Abstract
A biomarker for lifetime fluoride exposure would facilitate population-based research and policy making but currently does not exist. This study examined the suitability of primary tooth dentin as a biomarker by comparing dentin fluoride concentration and fluoride exposures. Ninety-nine children's exfoliated primary teeth were collected from 2 fluoridated and 2 fluoride-deficient communities in North Carolina. Coronal dentin was isolated by microdissection and fluoride concentration assayed using the microdiffusion, ion-specific electrode technique. Information on children's fluoride exposures since birth from drinking water, toothpaste, supplements, rinses, food and beverages was collected by a self-reported questionnaire administered to caregivers. Only a small portion of the variance (10%) in incisor dentin fluoride (mean 792, SD 402 mg/kg) was accounted for by the best linear regression model as evaluated by the adjusted R2. A moderate portion of the variance (60%) of molar dentin fluoride (mean 768, SD 489 mg/kg) was predicted by dietary fluoride supplement exposures, community of residence, and frequent tea consumption. Results for molars suggest that primary tooth dentin concentration may prove to be a satisfactory biomarker for fluoride exposure.
Key Words: Biomarker, Dentin, Fluoride exposure, Primary dentition
Fluoride is believed to be a major factor in the substantial decline in dental caries in the permanent dentition in many countries over the last several decades [Centers for Disease Control and Prevention, 2001]. It also is associated with dental fluorosis and has potential health risks such as bone fragility if consumed in excessive amounts [Kleerekoper, 1994]. Research and public health surveillance of chronic fluoride exposures and health outcomes are impeded by the lack of efficient, accurate methods to determine how much fluoride a person has ingested in his or her lifetime. A useful biomarker for fluoride exposure must: provide a cumulative record of exposures from birth; be a readily available biological substance; be collectable with noninvasive methods; and provide valid and reliable estimates of fluoride exposure amounts [Bonassi et al., 2001].
Past research has relied mostly on fluoride in hair, fingernails or toenails, urine, saliva, blood, bone and enamel as a biomarker of exposure [National Research Council, 2006]. However, most do not meet all the criteria necessary for measuring fluoride exposures over a substantial proportion of someone's lifetime. Tooth enamel acquires fluoride systemically and topically, and it is not possible to separate the two except in studies of unerupted third molars. Urine, saliva, plasma, hair and fingernails have been established as fluoride intake markers reflecting periods of hours to months, depending on which is used. Bone is the best marker for periods of a few years, depending on the age of the subject [Whitford, 2000]. It is regularly remodeled, more quickly in children than adults, especially older adults. However, bone biopsies are invasive and not practical for epidemiological studies. Thus, we are left without a useful, relatively long-term marker for fluoride uptake, especially in children during the time that the permanent teeth are susceptible to fluorosis.
Fluoride concentration of dentin has been suggested as a biomarker for cumulative fluoride exposures [Selwitz, 1994; WHO Expert Committee on Oral Health Status and Fluoride Use, 1994]. Dentin is a promising candidate because similar to bone, it continues to form throughout life but does not normally undergo physiologic resorption. Unlike enamel, it is not affected by topical fluoride exposures. Fluoride concentration of the dentin of permanent teeth of lifelong residents is correlated with the fluoride concentration of the community water supply [Jackson and Weidmann, 1959; Cutress et al., 1996] and consumption of high-fluoride containing foods [Elliott and Smith, 1960]. Dentin fluoride also is correlated with enamel fluorosis, known to be related to systematic exposures during certain periods of a child's life, while enamel fluoride shows a more variable correlation [Richards et al., 1989; Vieira et al., 2004]. Additionally, dentin fluoride concentrations increase with age [Jackson and Weidmann, 1959; Elliott and Smith, 1960; Nakagaki et al., 1987; Kato et al., 1997], suggesting accumulation from exposures within the dentin.
Primary tooth dentin in particular might be useful for monitoring fluoride exposures because unlike permanent teeth, primary teeth can be collected easily. Recent studies have used dentin fluoride in permanent teeth as a biomarker for cumulative fluoride exposures to study enamel fluorosis [Vieria et al., 2004] and bone fluoride concentration [Vieria et al., 2005]. Yet no study has compared lifetime systemic fluoride intake from multiple sources with dentin fluoride concentration, an analysis that is necessary to determine if dentin fluoride might serve as a valid biomarker of fluoride exposures. If cumulative fluoride exposures and other confounding or effect-modifying variables can explain a large proportion of the variation in dentin fluoride, then primary teeth might provide a valid biomarker of fluoride exposure during early and middle childhood. The purpose of this study is to characterize the fluoride concentration of exfoliated primary tooth dentin by determining its association with lifetime fluoride exposures up to the time of exfoliation and how it varies according to other variables that might affect the association.
Materials and Methods
The research was approved by the Institutional Review Board for the protection of human subjects at the University of North Carolina at Chapel Hill.
Exfoliated primary maxillary central incisors and mandibular second molars were collected from convenience samples of kindergarten (5 and 6 years of age) and fifth grade (10 and 11 years of age) students, respectively, living in four communities in North Carolina in 1995. One community with less than optimal fluoride concentration (<0.3 mg/l) in its drinking water (Hendersonville, elevation 656 m) was selected, one with an optimal amount of fluoride (1.0 mg/l; Lenoir, elevation 356 m) and one with fluoride (0.5 mg/l) below optimal levels (Jackson, elevation 31 m). A second fluoridated (1.0 mg/l) community (Asheville, elevation 650 m) was selected because a previous study had shown a higher than expected prevalence of fluorosis in this community and we hypothesized that dentin fluoride levels should be higher there than the other fluoridated community as well [Lalumandier and Rozier, 1995]. In each community, information describing the study, questionnaires and consent forms were distributed to parents of all public school students in the selected grades. Parents of enrolled parent-child dyads completed a questionnaire at enrollment and mailed their child's tooth to investigators at the time of its exfoliation. Teeth that showed evidence of caries or coronal dentin resorption were not included in the study.
