Abstract
The flavone apigenin has been previously selected as a potent pharmacological activator of the CFTR Cl− channel, however, its utility for the activation of CFTR in vivo is expected to be limited because flavonoids are readily metabolized. We therefore investigated the poorly metabolizable methylether of apigenin, 5,7,4′-trimethoxyflavone (TMF) as a CFTR activator using transepithelial short-circuit current measurements, whole cell and single cell patch clamp techniques, and nasal potential difference (PD) measurements. Transepithelial Cl− secretion by Calu-3 epithelia was stimulated by TMF with a halfmaximal concentration of 64±5 μM to 55±15% of maximal currents achieved by subsequent addition of cAMP agonist forskolin (10 μM). In forskolin-prestimulated tissues, TMF showed small effects and stimulated Cl− secretion by an additional 6%. Single channel and whole cell patch clamp techniques were used to verify these effects and identify CFTR as the target of TMF. TMF increased the open probability of silent CFTR (to 0.31±0.06) but showed small effects once CFTR had been prestimulated with forskolin. In nasal PD measurements in humans, perfusion of TMF onto the nasal mucosa activated nasal PD by −9.5±1.1 mV, which was 69% of the effect of TMF+isoproterenol (−13.8±3.9 mV). These data show that TMF is an activator of CFTR in both in vitro and in vivo assays that targets mainly the unstimulated CFTR.
Keywords: CFTR opener, Flavonoid, Electrophysiology, Patch clamp, Nasal potential difference
Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel is mutated and misfunctional in the inherited disease cystic fibrosis. Current drug development efforts are focused on identifying CFTR correctors (i.e., drugs that support proper intracellular processing of the protein) and CFTR openers (i.e., drugs that increases the open probability of membrane-resident CFTR) [1]. Initially, genistein has been identified as a CFTR opener [2,3] and from a screen of a small number of closely related flavonoids, apigenin was selected as the compound with the highest affinity and efficacy in stimulating CFTR [4,5]. By analyzing the dosedependent kinetics and structure-activity relationship of CFTR activation, it became apparent that apigenin binds to and activates both control (non-phosphorylated) and pre-stimulated (phosphorylated) CFTR by binding to selective sites with different affinities and potencies [5].
Most flavonoids, including apigenin, are quickly metabolized in vivo by a number of pathways, and the free hydroxyls are particularly prone to glucuronidation and sulfatation [6]. Metabolic degradation was previously shown to be largely reduced when the free hydroxyls were protected by methylation [7] suggesting that methylether derivatives may show improved bioavailability. The trimethylether of apigenin (5,7,4′-trimethoxyflavone, TMF) has been shown recently to be highly resistant to metabolic degradation by pooled human liver S9 fraction treatment [8].
In a previous study we tested the effects of a number of apigenin methylester derivatives on their kinetic interaction with the CFTR target. We found that TMF activated CFTR-mediated Cl− currents in Ussing chambers to a similar extent as apigenin, however, with a 5-times lower affinity [5]. In addition, TMF was one of the few flavonoids that we have tested that readily activated unstimulated CFTR, but had no appreciable effect on forskolin-stimulated CFTR. Owing to TMF’s advantages as a metabolically stable compound, we designed this study to investigate in more detail the effects of TMF on CFTR in tissue-based experiments in Ussing chambers, in single cell-based experiments using patch clamp techniques, and in nasal PD measurements in humans.
Material and Methods
Cell culture
Calu-3, a human airway epithelial cell line of adenocarcinoma origin, was cultured exactly as described [9]. Calu-3 cells were used for their high expression of CFTR and few detectable other types of Cl− channels as determined in transepithelial and patch clamp experiments [4,10] or by the pharmacological profile, the ion selectivity, and the physiological regulation of apical membrane Cl− currents [4,9,11,12]. Tissues were grown on permeable filter supports (Falcon, Becton Dickinson) and used 1–5 days after seeding. Cultures used in this study had a transepithelial resistance of Rt = 451±46 Ω·cm2 (n=16).
