Abstract
Streptococcus mutans, an agent of dental caries, was tested for growth in the presence or absence of manganese (Mn), since studies have linked Mn levels with cariogenic potential. Seven S. mutans serotype c strains were grown in chemically defined medium under different atmospheric conditions: 5% CO2, O2-enriched 5% CO2 (shaking) and anaerobic. There was significant strain variability with respect to Mn requirements under the various conditions tested. Both sucrose-dependent and sucrose-independent biofilm growth by strain UA159 were affected by the absence of Mn. S. mutans strains show highly variable responses to both high and low Mn concentrations.
Key Words: Biofilm, Caries, Manganese, Streptococcus mutans
Streptococcus mutans, a Gram-positive facultative anaerobic coccus that is part of the normal flora of the mouth, has been consistently associated with human dental caries [Loesche, 1986]. Trace metals have been studied for their roles in cariogenesis [Curzon, 1983]. While some elements such as aluminum, selenium and strontium have been associated with a low incidence of caries [Little and Barrett, 1976a, b], high concentrations of copper, manganese (Mn) and cadmium have been linked to a higher prevalence of dental caries [Beighton, 1982, 1983]. Furthermore, studies of trace metals in drinking water and tooth enamel have suggested a caries-promoting potential for Mn [Adkins and Losee, 1970; Glass et al., 1973]. Though availability of trace elements rarely limits microbial growth, since they are required in such minute amounts, there may be certain conditions under which the concentration of a trace element diminishes or enhances virulence. A rat model study revealed that Mn added to drinking water resulted in a significant increase in caries levels [Beighton, 1982]. Previous studies examining the effects of trace metals on S. mutans noted a requirement for Mn in some strains [Aranha et al., 1982] except under anaerobic conditions [Martin et al., 1984; Luengpailin, 2002; Paik et al., 2003]. Mn is essential for detoxification of reactive oxygen species in most bacteria. Furthermore, an increasing body of data indicates a role for Mn in the pathogenesis and virulence of a number of bacterial species [Jakubovics and Jenkinson, 2001; Kehres and Maguire, 2003; Paik et al., 2003; Zaharik and Finlay, 2004; Papp-Wallace and Maguire, 2006]. The objective of this study was to investigate how Mn affects biofilm formation and the growth of a panel of S. mutans serotype c strains, thereby defining the parameters appropriate for future investigations examining the effects of Mn on virulence gene expression.
Materials and Methods
Bacterial Strains and Media
S. mutans serotype c strains Ingbritt, LT11, 3209, UA130, UA159, ATCC 25175 and GS-5 were used in this study. Bacterial cultures were prepared in a modified chemically defined medium (SCDM) [Arirachakaran et al., 2007]. The medium was treated with Chelex 100 (Sigma, St. Louis, Mo., USA), a metal-chelating resin, to reduce trace metal contamination. Three independent ICP analyses indicated an average residual Mn concentration of 4 nM. Essential vitamins and minerals were added to complete the medium and, when desired, MnSO4 was added at varying concentrations.
Culture Conditions
Inocula were prepared by 3 serial subcultures in Mn-depleted media to eliminate the possibility of nutritional carryover from enriched media. For planktonic cultures 200 ml freshly prepared growth medium were placed aseptically into Erlenmeyer flasks and inoculated with 1% of mid-exponential phase inoculum. Cultures were incubated at 37°C in (1) 5% CO2; (2) O2-enriched 5% CO2 with shaking at 60 rpm for 10 s every hour, or (3) anaerobically (85% N2, 10% H2 and 5% CO2) (Forma Scientific, USA). Growth was monitored by measuring turbidity with a spectrophotometer at wavelength 540 nm. Brain-heart infusion (BHI) broth was used as the control medium, SCDM as the test medium without Mn or with MnSO4 added to a final concentration of 50, 100, 200 or 300 μM for each growth condition. All growth curves were performed in triplicate.
For biofilms, 70 μl of the inoculum preparation described above was added to 1.5 ml SCDM (4.7% inoculum), ± 5% sucrose and ± 50 μM Mn, in wells of a 2-well Lab-Tek Chamber slide (Nalgene Nunc International). For sucrose-independent biofilms the wells were first coated with artificial saliva (pH = 6.7) [Russell and Coulter, 1975; Landa et al., 1997]. For both sucrose and nonsucrose biofilms prewarmed medium, with or without Mn, was added along with the bacterial inocula. The biofilms were incubated overnight anaerobically at 37°C on a slowly rotating platform (approximately 5 rpm).