Sample Preparation and Fluoride Assay of Dentin
A single 300 μm-thick section was obtained from each tooth using a Gillings-Hamco thin sectioning machine. Buccal-lingual sections were taken at the midpoint of the maximum mesial-distal distance width of incisors. Buccal-lingual sections of molars were taken perpendicular to the enamel surface of the mesial-buccal cusp at the height of facial convexity. With the aid of a dissecting microscope, any remaining root structure in the section was cut from the crown at the level of the cervical extension of the enamel using a scalpel. The coronal dentin was then dissected from the enamel using a dissection microscope and micro-scalpels, oven dried at 100°C for 24 h to remove water and reach a constant weight, weighed, and dissolved in 2 N HCL. Three aliquots of the acid solution in which each sample was dissolved were assayed separately for fluoride as described by Bawden et al. [1989], a microdiffusion method that avoids electrode interference when assaying for fluoride that can result when proteins in a body fluid coat the electrode. The values for the three fluoride assays for any section were within ±1% of the mean value for that section. Therefore, the mean value of the three assay results for each section was used as the value for that section.
Main Sources of Fluoride Exposure
From 80 to 90% of fluoride intake is from water and beverages, toothpaste and fluoride supplement use [Levy et al., 2001]. Information on the donor children's fluoride exposure to these major sources was obtained from the parents via self-completed questionnaires. Information from the questionnaire on each child's place(s) of residence and length of residency in each place since birth was used to estimate exposure to fluoride in drinking water. The Fluoridation Census [Centers for Disease Control and Prevention, 1993] provided the fluoride concentration of public water supplies. For cases where public water supplies were not currently used, a sample of drinking water was obtained and analyzed for fluoride concentration. We then used a published model of fluid consumption and average body weight during various stages of life [Shulman et al., 1995] to estimate cumulative fluoride intake from drinking water alone in mg/kg body weight (b.w.) for each child from birth up to the age when the tooth was exfoliated.
Fluoride ingestion from fluoridated toothpaste was calculated using age at which the child began brushing and brushing frequency (times per day). Fluoride ingested per brushing episode was estimated using the mean amount (mg) of fluoride ingested at different ages [Naccache et al., 1992]. This value was divided by the estimated average weight of the child at each age range to obtain estimates of exposure in mg/kg b.w. for each brushing episode. We then totaled the episode estimates for the time from age that brushing was initiated until the tooth was exfoliated, to obtain an estimate of cumulative fluoride intake from brushing. An estimate of cumulative fluoride exposure from supplements was obtained in a similar manner using the 1979 American Dental Association guidelines for fluoride supplementation [American Dental Association, 1979].
Other Sources of Fluoride Exposure
Frequent consumption of fish, soda, and tea, foods and beverages possibly high in fluoride concentration, were self-reported in a brief dietary survey included in the parent questionnaire. Intake frequencies classified by parents as ‘a lot’ for each source were coded as a binary variable (yes vs. no). The fluoride concentration of the mother's drinking water at the time that the baby was born was used to create a binary variable for prenatal exposure to optimal fluoride levels (≥0.7 mg/l vs. other). Number of months of breastfeeding and a question on whether or not the child was bottle fed were included to estimate how early the child was exposed to fluoride from water sources. Use of fluoride mouthrinse in school and at home was recorded and also coded as a binary variable (any use vs. none). We reasoned that coding of these variables as binary would allow the detection of any effect of these other fluoride sources on dentin fluoride concentration without placing undue weight on a variable with multiple values that would increase the implied precision in measurement of use.
Control Variables
Illnesses and certain dietary practices such as vegetarianism can cause chronic acid-base disturbances in the body, affect fluoride levels in blood plasma, and consequently alter tissue concentrations of fluoride [Whitford, 1996]. Nonwhites and females can have earlier maturation and tooth eruption [Infante, 1974], which may affect the amount of fluoride that is retained within the teeth because the majority of the coronal dentin will already have been formed before the child begins using fluoride products. Demographic characteristics also can influence factors such as fluid intake [Sohn et al., 2001] and fluoride toothpaste use [Nourjah et al., 1994]. We chose to question parents about any prolonged illnesses of their children, vegetarianism, and two demographic variables (race and sex). Only one respondent reported a major illness and two a vegetarian diet, so these variables were excluded from the analysis. As a final control for fluoride exposures, we considered community of residence. Use of such a variable should control for any other potential unmeasured fluoride exposures associated with geographic locations, such as fluoride diffusion effects in which the benefits of water fluoridation are exported to nonfluoridated communities through beverages and foods manufactured in fluoridated communities, that are not accounted for in our individual-level assessments of ingestion [Griffin et al., 2001].