Transepithelial Ussing chamber recordings
Short-circuit current (Isc) measurements were done as described [5]. Briefly, filters were mounted in circulation-type Ussing chambers (World Precision Instruments) and shortcircuited (Physiologic Instruments); the resulting Isc was amplified, digitized, and recorded to a computer. Every 20 s (or 50 s), a 2-mV 1-s pulse was applied to continuously monitor Rt. Experiments were done with a serosal-to-mucosal Cl− gradient to generate a driving force for Cl− and to amplify the signal. The serosal solution contained (in mM) 120 NaCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2, and 5.6 glucose. In the mucosal solution, all Cl− salts were exchanged for gluconate salts. Both chamber compartments were gassed with 95% O2/5% CO2 (pH 7.4). Chamber aperture was 0.6 cm2, and Isc and Rt are reported normalized to 1 cm2. Positive currents were defined as Cl− movement from serosa to mucosa. Experiments were done at 37°C.
Patch-clamp recordings
Cells were patch clamped on the stage of an inverted microscope in a constantly perfused chamber at 37°C as described [4,13,14]. Solutions were optimized for the recording of Cl− currents. In the whole cell recording mode, a 29:150 mM Cl− gradient from pipette to bath was used as a driving force while the cell membrane potential was clamped to zero. Because there were no other significant gradients or driving forces, the resulting membrane current is a Cl− current. The bath solution had the following composition (in mM) 145 N-methyl-D-glucamine chloride (NMDG-Cl), 1.7 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and 10 sucrose, pH 7.4. The pipette solution contained (in mM) 27 NMDG-Cl, 2 EGTA, 1 MgCl2, 10 HEPES, 5 glucose, 110 NMDG-gluconate, 5 MgATP, and 0.1 LiGTP, pH 7.4. Outside-out recordings were done with the same solutions and were achieved by removing the pipette from the cell while in the whole cell mode. Recordings in the cell-attached patch clamp mode were performed with the 145 mM NMDG-Cl solution (as above) in both bath and pipette. In the whole cell and outsideout mode, the membrane potential is given as the pipette potential; in the cell-attached mode, potentials are given as the negative pipette potential. Recordings were analyzed with pClamp version 7 (Axon Instruments) using standard methods. If recordings contained a low number of channels, the number of channel (N) was determined from the maximum number of open levels observed in the total length of the recording, and open probability (Po) was calculated with standard techniques [14]. If the recording contained too many channels (as in all outsideout recordings) then the average current was used as a quantitative descriptor.
Measurements of nasal potential difference (PD)
This study on human volunteers was approved by the Internal Review Board of Children’s Hospital & Research Center Oakland and participants gave informed consent. Measurements were done exactly as described in ref [4]. The NaCl perfusion solution contained (in mM) 145 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.4. In Cl− free solutions all Cl− salts were replaced by the respective gluconate salts.
Chemicals
5,7,4′-trimethoxyflavone (TMF, also, apigenin trimethyl ether) was purchased from Indofine Chemical Co (reported purity 97%) and was made as a 100 mM stock solution in dimethylsulfoxide (DMSO). TMF is a homolog of apigenin (5,7,4′-trihydroxyflavone) where all three hydroxyls of apigenin are methylated (see structures in Fig. 4). The parent compound apigenin (Indofine Chemical Co) was used as a comparison and positive control, and was also prepared as a 100 mM stock in DMSO. The adenylate cyclase activator forskolin (Calbiochem) was made as a 20 mM stock in DMSO and used at 10 μM; the Cl− channel blocker diphenylaminecarboxylate (DPC, also N-phenyl antranylic acid, Sigma-Aldrich) was made as a 200 mM stock in ethanol and used at 2 mM at the end of all transepithelial experiments.
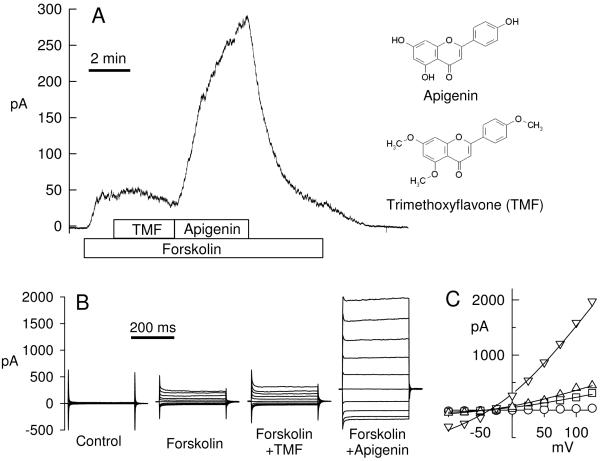
Fig. 4.
