Abstract
Neutrophil chemoattractant IL-8 is cleaved and inactivated by the Streptococcus pyogenes cell envelope protease, SpyCEP. A range of clinical S. pyogenes strains of differing emm-type demonstrated SpyCEP activity, although transcription of the SpyCEP gene cepA differed 1000-fold between isolates. Disruption of the 2-component regulatory system covR/S in pharyngeal isolates increased cepA transcription 100-fold; this finding is consistent with endogenous CovR/S-mediated cepA repression being responsible for low SpyCEP expression in some pharyngitis strains. Among patients with invasive S. pyogenes infection, disease severity and outcome was associated with the SpyCEP activity of the isolate. Lethal invasive isolate H292 (emm81) expressed more cepA than did other tested isolates. This strain carried a unique covR mutation that impaired binding to the cepA promoter. CovR/S sequence comparison in other clinical isolates revealed community-wide dissemination of covS but not covR mutations. The results highlight a potential hazard and underline the importance of continuing molecular epidemiological surveillance for community-wide dissemination of CovR/S mutant hyper-invasive strains.
Keywords: Streptococus pyogenes, Interleukin-8, Interleukin-6, CovR, SpyCEP protein, Virulence factors, Necrotizing fasciitis, Gene expression regulation, Neutrophils, Bacterial proteins
Introduction
Streptococcus pyogenes (group A streptococcus, GAS) causes a spectrum of infections, ranging from pharyngitis and impetigo, to more invasive, life threatening diseases such as necrotizing fasciitis and toxic-shock syndrome [1]. Lethal S. pyogenes soft tissue infections are associated with paucity of neutrophils at the site of infection [2-4]. The S. pyogenes cell envelope proteinase, SpyCEP can cleave and inactivate human interleukin-8 at Glu59-Arg60 removing the IL-8 C-terminal alpha-helix, thereby interrupting one of the fundamental human mechanisms of protective phagocyte recruitment [3].
SpyCEP is a cell wall-anchored subtilisin-like serine protease that can be released as a soluble enzyme [3, 5, 6]; although structurally similar to streptococcal C5a peptidases, it has no proteolytic activity against the C5a component of complement [3]. Invasive blood isolates of S. pyogenes have high SpyCEP activity, whilst lower activity is seen in non-invasive pharyngeal isolates [3]. In the present study we explored the basis for variation in SpyCEP expression between invasive and non-invasive isolates. In this article, we demonstrate that such variation is regulated at the level of transcription of the SpyCEP gene, cepA (also known as scpC) and that disease severity is associated with SpyCEP activity amongst invasive isolates. We confirm that cepA is subject to repression by the two-component control of virulence regulatory sensor CovR/S in pharyngeal strains that demonstrate low SpyCEP activity [7]. In contrast, strains with the greatest SpyCEP activity may have mutations within CovR/S. In this article, we show that a mutation in the covR locus underlies the extremely high expression of SpyCEP and associated virulence of one lethal invasive isolate, and we highlight the potential for harmful mutations to spread within the community.
Methods
Bacterial strains and media
Invasive S. pyogenes isolates (n = 17) were obtained from blood or muscle culture specimens from patients who, from 1994 through 2005, were referred to the infectious disease service at Hammersmith Hospital (London, United Kingdom), with a range of invasive disease manifestations including necrotizing fasciitis and pneumonia. Invasive isolate H292 was from a patient with bacteremia and lethal necrotizing fasciitis [3]. For measurement of gene expression, we used 9 invasive isolates that were included in a previously published report [3], although all activity analyses were repeated for the present study. Non-invasive isolates (n = 8) were obtained from patients with uncomplicated pharyngitis. Strain H305, an M1 scarlet fever throat strain (NCTC8198), was included as a well-characterized reference isolate [8]. For covR/S sequence analysis, isolates from a 1930-1940 S. pyogenes collection, as well as additional emm1 and emm81 blood culture isolates recovered in the United Kingdom, were also included.
S. pyogenes were grown in Todd-Hewitt broth (THB) (Oxoid) or Columbia horse blood agar. Escherichia coli strains were cultured in Luria-Bertani broth (Oxoid). Antibiotics were used at the following concentrations: erythromycin 1μg/mL, kanamycin 50μg/mL.
Patient serum samples
Serum samples were obtained from 15 patients at the time of admission to hospital and before antibiotics were administered; these patients were subsequently proven to have invasive S. pyogenes infection (5 had necrotising fasciitis, 2 had pneumonia, 3 had cellulitis, and 5 had other types of infection, Table 1). Clotted blood samples were drawn on admission to hospital for clinical chemistry analysis and residual serum samples were stored frozen at −80°C for research purposes. Serum samples were associated with the causal S. pyogenes isolates.
Table 1. Strains used in this study.
