Abstract
Background/Aims
Glucocorticoids effectively manage autoimmune hearing loss, although the cochlear mechanisms involved are unknown. Previous studies of steroid-responsive hearing loss in autoimmune (lupus) mice showed glucocorticoids and mineralocorticoids were equally effective, suggesting the ion homeostasis functions of glucocorticoids may be as relevant as immunosuppression for control of autoimmune-induced inner ear disease. Therefore, to better characterize the role of the glucocorticoid receptor in autoimmune hearing loss therapy, its function was blocked with the antagonist RU-486 (mifepristone) during glucocorticoid (prednisolone) treatments.
Methods
Following baseline auditory brainstem response (ABR) thresholds, MRL/MpJ-Faslpr autoimmune mice were implanted with pellets providing combinations of 1.25 mg/kg of RU-486, 4 mg/kg of prednisolone, or their respective placebos. After 1 month, animals were retested with ABR and blood was collected for immune complex analyses.
Results
Mice receiving no prednisolone (placebo + placebo and placebo + RU-486) showed continued declines in hearing. On the other hand, mice receiving prednisolone (prednisolone + placebo and prednisolone + RU-486) had significantly better hearing (p < 0.05) than the non-prednisolone groups. Immune complexes were significantly elevated in the placebo + RU-486 group, suggesting RU-486 effectively blocked glucocorticoid receptor-mediated immune suppression. These results showed that blockage of the glucocorticoid receptor with RU-486 did not prevent prednisolone's effects in the ear, suggesting its ion homeostasis actions via the mineralocorticoid receptor were more relevant in hearing control.
Conclusion
The mineralocorticoid receptor-mediated actions of glucocorticoids are potentially relevant in steroid-responsive hearing disorders, implying disrupted cochlear ion transport functions may underlie the vascular problems proposed in some forms of immune-mediated hearing loss.
Key Words: Autoimmune inner ear disease, Autoimmune mice, Inner ear, Prednisolone, RU-486, Steroid receptors
Introduction
Because hearing loss is often suspected to result from immune processes [1], glucocorticoids (prednisone, prednisolone, dexamethasone) are frequently used to treat such hearing disorders as sudden or rapidly progressive hearing loss [2,3,4], Ménière's disease [5], and autoimmune inner ear disease [6,7]. The glucocorticoids have traditionally been prescribed because of the perceived necessity for their immunosuppressive and anti-inflammatory functions in the ear. However, little is currently known about the molecular processes glucocorticoids control in the ear and whether their influence is limited to the immune system.
Glucocorticoids play a role in immunosuppression by reducing phosphorylation and nuclear binding of nuclear factor-κB (NF-kB), a transcription factor responsible for expression of numerous pro-inflammatory genes [8,9,10]. NF-kB is located in the ear [11,12] and is a target of glucocorticoid receptor activation [13]. However, glucocorticoids also bind to the mineralocorticoid receptor and have a significant impact on ion transport functions in the inner ear [14,15,16], suggesting disorders of cochlear ion homeostasis also may be reversed or influenced by such therapy [17]. How either of these receptor-driven processes in the ear responds to clinical glucocorticoid treatments is unknown, which makes the management of hearing disorders difficult and unpredictable.
The incidence of hearing loss in patients with various systemic autoimmune diseases is quite high, often reported to be 15–75% [18,19]. One autoimmune mechanism often proposed for hearing loss is the impact of circulating antibodies on the sensitive cochlear vasculature [20,21,22,23,24]. Hearing loss has been correlated with circulating autoantibodies against endothelial cells and their membrane phospholipids [18], such as cardiolipin and β2-glycoprotein 1. These autoantibodies have been shown to activate the inflammatory response of endothelial cells [25,26], which includes breaking tight junctions to facilitate intercellular movement of immune cells [27,28]. Such autoantibody impact on tight junctions of the blood labyrinth barrier is one proposed mechanism for hearing loss by disrupting the sensitive ion homeostatic functions within the stria vascularis. Thus, both functions of glucocorticoids could be at work to suppress inflammation via the glucocorticoid receptor and restore ion transport via the mineralocorticoid receptor.
In order to better understand these mechanisms of immune-mediated hearing loss and subsequent steroid control, this laboratory has investigated the ability of glucocorticoids to control inner ear disease in the autoimmune mouse model for lupus. Hearing loss in these mice is due to autoantibody disruption of endothelial cell tight junctions in the stria vascularis [29] and subsequent loss of the endocochlear potential [30]. They have elevated levels of autoantibodies against endothelial cell proteins [31], paralleling the antiphospholipid mechanisms of lupus patients with hearing loss. Hearing loss in these mice is prevented or reversed with the glucocorticoid prednisolone [32,33,34], providing significant parallels with human steroid-responsive hearing loss. However, studies also showed the mineralocorticoid aldosterone was equivalent to prednisolone in restoring hearing loss and stria vascularis pathology in the autoimmune mice [34]. This raised the question of whether the glucocorticoids were effective because of their mineralocorticoid receptor-mediated control of ion transport instead of their glucocorticoid receptor-mediated role in immune suppression. To begin differentiating these receptor-specific functions, glucocorticoids were given while blocking the mineralocorticoid receptor with spironolactone [16]. This prevented both the mineralocorticoid aldosterone and glucocorticoid prednisolone from restoring hearing, suggesting the mineralocorticoid action of glucocorticoids is significantly involved in steroid-responsive ear disease. However, in that study, the glucocorticoid receptor was still functional because blocking the mineralocorticoid receptor with spironolactone did not prevent glucocorticoid receptor-mediated immunosuppression, another potentially relevant factor.