Comparison of effect of TMF and apigenin on forskolin-stimulated Cl currents. A. Whole cell patch clamp recording from a Calu-3 cell; 10 μM forskolin, 100 μM TMF, and 50 μM apigenin were added to the bath as indicated. Holding potential is 0 mV and current is driven by a 29:150 mM Cl− gradient. The molecular structures of apigenin and TMF are shown for comparison. B. Current-voltage step protocols for every condition; y axis and time bar is same for all panels in B. C. Current-voltage relations were well fitted by the Goldman equation and yielded: control (open circles) 112 pS (at 0 mV); forskolin (squares) 508 pS; forskolin+TMF (triangles up) 674 pS, forskolin+apigenin (triangles down) 2884 pS, membrane capacitance of this cell was 41 pF
Statistics
Data are given as original recordings or as mean±SE and treatment effects were tested with paired or unpaired t tests as appropriate; p<0.05 was considered significant and calculated p values are given throughout. All calculations were done with the statistics routines implemented in Sigmaplot 11 (Systat Software).
Results
TMF-activated Cl− secretion across Calu-3 epithelia
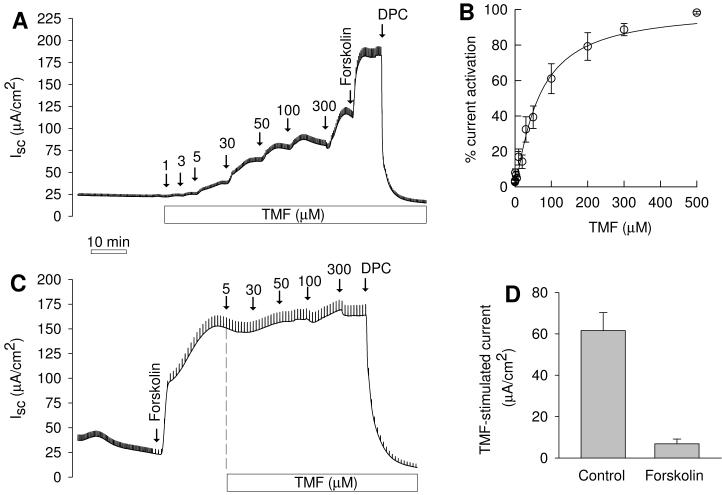
Cl− secretion by Calu-3 monolayers was investigated in Ussing chambers in presence of a serosa-to-mucosal Cl− gradient. In these recordings, CFTR was tentatively identified by using the Calu-3 cell line (which expresses CFTR as its major apical Cl− channel) and by using the blocker DPC. Addition of TMF to the mucosal compartment stimulated Cl− currents dose-dependently (Fig. 1A) and followed saturation kinetics that were well fitted by Michaelis-Menten-Hill kinetics (Fig. 1B, ref. [5]). Fits yielded a halfmaximal concentration of 63.9±5.0 μM and a Hill coefficient of 1.2±0.1 (n=6) indicating simple activation kinetics of CFTR by TMF. Maximal currents achieved by TMF treatment were 54.8±14.8% (n=6) of forskolin-stimulated currents (which were 178±23 μA/cm2). Currents activated by both TMF and forskolin were totally blocked by 2 mM DPC. When Calu-3 monolayers were first stimulated with forskolin (to 115±11 μA/cm2, n=7), TMF exerted a small additional effect of 6.9±2.3 μA/cm2 (Fig. 1C,D; p=0.026, paired t test). These data indicated that the major target for TMF is the unstimulated CFTR Cl− channel and once CFTR is activated TMF effects are small.
Fig. 1.
Effects of trimethoxyflavone (TMF) on transepithelial Cl− secretion by Calu-3 cells. A. Control tissue; mucosal addition of TMF dose-dependently stimulated Cl− secretion. Numbers given in panels are final concentrations of TMF in chamber. Addition of 10 μM forskolin (serosally) resulted in additional Cl− secretion; 2 mM mucosal DPC blocked currents. B. Dose-response relation of normalized currents. Fit to Michaelis-Menten-Hill kinetics [5] resulted in a half-maximal concentration of 63.9±5.0 μM and a Hill coefficient of 1.2±0.1. C. After forskolin stimulation the effects of TMF were small. D. Comparison of effect of 300 μM TMF in control (n=6) tissues and in tissues that were pre-stimulated with forskolin (n=7). Treatment effects were significantly different (p<0.001, t test) and TMF effect after forskolin was significant (p=0.026, paired t tests).