Statistical Methods
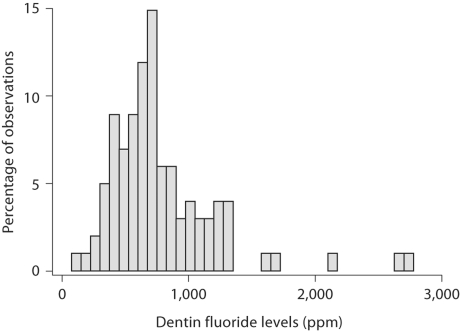
For descriptive analyses, we tested the association of dentin fluoride concentration with cumulative fluoride from the main fluoride sources (water, toothpaste, supplements) as well as with total fluoride from the three sources using Pearson's product moment correlation coefficient. To aid in interpretation of associations, we also created binary variables for the three primary fluoride sources and tested their associations as well as other variables, which were all binary, with dentin fluoride using ANOVA and a 0.05 level of significance. For these analyses, we dichotomized mg/kg b.w. of fluoride exposure into optimal or not (≥0.04 mg/kg/day vs. no) using the lower value of optimal fluoride exposure as described by Whitford [1994]. Optimal toothpaste and supplement use were defined according to frequency as at least 2 times per day or 5 days per week, respectively. For multivariable analyses, the log transformation of dentin fluoride was used to normalize the distribution of the dependent variable (fig. 1). Our primary analytical strategy was to use ordinary least squares regression to determine the association between dentin fluoride and lifetime fluoride exposures to drinking water, supplements and toothpaste, controlling for other variables (prenatal fluoride in drinking water, fluoride rinse, frequent tea, fish or soda consumption, sex, race and current community of residence), all coded 1 vs. 0.
Fig. 1.
Frequency distribution for all observations in parts per million.
We began the multivariable analysis with a base analytical model that included the main variables for fluoride exposure and geographic location. Because of sample size considerations, other control variables were added to the model one at a time. Variables with a significance level of p > 0.1 that decreased the adjusted R2 values were eliminated from the final models. Incisors and molars were analyzed separately because of differences in their life cycles and resulting fluoride exposures. Each model was examined for omitted variable bias using the Ramsey RESET test [Ramsey and Schmidt, 1976] and heteroskedasticity using the Cook-Weisberg test [Cook and Weisberg, 1982] and a significance level of 5%. These two tests yielded insignificant findings.
Results
Each of a total of 108 children provided a single primary tooth for fluoride analysis. Nine parents did not complete questionnaires, so these observations were removed leaving 99 observations (61 incisors; 38 molars). Summary statistics are presented in table 1. The average dentin fluoride concentrations per tooth were 792 mg/kg (SD 402) for incisors and 768 mg/kg (SD 489) for molars.
Table 1.
Summary descriptive statistics for fluoride concentration of dentin and fluoride exposure and control variables by tooth type
| Variables | Incisors (n = 61) |
Molars (n = 38) |
||||||
|---|---|---|---|---|---|---|---|---|
| mean | SD | min. | max. | mean | SD | min. | max. | |
| Response variables | ||||||||
| Dentin fluoride concentration, mg/kg | 792 | 402 | 106 | 2,753 | 768 | 489 | 231 | 2,699 |
| Log of dentin fluoride concentration | 6.56 | 0.48 | 4.66 | 7.92 | 6.49 | 0.54 | 5.44 | 7.90 |
| Explanatory variables | ||||||||
| Water fluoride exposure, mg/kg b.w.a | 83.3 | 55.6 | 6.03 | 185 | 122 | 93.1 | 11.1 | 242 |
| Toothpaste fluoride exposure, mg/kg b.w.a | 51.2 | 24.3 | 0 | 96.2 | 67.6 | 31.8 | 0 | 124 |
| Supplement fluoride exposure, mg/kg b.w.a | 10.6 | 20.9 | 0 | 90.4 | 14.0 | 32.7 | 0 | 172 |
| Optimal prenatal fluoride (60.7 mg/l) | 0.53 | 0.50 | 0 | 1 | 0.40 | 0.50 | 0 | 1 |
| School fluoride rinse (ever used) | 0.51 | 0.50 | 0 | 1 | 0.63 | 0.49 | 0 | 1 |
| Frequent tea consumption | 0.22 | 0.42 | 0 | 1 | 0.26 | 0.45 | 0 | 1 |
| Frequent soda consumption | 0.20 | 0.40 | 0 | 1 | 0.53 | 0.51 | 0 | 1 |
| Frequent fish consumption | 0.08 | 0.28 | 0 | 1 | 0.05 | 0.23 | 0 | 1 |
| Bottle fed | 0.80 | 0.40 | 0 | 1 | 0.81 | 0.39 | 0 | 1 |
| Demographic variables | ||||||||
| Female | 0.54 | 0.50 | 0 | 1 | 0.47 | 0.51 | 0 | 1 |
| Nonwhite | 0.08 | 0.28 | 0 | 1 | 0.13 | 0.34 | 0 | 1 |
| Asheville resident | 0.33 | 0.47 | 0 | 1 | 0.26 | 0.45 | 0 | 1 |
| Hendersonville resident | 0.33 | 0.47 | 0 | 1 | 0.24 | 0.43 | 0 | 1 |
| Lenoir resident | 0.28 | 0.45 | 0 | 1 | 0.37 | 0.49 | 0 | 1 |
| Jackson resident | 0.06 | 0.25 | 0 | 1 | 0.13 | 0.34 | 0 | 1 |
Values represent estimated cumulative exposure from birth to time of tooth exfoliation.
Dentin fluoride was positively correlated with lifetime total fluoride from water, toothpaste and supplements for all teeth (r = +0.198; p < 0.05), incisors (r = +0.116; p > 0.1) and molars (r = +0.336; p = 0.05). None of the correlations between dentin fluoride in incisors and lifetime fluoride from water (r = +0.095), toothpaste (r = +0.059) or supplements (r = −0.027) were statistically significant. For molars, only the association with supplements (r = +0.593; p < 0.01) was statistically significant (water: r = +0.093; toothpaste: r = +0.060).
Bivariate analyses of associations between dentin fluoride levels and dichotomized variables are presented in table 2. Of the main fluoride exposure variables (water, toothpaste and supplements), only subjects with optimal exposure to fluoridated water had increased mean fluoride concentration in dentin compared to those without optimal exposures, but this difference was not statistically significant for either incisors or molars. The only statistically significant difference in mean fluoride concentration was among geographic locations. Molar teeth from Asheville had a significantly higher fluoride concentration, on average, than other locations (p < 0.001). Teeth from Jackson had lower fluoride concentrations than other locations, on average, but this difference was significant only for incisors (p < 0.01).