Therefore, the present study was conducted to determine which glucocorticoid-mediated processes play a greater role in steroid-responsive hearing loss. Autoimmune mice were treated with the glucocorticoid prednisolone while blocking the glucocorticoid receptor with its antagonist RU-486 (mifepristone). This would effectively suppress glucocorticoid-receptor-mediated processes (immune suppression), while leaving the mineralocorticoid receptor fully functional (ion homeostasis). If hearing restoration occurs in the absence of glucocorticoid receptor function, this would provide significant new information regarding the importance of mineralocorticoid-receptor-mediated actions of the therapeutic glucocorticoids. Such pharmacologic information could be helpful in developing better targeted drug therapies for patients with hearing loss suspected to occur from elevated systemic immune complexes. This could include sudden hearing loss, labyrinthitis, Ménière's disease, and immune-mediated inner ear disease.
Materials and Methods
General Protocol
MRL/MpJ-Faslpr mice develop autoimmune disease and hearing loss at approximately 3–4 months of age. Therefore, mice (n = 40) were obtained from Jackson Laboratories at 2 months of age, tested for baseline auditory brainstem response (ABR) thresholds, and bled for serum immune complex analyses. Following baseline measurements, mice were implanted with various combinations of pellets to deliver the glucocorticoid prednisolone, the glucocorticoid receptor antagonist RU-486 (mifepristone), or placebo. Animals were retested by ABR after 1 month of treatment, and rebled for serum immune complex analyses.
Steroid Treatment
Mice were implanted with various combinations of time release pellets (Innovative Research, Sarasota, Fla., USA) designed to provide daily doses of 4.0 mg/kg prednisolone, 1.25 mg/kg RU-486 (mifepristone), or their respective placebos (table 1; n = 10 per treatment). Prednisolone was given with and without RU486 to determine the potential role of the glucocorticoid receptor in mediating their effects on the autoimmune hearing loss. RU486 is an antagonist of the glucocorticoid receptor with no affinity for the mineralocorticoid receptor. It has been shown to effectively block the glucocorticoid receptor in vivo at doses of 0.25 mg/kg, so the dose selected for the study was 5 times this demonstrated effective concentration [35,36,37]. While under ketamine-xylazine anesthesia, a small incision was made in the loose skin over the scapulae, the 2 pellets for each treatment combination (table 1) were inserted under the skin, and the incision was closed with tissue glue.
Table 1.
Drug treatment groups (n = 10 per group)
| RU-486 (1.25 mg/kg/day) |
||
|---|---|---|
| no | yes | |
| Placebo | placebo | placebo |
| placebo | RU-486 | |
| Prednisolone (4 mg/kg/day) | prednisolone | prednisolone |
| placebo | RU-486 | |
Cochlear Function
ABR audiometry to pure tones was used to evaluate cochlear function, and followed our standard protocol [38]. Prior to treatment, mice were anesthetized and their individual ears were stimulated with a closed-tube sound delivery system sealed into the ear canal. The ABR to tone-burst stimuli at 4, 8, 16, and 32 kHz were recorded and thresholds at each frequency were determined for each ear. All animals were tested the same day to minimize the impact of variations in instrumentation and calibration on thresholds. Following the respective pellet treatments for 1 month, ABR thresholds were again determined and the threshold shift at each of the 4 frequencies was determined for each ear. An analysis of variance (ANOVA) was conducted on the threshold data to determine if the glucocorticoid treatment was impacted by blocking its receptor with the antagonist RU-486.
Systemic Immune Complexes
The severity of systemic autoimmune disease was determined by measurement of serum immune complexes according to previous protocols [16,39]. Change in these serum immune complex levels is one way to quantify the progression of systemic disease, as well as the immune suppressive effects of the steroid treatments. RU-486, as an antagonist of the glucocorticoid receptor, would be expected to impair this receptor-mediated function. Blood was collected at baseline and after 1 month of treatment. Immune complex levels were compared by ANOVA among the treatment groups, and regression analyses were used to test for potential correlations between these immune complex measures and hearing levels for mice in each group.
The use of the animals reported in this study was approved by the Oregon Health & Science University Institutional Animal Care and Use Committee to ensure compliance with federal animal welfare guidelines.
Results
Steroid Treatment
All mice tolerated the surgery and pellet placements well, and incisions healed within 2 days. Throughout the treatment period, no skin pathology over the pellets was observed and mice did not appear to be in any discomfort. Attrition due to autoimmune disease was in line with previous studies. Two mice died in the placebo + placebo group, whereas all other groups had either 9 or 10 mice still alive at 1 month.