| emm type | Strain | Disease Focus | Source | Outcome |
|---|---|---|---|---|
| Invasive | ||||
| emm81 | H292 | Necrotizing fasciitis, STSS | Blood | Died |
| emm81 | H429 | Cellulitis | Blood | Survived |
| emm1 | H366 | Pneumonia, STSS | Blood | Survived |
| emm1 | H369 | Infected pelvic thrombosis | Blood | Survived |
| emm1 | H295 | Cellulitis | Blood | Survived |
| emm1 | H297 | Peritonitis, STSS | Blood | Survived |
| emm1 | H319 | Cellulitis | Blood | Survived |
| emm1 | H438 | Arm wound | Blood | Survived |
| emm3 | H325 | Necrotizing fasciitis, STSS | Blood | Died |
| emm3 | H330 | Puerperal sepsis, STSS | Blood | Died |
| emm5 | H386 | Necrotizing fasciitis, STSS | Blood | Survived |
| emm22 | H378 | Necrotizing fasciitis, STSS | Blood | Survived |
| emm28 | H360 | Occult bacteremia, STSS | Blood | Died |
| emm89 | H293 | Necrotizing fasciitis, STSS | Muscle | Survived |
| emm89 | H395 | Pneumonia | Blood | Survived |
| Non-Invasive | ||||
| emm3 | H356 | Pharyngitis | Throat | N/A |
| emm2 | H343 | Pharyngitis | Throat | N/A |
| emm12 | H357 | Pharyngitis | Throat | N/A |
| emm22 | H340 | Tonsillitis | Throat | N/A |
| emm28 | H342 | Pharyngitis | Throat | N/A |
| emm75 | H347 | Pharyngitis | Throat | N/A |
| emm75 | H353 | Pharyngitis | Throat | N/A |
| emm78 | H348 | Pharyngitis | Throat | N/A |
Enzyme- linked immunosorbent assay
Human IL-6 was measured in patient serum samples by means of the enzyme linked immunosorbent assay (ELISA) development kit Duoset (R&D). IL-8 cleaving activity was measured in late log (LL) phase (optical density measured at 600nm [OD600], 0.7-0.9) culture bacterial cell-free supernatant co-incubated at 37°C overnight with 5ng/ml human IL-8 (R&D) and either 50% normal (“nonimmunized”) rabbit serum or anti-SpyCEP rabbit serum raised against a recombinant protein representing residues 35 to 587 of the pre-pro SpyCEP sequence (ie, including signal sequence; Genbank accession no. DQ413032) [6]. Uncleaved IL-8 was measured using human IL-8 Duoset ELISA (R&D) (which does not detect cleaved IL-8), and IL-8 cleaving activity caused by SpyCEP was calculated in comparison to the control (IL-8 in Todd-Hewitt broth).
Gene sequence analysis
Bacterial DNA was extracted using the method of Pospiech and Neumann [9]. The primers used in the present study (Table 2) were designed using the available GenBank S. pyogenes genomes. Automated fluorescent DNA sequencing was provided by the Genomics Core Laboratory, Medical Research Council Clinical Sciences Centre, Hammersmith Hospital. emm typing was performed according to the protocol of the Centers for Disease Control and Prevention [10]. CovR/S mutations were assigned as non-synonymous variations that were not present in all the available S. pyogenes genome sequences.
Table 2. Primers used in this study.
| Target | Primer Name | Sequence (5′ to 3′) |
|---|---|---|
| cepA sequencing | CEP1-F | ACCTTGTCGAAGATAGACTG |
| CEP1-R | TCGTAGCACACGACAATAAC | |
| CEP2-F | AACATTTGTGGAGCGACAAG | |
| CEP2-R | AGGGACCGGTAAGTCTAATC | |
| CEP3-F | TTAGCAGTTGCAGAAGAGTC | |
| CEP3-R | GGTCTGATAATTACCGTCTG | |
| CEP4-F | ATCTGCTTTCATATCGCACG | |
| CEP4-R | TGGTCTCTGAACCAAGAGTG | |
| CEP5-F | ACACGGTATGCATGTGACAG | |
| CEP5-R | AATAGCCATCAGAAGTTAGG | |
| CEP6-F | AATCCAATAACATAGTTACAAGG | |
| CEP6-R | AAGAGTGATTCAGCTGATCC | |
|
cepA promoter sequencing |
PromF | GCTAGTGGTGCTGGTGATACC |
| PromR | TTCAGCTGAGCTCAACTCTG | |
| PromSeq | CGGTATGCCAATTGTTCAAG | |
| covR sequencing | Cov1F | CTTGCAAGGGTTGTTTGATG |
| Cov1R | TTGAACTATATGGCAATCAGTG | |
| covS sequencing | Cov2F | ATATCCAAACAGTGCGTGGC |
| Cov2R | CGTTGTAAGAGACCAATATGC | |
| Cov3F | CGCTCAGATATTTCGTCAGG | |
| Cov3R | ATCGCGATTGACAGTATTTC | |
| gyrA real time PCR | GyrA F | AGCGAGACAGATGTCATTGCTCAG |
| GyrA R | CCAGTCAAACGACGCAAACG | |
| cepA real time PCR | SpyCEPF | ACACGGTATGCATGTGACAG |
| SpyCEPR | GATAAAGAGTGATTCAGGTGATCC | |
| CovR/S knockout | CovR/S- KO F | CGGAATTCTCTGGCTAGATTCGTTTCTC |
| CovR/S- KO R | CGGAATTCATAGCACGAATACGGGCAAG | |
| CovR protein expression |
CovR-protein F | CACGTGACAAAGAAAATTTTA |
| CovR-protein R | TTATTTCTCACGAATAACGTATCC | |
|
cepA promoter region for EMSA |
CEPGS-F | TTCATCGATTCCATTTGATC |
| CEPGS-R | GTTCCTGATTTGTATTTTCTAA | |
|
hasA promoter region for EMSA |
HasAGS-F | CATAAATTCCTACAGTTGATGT |
| HasAGS-R | CATAAATTCCTACAGTTGATGT |
Quantitative real-time polymerase chain reaction
RNA was extracted from early log (EL; OD6000.2-0.3), mid log (ML; OD6000.5) and late log (LL; OD6000.7-0.9) phases of growth, on three separate occasions (Protocol in Appendix). Complementary DNA was synthesised from 5μg of Turbo DNA-free (Ambion) treated RNA (Transcriptor reverse transcriptase, Roche Diagnostics). Real time polymerase chain reaction (PCR) was performed on 200 ng of complementary DNA template in triplicate, by use of SYBR Green Jumpstart Taq Readymix (Sigma). A standard-curve method was applied to calculate the copy number for each transcript by use of BamH1 linearized plasmid (RTpCR2.1) of known concentration (2 × 108 to 2 × 102 copies). RTpCR2.1 was constructed using the TA cloning kit (Invitrogen), to contain single copies of blunt-end ligated 100- to 150-bp regions of cepA and hasA, and reference gene gyrA, generated by PCR (Table 2). Each standard reaction also contained 5ng of λ DNA to stabilize the plasmid and mimic amplification conditions of the sample reactions. Copies of sample transcript were measured against the standard and normalized to 10,000 copies of gyrA.