Confocal Scanning Laser Microscopy and Image Processing
For the study of both sucrose-dependent and sucrose-independent S. mutans biofilms, 3 independent experiments were performed by generating biofilms as described above. The mature biofilms were rinsed twice with PBS buffer. Live Baclight Bacterial Gram Stain fluorescent dye mixture (5 mM SYTO9, 7 mM hexidium iodide and 0.3% dimethyl sulfoxide in PBS buffer; Molecular Probes, Eugene, Oreg., USA) at a 1:1000 dilution was added onto the biofilm and incubated for 1 h in the dark, followed by washing twice with PBS. Using confocal scanning laser microscopy (Carl Zeiss, LSM 510 META-NLO), at least 5 three-dimensional biofilm image stacks of each sample were acquired from random positions. Images were acquired at 1.0-μm intervals through the entire thickness of the biofilm. Images were processed by the COMSTAT program and analyzed in Matlab 5.3 (MathWorks Inc., Natick, Mass., USA) [Heydorn et al., 2000].
Statistical Analysis
Biofilm characteristics in the presence or absence of Mn were compared using paired t tests performed in SPSS version 11.1. A p value of <0.05 was considered significant.
Results
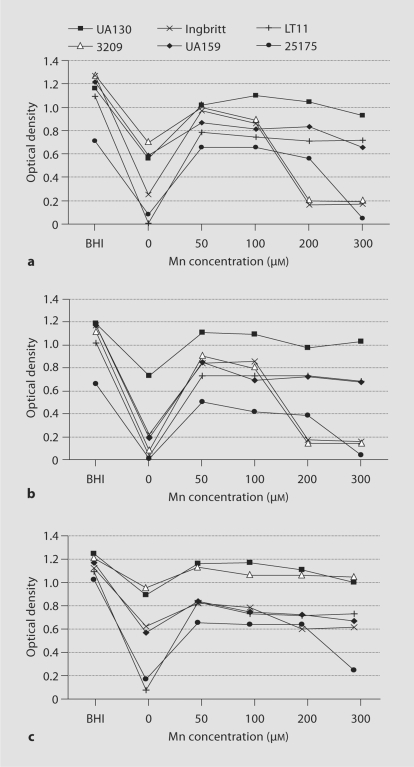
Figure 1 presents the growth data for each strain, with and without Mn, under each atmospheric condition. All strains except GS-5 grew in Mn-depleted media in a 5% CO2 atmosphere. For this reason S. mutans GS-5 was not included in subsequent experiments. Growth yield was enhanced for all strains when supplemented with any concentration of Mn relative to Mn-depleted media, regardless of the atmosphere used for incubation. Mn supplementation began at a concentration of 50 μM, since preliminary experiments with lower concentrations did not yield higher growth. All strains reached a maximal optical density in either 50 or 100 μM Mn. In none of the strains was the yield as high in Mn-SCDM as in BHI: most likely because of the more limited amounts of glucose and carbon sources in the SCDM.
Fig. 1.
Growth curves of S. mutans in media with varying concentrations of Mn under aerobic and anaerobic atmospheres. Growth was measured in BHI broth as a control and in SCDM with 0–300 μM Mn (x-axis) and expressed as optical density at wavelength 540 nm (y-axis). a Growth in a 5% CO2 aerobic atmosphere. b Growth in a 5% CO 2 aerobic atmosphere with shaking. c Growth under anaerobic conditions.
Strains LT11 and ATCC 25175 were most affected by Mn depletion, regardless of atmosphere, though an anaerobic environment somewhat moderated the effect. In an O2-enriched 5% CO2 atmosphere growth of 3209, UA159 and Ingbritt was also highly repressed when Mn deprived. A trend towards lower growth yields was also observed at higher concentrations of Mn, particularly for strains 3209, Ingbritt and ATCC 25175 under aerobic atmospheres. Strain UA130 was the least susceptible to variations in Mn concentration.