Cochlear Function
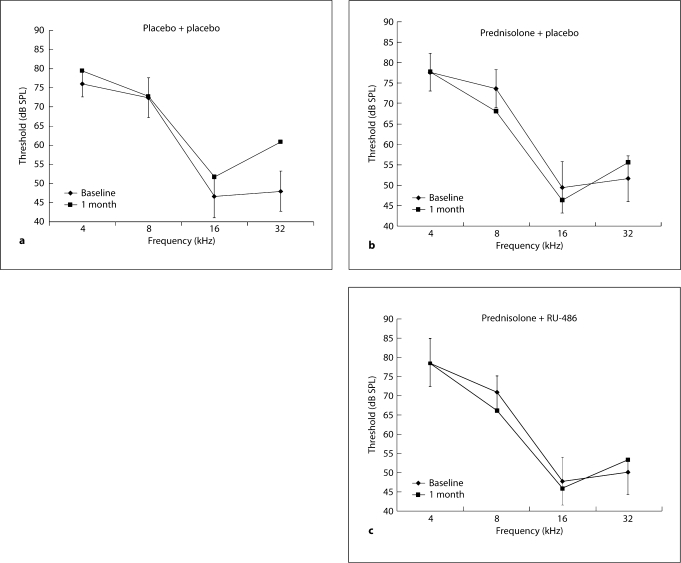
Autoimmune mice receiving no drugs (placebo + placebo) showed the progression of hearing loss normally seen in untreated autoimmune mice. Average thresholds were higher after 1 month, particularly in the higher frequencies (fig. 1a). Their 32-kHz threshold at 1 month was more than 2.0 SD from baseline. In contrast, the 2 groups receiving prednisolone showed little or no threshold elevation at any frequency after 1 month of treatment. The prednisolone + placebo mice (fig. 1b) and the prednisolone + RU-486 mice (fig. 1c) were virtually identical in their audiograms, and in fact showed a trend for better thresholds. Both showed a slight elevation at 32 kHz, the frequency most affected by systemic autoimmune disease.
Fig. 1.
a Threshold elevations in placebo + placebo mice (8 mice, 15 ears) after 1 month are typical for untreated autoimmune-diseased mice. The major shift in thresholds occurs at 32 kHz. Vertical bars represent 8 1 SD of the baseline measures. b The prednisolone + placebo group (10 mice, 20 ears) did not show the typical rise in thresholds at any frequency. In fact, thresholds at 8 and 16 kHz trended lower. c The prednisolone + RU-486 group (10 mice, 20 ears) also did not show the typical rise in thresholds at any frequency and trended lower at 8 and 16 kHz.
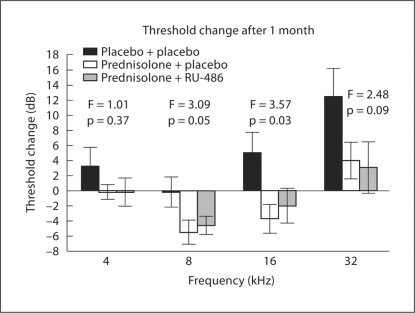
An ANOVA of the total threshold shift across the 4 frequencies for mice in the 4 groups showed a significant treatment effect (table 2; p = 0.03). The placebo + placebo group averaged a threshold rise of 20.4 dB (approximately 5 dB per frequency), with individual mice ranging from an improvement of 33 dB to a worsening of 137 dB. This was a much higher total threshold shift than that was seen in the 2 prednisolone groups. The prednisolone + placebo group, which represents the standard glucocorticoid treatment of previous studies, averaged a total threshold improvement of 5.4 dB (more than 1 dB per frequency), essentially no change from baseline. The range of hearing shifts in this prednisolone group was an improvement of 35 dB to a worsening of only 17 dB (slightly more than 4 dB per frequency). When RU-486 was added to the prednisolone treatment, there was little effect (table 2). Mice averaged a 3.7-dB improvement across the frequencies, essentially the same as the prednisolone + placebo group. Thus, the ANOVA provides statistical evidence that the mice receiving prednisolone, with or without RU-486, changed very little from pretreatment baseline and were significantly better than those mice receiving no drug.
Table 2.
ANOVA of total threshold change after 1-month treatment period
| Total threshold shift, dB SPL | ANOVA | ||
|---|---|---|---|
| mean ± SD | range | ||
| Placebo + placebo | 20.4±36.2 | −33.0 to 137.0 | F = 3.16; p = 0.03 |
| Placebo + RU486 | 5.5±24.3 | −48.6 to 46.5 | |
| Prednisolone + placebo | −5.4±14.4 | −35.3 to 17.0 | |
| Prednisolone + RU486 | −3.7±30.6 | −51.6 to 52.0 | |
Negative values are improving thresholds.
To further evaluate the impact of RU-486 on prednisolone-mediated hearing preservation, the 2 prednisolone groups were compared to the placebo + placebo mice in frequency-specific ANOVAs. This permitted the assessment of the 2 steroid groups to determine if prednisolone + RU-486 mice paralleled the prednisolone + placebo mice across the frequency spectrum. If thresholds in these 2 prednisolone groups were statistically similar, it would offer evidence the glucocorticoid receptor is not the sole mediator of glucocorticoid treatments. The frequency-specific shift in thresholds for these groups showed that the prednisolone + RU-486 group consistently paralleled the prednisolone + placebo group (fig. 2). The 2 prednisolone groups were essentially unchanged at 4 kHz, but the minor rise of only 3 dB in the mice receiving no drugs (placebo + placebo) led to no significant difference among the groups (F = 1.01; p = 0.37). At 8 kHz, the placebo + placebo group showed no change, but the 2 prednisolone groups showed similarly improved thresholds, causing them to be statistically different from the controls (F = 3.09; p = 0.05). The ANOVA comparison at 16 kHz also showed the prednisolone groups had significantly better thresholds compared to the rise in thresholds in the placebo + placebo mice (F = 3.57; p = 0.03). The greatest shift in hearing in autoimmune mice is generally seen at the higher frequencies, as evidenced by the average threshold increase of 13 dB at 32 kHz in the placebo + placebo mice (fig. 2). The 2 prednisolone groups also showed a slight rise in thresholds, although the trend was for less hearing loss (3–4 dB). This led to a difference among the groups that was significant at only the p = 0.09 level, outside the generally accepted limit of p = 0.05. Thus, the consistent threshold similarity between the 2 prednisolone groups suggested that the RU-486 treatment did not appreciably interfere with prednisolone's preservation of hearing.