Construction of CovR/S mutant pharyngeal GAS strains
A 39- to 377-bp region of the covR coding sequence amplified using CovR-knockout (KO) primers (Table 2) was cloned into the EcoRI site of the temperature-sensitive shuttle vector pGhostaph1 [11]. The plasmid, which was designated “pGhostcovR”, was used to transform 2 pharyngeal S. pyogenes isolates (emm75 and emm2) by electroporation. Transformants were selected using erythromycin at 30°C and then were moved to 37°C in liquid culture to obtain plasmid integration. Transformants were analyzed by PCR and Southern blot hybridisation.
Western blot analysis of culture supernatant and cell wall fractions
Supernatant and cell pellets were obtained from isolates cultured to the EL, ML and LL phases of growth. Cell wall preparations were made using an enzymatic method described by Biswas et al [12]. Proteins were separated by 10% Bis-Tris sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (Invitrogen) and underwent immunoblotting performed using rabbit polyclonal anti-SpyCEP serum. Blots were developed using the ECL system (GE Healthcare).
IL-8 cleavage SDS-PAGE & immunoblotting
Cell-free bacterial culture supernatant was incubated at 37°C overnight with 100μg/mL human IL-8 (R&D). IL-8 was detected by 12% Bis-Tris SDS-PAGE (Invitrogen) stained with Colloidal Blue staining kit (Invitrogen) or immunoblotted with anti-human IL-8 horse-radish peroxidise-labeled antibody (R&D) and streptavidin (R&D).
Electromobility shift assay
Wild-type CovR (from reference strain H305) and mutant CovR from H292 were expressed and purified from E. coli (Appendix). Electromobility shift assay primers (Table 2) yielded 295-bp and 336-bp products for the cepA and hasA promoter regions, respectively and were labeled with digoxigenin (DIG) by use of the Non-radioactive Gel Mobility Shift Assay kit (Roche Diagnostics). CovR protein was diluted in binding buffer (50 mmol/L potassium chloride, 25 mmol/L Tris pH8, 5 mmol/L magnesium chloride, 3 mmol/L calcium chloride, 4% glycerol, 1 mg/mL bovine serum albumin, 10 μg/mL salmon sperm DNA, 0.5 mmol/L ethylenediaminetetraacetic acid [EDTA], 0.5 mmol/L dithiothreitol) [13] with 0.8 ng of labelled promoter and incubated at room temperature for 20 min before it was loaded onto a 0.5 × Tris-borate-EDTA DNA retardation gel (Invitrogen). DNA was electro-transferred onto Hybond-N (GE Healthcare) membrane and DIG labelled DNA was detected using anti-DIG–alkaline phosphatase antibody and developed with CDP-Star (Roche Diagnostics).
Ethics
The collection, storage, and testing of anonymized serum samples and bacterial strains obtained from patients identified as having invasive S. pyogenes infection were approved by the local research ethics committee of the Hammersmith Hospitals National Health Trust.
Statistical analysis
All statistics were performed using non-parametric analysis with GraphPad Prism for Windows, (version 4.03; GraphPad Software).
Results
SpyCEP- dependent IL-8 cleaving activity in all clinical isolates
IL-8 cleaving activity was tested in a range of clinical isolates, both invasive (n=15) and non-invasive (n=8) and of different emm types (Figure 1, Table 1). Consistent with the findings of a previously published report [3], invasive isolates had greater IL-8 cleaving activity (86.0%; range, 54.3%-93.6%) than did non-invasive isolates (60.7%; range, 23.5%-87.3%) (P<0.011). IL-8 cleaving activity was specifically caused by proteolytic activity of SpyCEP, because IL-8 cleavage was abrogated by co-incubation with specific anti-SpyCEP antibody.
Figure 1.
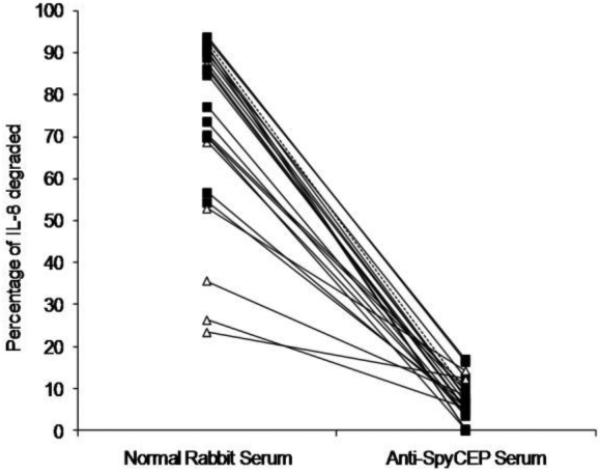
Inhibition of interleukin(IL)-8 degrading activity by anti-Streptococcus pyogenes cell envelope proteinase (SpyCEP) serum. IL-8 degrading activity was measured in a range of isolates of different emm-types (emm 81, 1, 2 3, 5, 12, 22, 28, 44, 75, 78, 89). Bacterial supernatants were co-incubated with 5ng/ml IL-8 and either normal rabbit serum or anti-SpyCEP serum. The percentage of IL-8 degraded represents comparison with IL-8 incubated with serum only. Data are the mean of 3 separate experiments measured in duplicate. Invasive isolates (black squares) had higher cleaving activity compared to non-invasive isolates (white triangles) (P<0.011, Mann-Whitney U test,). Dotted line: reference emm1 strain H305
Variation in IL-8 cleaving activity and cepA transcription
Sequence analysis of the SpyCEP gene cepA in S. pyogenes isolates of different emm-types (8 invasive, 8 non-invasive and 1 reference strain) showed that variation in IL-8 cleaving activity was not associated with an alteration in cepA sequence (Genbank accession nos. DQ413032-DQ413046, EU730694 and EU730695).
SpyCEP was expressed continuously throughout exponential growth both in supernatant and in the cell wall (data not shown). Separate ELISA studies performed on whole cells confirmed that cell wall SpyCEP was biologically active (not shown).
