvinylidene difluoride membranes (Biorad). Membranes were blocked with 5% nonfat milk or 5% bovine serum albumin in 20 mM Tris-HCl (pH 7.5), 137 mM NaCl, and 0.1% Tween-20. Membranes were then probed with anti-phospho-Akt (Ser473, #9271, Cell Signaling) or anti-phospho-Akt (Thr308, #4056, Cell Signaling). The membranes were then probed with horseradish-peroxidase-conjugated anti-rabbit secondary antibody (Amersham Pharmacia Biotech) and visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech) on a Kodak Image Station (Perkin Elmer). Densitometry was performed using molecular imaging software (Kodak). Band area net intensities were normalized to the 5 minute (for HT-29 cells) or 10 minute (for CHO-EGFR cells) value to produce the time series presented in Figures 1-4. Linearity for each antibody was established using serial dilutions of an insulin-stimulated 5 min. lysate from HT-29 cells (see Supplementary Figures 1 and 2).
Figure 1. HT-29 cells treated with EGF exhibit transient Akt activation and phosphorylation.
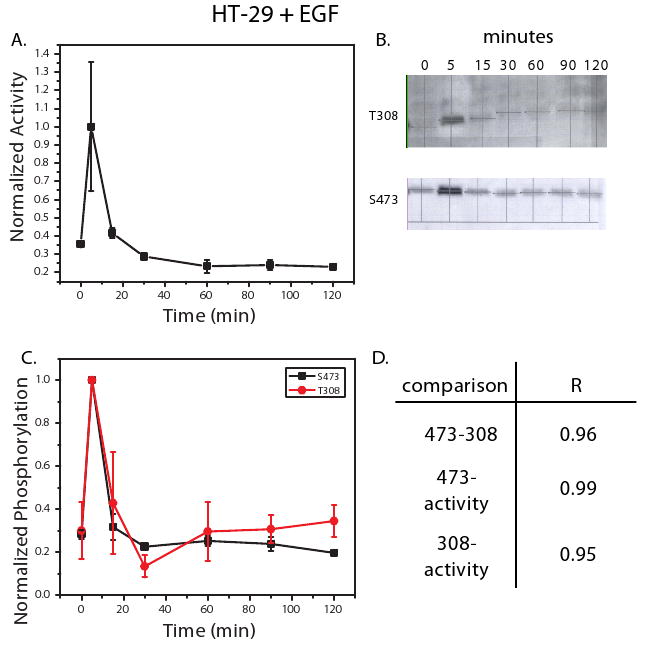
An in vitro kinase activity assay was used to measure Akt activity in HT-29 cells treated with EGF (100 ng/ml) at 0, 5, 15, 30, 60, 90, and 120 minutes (A). Phosphorylation at T308 and S473 was also measured under these conditions using western blot analysis. Shown in (B) are representative blots for T308 and S473 from the three biological replicates measured. Densitometry was used to quantify the net band intensity for all western blots (C). Calculation of the Pearson's correlation between phosphorylation of T308, S473, and kinase activity is shown in (D). All points in the time courses are the average of three biological replicates ± SEM. Time points were normalized to 5 minute kinase activity or phosphorylation levels.
Figure 4. CHO-EGFR cells treated with insulin exhibit sustained Akt activation mirrored by S473 but not T308 phosphorylation.