Fig. 2.
Changes in hearing thresholds after 1 month of treatment. At 4 kHz, the 2 prednisolone groups were unchanged from baseline, but the small elevation in the placebo + placebo group lead to no statistical difference among the 3 groups (p = 0.37). In contrast, the improvement in thresholds of the 2 prednisolone groups at 8 and 16 kHz led to their being significantly better than the placebo + placebo group. Although no statistical group differences were seen at 32 kHz, the placebo + placebo mice showed a trend towards higher thresholds than the 2 prednisolone groups. However, the slight rise in thresholds in the 2 latter groups was sufficient to make the overall group difference significant at only the p = 0.09 level. However, across all frequencies, the prednisolone + RU-486 mice did not differ from the prednisolone + placebo mice, suggesting that blockage of the glucocorticoid receptor did not prevent prednisolone from preserving hearing levels.
Serum Immune Complexes
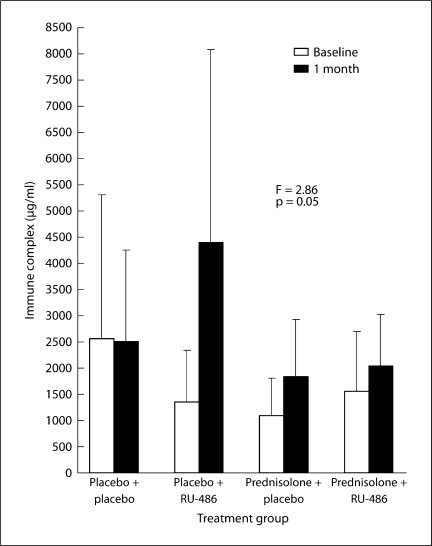
Serum immune complexes and/or immunoglobulin in a normal mouse are present at a concentration of less than 500 μg/ml. Levels in autoimmune mice can reach 5000–10000 μg/ml due to systemic disease. Suppression of immune complexes is the hallmark of glucocorticoid treatments and this function is mediated through the glucocorticoid receptor. Thus, RU-486 as an antagonist to the glucocorticoid receptor should block this immunosuppression function of prednisolone. There was a trend for this to be the case. ANOVA of the change in immune complex levels between baseline and 1 month of treatment showed the groups were at the statistical cutoff for significance (fig. 3; p = 0.05). There was considerable variability in the immune complex levels at baseline and after 1 month. Comparison of the placebo + placebo and placebo + RU-486 groups suggested a considerable rise in immune system activity due to blockage of glucocorticoid receptor access by the natural glucocorticoids (cortisol). If a comparison is made of actual levels of immune complexes in these 2 groups, 57% (4/7) of the placebo + placebo mice had levels less than 2000 μg/ml, while only 12% (1/8) of those getting RU-486 were below this critical level; χ2 analysis of these ratios showed they were significant (p = 0.006).
Fig. 3.
An ANOVA of the level of immune complexes revealed an overall group difference. The biggest increase in immune complexes was seen in the mice receiving RU-486, presumably due to its blockage of the glucocorticoid receptor and prevention of immune suppressive functions.
The differences between the 2 prednisolone-treated groups were less dramatic (fig. 3). There was a trend for the prednisolone + RU-486 mice to have higher immune complex levels due to blockage of the receptor, but the lack of statistical significance of the ANOVA did not support this. However, comparison of the actual levels in the 2 prednisolone groups suggested the RU-486 did prevent immunosuppression. Two of the ten prednisolone + placebo mice had immune complexes under 1000 μg/ml, while none of the prednisolone + RU-486 mice did. Also, 70% (7/10) of the prednisolone + placebo mice had levels under 2000 μg/ml, while less than half (4/9) of those getting prednisolone with RU-486 were under this amount. Although statistically inconclusive, these trends in prevention of immunosuppression suggest RU-486 was effective in blocking the glucocorticoid receptor.
Immune Complex-Threshold Relationship
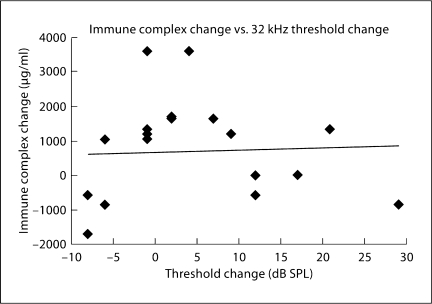
The critical question regarding glucocorticoid treatments in these mice is whether hearing restoration is due to immunosuppression or re-establishing cochlear ion homeostasis. The hearing and immune complex results above suggest prednisolone was effective in spite of glucocorticoid receptor block, although both mechanisms may be involved. To further evaluate this potential relationship between cochlear function and circulating immune complexes, a series of regression analyses compared these factors. Thresholds at 32 kHz were chosen for the comparisons because it is the frequency most affected by the systemic disease (fig. 1a).