Expression of cepA at ML phase was highly variable between isolates, ranging from 10 to 10,000 copies of cepA per 10,000 copies of the housekeeping gene, gyrA (Figure 2A). Invasive isolates (n = 9) expressed levels of cepA that were higher than those expressed by non-invasive isolates (n = 8) (P=0.046, Mann-Whitney U test). Expression of capsule operon member hasA was also increased in invasive isolates, compared with non-invasive isolates, and it correlated positively with cepA expression (Figure 2B) (r = 0.67, Spearman rank correlation; P<0.003), suggesting that both genes were co-regulated. hasA is known to be negatively controlled by the 2-component system CovR/S [14, 15]. This finding is consistent with 2 putative ATTARA CovR-binding sites located within the cepA promoter region, which were present in all isolates sequenced (DQ413032-DQ412047, EU730694 and EU730695).
Figure 2.
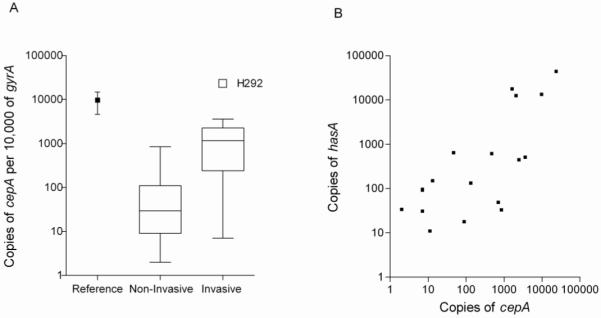
A, Comparison of cepA transcription between invasive and non-invasive isolates. Target gene transcripts were measured as the no. of copies per 10,000 copies of the housekeeping gene gyrA, with reference to a plasmid of known copy number. Data are grouped by isolate source. Reference strain: H305, M1T1 scarlet fever isolate. Strain H292 is shown as an outlier. Invasive isolates expressed more cepA than did non-invasive isolates, (P<0.046, Mann-Whitney U test). B, Correlation between copies of cepA and copies of hasA. The expression of cepA correlated positively with the expression of hasA (r= 0.67, Spearman rank correlation; P<0.003).
Pharyngeal isolates and experimental increases in enhanced IL-8 cleaving activity through mutation of covR/S
We hypothesized that if CovR/S represses SpyCEP expression in non-invasive pharyngeal isolates, then disruption of the covR/S locus in these isolates would de-repress cepA. Mutagenesis of covR/S in an emm75-type low SpyCEP-producing pharyngeal isolate resulted in a mutant covR/S strain (ΔcovR/S). Western blot analysis demonstrated that, although SpyCEP was undetectable in the parent strain, it was clearly present both in the supernatant and the cell wall of ΔcovR/S (Figure 3A). This finding was associated with clear acquisition of IL-8 cleaving activity in the culture supernatant of ΔcovR/S, which was absent in the culture supernatant of the parent strain (Figure 3B). Real time PCR analysis confirmed an ~200-fold increase in cepA expression in the EL and ML phases and 10-fold in the LL phase (Figure 3C). To verify that this increase was not an emm-type specific phenomenon, covR/S mutagenesis was repeated in another low SpyCEP-producing pharyngeal isolate of different emm-type, emm2. Similar increased expression of SpyCEP was seen with 200-, 100- and 10-fold increases in the EL, ML and LL phases, respectively. The data confirmed that CovR/S represses cepA in non-invasive isolates and that repression is more pronounced in the EL and ML phases of logarithmic growth.
Figure 3.
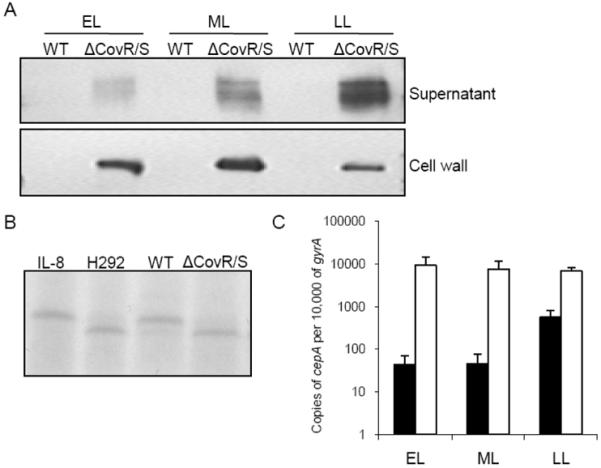
De-repression of cepA in a low producer of Streptococcus pyogenes cell envelope proteinase (SpyCEP), by covR/S mutation. A, Western blot analysis for SpyCEP in culture supernatant and cell wall extract from wild type (WT) emm75 pharyngeal isolate and ΔcovR/S mutant grown to early-log (EL), mid-log (ML) and late-log (LL) phases of growth. B, Coomassie blue-stained sodium dodecyl sulphate-polyacrylamide gel electrophoresis showing IL-8 cleavage after incubation with supernatant from WT or ΔcovR/S mutant. IL-8 incubated in Todd-Hewitt broth alone was used as a control. As a positive control for cleavage, IL-8 was incubated with supernatant from the high SpyCEP producer, H292. C, No. of copies of cepA transcript per 10,000 copies of gyrA transcript measured by real-time polymerase chain reaction comparing WT (black bars) and ΔcovR/S mutant (white bars) at EL, ML and LL phase. Each bar denotes the mean of 3 independent samples measured in triplicate (+standard deviation). The effect of the CovR/S mutation was reproduced in another pharyngeal emm2 isolate.
Association of SpyCEP activity in invasive disease with severity of disease
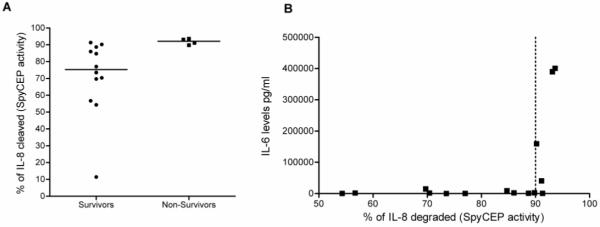
Focusing on invasive disease isolates, bacterial SpyCEP activity was determined for each of 15 clinical cases for which paired serum and isolates were available; 14 of 15 patients had bacteremia. Although numbers were small, non-survivors yielded isolates that had higher IL-8 cleaving activity (median, 92.1%, range 89.8%-93.60%) than did those from survivors (median, 77.0%, range 54.3%-91.3%) (P<0.016) (Figure 4A). Consistent with this finding, isolates with the greatest SpyCEP activity (>90%) were associated with the highest serum IL-6 levels in patients (Figure 4B).