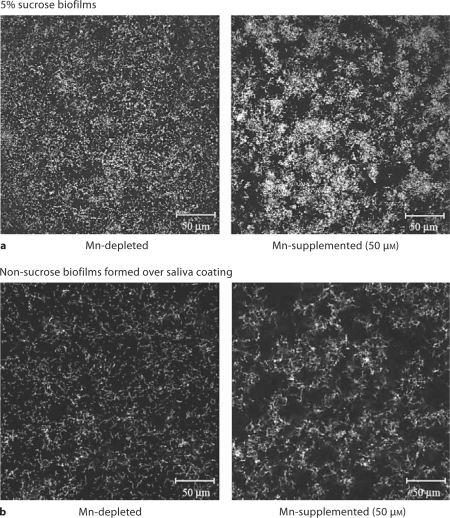
Strain UA159 was chosen to investigate the effects of Mn on biofilm formation, since this was one of the strains capable of growth in the absence of Mn and in anticipation of subsequent investigations that would take advantage of the fact that its genome sequence is known. Biofilms generated in the absence of Mn displayed differences from those grown in the presence of Mn, whether or not sucrose was present. Macroscopically the Mn-depleted biofilms showed formation of extensive clumps or aggregates of bacteria that could easily be washed away. Confocal microscopy indicated that Mn-depleted bacteria adhered to the substratum more evenly than Mn-supplemented bacteria (fig. 2). Computer analysis did not detect significant differences between Mn-supplemented and Mn-depleted biofilms when grown with sucrose (table 1). The lone exception was the surface/biovolume ratio, which was higher for the Mn-depleted biofilm. In contrast, this ratio was the only parameter that was not significantly different when comparing biofilms generated in the absence of sucrose (table 1).
Fig. 2.
Confocal microscopic images of fluorescently stained bacteria adherent to the substratum. a Biofilm at the substratum after growth in SCDM plus 5% sucrose and without Mn (left) or with 50 μM Mn (right). b Biofilms grown in SCDM within saliva-coated wells without Mn2+ (left) or with 50 μM Mn (right).
Table 1.
COMSTAT comparisons of quantitative differences in S. mutans UA159 biofilms formed in the presence and absence of Mn
| Mn-depleted | 50 μM Mn | p value | |
|---|---|---|---|
| Sucrosebiofilms | |||
| Biomass,μm3/μm 2 | 7.37±0.12 | 8.50±1.36 | 0.36 |
| Substratum occupied, % | 30.3±16.1 | 39.3±16.7 | 0.12 |
| Average thickness, μm | 14.1±2.4 | 14.2±1.0 | 0.98 |
| Roughness coefficient | 0.77±0.07 | 0.70±0.08 | 0.33 |
| Surface/biovolume ratio, μm 2/μm 3 | 1.79±0.04 | 1.34±0.12 | 0.004 |
| Nonsucrose biofilms | |||
| Biomass,μm3/μm 2 | 0.73±0.68 | 2.62±1.55 | <0.001 |
| Substratum occupied, % | 5.9±3.4 | 10.0±4.8 | 0.001 |
| Average thickness, μm | 1.8±2.1 | 9.9±7.2 | <0.001 |
| Roughness coefficient | 1.75±0.16 | 1.21±0.31 | <0.001 |
| Surface/biovolume ratio, μm 2/μm 3 | 2.56±0.49 | 2.77±0.48 | 0.13 |
Mean ± standard error of the mean (n = 3). Biomass: estimated value from total biovolume divided by the field area. Roughness coefficient is a unitless measure of the variation in biofilm thickness.
Discussion
Bacteria grown in an oxygenated environment use enzymes such as superoxide dismutases, catalases and peroxidases for protection against damage by reactive oxygen species such as superoxide radicals (O2–), H2O2 and hydroxyl radicals (·OH). Mn is a cofactor for superoxide dismutase [Martin et al., 1986]. The very low population densities measured in most cultures grown in Mn-depleted media under aerobic conditions (except UA130) strongly support a role for Mn in enzymic activity against oxygen stress. We can only speculate on why strains such as UA130 could grow under aerobic conditions in Mn-depleted media. It is probable that other enzymes, such as peroxidases and oxidases, can contribute to the capacity for aerobic growth [Thomas and Pera, 1983; Higuchi, 1984] or that UA130 may be able to use iron to replace the Mn requirement as a co-factor for superoxide dismutase [Martin et al., 1984; Martin et al., 1986].
Our study indicated that all strains showed maximal growth when supplemented with 50–100 μM Mn, whereas some strains began to be inhibited at an Mn concentration of 200 μM. Salivary Mn concentrations have most often been found to be between 1 nM [Green, 1970] and 4 μM [Duggal, 1991], though concentrations as high as 36 μM have been reported [Chicharro et al., 1999]. In preliminary trials we measured salivary Mn concentrations in the range 0.01–0.42 μM (n = 44), but Mn concentrations within dental plaque were significantly higher, averaging 6.2 mM (n = 16; data not shown). The concentration of dissolved Mn in natural waters can range from 0.18 to 182 μM) [Howe et al., 2004]. Transient exposure to high levels of Mn may affect S. mutans physiology by analogy with the anti-cariogenic effect of fluoride added to drinking water.