At baseline, prior to any treatment, 32-kHz thresholds were compared to serum immune complex levels for all mice in a regression analysis. This regression was not significant (r = 0.04; p = 0.71), suggesting that the degree of hearing loss was not correlated with systemic disease. After 1 month of treatment, a similar non-significant relationship between hearing levels and serum immune complexes was observed (r = 0.20; p = 0.10). If those mice treated with steroids were specifically analyzed, again no correlation was seen between immune complex levels and 32-kHz thresholds. The change in threshold versus the change in immune complex levels was also not significant, neither for the prednisolone + placebo mice (fig. 4; r = 0.04; p = 0.87) nor the prednisolone + RU-486 mice. These steroid-treated groups would presumably be the most likely to show a correlation if the level of hearing was due mainly to immune suppression. Thus, the immune complex data support the conclusion that cochlear physiologic factors other than systemic immune disease are responsible for hearing levels. These data, coupled with the threshold differences above, implicate the restoration of ion homeostasis as a significantly relevant mechanism in glucocorticoid-induced hearing recovery.
Fig. 4.
The prednisolone + placebo mice showed virtually no correlation (r = 0.04; p = 0.87) between hearing change and immune complex levels, suggesting the latter was not a major factor in the level of hearing following steroid treatment.
Discussion
The results of the present study suggest that RU-486 effectively blocked the glucocorticoid receptor, and hearing recovery or preservation occurred via the glucocorticoid prednisolone binding to the mineralocorticoid receptor. It cannot be stated with certainty that the RU-486 blocked the receptor 100%. Numerous studies [35,36,37] have shown measurable effects with this antagonist at concentrations of only 0.25 mg/kg, so it is assumed that this study's use at 5 times that concentration was more than adequate to compromise glucocorticoid-receptor-driven processes. Further studies designed to isolate the specific cellular processes affected by this antagonist will need to establish its dose-response effects. However, based on this study and those in other organ systems, it is assumed there was significant blockage of the glucocorticoid receptor.
The objective of studies in this laboratory is to elucidate the steroid-responsive mechanisms of the ear, particularly those related to hearing restoration therapies. Toward this end, efforts have been made to better delineate the specific roles of the glucocorticoid and mineralocorticoid receptors in glucocorticoid treatments for hearing loss. Both mineralocorticoid [40,41] and glucocorticoid [42,43] receptors occur in the ear, and recent studies of cochlear pharmacokinetics of glucocorticoids have begun to clarify some parameters of steroid flow and scalar distribution, particularly after transtympanic delivery [44,45,46,47,48,49]. It is well established that glucocorticoids have an affinity for the mineralocorticoid receptor that is equal to or higher than affinity for the glucocorticoid receptor [50,51,52]. The glucocorticoid immunosuppressive and anti-inflammatory functions are mediated through the glucocorticoid receptor, whereas sodium and potassium exchange is controlled by glucocorticoid activation of the mineralocorticoid receptor [53,54,55,56]. The data of the present study parallel the previous finding that glucocorticoid-induced hearing recovery was mediated through the mineralocorticoid receptor [16]. Spironolactone blocked the mineralocorticoid receptor-mediated actions of both glucocorticoids and mineralocorticoids, but did not prevent the glucocorticoid-receptor-mediated function of systemic immune suppression. It was concluded from that study as well that both steroid classes directly affected inner ear ion homeostasis, while systemic immune suppression had little functional impact on the recovery of hearing loss. Thus, the mineralocorticoid-receptor-driven processes of cochlear ion homeostasis that are directly regulated by glucocorticoids may be as responsible for hearing restoration as the immune suppression function of glucocorticoids mediated through the glucocorticoid receptor.
Hearing Disorders of Ion Homeostasis
These various studies demonstrate that both immunosuppression and ion homeostasis mechanisms are potential targets of glucocorticoids in the cochlea, but these 2 processes may be affected differentially by the various types of inner ear trauma. Although circulating immune factors (cytokines, autoantibodies) due to systemic disease may underlie the initial development of hearing loss, the systemic immunosuppression by steroids may not be the primary mechanism that restores cochlear function. This emphasizes the need to better understand the actual cochlear dysfunction underlying the various types of hearing loss.
If current theories are correct that the vascular elements of the stria vascularis are susceptible in many cases of immune-related hearing loss, then the glucocorticoids may be restoring normal endolymph sodium and potassium balances. This potential impact on ion homeostasis in the ear has largely been ignored in explaining steroid-responsive ear disease. Recently, presbycusis has been shown to correlate with reduced levels of aldosterone, the natural mineralocorticoid [57]. Also, supplementing low aldosterone patients with this hormone appears to be effective in reversing auditory and vestibular problems [Wright, J., pers. commun.]. Thus, the ion homeostasis mechanisms within the ear are at risk and responsive to mineralocorticoids.