Figure 4.
Association of Streptococcus pyogenes cell envelope proteinase (SpyCEP) activity with disease outcome. A, SpyCEP activity was measured in the culture supernatant of clinical invasive S. pyogenes disease isolates by means of enzyme-linked immunosorbent assay (ELISA) performed to calculate interleukin(IL)-8 degrading activity as a percentage of the control, averaged over 3 separate experiments. Isolates were matched to the disease outcome of the patient from which they originated (survivors or non-survivors). The median value of the data is denoted by a horizontal line. SpyCEP activity was significantly higher in strains from non-survivors (P< 0.016, Mann-Whitney U test). B, Association of patient serum IL-6 levels with the SpyCEP activity of the causative isolate. IL-6 was measured by ELISA performed on patient serum samples obtained at the onset of bacteremia. High IL-6 levels were associated with high SpyCEP activity apparent at SpyCEP activity >90% (dotted line).
Invasive isolates and acquisition of marked IL-8 cleaving activity through natural mutation of covR
In recent years, emm81 strains have emerged as important causes of invasive GAS disease in Europe [16-19]. Of the 6 invasive emm81 isolates studied, strain H292 was the only isolate to cause lethal disease with rapidly progressive lethal necrotizing fasciitis. H292 expressed more cepA than any other isolate analyzed. To confirm that high cepA expression was unique to H292, we compared the expression of SpyCEP in 5 contemporaneous clinical emm81 isolates recovered from patients with nonfatal cases of invasive disease. Strain H292 expressed approximately 20- to 800- fold, 20- to 3,000-fold, and 2- to 400- fold more copies of cepA than the other 5 emm81 isolates (a-e) at EL, ML and LL phases, respectively (Figure 5A). Transcription of hasA in these isolates was consistent with cepA; H292 expressed >60-fold more copies of hasA than all the 5 other emm81 isolates (Figure 5B). Taken together the results suggest that enhanced disease severity can be associated with specific and detectable polarization of phenotype.
Figure 5.
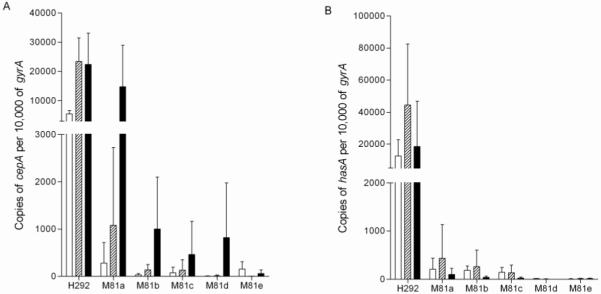
Association of CovR substitution with high cepA and hasA transcription. Copies of cepA (A) and hasA (B) transcript expressed by H292, compared to other contemporaneous emm81 isolates (M81a-e) measured as copies per 10,000 copies of gyrA. Each bar denotes the mean (+standard deviation) of 3 independent samples obtained at early log (white bar), mid log (stripe bar) and late log (black bar) phase, measured in triplicate.
We hypothesized that CovR/S mutations may be responsible for the high cepA expression in strain H292; therefore we sequenced the covR/S locus in this isolate and 5 other emm81 isolates. Although other emm81 isolates were wild type for CovR/S, strain H292 had a substitution mutation in CovR of alanine (Ala) to valine (Val) at amino acid (aa) residue 111 (Table 3). This mutation was stable despite passage in vitro and in vivo.
Table 3.
CovR/S sequence analysis in emm1 and emm81 isolates
|
emm- type |
Source | Date | SpyCEP Copy No.a |
CovR/S | Sequence Analysis |
|---|---|---|---|---|---|
| emm1 | Reference | 1880 | 9669 | CovS | 1bp insert=stop at 318aa |
| Blood | 1932 | 3505 | Wild type sequence | ||
| Blood | 1934 | 1334 | Wild type sequence | ||
| HVS | 1938 | 56 | CovS | Arg to Cys at 216aa Leu to Val at 458aa |
|
| Blood | 1999 | 1363 | CovS | Ala to Thr at 38aa Ile to Val at 332aa |
|
| Blood | 2000 | 700 | CovS | Ala to Thr at 38aa Ile to Val at 332aa |
|
| Blood | 2000 | 472 | CovS | Ile to Val at 332aa | |
| Blood | 2000 | 3594 | CovS | Ile to Val at 332aa† | |
| Throat | 2000 | 786 | CovS | Ala to Thr at 38aa Ile to Val at 332aa |
|
| emm81 | Bloodb | 1994 | 23447 | CovR | Ala to Val at 111aa |
| Blood | 2001 | 1152 | Wild type sequence | ||
| Blood | 2001 | 138 | Wild type sequence | ||
| Blood | 2001 | 131 | Wild type sequence | ||
| STSS | 2003 | 12 | Wild type sequence | ||
| STSS | 2003 | 5 | Wild type sequence | ||
Note. Genbank accession numbers EU726232, EU726234, EU26247-EU26249, EU730696-EU730705. Aa, amino acid; Ala, alanine; Arg, arginine; Cys, cysteine; HVS, high vaginal swab; Ile, isoleucine; Leu, leucine; PCR, polymerase chain reaction; STSS, streptococcal toxic-shock syndrome; Thr, threonine; Val, valine; WT, wild type.
copies per 10,000 copies of gyrA measured by real time PCR at mid log phase
Strain H292
Electromobility shift assays were performed to examine binding of recombinantly-expressed CovR protein from the reference strain or strain H292 to a 295-bp region of the cepA promoter (pcepA). Without phosphorylation, dose-dependent binding of CovR occurred, shifting the CovR-pcepA complex to form I (Figure 6A). On phosphorylation the CovR-pcepA complex further shifted to form II confirming binding of CovR directly to the cepA promoter. Without phosphorylation, mutant H292-CovR demonstrated binding similar to that demonstrated by the wild type; in contrast, with phosphorylation the mutant H292-CovR was unable to shift the complex further to form II. Binding to the has operon promoter was similarly affected by the single residue substitution (Figure 6B), confirming that the mutation in CovR impaired its phosphorylation-enhanced ability to bind to both the SpyCEP and capsule operon promoters.