Single channel and whole cell patch clamp recording of TMF-stimulated CFTR activity
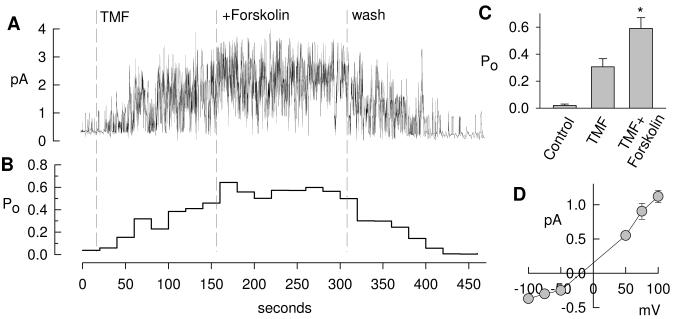
To further test the effect of TMF on CFTR, we investigated single Calu-3 cells using patch clamp techniques. Fig. 2A shows a typical cell-attached recording of CFTR in Calu-3 cells at a holding potential of 75 mV, and Fig. 2B shows the corresponding open probability (Po) for consecutive 20-s intervals assuming this recording contained 6 active channels. Addition of 100 μM TMF to the bath activated CFTR gating and increased its Po to, on average, 0.31±0.06 (n=3, Fig. 2C), and addition of 10 μM forskolin to the bath increased Po to 0.59±0.08 (p=0.047, t test). CFTR activity readily returned to baseline after washout of drugs indicating a reversible stimulation of CFTR by TMF. The current-voltage relation of cell-attached CFTR typically rectified outwardly [15] with a single channel conductance in the positive voltage range (50 to 100 mV) of 11.4±2.4 pS and in the negative voltage range (−100 to −50 mV) of 2.4±0.5 pS. No difference was noted between single channel currents activated by TMF or by forskolin.
Fig. 2.
Activation of CFTR in a cell-attached patch clamp recording on a Calu-3 cell. A. 100 μM TMF activated basally active CFTR and forskolin shows additional small effects. Washout of drugs restores baseline activity. This recordings likely contains 6 channels. B. Average open probability (Po) calculated for 20-s intervals from recording in A according to Po=I/(i·N), where I is the time-averaged current, i is the single channel current, and N=6 channels. C. Average Po determined from three similar recordings, addition of forskolin to TNF-treated cells was significant (*, p=0.047, t test). D. Single channel current voltage relation typically rectifies outwardly in the cell-attached mode resulting in a single channel conductance of 11.4±2.4 pS in the positive and 2.4±0.5 pS in the negative range (n=3).
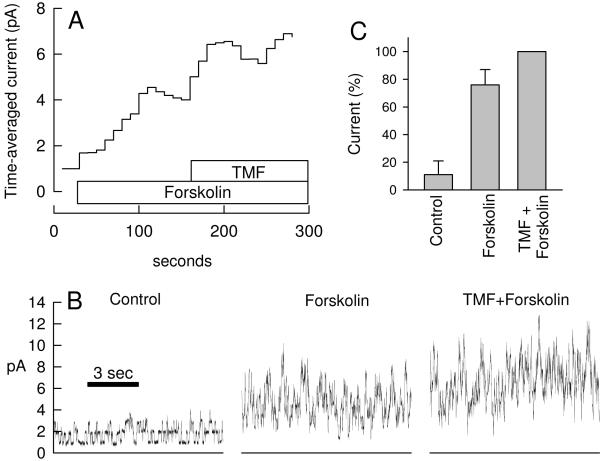
Recordings in the cell-attached mode have the advantage that the cell is intact and functional, however, the membrane potential is not well controlled, and thus recorded currents may be affected by drug effects on the membrane potential. Therefore, a different set of recordings was performed in the outside-out patch clamp configuration. Fig. 3A shows the average current and Fig. 3B shows selected 10-s intervals at a resolution that allows identification of channel events. Basal CFTR channel activity was noted in all outside-out recordings. Addition of forskolin (10 μM) increased channel activity and average currents. This recording contained >10 channels and discrete channel events are difficult to discern after stimulation. Subsequent addition of 100 μM TMF to forskolin-stimulated cells showed a small effect on the mean current in the recording in Fig. 3A, however, these effects were on average not statistically significant (Fig. 3C, p=0.16, paired t test) possibly owing to the high variability of current in this recording mode.