The fact that underlying disease is not determinable in many cases of hearing loss precludes targeting therapy to the specific cochlear processes involved and probably underlies the unpredictable, and often inconsistent, outcome of glucocorticoid treatment [7]. While convincingly beneficial to some, other studies have concluded glucocorticoids lack efficacy, and their use is still debated for some forms of hearing loss [58,59,60,61,62]. Inconsistent treatment outcomes reflect our lack of understanding of underlying disease and the cellular and molecular processes that are regulated by steroids in the ear. If the immune suppressive functions of steroids are secondary to their impact on cochlear ion (K+, Na+) transport, then alternative therapies may be more suitable for some forms of hearing loss. The fact that many types of hearing loss are steroid-responsive suggests the underlying pathology occurs in cochlear regions that are capable of regeneration, such as the stria vascularis. Thus, it is imperative to develop a better understanding of steroid-driven mechanisms within the normal ear and how these are impacted by various diseases. The steroid control of cochlear ion homeostatic functions must be considered when diagnosing and treating some forms of hearing loss. Because the long-term management of hearing loss with glucocorticoids is difficult because of their severe side effects, any alternative therapy to glucocorticoids could be of significant clinical benefit. Therefore, matching ear disease with the most effective therapy must be more critically evaluated in order to significantly advance management of hearing loss in patients.
Acknowledgment
Supported by grant numbers R01 DC 05593 and P30 DC005983 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
References
- 1.Dornhoffer JL, Arenberg JG, Arenberg IK, Shambaugh GE., Jr Pathophysiological mechanisms in immune inner ear disease. Acta Otolaryngol (Stockh) 1997;Suppl 526:30–36. doi: 10.3109/00016489709124018. [DOI] [PubMed] [Google Scholar]
- 2.Alexiou C, Arnold W, Fauser C, Schratzenstaller B, Gloddek B, Fuhrmann S, Lamm K. Sudden sensorineural hearing loss: does application of glucocorticoids make sense? Arch Otolaryngol Head Neck Surg. 2001;127:253–258. doi: 10.1001/archotol.127.3.253. [DOI] [PubMed] [Google Scholar]
- 3.Rauch SD. Intratympanic steroids for sensorineural hearing loss. Otolaryngol Clin North Am. 2004;37:1061–1074. doi: 10.1016/j.otc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Aoki D, Takegoshi H, Kikuchi S. Evaluation of super-high-dose steroid therapy for sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2006;134:783–787. doi: 10.1016/j.otohns.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Barrs DM. Intratympanic corticosteroids for Meniere's disease and vertigo. Otolaryngol Clin North Am. 2004;37:955–972. doi: 10.1016/j.otc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Niparko JK, Wang N-Y, Rauch SD, Russell GB, Espeland MA, Pierce JJ, Bowditch S, Masuda A, Gulya AJ, Gantz BJ, Hughes GB, Brookhouser PE, Hannley MT, Telian SA, Harris JP, AIED Study Group Serial audiometry in a clinical trial of AIED treatment. Otol Neurotol. 2005;26:908–917. doi: 10.1097/01.mao.0000185081.28598.5c. [DOI] [PubMed] [Google Scholar]
- 7.Loveman DM, de Comarmond C, Cepero R, Baldwin DM. Autoimmune sensorineural hearing loss: clinical course and treatment outcome. Sem Arthrit Rheumat. 2004;34:538–543. doi: 10.1016/j.semarthrit.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Age Develop. 2004;125:697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 9.de Bosscher K, vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kB or activator protein-1: molecular mechanisms for gene repression. Endocrin Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 10.Ghaheri B, Kempton JB, Pillers DM, Trune DR. Cochlear cytokine gene expression in murine acute otitis media. Laryngoscope. 2007;117:22–29. doi: 10.1097/01.mlg.0000240170.48584.73. [DOI] [PubMed] [Google Scholar]
- 11.Adams JC. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otol Neurotol. 2002;23:316–322. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Nagashima R, Sugiyama C, Yoneyama M, Ogita K. Transcriptional factors in the cochlea within the ear. J Pharmacol Sci. 2005;99:301–306. doi: 10.1254/jphs.cpj05004x. [DOI] [PubMed] [Google Scholar]
- 13.Canlon B, Meltser I, Johansson P, Tahera Y. Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hear Res. 2007;226:61–69. doi: 10.1016/j.heares.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Marcus DC. Nongenomic effects of corticosteroids on ion transport by stria vascularis. Audiol Neurootol. 2002;7:100–106. doi: 10.1159/000057657. [DOI] [PubMed] [Google Scholar]
- 15.Pondugula SR, Sanneman JD, Wangemann P, Milhaud PG, Marcus DC. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Renal Physiol. 2004;286:F1127–F1135. doi: 10.1152/ajprenal.00387.2003. [DOI] [PubMed] [Google Scholar]