Figure 6.
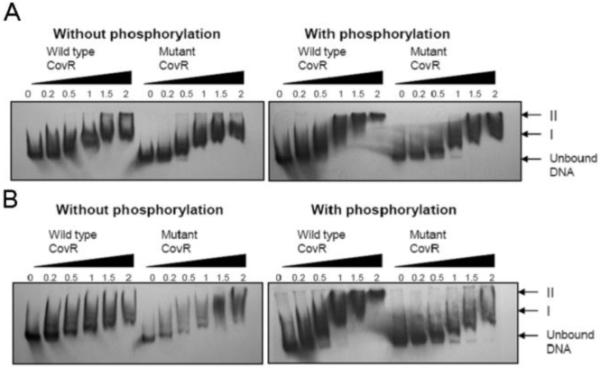
Reduction in binding associated with mutant CovR, compared with wild-type CovR. Electromobility shift assays with wild-type CovR or mutant CovR either without phosphorylation (left) or with phosphorylation by preincubation with acetyl phosphate (right). The amount of protein added is expressed in micrograms above each lane. Positions of unbound DNA and CovR-promoter complexes are denoted by arrows. A Binding of wild-type and mutant CovR to a 295bp region of the cepA promoter. B Binding of wild-type or mutant CovR to a 344bp region of the hasA promoter.
Commonality of variations in CovS but not CovR among isolates of different emm-types and evidence of spread in the community
CovS mutations have recently been highlighted to underlie the invasiveness of modern emm1 isolates [20, 21]. Sequencing of covR/S in a broadened range of clinical isolates of various emm-types obtained from 2 collections of isolates at Hammersmith Hospital revealed a number of variations in covS but not covR (Tables 3 and 4). In contrast to the covR mutation seen in strain H292, covS single amino acid changes were found in both invasive and non-invasive isolates and were not necessarily associated with high expression of cepA. Reference emm1 strain H305, a scarlet fever throat isolate (NCTC8198) and a high expresser of cepA, had a single-base insert mutation in covS that results in a premature stop codon. A single consistent variation (I332 to V332) was observed in modern emm1 isolates originating from patients in West London in 1999-2000, in some cases coupled with an additional variation (Ala to threonine [Thr] at aa 38); this variation was observed in both blood and throat isolates.
Table 4.
CovR/S sequence analysis in a range of M-types
|
emm- type |
Source | Date | SpyCEP Copy No.a |
CovR/S | Sequence Analysis |
|---|---|---|---|---|---|
| emm2 | Throat | 1998 | 7 | Wild type sequence | |
| emm3 | Blood | 1998 | 2047 | CovS | Pro to Ser at 285aab |
| Throat | 1998 | 13 | Wild type sequence | ||
| Blood | 1998 | 7 | Wild type sequence | ||
| emm12 | Throat | 1998 | 2 | Wild type sequence | |
| emm22 | Throat | 1998 | 11 | Wild type sequence | |
| emm28 | Blood | 1999 | 2450 | CovS | 1bpc insert = stop at 85aa |
| Throat | 1998 | 132 | Wild type sequence | ||
| emm58 | Blood | 1998 | 7 | CovS | Thr to Ile at 42aa |
| emm75 | Throat | 1998 | 846 | CovS | Ser to Leu at 337aa |
| Throat | 1998 | 46 | CovS | Ser to Leu at 337aa | |
| emm78 | Throat | 1998 | 88 | Wild type sequence | |
| emm89 | Muscle | 1995 | 1613 | Wild type sequence | |
copies per 10,000 copies of gyrA measured by quantitative real time PCR at mid log phase
aa, amino acid.
bp, base pair
Genbank accession numbers EU726233, EU726235-EU726246
Discussion
Streptococcus pyogenes cell envelope proteinase, SpyCEP cleaves and inactivates IL-8 and other CXC chemokines, disrupting IL-8 mediated neutrophil recruitment [3, 6, 7, 22]. In the present study, all clinical isolates tested were able to cleave IL-8 to some degree, and this activity could be specifically prevented by SpyCEP-targeted anti-serum, confirming that SpyCEP is responsible for IL-8 cleaving activity in all GAS strains. Consistent with a previous report by Edwards et al [3], SpyCEP activity was higher in clinical invasive isolates than in non-invasive isolates, regardless of emm-type.
In the present study, we had a unique opportunity to measure serum cytokine levels and bacterial SpyCEP activity in samples obtained from the same patients at the time of admission to the hospital. S. pyogenes SpyCEP activity was associated with a poor outcome and elevated serum IL-6, suggesting that SpyCEP plays an important role in determining the severity of human disease. This finding is consistent with the findings of Zinkernagel et al. [6] and Hidalgo-Grass et al. [22] who demonstrated that mutagenesis of cepA (or scpC) led to attenuation of infection severity in mouse models. Elevated IL-6 levels have been associated with severe sepsis, toxic shock syndrome, and poor disease outcome [23-27]. In the present study, high serum levels of IL-6 at the time of bacteremia were associated failure to survive; patients who survived (n = 11) had lower IL-6 levels (median, 1.9ng/mL, range 0.13-159ng/mL) than did patients who did not survive (n = 4) (median, 215ng/mL, range 2.7-400ng/mL) (P<0.022). Interestingly, serum IL-8 levels did not correlate inversely with SpyCEP activity. We speculate that, during sepsis, serum IL-8 levels may reflect the severity of the disease and substrate abundance rather than SpyCEP abundance. It is also possible that serum IL-8 levels may not reflect tissue levels of IL-8, as supported by findings in mouse models [22].