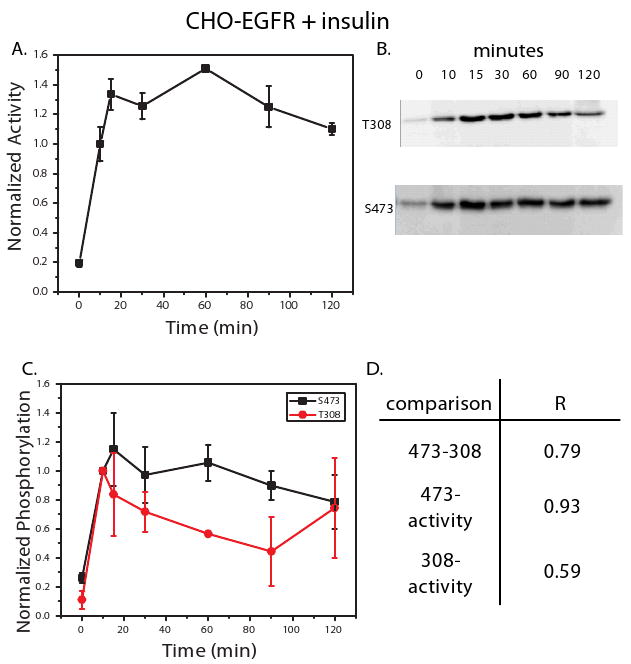
An in vitro kinase activity assay was used to measure Akt activity in CHO-EGFR cells treated with insulin (500 ng/ml) at 0, 10, 15, 30, 60, 90, and 120 minutes (A). Phosphorylation at T308 and S473 was also measured under these conditions using western blot analysis. Shown in (B) are representative blots for T308 and S473 from the three biological replicate measured. Densitometry was used to quantify the net band intensity for all western blots (C). Calculation of the Pearson's correlation between phosphorylation of T308, S473, and kinase activity is shown in (D). All points in the time courses are the average of three biological replicates ± SEM. Time points were normalized to 10 minute kinase activity or phosphorylation levels.
Kinase Activity Assay
Kinase activity assays were performed as previously described [16]. Briefly, anti-Akt antibody (Upstate Biotech) was incubated in 96-well protein G-coated plates (Pierce) overnight. Lysates were then added and incubated overnight as well. Subsequent exposure to [γ32-P]ATP and Aktide substrate initiated an in vitro reaction that was subsequently terminated after 30 minutes by addition of phosphoric acid. Reaction mixtures were then transferred to a phosphocellulose filter plate and filter bound [γ32-P]-substrate was quantified using a scintillation counter. Linearity of the assay in each cell type has been established ([16], Supplementary Figure 3). Count per minute readings were normalized to lysate concentrations and then to the 5 minute (for HT-29 cells) or 10 minute (for CHO-EGFR cells) value to produce the time series presented in Figures 1-4.
Statistical Analysis
Pearson correlation (R) values and p-values using student's t-test (95% confidence intervals) were obtained in Microsoft Excel.
Results
An experimental strategy for the quantitative comparison of Akt phosphorylation and activity
To directly compare phosphorylation and kinase activity, we conducted quantitative western blots (T308 and S473) and a kinase activity assay from individual lysates corresponding to one of three biological replicates for a particular cellular treatment (Supplementary Figure 4). Each measurement technique was validated for linearity as described in the Methods section (Supplementary Figures 1-3).
EGF treatment stimulates a transient Akt response in HT-29 cells and a sustained Akt response in CHO-EGFR cells
When HT-29 cells were treated with EGF (100 ng/ml), a transient ∼3-fold activation was observed (Figure 1A). Quantification of T308 and S473 phosphorylation revealed a similar trend, with phosphorylation and subsequent dephosphorylation occurring rapidly within 15 minutes of ligand treatment (Figures 1B, C). The correlation between kinase activity and phosphorylation over the 2 hour time course was high, with R ≥ 0.95 in both cases (Figure 1D). The phosphorylation and dephosphorylation trends for T308 and S473 correlated strongly with each other, yielding an R = 0.96 (Figure 1D). In contrast to HT-29 cells, CHO-EGFR cells treated with EGF exhibited sustained kinase activity that peaked after approximately 30 minutes (Figure 2A). Concomitant phosphorylation at the T308 and S473 was also observed (Figure 2B), as captured by the strong correlation between each site and kinase activity (Figure 2C, D). As was the case in the HT-29 cells, correlation between the two phosphorylation sites was high (R = 0.94, Figure 2D). Thus, the phosphorylation levels of T308 and S473 each accurately reflect kinase activity in two cell lines exhibiting unique temporal responses to EGF stimulation.
Figure 2. CHO-EGFR cells treated with EGF exhibit sustained Akt activation and phosphorylation.