- 16.Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear Res. 2006;212:22–32. doi: 10.1016/j.heares.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Trune DR. Ion homeostasis and inner ear diseases. In: Hamid MA, Sismanis A, editors. Medical Otology and Neurotology: A Medical Guide to Auditory and Vestibular Disorders. New York: Thieme; 2006. pp. 21–32. [Google Scholar]
- 18.Mouadeb DA, Ruckenstein MJ. Antiphospholipid inner ear syndrome. Laryngoscope. 2005;115:879–883. doi: 10.1097/01.MLG.0000158666.15447.37. [DOI] [PubMed] [Google Scholar]
- 19.Barkhuizen A, Lim L, Trune D, Rosenbaum J. Ocular, aural, and oral manifestations. In: Wallace DJ, Hahn BH, editors. Dubois' Lupus Erythematosus. Baltimore: Lippincott William & Wilkins; 2006. pp. 789–800. [Google Scholar]
- 20.Mathews J, Kumar BN. Autoimmune sensorineural hearing loss. Clin Otolaryngol. 2003;28:479–488. doi: 10.1046/j.0307-7772.2003.00738.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris JP, Ryan AF. Fundamental immune mechanisms of the brain and inner ear. Otolaryngol Head Neck Surg. 1995;112:639–653. doi: 10.1016/s0194-5998(95)70170-2. [DOI] [PubMed] [Google Scholar]
- 22.Barna BP, Hughes GB. Autoimmune inner ear disease – a real entity? Clin Lab Med. 1997;17:581–594. [PubMed] [Google Scholar]
- 23.Yehudai D, Schoenfeld Y, Toubi E. The autoimmune characteristics of progressive or sudden sensorineural hearing loss. Autoimmunity. 2006;39:153–158. doi: 10.1080/08916930500499599. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Berrocal JR, Ramirez-Camacho R. Sudden sensorineural hearing loss: supporting the immunologic theory. Ann Otol Rhinol Laryngol. 2002;111:989–997. doi: 10.1177/000348940211101107. [DOI] [PubMed] [Google Scholar]
- 25.Meroni PL, Raschi E, Testoni C, Borghi MO. Endothelial cell activation by antiphospholipid antibodies. Clin Immunol. 2004;112:169–174. doi: 10.1016/j.clim.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Pierangeli SS, Chen PP, González EB. Antiphospholipid antibodies and the antiphospholipid syndrome: an update on treatment and pathogenic mechanisms. Curr Opin Hematol. 2006;13:366–375. doi: 10.1097/01.moh.0000239710.47921.d2. [DOI] [PubMed] [Google Scholar]
- 27.Hamid C, Norgate K, D'Cruz DP, Khamashta MA, Arno M, Pearson JD, Frampton G, Murphy JJ. Anti-beta2GPI-antibody-induced endothelial cell gene expression profiling reveals induction of novel pro-inflammatory genes potentially involved in primary antiphospholipid syndrome. Ann Rheum Dis. 2007;66:1000–1007. doi: 10.1136/ard.2006.063909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 29.Lin DW, Trune DR. Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol Head Neck Surg. 1997;117:530–534. doi: 10.1016/S0194-59989770026-3. [DOI] [PubMed] [Google Scholar]
- 30.Ruckenstein MJ, Milburn M, Hu L. Strial dysfunction in the MRL-Faslpr mouse. Otolaryngol Head Neck Surg. 1999;121:452–456. doi: 10.1016/S0194-5998(99)70236-6. [DOI] [PubMed] [Google Scholar]
- 31.Hefeneider SH, McCoy SL, Hausman FA, Trune DR. Autoimmune mouse antibodies recognize multiple antigens proposed in human immune-mediated hearing loss. Otol Neurotol. 2004;25:250–256. doi: 10.1097/00129492-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Trune DR, Wobig RJ, Kempton JB, Hefeneider SH. Steroid therapy improves cochlear function in the C3H.MRL-Faslpr autoimmune mouse. Hear Res. 1999;137:160–166. doi: 10.1016/s0378-5955(99)00147-1. [DOI] [PubMed] [Google Scholar]
- 33.Trune DR, Wobig RJ, Kempton JB, Hefeneider SH. Steroid treatment in young MRL.MpJ-Faslpr autoimmune mice prevents cochlear dysfunction. Hear Res. 1999;137:167–173. doi: 10.1016/s0378-5955(99)00148-3. [DOI] [PubMed] [Google Scholar]
- 34.Trune DR, Kempton JB. Aldosterone and prednisolone control of auditory dysfunction in MRL/MpJ-Faslpr autoimmune mice. Hear Res. 2001;155:9–20. doi: 10.1016/s0378-5955(01)00240-4. [DOI] [PubMed] [Google Scholar]
- 35.Daynes RA, Araneo BA, Dowell TA, Huang K, Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990;171:979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Chua SS, Burcin MM, Reynolds SD, Stripp BR, Edwards RA, Finegold MJ, Tsai SY, DeMayo FJ. Phenotypic consequences of lung-specific inducible expression of FGF-3. PNAS. 2001;98:5898–5903. doi: 10.1073/pnas.101116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierson TM, Wang Y, DeMayo FJ, Matzuk MM, Tsai SY, O'Malley BW. Regulable expression of inhibin A in wild-type and inhibin α null mice. Mol Endocrin. 2000;14:1075–1085. doi: 10.1210/mend.14.7.0478. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell CR, Kempton JB, Creedon TA, Trune DR. Using a 56-stimulus train for the rapid acquisition of auditory brainstem responses. Audiol Neurootol. 1999;4:80–87. doi: 10.1159/000013824. [DOI] [PubMed] [Google Scholar]