Table 2.
Bivariate analysis of predictor variables and dentin fluoride concentration, mg/kg by tooth type
| Category | Incisors | Molars | ||||
|---|---|---|---|---|---|---|
| n | mean (95% CI) | n | mean (95% CI) | |||
| Fluoride exposures | Optimal water (average ≥ 0.04 mg/kg/day) | No | 29 | 763 (587-938) | 24 | 739 (497-982) |
| Yes | 32 | 819 (695-944) | 14 | 816(637-994) | ||
| Optimal toothpaste (at least 2 times per day) | No | 39 | 792 (652-932) | 28 | 817(609-1,025) | |
| Yes | 22 | 792 (635-949) | 10 | 629 (413-844) | ||
| Optimal supplements (at least 5 days per week) | No | 58 | 792 (686-898) | 37 | 715 (591-840) | |
| Yes | 3 | 793 (−373 to 1,959) | 1 | 2,699 (−) | ||
| Prenatal fluoride (water ≥0.7 mg/1) | No | 29 | 728 (610-845) | 23 | 736 (482-991) | |
| Yes | 32 | 851 (682-1,020) | 15 | 816 (654-977) | ||
| School fluoride rinse (ever used) | No | 30 | 766 (590-942) | 14 | 765 (491-1,039) | |
| Yes | 31 | 818 (698-938) | 24 | 769 (554-983) | ||
| Diet | Frequent tea consumption | No | 47 | 780 (659-901) | 28 | 682 (498-866) |
| Yes | 14 | 833 (613-1,054) | 10 | 1,007 (667-1,346) | ||
| Frequent soda consumption | No | 49 | 813 (689-936) | 18 | 850(531-1,169) | |
| Yes | 12 | 710 (542-878) | 20 | 694(557-830) | ||
| Frequent fish consumption | No | 56 | 778 (673-884) | 36 | 774 (605-943) | |
| Yes | 5 | 950(311-1,589) | 2 | 651 (−1,522 to 2,824) | ||
| Demographics | Race | White | 56 | 801 (692-910) | 33 | 816 (638-993) |
| Nonwhite | 5 | 694(253-1,135) | 5 | 450 (144-755) | ||
| Sex | Male | 28 | 796 (618-973) | 20 | 708 (572-845) | |
| Female | 33 | 589 (663-915) | 18 | 833(512-1,155) | ||
| Residency city | Asheville | 20 | 770 (644-895) | 10 | 1,204(711-1,698)∗∗ | |
| Lenoir | 17 | 818(612-1023) | 14 | 586 (436-737) | ||
| Jackson | 4 | 372 (200-543)∗ | 5 | 398 (222-575) | ||
| Hendersonville | 20 | 878(640-1,115) | 9 | 769 (564-975) | ||
Significant at the 5% level;
Significant at the 0.1% level.
The results of the regression analyses are presented in table 3. The regression model for incisors accounted for a small proportion of variation in dentin fluoride (10.5%). It included only one significant variable, residence in Jackson, which resulted in a decrease in dentin fluoride of 68% (p < 0.05) compared to residents of the optimally fluoridated town of Lenoir. The best fitting regression model for molars accounted for 60.3% of the variance in dentin fluoride. Fluoride supplements (p < 0.001) and frequent tea consumption (p < 0.01) were significant in predicting increased molar dentin fluoride levels. Living in Asheville increased predicted molar dentin fluoride by 53% compared to the optimally fluoridated town of Lenoir (p < 0.001). Molar dentin fluoride levels in Hendersonville did not differ from Lenoir, but Jackson residents had less fluoride by a statistically nonsignificant amount (p < 0.07).
Table 3.
Results of ordinary least squares regression analysis of log(dentin fluoride concentration) by tooth type
| Incisors (n = 61) |
Molars (n = 38) |
|||
|---|---|---|---|---|
| effect | SE | effect | SE | |
| Intercept | 6.3604∗∗∗ | 0.2546 | 6.1923∗∗∗ | 0.1866 |
| Water fluoride exposure (mg/kg b.w.)a | 0.0018 | 0.0017 | 0.0001 | 0.0008 |
| Toothpaste fluoride exposure (mg/kgb.w.)a | 0.0003 | 0.0025 | −0.0013 | 0.0018 |
| Supplement fluoride exposure (mg/kg b.w.)a | −0.0016 | 0.0036 | 0.0071∗∗∗ | 0.0019 |
| Asheville resident (vs. Lenoir) | −0.0794 | 0.1539 | 0.5336∗∗∗ | 0.1518 |
| Jackson resident (vs. Lenoir) | −0.6822∗ | 0.2776 | −0.3460∗ | 0.1887 |
| Hendersonville resident (vs. Lenoir) | 0.2752 | 0.2136 | 0.2395 | 0.1563 |
| Frequent fish consumption | 0.3501 | 0.2221 | ||
| Frequent tea consumption | 0.4728∗∗ | 0.1429 | ||
| Adjusted R2 | 0.1051 | 0.6031 | ||
Statistically significant at the 5% level
statistically significant at the 1% level
statistically significant at the 0.1% level.
Values represent estimated cumulative exposure from birth to time of tooth exfoliation.