Fig. 3.
Activation of CFTR by forskolin and TMF in outside-out patch clamp recordings. A. Stimulation of currents across an outside-out patch excised from a Calu-3 cell. Shown is the time-averaged current for consecutive 10-s intervals at 80 mV. B. Selected 10-s recordings from record in A before drug addition (Control), in presence of 10 μM forskolin, and in presence of 100 μM TMF+10 μM forskolin. This recording contained >10 channels. The bottom lines represents zero current. C. Average currents from three similar recordings as in A normalized to maximal currents (=100%). Forskolin-stimulated currents were not different from maximal currents in presence of TMF+forskolin (paired t test, p=0.16).
This was further tested using the whole cell recording configuration and TMF’s effect was compared to its parent compound apigenin (Fig. 4). Forskolin-activated currents were slightly but significantly increased by TMF (Fig. 4A) immediately after addition. On average, currents increased by 6.7±2.4% (n=9, p=0.022, t test) over forskolin-stimulated currents (=100%). These small TMF-activated currents typically were transient and inactivated over several minutes, as shown in Fig. 4A. For comparison, treatment with apigenin resulted in very large additional current stimulations (Fig. 4A) of, on average, 319±60% (n=9, significantly larger than TMF effects, p<0.001). Current step protocols are shown for every condition in Fig. 4B at maximal stimulation. Currents were largely time and voltage independent and showed Goldman-type outward rectification as expected for the recording conditions (i.e., a pipette-to-bath 29:150 mM Cl− gradient). Current-voltage relations (Fig. 4C) were well fitted by the Goldman equation [4] indicative for a linear single channel conductance.
These in vitro electrophysiological recordings showed that TMF effectively stimulated inactive CFTR in Ussing chambers and cell-attached patch clamp recordings. The effect of TMF on forskolin-stimulated CFTR was small but significant in Ussing chambers and whole cell patch clamping, but not in outside-out patch clamp recordings, possibly owing to the high variability of the latter recording technique.
TMF activates Cl−-selective potentials in nasal PD measurements
In vivo effects of TMF were investigated in nasal PD measurements in healthy volunteers (2 male, 1 female, all Caucasian, age 36-40 years). The standard nasal PD protocol was used [16], which is an optimized protocol to determine the effects of CFTR activators by blocking the Na+-selective PD with amiloride and amplifying the Cl−-selective PD by use of a Cl− free perfusate. All drugs were acutely perfused onto the nasal mucosa. Fig. 5 shows a typical recording and the corresponding averages for all conditions. 100 μM amiloride depolarized nasal PD from −13.3±4.8 mV (baseline) by 6.9±2.4 mV. Perfusion with Cl− free solution hyperpolarized nasal PD by −19.2±4.6 mV. Addition of the test drug TMF (100 μM) resulted in a further hyperpolarization by −9.5±1.1 mV (p=0.031, paired t test), which was 69% of the effect observed by treatment with TMF+isoproterenol (−13.8±3.9 mV). Isoproterenol is used as a cAMP agonist in nasal PD measurements. These data show that in vitro effects of TMF were readily reproduced in vivo. For comparison, we also tested the effects of ATP in nasal PD measurements, which further hyperpolarized nasal PD by −5.4±2.4 mV, and all effects were reversed when washed with NaCl-containing perfusate.
Fig. 5.
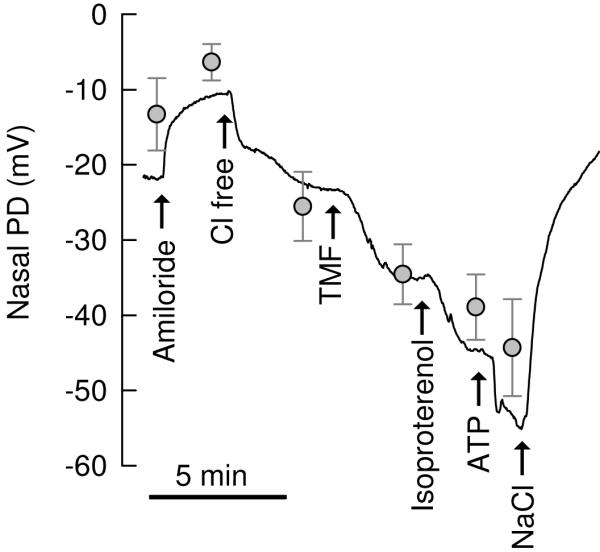
Effects of TMA in nasal PD measurements in humans. A. Line shows a typical recording of nasal PD and grey symbols show the average±SE of 3 recordings at the respective conditions. All drugs were acutely perfused onto the nasal mucosa as indicated. 100 μM amiloride, 100 μM TMF, 10 μM isoproterenol, 100 μM ATP. Effects of TMF were significant (hyperpolarization by −9.5±1.1 mV, p=0.031, paired t test), which was 69% of the effect of TMF+isoproterenol (−13.8±3.9 mV).