SpyCEP is part of a family of lactococcal and streptococcal serine proteases that have adapted to cleave host chemokines; in addition to the C5a peptidases, homologues exist in many other pathogenic streptococci including, Streptococcus agalactiae (CspA), Streptococcus pneumoniae (PrtA), Streptococcus equi (SeCEP), and Streptococcus iniae (CepI) [6, 22, 28, 29]. In the present study, sequence analysis of different emm-types, showed the SpyCEP gene (cepA) to be highly conserved and unlikely to underlie the observed variation in SpyCEP activity between invasive and non-invasive isolates. Recently, invasive M1 S. pyogenes isolates were reported to demonstrate distinct transcriptomes, compared to non-invasive isolates [20]; upregulation of virulence factors including SpyCEP were attributed to mutations in the sensory component of the 2-component gene regulator system CovR/S. CovR/S represses approximately ~15% of the genome [30, 31] including hasA, a component of the hyaluronic acid capsule operon [15]. In the present study, expression of hasA, correlated positively with cepA expression suggesting that both genes are under the same regulatory influence. Deletion of the covR/S locus up-regulated cepA and conferred IL-8 degrading activity on 2 non-IL-8-degrading pharyngeal isolates thus demonstrating that cepA is under the negative influence of CovR/S even in non emm1 strains. Expression of cepA was growth phase dependent and, unlike other cell wall proteins, increased towards late logarithmic growth.
Strain H292 is an invasive emm81 isolate obtained from the blood of a patient who rapidly died from necrotizing fasciitis [3]. emm81 strains are increasingly recognised in Europe and represented 10-20% of invasive isolates in the United Kingdom and Sweden [16-19]. Strain H292 had very high SpyCEP activity and expressed the highest cepA transcript copy number compared to other isolates tested, including other emm81 isolates. The high expression of cepA was attributed to a mutation within covR comprising a single amino acid change of alanine to valine at aa 111; the in vivo effect of this CovR mutation could be further investigated by gene replacement of CovR. Nonetheless the in vitro studies reported herein demonstrate that this mutation affects phosphorylation-dependent binding of CovR not only to the cepA promoter but also to the hasA promoter. Wild-type CovR demonstrated binding to the cepA promoter consistent with the recently published findings of Sumby et al. [7]. Mutant H292-CovR was unable to shift to form II upon phosphorylation indicating reduced and/or unstable binding of CovR to the cepA and hasA promoters. This finding suggests that the variant H292-CovR would lead to reduced repression of target promoters [13]. Whether the H292-CovR mutation altered CovS-mediated phosphorylation directly or interfered with phosphorylation-dependent dimerization of CovR is unknown. Interestingly, the mutation in H292-CovR is in a region that is highly conserved amongst response regulators from other bacterial species but is not a recognised region for phosphorylation [32].
It was recently proposed that CovS mutations resulting in invasive GAS arose solely through selective pressure from a novel DNAse present in modern M1 strains only [21]. Although mutations that impede the function of CovS may contribute to virulence, our data show that some CovS sequence variations were observed both in invasive isolates as well as non-invasive isolates without any measurable effect on cepA expression. This may either be because the amino acid changes do not impede kinase activity or, perhaps, because CovS mutations can only have an indirect effect on CovR; CovR may be phosphorylated through other as yet undefined mechanisms. Many of the observed differences in CovS may reflect allelic variants that do not alter function.
Reference emm1 strain H305, a scarlet fever isolate dating from the 1880s had a mutation in covS that resulted in a premature stop codon. This finding potentially explains the high expression of exotoxins, cepA and hasA by this isolate and confirms that selective pressure for mutations in covR/S is not associated with modern strains alone.
Other CovR mutations were not found in our small collection of strains, though a CovR mutation in an emm3 isolate has previously been reported to affect capsule expression [33]. In the present article we have shown that CovR mutations can exert stable and dramatic effects on virulence factor expression but these mutations are rare. Reduced fitness to spread may be the reason behind the rarity of CovR mutations. Potentially CovR/S impaired-function mutations select against the carriage/pharyngeal state; whether major CovR/S mutations can confer or impede fitness to spread within a population now deserves focussed consideration as does the possibility that such mutations might explain rare clusters of invasive S. pyogenes disease [34].
Supplementary Material
Acknowledgments
The authors wish to thank Dr Ian Teo for advice on the quantitative real time PCR.
Funding: The Wellcome Trust, Lee Spark Foundation, Conor Kerin Fund, and the Hammersmith Hospital Trustees Research Committee. S.S. is grateful for the support from the UK NIHR Biomedical Research Centre funding scheme. C.E.T was supported by an MRC studentship award.
Footnotes
Potential conflicts of interest: C.E.T., P.K., M.J.D., R.J.E., S.S., No conflict.
Presented in part at: XVII Lancefield International Symposium on Streptococci and Streptococcal Diseases, Porto Heli, Greece, June 2008 (abstract 10.5).
Reference List
- (1).Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13(3):470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Taylor FB, Jr., Bryant AE, Blick KE, et al. Staging of the baboon response to group A streptococci administered intramuscularly: a descriptive study of the clinical symptoms and clinical chemical response patterns. Clin Infect Dis. 1999;29(1):167–77. doi: 10.1086/520147. [DOI] [PubMed] [Google Scholar]
- (3).Edwards RJ, Taylor GW, Ferguson M, et al. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J Infect Dis. 2005;192(5):783–90. doi: 10.1086/432485. [DOI] [PubMed] [Google Scholar]
- (4).Hidalgo-Grass C, Dan-Goor M, Maly A, et al. Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet. 2004;363(9410):696–703. doi: 10.1016/S0140-6736(04)15643-2. [DOI] [PubMed] [Google Scholar]
- (5).Middleton J, Neil S, Wintle J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91(3):385–95. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- (6).Zinkernagel AS, Timmer AM, Pence MA, et al. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe. 2008;4(2):170–8. doi: 10.1016/j.chom.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sumby P, Zhang S, Whitney AR, et al. A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect Immun. 2008;76(3):978–85. doi: 10.1128/IAI.01354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sriskandan S, Unnikrishnan M, Krausz T, Cohen J. Molecular analysis of the role of streptococcal pyrogenic Exotoxin A (SPEA) in invasive soft-tissue infection resulting from Streptococcus pyogenes. Mol Microbiol. 1999;33(4):778–90. doi: 10.1046/j.1365-2958.1999.01525.x. [DOI] [PubMed] [Google Scholar]
- (9).Pospiech A, Neumann B. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 1995;11(6):217–8. doi: 10.1016/s0168-9525(00)89052-6. [DOI] [PubMed] [Google Scholar]
- (10).Streptococcus pyogenes emm sequencing protocol. [Accessed 31 Aug 2008]. CDC.gov. Available at http://www.cdc.gov/ncidod/biotech/strep/M-ProteinGene_typing.htm.