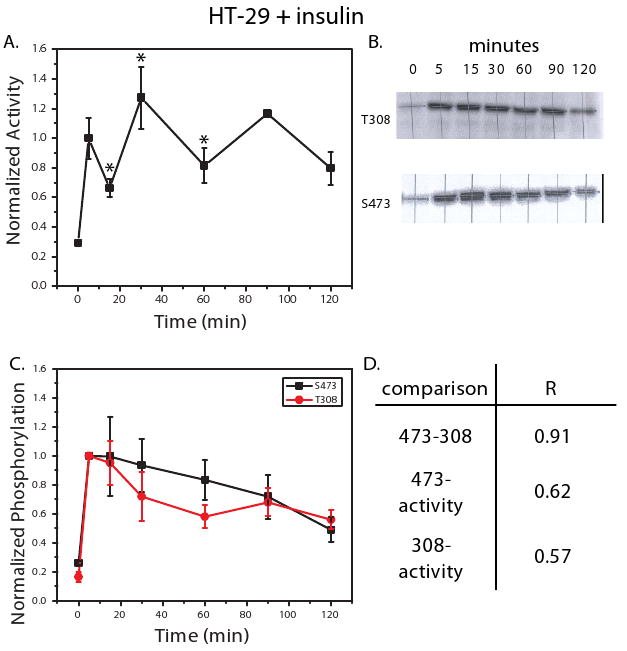
An in vitro kinase activity assay was used to measure Akt activity in CHO-EGFR cells treated with EGF (100 ng/ml) at 0, 10, 15, 30, 60, 90, and 120 minutes (A). Phosphorylation at T308 and S473 was also measured under these conditions using western blot analysis. Shown in (B) are representative blots for T308 and S473 from the three biological replicates measured. Densitometry was used to quantify the net band intensity for all western blots (C). Calculation of the Pearson's correlation between phosphorylation of T308, S473, and kinase activity is shown in (D). All points in the time courses are the average of three biological replicates ± SEM. Time points were normalized to 10 minute kinase activity or phosphorylation levels.
Insulin treatment induces sustained AKT kinase activity in both HT-29 and CHO-EGFR cell lines that is not fully captured by T308 and S473 phosphorylation
HT-29 cells treated with insulin exhibited sustained Akt activity throughout the two hour time course. Interestingly, a statistically significant oscillatory behavior was observed, with the differences between subsequent time points from 5 to 60 minutes significant at p < 0.05 (Figure 3A). These oscillations were not reflected in the phosphorylation patterns of either S473 or T308 (Figure 3B, C), as captured by the low correlation between phosphorylation and activity [R = 0.62 and 0.57 for S473 and T308, respectively (Figure 3D)]. Despite low correlation with activity, the phosphorylation levels at T308 and S473 correlated strongly with each other, as reflected by the high correlation coefficient (R = 0.91, Figure 3D).
Figure 3. HT-29 cells treated with insulin exhibit oscillatory Akt activation and sustained phosphorylation.
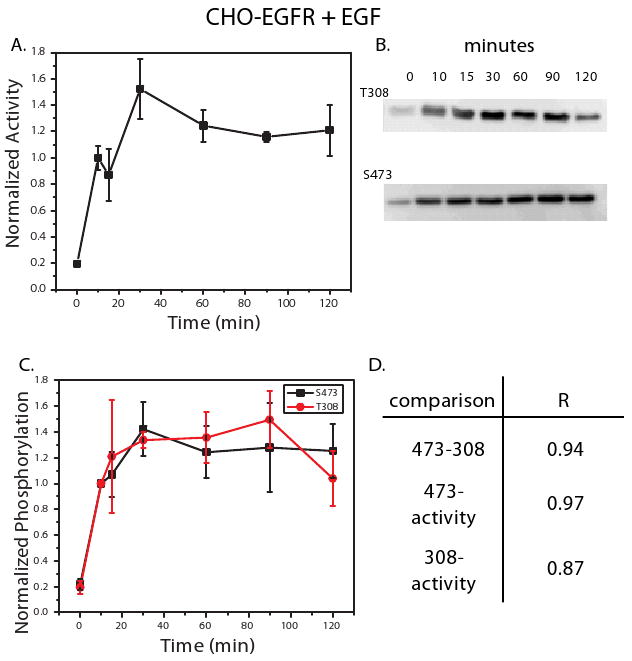
An in vitro kinase activity assay was used to measure Akt activity in HT-29 cells treated with insulin (500 ng/ml) at 0, 10, 15, 30, 60, 90, and 120 minutes (A). Phosphorylation at T308 and S473 was also measured under these conditions using western blot analysis. Shown in (B) are representative blots for T308 and S473 from the three biological replicates measured. Densitometry was used to quantify the net band intensity for all western blots (C). Calculation of the Pearson's correlation between phosphorylation of T308, S473, and kinase activity is shown in (D). All points in the time courses are the average of three biological replicates ± SEM. Time points were normalized to 5 minute kinase activity or phosphorylation levels. * indicates that the difference between the time point and the one previous to it is significant (P < 0.05) at a 95% confidence interval.