Discussion
This study aimed to investigate the utility of the poorly metabolizable flavone TMF as a CFTR activator. We found that it consistently stimulated CFTR both in vitro and in vivo. In tissue studies in Ussing chambers CFTR-mediated currents across Calu-3 cells were stimulated by maximal TMF concentrations to 55% of currents achieved by subsequent forskolin stimulation. This observation was consistently replicated in cell-attached single channel studies, where the open probability of CFTR was stimulated by TMF to 52% of forskolin-stimulated activity. Similarly, in nasal PD studies, TMF-induced hyperpolarization resulted on average in 69% of maximal stimulation in the additional presence of isoproterenol. Thus, TMA is a reliable CFTR activator across different recording techniques both in vitro and in vivo in humans.
Previously we have found that most tested flavonoids (including genistein, apigenin, kaempferol, quercetin, resveratrol) show two distinct types of activation kinetics of CFTR [4,5,17]: low-affinity binding and activation of silent (likely non-phosphorylated) CFTR and high-affinity binding and potentiation of forskolin (or cAMP) pre-stimulated (likely phosphorylated) CFTR. This suggests that CFTR activation and CFTR potentiation are governed by selective and distinct binding sites whose availability is governed by the level of phosphorylation. Recently, genistein was similarly found to bind and stimulate CFTR with distinct affinities dependent on the cAMP concentrations used [18].
In contrast, in the current study, TMF potently activated CFTR but showed only very small effects after forskolin-stimulation. This is consistent with the notion that TMF binds to the activating binding site, but only poorly to the potentiating binding site of CFTR. Likely the affinity to the potentiating site is governed by the free hydroxyls (for example, in apigenin) because when the hydroxyls of apigenin were methylated (this study) or removed (to yield flavone, ref. [5]) the potentiating effects were very small.
Most naturally occurring flavonoids are quickly and extensively metabolized and degraded by a number of pathways including conjugation to glucuronate or sulfate and oxidative metabolism [6,19]. Effective metabolism limits their use as a CFTR activator in vivo or any in vitro long term study. The free hydroxyls of flavonoids are the primary targets for conjugating enzymes. Recently, the protection of the free hydroxyls of several flavonoids, including apigenin, by methylation has been found to result in stable, poorly metabolizable compounds when exposed to degradation by human liver S9 fraction extract [8]. Thus, despite the fact that TMF shows activation kinetics of CFTR with 7-fold reduced affinity (64 μM) compared to apigenin (9 μM, ref. [5]), its use is likely preferred under conditions where active metabolizing enzymes will reduce the availability of the drug.
Springsteel and colleagues [20] recently used a different approach by generating a synthetic compound library based on the flavonoid backbone omitting any free hydroxyls on the compound. That study resulted in the identification of a synthetic benzoflavone (UCCF-339) that showed 2.3-fold improved affinity of activation kinetics over apigenin [20].
In summary our study shows that the trimethyl-derivative of apigenin 1) is a CFTR activator with a halfmaximal stimulator concentration of 64±5 μM, 2) has 7-fold reduced affinity compared to apigenin, 3) shows very little potentiating activity in the presence of the cAMP agonist forskolin, and 4) is an effective CFTR activator in nasal PD measurements in human subjects. We suggest that TMF has its major use in experimentation where long-term flavonoid effects in vitro or in vivo are investigated owing to its relative metabolic stability.
Acknowledgement
Supported by the Cystic Fibrosis Foundation (Fische07G0, Illek08G0) and the National Institutes of Health (HL086323, HL089196, AT002620).