- 39.Trune DR, Craven JP, Morton JI, Mitchell C. Autoimmune disease and cochlear pathology in C3H/lpr strain mouse. Hear Res. 1989;38:57–66. doi: 10.1016/0378-5955(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 40.Furuta H, Mori N, Sato C, Hoshikawa H, Sakai S, Iwakura S, Doi K. Mineralocorticoid type I receptor in the rat cochlea: mRNA identification by polymerase chain reaction (PCR) and in situ hybridization. Hear Res. 1994;78:175–180. doi: 10.1016/0378-5955(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 41.Yao X, Rarey KE. Localization of the mineralocorticoid receptor in rat cochlear tissue. Acta Otolaryngol. 1996;116:493–496. doi: 10.3109/00016489609137879. [DOI] [PubMed] [Google Scholar]
- 42.Shimazaki T, Ichimiya I, Suzuki M, Mogi G. Localization of glucocorticoid receptors in the murine inner ear. Ann Otol Rhinol Laryngol. 2002;111:1133–1138. doi: 10.1177/000348940211101213. [DOI] [PubMed] [Google Scholar]
- 43.Erichsen S, Bagger-Sjöbäck D, Curtis L, Zuo J, Rarey K, Hultcrantz M. Appearance of glucocorticoid receptors in the inner ear of the mouse during development. Acta Otolaryngol. 1996;116:721–725. doi: 10.3109/00016489609137913. [DOI] [PubMed] [Google Scholar]
- 44.Hargunani CA, DeGagne JM, Kempton JB, Trune DR. Inner ear uptake and distribution of dexamethasone injected into the middle ear. Otol Neurotol. 2006;27:564–569. doi: 10.1097/01.mao.0000194814.07674.4f. [DOI] [PubMed] [Google Scholar]
- 45.Parnes LS, Sun A-H, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999;91(suppl):1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- 46.Hahn H, Kammerer B, DiMauro A, Salt AN, Plontke SK. Cochlear microdialysis for quantification of dexamethasone and fluorescein entry into scala tympani during round window administration. Hear Res. 2006;212:236–244. doi: 10.1016/j.heares.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plontke SK, Siedow N, Wegener R, Zenner HP, Salt AN. Cochlear pharmacokinetics with local inner ear drug delivery using a three-dimensional finite-element computer model. Audiol Neurootol. 2007;12:37–48. doi: 10.1159/000097246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008;29:401–406. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandrasekhar SS, Rubinstein RY, Kwartler JA, Gatz M, Connelly PE, Huang E, Bareded S. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngol Head Neck Surg. 2000;122:521–528. doi: 10.1067/mhn.2000.102578. [DOI] [PubMed] [Google Scholar]
- 50.Claire M, Faraj H, Grassy G, Aumelas A, Rondot A, Auzou G. Synthesis of new 11 β-substituted spironolactone derivatives. Relationship with affinity for mineralocorticoid and glucocorticoid receptors. J Med Chem. 1993;36:2404–2407. doi: 10.1021/jm00068a018. [DOI] [PubMed] [Google Scholar]
- 51.Munck A, Mendel DB, Smith LI, Orti E. Glucocorticoid receptors and actions. Am Rev Respir Dis. 1990;141:S2–S10. [PubMed] [Google Scholar]
- 52.Rupprecht R, Reul JMHM, van Steensel B, Spengler D, Söder M, Berning B, Holsboer F, Damm K. Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur J Pharm Mol Pharm. 1993;247:145–154. doi: 10.1016/0922-4106(93)90072-h. [DOI] [PubMed] [Google Scholar]
- 53.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 54.Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharm Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 55.Smith PJ, Cousins DJ, Jee Y-K, Staynov DZ, Lee TH, Lavender P. Suppression of granulocyte-macrophage colony-stimulating factor expression by glucocorticoids involves inhibition of enhancer function by the glucocorticoid receptor binding to composite NF-AT/activator protein-1 elements. J Immunol. 2001;167:2502–2510. doi: 10.4049/jimmunol.167.5.2502. [DOI] [PubMed] [Google Scholar]
- 56.Funder JW. Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Ann Rev Med. 1997;48:231–240. doi: 10.1146/annurev.med.48.1.231. [DOI] [PubMed] [Google Scholar]
- 57.Tadros SF, Frisina ST, Mapes F, Frisina DR, Frisina RD. Higher serum aldosterone correlates with lower hearing thresholds: a possible protective hormone against presbycusis. Hear Res. 2005;209:10–18. doi: 10.1016/j.heares.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss. II. A Meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582–586. doi: 10.1001/archotol.133.6.582. [DOI] [PubMed] [Google Scholar]
- 59.Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss. I. A systematic review. Arch Otolaryngol Head Neck Surg. 2007;133:573–581. doi: 10.1001/archotol.133.6.573. [DOI] [PubMed] [Google Scholar]
- 60.Eisenman DJ, Arts HA. Effectiveness of treatment for sudden sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 2000;126:1161–1166. doi: 10.1001/archotol.126.9.1161. [DOI] [PubMed] [Google Scholar]
- 61.Doyle KJ, Bauch C, Battista R, Beatty C, Hughes GB, Mason J, Maw J, Musiek FL. Intratympanic steroid treatment: a review. Otol Neurotol. 2004;25:1034–1039. doi: 10.1097/00129492-200411000-00031. [DOI] [PubMed] [Google Scholar]
- 62.Rahman MU, Poe DS, Choi HK. Autoimmune vestibulo-cochlear disorders. Curr Opin Rheumatol. 2001;13:184–189. doi: 10.1097/00002281-200105000-00006. [DOI] [PubMed] [Google Scholar]