CHO-EGFR cells stimulated with insulin exhibited sustained Akt activity, but do not show any of the oscillatory behavior identified in the HT-29 cells (Figure 4A). Phosphorylation levels of both T308 and S473 reflect sustained kinase activity, although T308 phospho-levels in particular decline on average, a trend not seen in kinase activity. The correlation between the average phosphorylation measurements for T308 and S473 as shown in Figure 4C is relatively high (R = 0.79), but still significantly lower than seen in any of the other cellular conditions (all R's > 0.90), due to the previously mentioned T308 dephosphorylation trend that is not present for S473 phosphorylation. The S473 phosphorylation levels correlate strongly with activity (R = 0.93), whereas phosphorylation levels of T308 correlate weakly with kinase activity (R = 0.59). Thus, the measurement of S473 or T308 phosphorylation in response to insulin may not be enough to infer quantitative changes in kinase activity for HT-29 cells and only S473 phosphorylation correlates strongly with kinase activity in the CHO-EGFR cells.
Discussion
The relationship between the phosphorylation state of Akt and its catalytic activity is not precisely understood. In this work, we endeavored to understand the accuracy associated with the most common tools used to interrogate Akt function, namely the phospho-specific antibodies against S473 and T308. To do this, we compiled quantitative phosphorylation and kinase activity data spanning two cell lines and as many ligand treatments for seven time points over the course of two hours.
Since, as mentioned in the introductory section, novel activity-regulating tyrosine phosphorylation sites have been identified in cells stimulated with EGF, we hypothesized that quantitative correlation between T308 or S473 phosphorylation and kinase activity may not be accurate. For cells stimulated with insulin, however, we expected that the correlation would be more accurate, given the fact that much of the seminal work linking phosphorylation and kinase activity was done in the presence of insulin or IGF-1. Counterintuitively, we observed a tight correlation between T308 and S473 phosphorylation and kinase activity in response to EGF, but under insulin treatment the correlation was weaker, with phosphorylation trends generally failing to match long term kinase activity profiles, particularly in the case of the HT-29 cells where oscillations were observed throughout much of the two hour time course. These data suggest that Akt activity in response to insulin treatment may be regulated through other phosphorylation sites or perhaps through protein-protein interactions that were not disrupted by the wash steps in the in vitro kinase assays. Candidate phosphorylation sites might include the afore mentioned tyrosine phosphorylation sites or the autophosphorylated residues T72 and S246, which have been shown to regulate kinase activity in response to insulin stimulation [14]. The data under EGF treatment suggests that although tyrosine phosphorylation is necessary for kinase activity, it may be tightly coupled to S473 and T308 phosphorylation, at least at the level of temporal resolution presented in this study. It is worth noting that under all treatment conditions measurements of either S473 or T308 captured the qualitative activation of Akt kinase such that S473 or T308 phosphorylation measurement may be sufficient depending on the quantitative accuracy one needs for biological interpretation. Finally, in three of the four cellular conditions studied, both T308 and S473 phosphorylation levels correlated strongly, suggesting that measurement of only one of the sites is necessary.