The variations between strains in maximal Mn concentration for growth, and the growth inhibition for some strains at Mn concentrations >200 μM, may reflect varying efficiencies in Mn transport. Another possible explanation is that excess Mn may interfere with uptake of other beneficial trace metals. Yet another possibility is that Mn affected the overall growth of the organism via an effect on gene expression. Several Mn-dependent genes have been identified in Streptococcus pneumoniae [Johnston, 2006], and the SloR Fe3+-Mn2+ metalloregulator in S. mutans reportedly influences virulence gene expression including genes that affect sucrose-dependent (gtfB) and sucrose-independent (spaP) biofilm formation [Rolerson, 2006]. The observation that S. mutans ATCC 25175 and LT11 displayed poor growth in the absence of Mn even under anaerobic conditions strongly suggested another role for this trace metal beyond oxygen intermediate detoxification.
The effects of Mn on biofilm formation were interesting and may indicate how S. mutans responds when nutrient-deprived. Anaerobic conditions were used so that differences in biofilm morphology could be attributed to the presence or lack of Mn independent of an effect on growth which if included would have likely resulted in even more profound differences. A lack of Mn-induced formation of easily removed clumps and a biofilm lacking bacterial organization (fig. 2). Ordinarily, clumping or aggregation of S. mutans is associated with the synthesis of glucans from sucrose but in this instance macroscopic clumps were more pronounced in the absence of sucrose. Clumping was also reported for a sloR mutant [Rolerson, 2006] but this effect occurred in the presence of adequate Mn. The clumping may be the result of removal of SloR repression leading to higher levels of SloC, which has been proposed to play a role in adhesion as well as transport of Mn2+ [Kitten et al., 2000; Spatafora et al., 2001]. Bacterial clumping may be a means whereby the bacteria pool their nutritional resources, perhaps by parasitizing some members of the aggregate. The larger surface/biovolume ratios of nonsucrose biofilms, and in Mn-depleted sucrose biofilm compared to the Mn-supplemented sucrose biofilm, might facilitate acquisition of trace minerals from the planktonic phase. At the same time the formation of large aggregates may preclude efficient biofilm formation allowing the bacteria to more easily move to new sites that might be more nutritionally abundant.
Dental plaque is a complex community comprising many oral bacteria. The Mn concentrations in saliva and dental plaque can vary widely. The strain-specific responses documented in this study suggest that the relative availability of Mn may influence colonization of plaque by particular strains of S. mutans.
Acknowledgments
This research was supported by NIDCR grant DE10058 (J.A.B.) and the Dental School Research Fund, Chulalongkorn University.
References
- Adkins BL, Losee FL. A study of the covariation of dental caries prevalence and multiple trace element content of water supplies. NY State Dent J. 1970;36:618–622. [PubMed] [Google Scholar]
- Aranha H, Strachan RC, Arceneaux JE, Byers BR. Effect of trace metals on growth of Streptococcus mutans in a teflon chemostat. Infect Immun. 1982;35:456–460. doi: 10.1128/iai.35.2.456-460.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arirachakaran P, Benjavongkulchai E, Luengpailin S, Ajdić D, Banas JA. Manganese affects Streptococcus mutans virulence gene expression. Caries Res. 2007;41:503–511. doi: 10.1159/000110883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton D. The influence of manganese on carbohydrate metabolism and caries induction by Streptococcus mutans strain Ingbritt. Caries Res. 1982;16:189–192. doi: 10.1159/000260596. [DOI] [PubMed] [Google Scholar]
- Beighton D. Manganese. In: Gardner AF, editor. Trace Elements and Dental Disease. Postgraduate dental handbook series. Boston: Wright PSG Inc; 1983. pp. 237–244. [Google Scholar]
- Chicharro JL, Serrano V, Urena R, Gutierrez AM, Carvajal A, Fernandez-Hernando P, Lucia A. Trace elements and electrolytes in human resting mixed saliva after exercise. Br J Sports Med. 1999;33:204–207. doi: 10.1136/bjsm.33.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon MEJ. Epidemiology of trace elements and dental caries. In: Gardner AF, editor. Trace Elements and Dental Disease. Postgraduate dental handbook series. Boston: Wright PSG Inc; 1983. pp. 11–30. [Google Scholar]