Discussion
This study is the first to quantify fluoride exposures from multiple sources and relate them to fluoride concentration in dentin of primary teeth using multivariable analysis techniques. Early studies of primary tooth dentin fluoride concentration were hampered by the inability to detect low levels of fluoride in samples because of crude assay techniques or incomplete statistical analysis. Almost all studies had very small sample sizes. Furthermore, most of these studies did not include fluoride intake from sources other than drinking water. Nevertheless, the majority of studies have shown that the fluoride concentration of primary tooth dentin is greater, on average, for teeth from individuals who are exposed to higher levels of dietary fluoride or optimally fluoridated water. Our study found that dentin fluoride regressed on cumulative lifetime fluoride intake estimated from exposures to three primary sources (water, toothpaste, and supplements), selected secondary fluoride sources, and demographic control variables explained a relatively large percentage of the variance in analytical regression models for molars, but not incisors. In light of our overall findings and those of previous investigators, primary tooth dentin appears to have potential as a biomarker for chronic fluoride intake.
Two aspects of our findings qualify this conclusion, however, and suggest that continued research is necessary. First, only one of the two tooth types performed adequately as a biomarker using the coefficient of determination as the primary measure of success. The incisor analytical model predicted dentin fluoride poorly. This finding may be because primary incisors complete formation soonest [Logan and Kronfeld, 1933], so the majority of incisor coronal dentin may have been formed by the time the child is exposed to fluoride from diet, supplements or toothpaste. This finding also may be associated with difficulties in obtaining accurate samples of incisor teeth for testing of fluoride concentrations. The coronal dentin of incisors is thinner from the dentin-enamel junction to the pulpal surface than it is for molar dentin. Therefore, the pulpal area of incisor dentin makes up a greater proportion of the total dentin sample than it does for molars. Also, their smaller size makes dissection of incisors more difficult than molars, and increases the probability of losing a portion of the sample from the dentin-enamel junction area. Finally, the occlusal surfaces of primary teeth, particularly those of maxillary incisors, are prone to wear, which can remove some dentin [Warren et al., 2002]. For these reasons, incisor dentin samples may have contained a greater proportion of total dentin from the fluoride-rich pulpal region than molars, artificially inflating dentin fluoride concentrations and biasing results toward the null.
A second aspect of this study that influences any conclusions about the potential of dentin fluoride to serve as a biomarker is the finding that estimated fluoride intake demonstrated only a weak association with dentin fluoride. Although we found total fluoride ingestion to be associated with dentin fluoride at a statistically significant level for incisors and molars combined in the bivariate analysis, we did not find this association in models analyzed by tooth type. Further, of the separate exposures estimated for the three major sources of fluoride, only dietary fluoride supplements were associated with dentin fluoride in the final regression models at a statistically significant level. Confidence in the validity of fluoride dentin as a biomarker for chronic fluoride ingestion would be increased if fluoride intake from pathways known to be major sources of fluoride had been associated with dentin fluoride levels.
Several factors could explain the lack of definitive findings of an association between fluoride in water or toothpaste and dentin fluoride. The American diet is complex, particularly during the first few years of life, and fluoride can be incorporated into the diet through multiple pathways. Fluoride intake by infants depends almost entirely on their diet and how they are fed. Human breast milk or cow's milk contains little fluoride (<0.07 mg/l), while soy milk has much more (0.5 mg/l) [National Research Council, 2006]. Since 1979, manufacturers have attempted to decrease the amount of fluoride in soy-based infant formulas, but ready-to-feed soy formula still contains more fluoride (0.30 mg/l) than milk-based formula (0.17 mg/l). The concentration of fluoride in infant formulas depends primarily on whether it is reconstituted with fluoridated water or not. More than 80% of total fluoride exposure in infants living in a fluoridated community can come from drinking water [National Research Council, 2006]. Our questionnaire only asked if the child was bottlefed, so we were unable to include in our estimates the wide degree of variation in dietary fluoride exposure that can occur between ready-to-feed milk-based formula compared to powdered soy formula that is reconstituted with fluoridated water [Fomon et al., 2000].
Drinking water alone contributes much less to the total intake of fluoride in children than it does in infants, and it can provide as little as 20–30% of total fluoride ingestion between the ages of 36 and 72 months [Levy et al., 2003]. The amount of fluoride from water alone can depend on the use of bottled water and home purification systems. However, most bottled water at the time of this study contained little fluoride, although variable [Van Winkle et al., 1995]. Another 35% of total fluoride ingestion can come from other beverages [Levy et al., 2003]. Pang et al. [1992] reported the fluoride concentration of sodas, juices, punches, tea, and Gatorade available for purchase in North Carolina to be highly variable (<0.1 to 6.7 mg/l), but to constitute 58.8, 62.8, and 59.6% of total liquid consumption among 2- to 3-, 4- to 6- and 7- to 10-year-olds, respectively, thus potentially being a major source of fluoride. Fluoride ingestion from foods or beverages depends on whether it is prepared with fluoridated water or not [Jackson et al., 2002]. On average, toothpaste is a major source of fluoride in children, estimated to contribute from about 30% [Levy et al., 2003] to 60% [Maguire et al., 2007; Rojas-Sanchez et al., 1999] of total fluoride ingestion.
The different sources of fluoride and the choices that parents and children make about diet and use of dental products can result in substantial variation in fluoride intake [Levy et al., 2003]. This individual variability can lead to overlap in the amount of fluoride ingestion among individuals living in fluoridated and nonfluoridated communities. Maguire et al. [2007] estimated total fluoride ingestion at 0.736 mg per day among 6- to 7-year-old children with nonfluoridated home drinking water and 1.043 mg per day among those with optimally fluoridated home drinking water, differences that are much smaller than would be expected based on earlier studies [Ophaug et al., 1985]. They also found that total fluoride intake was not correlated with the concentration of fluoride in individuals’ drinking water. Rojas-Sanchez et al. [1999] found no difference in total fluoride intake in 1- to 3-year-old children residing in a nonfluoridated community (0.073 mg/kg/day) compared to a fluoridated community (0.070 mg/kg/day). In a third study that relied on secondary data to estimate a mathematical model of fluoride intake based on a framework suggested by the US Environmental Protection Agency, Erdal and Buchanan [2005] found total fluoride intake to be identical (0.06 mg/kg/day) in 3- to 5-year-old children in nonfluoridated and fluoridated communities. Unlike in the past, residency status in a fluoridated community appears to be weakly correlated with fluoride ingestion.