We noted a strong correlation between T308 and S473 phosphorylation during the initial onset of kinase activity. This result was expected, as it is generally accepted that the initial phosphorylation of these two sites is strongly coupled. Nevertheless, studies have also indicated that S473 phosphorylation precedes and promotes T308 phosphorylation to achieve kinase activation [9]. We do not see any evidence of differential S473 versus T308 early phase phosphorylation, but this may be due to a lack in temporal resolution at early times in our experiment. Although the phosphorylation of T308 and S473 is expected to be tightly coupled, several studies have shown that the dephosphorylation of these two sites is uncoupled [10, 11]. In particular, prior studies using the shellfish toxin okadaic acid suggest that phosphatase activity at the T308 site is not connected to dephosphorylation at the S473 site, with further work suggesting that the phosphatase PP2A is responsible for T308 dephosphorylation [10, 11, 17]. Gao et al. recently showed that a novel phosphatase, PHLPP, is responsible for dephosphorylating the S473 site [10]. Interestingly, the gene for PHLPP is found near a commonly mutated chromosomal region in colon cancers, and Gao et al. showed that the HT-29 colon carcinoma cell line had decreased expression of PHLPP. Accordingly, we hypothesized that the dephosphorylation of T308 and S473 might be decoupled in HT-29 cells. However, under EGF stimulation, where we observed a transient spike of Akt activity and phosphorylation levels, the rapid dephosphorylation of both S473 and T308 is tightly coupled (Figure 1). In the case of insulin treatment, where we observed sustained kinase activity with only partial dephosphorylation over two hours, we again observed a high correlation between T308 and S473 phosphorylation levels (R = 0.91), although on average T308 was dephosphorylated more from 15 to 30 minutes (Figure 3). Our results in EGF treated HT-29 cells, where dephosphorylation was significant, indicate that the coordinated dephosphorylation of both T308 and S473 can occur in cell systems where individual phosphatase levels are abnormal. In contrast, CHO-EGFR cells stimulated with insulin do show evidence of decoupled dephosphorylation, where T308 levels decline more rapidly than both S473 or activity levels (Figure 4). This finding is consistent with Yamada et al. observations in insulin treated CHO cells, where they observed rapid T308 dephosphorylation not reflected in either activity or S473 levels.
Taken together, our data help to delineate the confidence with which researchers can use commercially available phospho-specific antibodies to understand signaling downstream of the Akt kinase. In addition, the quantitative approach taken allows for a greater understanding of the coordinate regulation of S473 and T308 phosphorylation levels. Future work focused on the measurement of more phosphorylation sites in the case of insulin treatment and with more accurate measurement technologies should enable further insights from this type of experimental approach.
Supplementary Material
Acknowledgments
The authors thank Kevin Janes and Forest White for enlightening discussions and technical assistance. This work was partially funded by NIH Grant CA096504. NK is supported by a fellowship from the NIGMS Biotechnology Training Program at MIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29(5):233–42. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697(12):3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 4.Feng J, et al. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279(39):41189–96. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 5.Persad S, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276(29):27462–9. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 6.Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275(12):8271–4. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15(23):6541–51. [PMC free article] [PubMed] [Google Scholar]
- 9.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22(17):6247–60. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Yamada T, et al. 3-phosphoinositide-dependent protein kinase 1, an Akt1 kinase, is involved in dephosphorylation of Thr-308 of Akt1 in Chinese hamster ovary cells. J Biol Chem. 2001;276(7):5339–45. doi: 10.1074/jbc.M005685200. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, et al. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276(34):31858–62. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 13.Conus NM, et al. Direct identification of tyrosine 474 as a regulatory phosphorylation site for the Akt protein kinase. J Biol Chem. 2002;277(41):38021–8. doi: 10.1074/jbc.M203387200. [DOI] [PubMed] [Google Scholar]
- 14.Li X, et al. Autophosphorylation of Akt at threonine 72 and serine 246. A potential mechanism of regulation of Akt kinase activity. J Biol Chem. 2006;281(19):13837–43. doi: 10.1074/jbc.M602060200. [DOI] [PubMed] [Google Scholar]
- 15.Harms BD, et al. Directional persistence of EGF-induced cell migration is associated with stabilization of lamellipodial protrusions. Biophys J. 2005;88(2):1479–88. doi: 10.1529/biophysj.104.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janes KA, et al. A high-throughput quantitative multiplex kinase assay for monitoring information flow in signaling networks: application to sepsis-apoptosis. Mol Cell Proteomics. 2003;2(7):463–73. doi: 10.1074/mcp.M300045-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Andjelkovic M, et al. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci U S A. 1996;93(12):5699–704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