- (11).Unnikrishnan M, Altmann DM, Proft T, et al. The bacterial superantigen streptococcal mitogenic exotoxin Z is the major immunoactive agent of Streptococcus pyogenes. J Immunol. 2002;169(5):2561–9. doi: 10.4049/jimmunol.169.5.2561. [DOI] [PubMed] [Google Scholar]
- (12).Biswas I, Germon P, McDade K, Scott JR. Generation and surface localization of intact M protein in Streptococcus pyogenes are dependent on sagA. Infect Immun. 2001;69(11):7029–38. doi: 10.1128/IAI.69.11.7029-7038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Miller AA, Engleberg NC, DiRita VJ. Repression of virulence genes by phosphorylation-dependent oligomerization of CsrR at target promoters in S. pyogenes. Mol Microbiol. 2001;40(4):976–90. doi: 10.1046/j.1365-2958.2001.02441.x. [DOI] [PubMed] [Google Scholar]
- (14).Levin JC, Wessels MR. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol Microbiol. 1998;30(1):209–19. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- (15).Bernish B, van dR I. Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J Biol Chem. 1999;274(8):4786–93. doi: 10.1074/jbc.274.8.4786. [DOI] [PubMed] [Google Scholar]
- (16).Curtis SJ, Tanna A, Russell HH, et al. Invasive group A streptococcal infection in injecting drug users and non-drug users in a single UK city. J Infect. 2007;54(5):422–6. doi: 10.1016/j.jinf.2006.10.004. [DOI] [PubMed] [Google Scholar]
- (17).Lamagni TL, Neal S, Keshishian C, et al. Severe Streptococcus pyogenes infections, United Kingdom, 2003-2004. Emerg Infect Dis. 2008;14(2):202–9. doi: 10.3201/eid1402.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Darenberg J, Luca-Harari B, Jasir A, et al. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis. 2007;45(4):450–8. doi: 10.1086/519936. [DOI] [PubMed] [Google Scholar]
- (19).Jasir A, Schalen C. Strep-EURO: progress in analysis and research into severe streptococcal disease in Europe, 2003-2004. Euro Surveill. 2005;10(2):E050203. doi: 10.2807/esw.10.05.02635-en. [DOI] [PubMed] [Google Scholar]
- (20).Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2(1):e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Walker MJ, Hollands A, Sanderson-Smith ML, et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13(8):981–5. doi: 10.1038/nm1612. [DOI] [PubMed] [Google Scholar]
- (22).Hidalgo-Grass C, Mishalian I, Dan-Goor M, et al. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. EMBO J. 2006;25(19):4628–37. doi: 10.1038/sj.emboj.7601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).O’Dwyer MJ, Mankan AK, Stordeur P, et al. The occurrence of severe sepsis and septic shock are related to distinct patterns of cytokine gene expression. Shock. 2006;26(6):544–50. doi: 10.1097/01.shk.0000235091.38174.8d. [DOI] [PubMed] [Google Scholar]
- (24).Norrby-Teglund A, Pauksens K, Norgren M, Holm SE. Correlation between serum TNF alpha and IL6 levels and severity of group A streptococcal infections. Scand J Infect Dis. 1995;27(2):125–30. doi: 10.3109/00365549509018991. [DOI] [PubMed] [Google Scholar]
- (25).Norrby-Teglund A, Chatellier S, Low DE, McGeer A, Green K, Kotb M. Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur J Immunol. 2000;30(11):3247–55. doi: 10.1002/1521-4141(200011)30:11<3247::AID-IMMU3247>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- (26).Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119(8):771–8. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- (27).Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–63. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Shelver D, Bryan JD. Expression of the Streptococcus agalactiae virulence-associated protease CspA in a soluble, active form utilizing the Gram-positive host, Lactococcus lactis. Journal of Biotechnology. 2008;136(3-4):129–34. doi: 10.1016/j.jbiotec.2008.06.002. [DOI] [PubMed] [Google Scholar]
- (29).Bethe G, Nau R, Wellmer A, et al. The cell wall-associated serine protease PrtA: a highly conserved virulence factor of Streptococcus pneumoniae. FEMS Microbiology Letters. 2001;205(1):99–103. doi: 10.1111/j.1574-6968.2001.tb10931.x. [DOI] [PubMed] [Google Scholar]
- (30).Churchward G. The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol Microbiol. 2007;64(1):34–41. doi: 10.1111/j.1365-2958.2007.05649.x. [DOI] [PubMed] [Google Scholar]
- (31).Graham MR, Smoot LM, Migliaccio CA, et al. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc Natl Acad Sci U S A. 2002;99(21):13855–60. doi: 10.1073/pnas.202353699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Dalton TL, Scott JR. CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J Bacteriol. 2004;186(12):3928–37. doi: 10.1128/JB.186.12.3928-3937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Miyoshi-Akiyama T, Ikebe T, Watanabe H, Uchiyama T, Kirikae T, Kawamura Y. Use of DNA arrays to identify a mutation in the negative regulator, csrR, responsible for the high virulence of a naturally occurring type M3 group A streptococcus clinical isolate. J Infect Dis. 2006;193(12):1677–84. doi: 10.1086/504263. [DOI] [PubMed] [Google Scholar]
- (34).Cordery RJ, Efstratiou A, George R, Cohuet S, Lamagni T. Invasive group A streptococcal disease in maternity settings: Time to reassess case and cluster management? [abstract P46]; Abstract book of XVII Lancefield International Symposium on Streptococcal & Streptococcal Diseases; Porto Heli, Greece. 2008.p. 140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.