The complex and changing diet, multiple pathways for exposure that make fluoride available in fluoridated and nonfluoridated communities and the choices the public makes can lead to substantial difficulties in accurately estimating fluoride exposures for individuals. Erdal and Buchanan [2005] concluded that a ‘moderate to high’ level of uncertainty is associated with estimating fluoride ingestion from known pathways for infants and children living in fluoridated and nonfluoridated communities, particularly from beverages, foods, infant formula and toothpaste ingestion. We used estimates of fluoride ingestion from water that are based on a national survey of food frequencies [Shulman et al., 1995]. Their estimates of mg fluoride intake per kg of body weight per day seem to have face validity because they are similar to findings from other studies of fluoride intake. However, construction of lifetime fluoride exposures in our study used parent-reported residency histories to determine exposures to fluoride in drinking water and the application of average intakes derived from the model of Shulman et al. [1995] to the individuals included in this study. These estimates are subject to recall bias and do not account for the potential for large individual variability in fluoride ingestion.
Geographic location explained a larger percentage of the variation in dentin fluoride than did fluoride exposures from what we considered to be the major sources. Many studies demonstrate that the characteristics of where people live can influence health outcomes independent of their individual characteristics [Kawachi and Berkman, 2003]. Only recently have studies of oral health outcomes been done using analytical methods that permit separation of individual risk factors from those associated with place of residency. They show that characteristics of where people live can be important over and above the characteristics of individuals who live in these areas [Tellez et al., 2006; Turrell et al., 2007]. The four communities included in our study could differ in environmental, cultural, dietary or healthcare features that result in large variation in total fluoride ingestion across communities.
Except in areas with heavy pollution, environmental exposures from air and soil result in only about 5–10% of total ingestion [US Environmental Protection Agency, 1988]. Three of the four communities (Asheville, Hendersonville, and Lenoir) are within 60 miles of each other, and are located in the Great Smokey Mountains (GSM) or its foothills. This area was chosen because a previous study found a high prevalence of fluorosis in one of the cities (Asheville) and surrounding counties that could not be explained completely by water fluoridation, dentifrice use or dietary fluoride supplements [Lalumandier and Rozier, 1995]. The fourth community (Jackson) is located 300 miles east of the other three. The GSM are located downwind of coal-burning power plants that generate large amounts of air pollutants [Shaver et al., 1994] that make exposures the highest in the eastern United States [Mueller, 1994; US Environmental Protection Agency, 2001]. Additionally, the GSM area is surrounded by numerous aluminum, glass, ceramic, fiberglass or chemical manufacturing industries, which release high amounts of fluoride emissions as well. Although a potential environmental source of large amounts of fluoride, the fluoride concentration of air or soil is unknown in the study areas.
Altitude is another environmental factor that has been shown to affect plasma fluoride concentration. Increased altitude decreases metabolism and excretion of ingested fluoride, and thereby increases the fluoride concentration of mineralized tissues [Whitford, 1996]. Existing studies of this phenomenon have examined differences due to altitudes much higher than those in this study, so the effects of relatively small variations in altitude seen in our study (31 m for Jackson vs. 656 m for Hendersonville) are unknown.
The major limitation of this study is that fluoride exposures are based on self-report of parents without any validation. Therefore, we do not know the extent to which results might be influenced by recall biases. Further, age-specific, lifetime fluoride ingestion from water was estimated using the fluoridation status of the drinking water for each child's reported place of residence and published estimates for the average daily dose of fluoride, which themselves were based on average daily fluid consumption in the American diet. As already discussed, these aggregate estimates do not account for the large individual variation in fluoride ingestion reported in the literature that might be independent of residence in a fluoridation community. Basing fluoride ingestion on the fluoridation status of drinking water can introduce inaccuracies because drinking water alone contributes much less fluoride to the diet of many children than it did before the widespread incorporation of commercially processed foods and beverages into the diet.
The small sample size, particularly for some of the locations, prevented us from fully testing the effects of some variables on dentin fluoride. The strong effect of location implies that these communities have unique characteristics that are important determinants of dentin fluoride independent of individual characteristics. However, we were unable to use multilevel modeling techniques to explore the separate contributions of individual and community level variables to dentin fluoride. Characteristics of individuals that are omitted from our regression models might further bias our results. At least two studies have demonstrated that socioeconomic status, which was not included in our study, can affect fluoride ingestion [Rojas-Sanchez et al., 1999; Levy et al., 2003].
To summarize, the association between fluoride exposures and dentin fluoride was weak in this study, particularly for incisor teeth. The lack of an effect of fluoride exposures in water and toothpaste on dentin fluoride was unexpected. However, the regression model for molars accounted for a large proportion of the variance in dentin fluoride, which seems to indicate that primary molar dentin may be a promising biomarker for fluoride exposure. This study suggests that a major source of variability in dentin fluoride is the community itself, independent of water fluoridation status. Future studies will need to account for community residency status, which will require a large sample to separate individual and community level contributions to dentin fluoride.
Validation of dentin fluoride as a biomarker for lifetime fluoride ingestion may be difficult, particularly if the study accounts for the potential for community variation. Such a study will require accurate measurement of longitudinal exposures to fluoride from a complex array of pathways that can have large individual variability. No gold standard for total fluoride exposure is available that can be used to validate dentin fluoride as a biomarker of cumulative lifetime exposures, particularly one that is easily obtainable for a large population over a number of years. These challenges in validating dentin fluoride as a biomarker of cumulative lifetime fluoride exposures increase the significance of findings from this study, which is the first to examine multiple factors predicting dentin fluoride.