- Duggal MS, Chawla HS, Curzon MEJ. A study of the relationship between trace elements in saliva and dental caries in children. Arch Oral Biol. 1991;36:881–884. doi: 10.1016/0003-9969(91)90118-e. [DOI] [PubMed] [Google Scholar]
- Glass RL, Rothman KJ, Espinal F, Velez H, Smith NJ. The prevalence of human dental caries and water-borne trace metals. Arch Oral Biol. 1973;18:1099–1104. doi: 10.1016/0003-9969(73)90083-6. [DOI] [PubMed] [Google Scholar]
- Green I. Copper and manganese in saliva of children. J Dent Res. 1970;49:776–782. doi: 10.1177/00220345700490041201. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Higuchi M. The effect of oxygen on the growth and mannitol fermentation of Streptococcus mutans. J Gen Microbiol. 1984;130:1819–1826. doi: 10.1099/00221287-130-7-1819. [DOI] [PubMed] [Google Scholar]
- Howe PD, Malcolm HM, Dobson S. United Nations Environment Programme. Geneva: International Labour Organization and World Health Organization; 2004. Manganese and its compounds: environmental aspects. [Google Scholar]
- Jakubovics NS, Jenkinson HF. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology. 2001;147:1709–1718. doi: 10.1099/00221287-147-7-1709. [DOI] [PubMed] [Google Scholar]
- Johnston JW, Briles DE, Myers LE, Hollingshead SK. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect Immun. 2006;74:1171–1180. doi: 10.1128/IAI.74.2.1171-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Maguire ME. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27:263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Kitten T, Munro CL, Michalek SM, Macrina FL. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect Immun. 2000;68:4441–4451. doi: 10.1128/iai.68.8.4441-4451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa AS, van der Mei HC, Busscher HJ. Detachment of linking film bacteria from enamel surfaces by oral rinses and penetration of sodium lauryl sulphate through an artifical oral biofilm. Adv Dent Res. 1997;11:528–538. doi: 10.1177/08959374970110042201. [DOI] [PubMed] [Google Scholar]
- Little MF, Barrett K. Strontium and fluoride content of surface and inner enamel versus caries prevalence in the Atlantic coast of the United States of America. Caries Res. 1976a;10:297–307. doi: 10.1159/000260210. [DOI] [PubMed] [Google Scholar]
- Little MF, Barrett K. Trace element content of surface and subsurface enamel relative to caries prevalence of the west coast of the United States of America. Arch Oral Biol. 1976b;21:651–657. doi: 10.1016/0003-9969(76)90139-4. [DOI] [PubMed] [Google Scholar]
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengpailin S: Manganese Regulation of Glucan-Binding Protein C and Growth of Streptococcus mutans in Biofilm and Planktonic Conditions (PhD thesis). Louisville, Department of Microbiology and Immunology, University of Louisville, 2002.
- Martin ME, Byers BR, Olson MOJ, Salin ML, Arceneaux JEL, Tolbert C. A Streptococcus mutans superoxide dismutase that is active with either manganese or iron as a cofactor. J Biol Chem. 1986;261:9361–9367. [PubMed] [Google Scholar]
- Martin ME, Strachan RC, Aranha H, Evans SL, Salin ML, Welch B, Arceneaux JEL, Byers BR. Oxygen toxicity in Streptococcus mutans: manganese, iron, and superoxide dismutase. J Bacteriol. 1984;159:745–749. doi: 10.1128/jb.159.2.745-749.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J Bacteriol. 2003;185:5967–5975. doi: 10.1128/JB.185.20.5967-5975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Ann Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- Rolerson E, Swick A, Newlon L, Palmer C, Pan Y, Keeshan B, Spatafora G. The SloR/Dlg metalloregulator modulates Streptococcus mutans virulence gene expression. J Bacteriol. 2006;188:5033–5044. doi: 10.1128/JB.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C, Coulter WA. Continuous monitoring of pH and Eh in bacterial plaque grown on a tooth in an artificial mouth. Appl Microbiol. 1975;29:141–144. doi: 10.1128/am.29.2.141-144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatafora G, Moore M, Landgren S, Stonehouse E, Michalek S. Expression of Streptococcus mutans fimA is iron-responsive and regulated by a DtxR homologue. Microbiology. 2001;147:1599–1610. doi: 10.1099/00221287-147-6-1599. [DOI] [PubMed] [Google Scholar]
- Thomas EL, Pera KA. Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol. 1983;154:1236–1244. doi: 10.1128/jb.154.3.1236-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharik ML, Finlay BB. Mn2+ and bacterial pathogenesis. Frontiers Biosci. 2004;9:1035–1042. doi: 10.2741/1317. [DOI] [PubMed] [Google Scholar]