References
- 1.Van Goor F, Straley KS, Cao D, Gonzalez J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PDJ, Negulescu P. Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 2.Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol Cell Physiol. 1995;268:C886–C893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- 3.Sears CL, Firoozmand F, Mellander A, Chambers FG, Eromar IG, Bot AG, Scholte B, De Jonge HR, Donowitz M. Genistein and tyrphostin 47 stimulate CFTR-mediated Cl secretion in T84 cell monolayers. Am J Physiol Gastrointest Liver Physiol. 1995;269:G874–G882. doi: 10.1152/ajpgi.1995.269.6.G874. [DOI] [PubMed] [Google Scholar]
- 4.Illek B, Fischer H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 1998;275:L902–L910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- 5.Illek B, Lizarzaburu ME, Lee V, Nantz MH, Kurth MJ, Fischer H. Structural determinants for activation and block of CFTR-mediated chloride currents by apigenin. Am J Physiol Cell Physiol. 2000;279:C1838–C1846. doi: 10.1152/ajpcell.2000.279.6.C1838. [DOI] [PubMed] [Google Scholar]
- 6.Otake Y, Walle T. Oxidation of the flavonoids galangin and kaempferide by human liver microsomes and CYP1A1, CYP1A2, and CYP2C9. Drug Metab Dispos. 2002;30:103–105. doi: 10.1124/dmd.30.2.103. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji P, Winn R, Walle T. Accumulation and metabolism of the anticancer flavonoid 5,7-dimethoxyflavone compared to its unmethylated analog chrysin in the atlantic killifish. Chem Biol Interact. 2006;164:85–92. doi: 10.1016/j.cbi.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos. 2006;34:1786–1792. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 9.Illek B, Tam AW-K, Fischer H, Machen TE. Anion selectivity of apical membrane conductance of Calu 3 human airway epithelia. Pflugers Arch. 1999;437:812–822. doi: 10.1007/s004240050850. [DOI] [PubMed] [Google Scholar]
- 10.Haws C, Krouse ME, Xia Y, Gruenert DC, Wine JJ. CFTR channels in immortalized human airway cells. Am J Physiol Lung Cell Mol Physiol. 1992;263:L692–707. doi: 10.1152/ajplung.1992.263.6.L692. [DOI] [PubMed] [Google Scholar]
- 11.Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 1997;272:L752–761. doi: 10.1152/ajplung.1997.272.4.L752. [DOI] [PubMed] [Google Scholar]
- 12.Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: A human airway epithelial cell line that shows cAMP-dependent Cl-secretion. Am J Physiol Lung Cell Mol Physiol. 1994;266:L493–501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- 13.Illek B, Zhang L, Lewis NC, Moss RB, Dong J-Y, Fischer H. Defective function of the cystic fibrosis-causing mutation G551D is recovered by genistein. Am J Physiol. 1999;277:C833–C839. doi: 10.1152/ajpcell.1999.277.4.C833. [DOI] [PubMed] [Google Scholar]
- 14.Fischer H. Electrophysiologic approch to study CFTR. In: Skatch WR, Walker JM, editors. Methods in Molecular Medicine. Humana Press Inc; Totowa: 2002. pp. 49–66. [Google Scholar]
- 15.Fischer H, Machen TE. CFTR displays voltage dependence and two gating modes during stimulation. J Gen Physiol. 1994;104:541–566. doi: 10.1085/jgp.104.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: Techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther. 1995;6:445–455. doi: 10.1089/hum.1995.6.4-445. [DOI] [PubMed] [Google Scholar]
- 17.Illek B, Fischer H, Machen TE. Alternate stimulation of apical CFTR by genistein in epithelia. Am J Physiol Cell Physiol. 1996;270:C265–C275. doi: 10.1152/ajpcell.1996.270.1.C265. [DOI] [PubMed] [Google Scholar]
- 18.Moran O, Galietta L, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–460. doi: 10.1007/s00018-004-4422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos. 2002;30:576–581. doi: 10.1124/dmd.30.5.576. [DOI] [PubMed] [Google Scholar]
- 20.Springsteel M, Galietta L, Ma T, By K, Berger G, Yang H, Dicus C, Choung W, Quan C, Shelat A, Guy R, Verkman A, Kurth M, Nantz M. Benzoflavone activators of the cystic fibrosis transmembrane conductance regulator: Towards a pharmacophore model for the nucleotide-binding domain. Bioorg Med Chem. 2003;11:4113–4120. doi: 10.1016/s0968-0896(03)00435-8. [DOI] [PubMed] [Google Scholar]