Acknowledgements
We acknowledge the contributions of T.G. Deaton who conducted the fluoride assays of dentin. This investigation was supported in part by Grant No. R01-DE11294 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD.
References
- American Dental Association, Council on Dental Therapeutics . Accepted Dental Therapeutics. ed 38. Chicago: American Dental Association; 1979. Fluoride compounds; pp. 316–338. [Google Scholar]
- Bawden JW, Deaton TG, Koch GG, Crawford BP. Effect of an acute maternal fluoride dose on fetal plasma fluoride levels and enamel fluoride uptake in guinea pigs. J Dent Res. 1989;68:1169–1172. doi: 10.1177/00220345890680070601. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Neri M, Puntoni R. Validation of biomarkers as early predictors of disease. Mutat Res. 2001;481:349–358. doi: 10.1016/s0027-5107(01)00194-4. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Recommendations for using fluoride to prevent and control dental caries in the United States. MMWR Recomm Rep. 2001;50:1–42. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Fluoridation Census. Atlanta: US Department of Health & Human Services, Public Health Service, Division of Oral Health; 1993. [Google Scholar]
- Cook RD, Weisberg S. Residuals and Influence in Regression. New York: Chapman & Hall; 1982. [Google Scholar]
- Cutress TW, Coote GE, Shu M, Pearce EIF. Fluoride content of the enamel and dentine of human premolars prior to and following the introduction of fluoridation in New Zealand. Caries Res. 1996;30:204–212. doi: 10.1159/000262161. [DOI] [PubMed] [Google Scholar]
- Elliot CG, Smith MD. Dietary fluoride related to fluoride content of teeth. J Dent Res. 1960;39:93–98. doi: 10.1177/00220345600390012601. [DOI] [PubMed] [Google Scholar]
- Erdal S, Buchanan SN. A quantitative look at fluorosis, fluoride exposure, and intake in children using a health risk assessment approach. Environ Health Perspect. 2005;113:111–117. doi: 10.1289/ehp.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomon SJ, Ekstrand J, Ziegler EE. Fluoride intake and prevalence of dental fluorosis: trends in fluoride intake with special attention to infants. J Public Health Dent. 2000;60:131–146. doi: 10.1111/j.1752-7325.2000.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Griffin SO, Gooch BF, Lockwood SA, Tomar SL. Quantifying the diffused benefit from water fluoridation in the United States. Community Dent Oral Epidemiol. 2001;29:120–129. doi: 10.1034/j.1600-0528.2001.290206.x. [DOI] [PubMed] [Google Scholar]
- Infante PF. Sex differences in the chronology of deciduous tooth emergence in white and black children. J Dent Res. 1974;53:418–421. doi: 10.1177/00220345740530024001. [DOI] [PubMed] [Google Scholar]
- Jackson RD, Brizendine EJ, Kelly SA, Hinesley R, Stookey GK, Dunipace AJ. The fluoride content of foods and beverages from negligibly and optimally fluoridated communities. Community Dent Oral Epidemiol. 2002;30:382–391. doi: 10.1034/j.1600-0528.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- Jackson D, Weidmann SM. The relationship between age and the fluorine content of human dentine and enamel: a regional survey. Br Dent J. 1959;107:303–306. [Google Scholar]
- Kato S, Nakagaki H, Toyama Y, et al. Fluoride profiles in the cementum and root dentine of human permanent anterior teeth extracted from adult residents in a naturally fluoridated and a non-fluoridated area. Gerodontology. 1997;14:1–8. doi: 10.1111/j.1741-2358.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Berkman LF. Neighborhoods and Health. New York: Oxford University Press; 2003. [Google Scholar]
- Kleerekoper M. Non-dental tissue effects of fluoride. Adv Dent Res. 1994;8:32–38. doi: 10.1177/08959374940080010801. [DOI] [PubMed] [Google Scholar]
- Lalumandier JA, Rozier RG. The prevalence and risk factors of fluorosis among patients in a pediatric dental practice. Pediatr Dent. 1995;17:19–25. [PubMed] [Google Scholar]
- Levy SM, Warren JJ, Broffitt B. Patterns of fluoride intake from 36–72 months of age. J Public Health Dent. 2003;63:211–220. doi: 10.1111/j.1752-7325.2003.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Levy SM, Warren JJ, Davis DS, Kirchner HL, Kanellis MJ, Wefel JS. Patterns of fluoride intake from birth to 36 months. J Public Health Dent. 2001;61:70–77. doi: 10.1111/j.1752-7325.2001.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Logan WHG, Kronfeld R. Development of the human jaws and surrounding structures from birth to the age of fifteen years. J Am Dent Assoc. 1933;20:379–427. [Google Scholar]
- Maguire A, Zohouri FV, Hindmarch PN, Hatts J, Moynihan PJ. Fluoride intake and urinary excretion in 6- to 7-year-old children living in optimally, sub-optimally and non-fluoridated areas. Community Dent Oral Epidemiol. 2007;35:479–488. doi: 10.1111/j.1600-0528.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- Mueller SF. Characterization of ambient ozone levels in the Great Smoky Mountains National Park. J Appl Meterolol. 1994;33:465–472. [Google Scholar]
- Naccache H, Simard PL, Trahan L, et al. Factors affecting the ingestion of fluoride dentifrice by children. J Public Health Dent. 1992;52:222–226. doi: 10.1111/j.1752-7325.1992.tb02277.x. [DOI] [PubMed] [Google Scholar]
- Nakagaki H, Koyama Y, Sakakibara Y, Weatherell JA, Robinson C. Distribution of fluoride across human dental enamel, dentine and cementum. Arch Oral Biol. 1987;32:651–654. doi: 10.1016/0003-9969(87)90039-2. [DOI] [PubMed] [Google Scholar]
- National Research Council, Committee on Fluoride in Drinking Water . Fluoride in Drinking Water: A Scientific Review of EPA's Standards. Washington: National Academies Press; 2006. available at: http://books.nap.edu/catalog/11571.html. [Google Scholar]
- Nourjah P, Horowitz AM, Wagener DK. Factors associated with the use of fluoride supplements and fluoride dentifrice by infants and toddlers. J Public Health Dent. 1994;54:47–54. doi: 10.1111/j.1752-7325.1994.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Ophaug RH, Singer L, Harland BF. Dietary fluoride intake of 6-month and 2-year-old children in four dietary regions of the United States. Am J Clin Nutr. 1985;42:701–707. doi: 10.1093/ajcn/42.4.701. [DOI] [PubMed] [Google Scholar]
- Pang DTY, Phillips CL, Bawden JW. Fluoride intake from beverage consumption in a sample of North Carolina children. J Dent Res. 1992;71:1382–1388. doi: 10.1177/00220345920710070601. [DOI] [PubMed] [Google Scholar]
- Ramsey JB, Schmidt B. Some further results on the use of OLS and BLUS residuals in specification error tests. J Am Stat Assoc. 1976;71:389–390. [Google Scholar]
- Richards A, Fejerskov O, Baelum V. Enamel fluoride in relation to severity of human dental fluorosis. Adv Dent Res. 1989;3:147–153. doi: 10.1177/08959374890030021301. [DOI] [PubMed] [Google Scholar]
- Rojas-Sanchez F, Kelly SA, Drake KM, Eckert GJ, Stoodey GK, Dunipace AJ. Fluoride intake from foods, beverages and dentifrice by young children in communities with negligibly and optimally fluoridated water: a pilot study. Community Dent Oral Epidemiol. 1999;27:288–297. doi: 10.1111/j.1600-0528.1998.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Selwitz RH. Summary of session III: strategies for improving methods of assessing fluoride accumulation in body fluids and tissues. J Dent Res. 1994;8:111–112. doi: 10.1177/08959374940080010301. [DOI] [PubMed] [Google Scholar]
- Shaver CL, Tonnessen KA, Maniero TG. Clearing the air at Great Smoky Mountains National Park. Ecol Appl. 1994;4:690–701. [Google Scholar]
- Shulman JD, Lalumandier JA, Grabenstein JD. The average daily dose of fluoride: a model based on fluid consumption. Pediatr Dent. 1995;17:13–18. [PubMed] [Google Scholar]
- Sohn W, Heller KE, Burt BA. Fluid consumption related to climate among children in the United States. J Public Health Dent. 2001;61:99–106. doi: 10.1111/j.1752-7325.2001.tb03373.x. [DOI] [PubMed] [Google Scholar]
- Tellez M, Sohn W, Burt BA, Ismail AI. Assessment of the relationship between neighborhood characteristics and dental caries severity among low-income African-Americans: a multilevel approach. J Public Health Dent. 2006;66:30–36. doi: 10.1111/j.1752-7325.2006.tb02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrell G, Sanders AE, Slade GD, Spencer AJ, Marcenes W. The independent contribution of neighborhood disadvantage and individual-level socioeconomic position to self-reported oral health: a multilevel analysis. Community Dent Oral Epidemiol. 2007;35:195–206. doi: 10.1111/j.1600-0528.2006.00311.x. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency: Summary Review of Health Effects Associated with Hydrogen Fluoride and Related Compounds. Health Issue Assessment. EPA/ 600/8-89/002F. Research Triangle Park, Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development, US Environmental Protection Agency, 1988.
- US Environmental Protection Agency: Latest findings on national air quality: 2000 status and trends. EPA 454/K-01-002. Research Triangle Park, Office of Air Quality, Planning and Standards, US Environmental Protection Agency, 2001.
- Van Winkle S, Levy SM, Kiritsy MC, Heilman JR, Wefel JS, Marshall T. Water and formula fluoride concentrations: significance for infants fed formula. Pediatr Dent. 1995;17:305–310. [PubMed] [Google Scholar]
- Vieira A, Hancock R, Limeback H, Maia R, Grynpas MD. Is fluoride concentration in dentin and enamel a good indicator of dental fluorosis? J Dent Res. 2004;83:76–80. doi: 10.1177/154405910408300115. [DOI] [PubMed] [Google Scholar]
- Vieira AP, Mousny M, Maia R, Hancock R, Everett ET, Grynpas MD. Assessment of teeth as biomarkers for skeletal fluoride exposure. Osteoporos Int. 2005;16:1576–1582. doi: 10.1007/s00198-005-1870-z. [DOI] [PubMed] [Google Scholar]
- Warren JJ, Yonezu T, Bishara SE. Tooth wear patterns in the deciduous dentition. Am J Orthod Dentofacial Orthop. 2002;122:614–618. doi: 10.1067/mod.2002.129193. [DOI] [PubMed] [Google Scholar]
- Whitford GM. Intake and metabolism of fluoride. Adv Dent Res. 1994;8:5–14. doi: 10.1177/08959374940080011001. [DOI] [PubMed] [Google Scholar]
- Whitford GM. The Metabolism and Toxicity of Fluoride, ed 2 rev. Basel: Karger; 1996. [PubMed] [Google Scholar]
- Whitford GM. International collaborative research on fluoride. J Dent Res. 2000;79:893–904. doi: 10.1177/00220345000790040301. [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee on Oral Health Status and Fluoride Use Fluorides and oral health. World Health Organ Tech Rep Ser. 1994;846:1–37. [PubMed] [Google Scholar]